Introduction
Multicentric carpotarsal osteolysis (MTCO) syndrome is a rare skeletal disorder characterized by osteolysis, or bone resorption, particularly affecting the carpal and tarsal bones. It is frequently associated with generalized osteoporosis, scoliosis, and protrusio acetabuli, with destruction of larger joints including the elbows and hips, and can be associated with progressive renal failure [3, 14, 17]. Because its initial presentation typically includes joint pain and swelling that can mimic symptoms of juvenile idiopathic arthritis (JIA), patients may be misdiagnosed and treated with anti-rheumatic therapy, which has no effect on the progressive joint destruction [17].
This autosomal dominant disorder is attributed to missense mutations in the gene for MAFB (v-maf musculoaponeurotic fibrosarcoma oncogene ortholog B), a transcription factor that plays an important role in bone remodeling through the regulation of osteoclast generation [18]. Osteoclasts are multinucleated cells formed by fusion of mononuclear precursors of the monocyte/macrophage lineage at or near the bone surface, and they have the important function of bone resorption [2]. One of the cytokines essential for the development and activation of osteoclasts is receptor activator of nuclear factor kB ligand (RANKL), which is expressed on the surface of osteoblasts and other cells and binds to RANK, a transmembrane receptor on the surface of osteoclasts and their precursors [1, 2]. Normally, MAFB inhibits osteoclast generation mediated by RANKL; however, this negative control is lost in MAFB mutations, leading to increased osteoclastogenesis and bone resorption [1, 18]. Therapy using anti-RANKL antibody to treat MCTO has been proposed but its use in this setting has not been reported in the literature.
Since the initial discovery of mutations of the MAFB gene, additional cases confirmed by genetic testing have been reported [6, 9, 11, 13]. The literature regarding surgical management for severe osteolysis is sparse [4, 9]. We here report a case of an adolescent patient with MTCO undergoing complicated hip replacement surgery.
Case Report
Our patient was born in Wenzhou, China. At age 8 years, she developed pain and decreased range of motion in her wrists, elbows and shoulders, and scoliosis. At age 12 years, she underwent multiple medical and surgical consultations in the USA and was found to have severe thoracic scoliosis and severe Chiari I malformation with herniation and underwent decompression and spinal fusion from T2 to L3. During her preoperative workup, she was found to have mild dilatation of the aortic root and a restrictive pattern on pulmonary function tests without parenchymal lung disease. She also had mild central obstructive sleep apnea, bilateral renal cysts, and non-nephrotic range proteinuria. Radiographs at age 14 years revealed bony deformities of various joints including both hips and both elbows. The wrist joints were severely affected and exhibited notable osteopenia imaging (Fig. 1a–b). Rheumatologic workup revealed 2+ ANA, low anticardiolipin antibody titers, and the differential diagnosis of JIA, infection, or skeletal dysplasia was considered. She was later seen by a geneticist, and a clinical diagnosis of MCTO was made. The patient and her parents underwent genetic testing for mutation in the MAFB gene, and a heterozygous mutation c.176C>T, Prot59Leu was detected in the patient but not her parents.
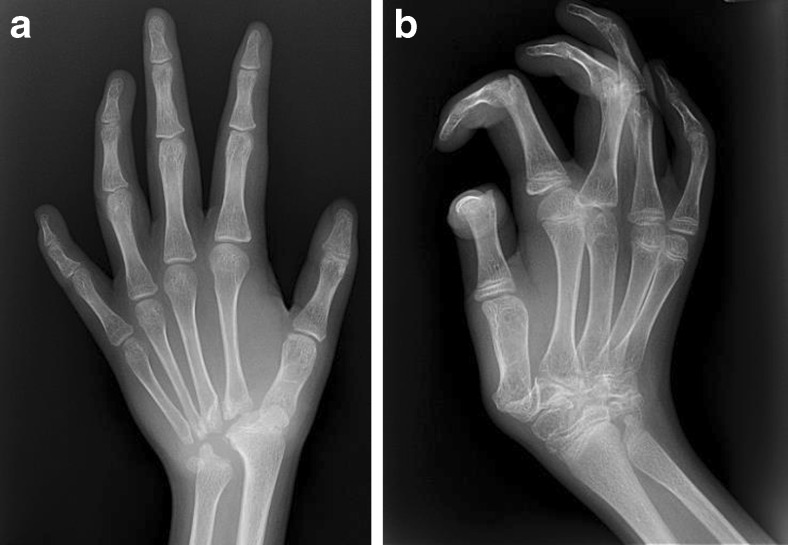
Fig. 1.
a–b Hand X-rays showing osteolysis of the carpus with relative sparing of the digits characteristic of multifocal tarsal carpal osteolysis (MTCO).
At age 16 years, the patient presented to the Combined Arthritis Program (CAP) with progressive right hip pain over 2 years and an associated limb-length discrepancy. She was attending school and was interviewed through an interpreter as she spoke only Mandarin. Despite a marked limp, she was not using assistive devices. She was referred to physical therapy which provided no relief of her hip pain. Due to the known association of MCTO syndrome with nephropathy, the use of nonsteroidal anti-inflammatory therapy was limited.
Her physical exam was notable for a thin female of Chinese ancestry with short stature, and she appeared younger than stated age and appeared of normal cognition. Her height was 1.41 m and weighed 24.9 kg with a BMI of 12.5. She ambulated without any assistive devices, and her gait demonstrated an adductor lurch pattern. Patient was flexible, but did not demonstrate Wynne-Davies signs of hyperlaxity. She had limb-length inequality of 4 cm on clinical exam. The range of motion of the right hip was 0–90° of flexion and extension with no flexion contracture. Right hip internal rotation was to 30° and external rotation to 60°. Her right hip passive abduction was limited to 10°, and adduction was similarly limited to 10°. The unaffected left hip had normal range of motion. She was only able to perform a straight leg raise to 6 in off the exam table with associated pain. Patient had a normal neurovascular exam of the lower extremities.
The remainder of her physical examination revealed mid-face hypoplasia, exophthalmos, micrognathia, limited neck range of motion following cervical fusion for Chiari malformation, and Tanner 1 secondary sex characteristics. Her upper extremity exam was significant for right elbow radiocapitellar joint congenital dislocation, bilateral wrist ulnar deviation, camptodactyly of right index and long fingers, and brachydactyly of right index and small fingers. Her right elbow range of motion was severely limited, and her hands appeared narrow with limited motion, especially at the proximal interphalangeal joints of the index and long fingers.
There is no family history of rheumatologic disease, metabolic bone disease, or other boney deformities, but her family history is significant for consanguinity; her maternal grandparents are first cousins.
Her radiographs and advanced imaging of the right hip revealed a marked dilation of the acetabulum with a noncongruent and diminutive femoral head (Fig. 2). The femoral head was displaced proximally by 30 mm. In addition to the femoral head deformity, the femoral neck was gracile, and the femoral diaphyseal cortical diameter of the right hip was approximately 50% of the left side. The acetabulum had remarkable deformity. The acetabulum was dilated with thin, sclerotic walls. Kohler’s line was violated and there was acetabular protrusio. The acetabular rim anterior-posterior diameter was 33 mm using axial computed tomography imaging (Fig. 3). A limb length inequality of 1.8 cm was seen on lower extremity radiographs when measured from the center of femoral head to midpoint of the tibial plafond.
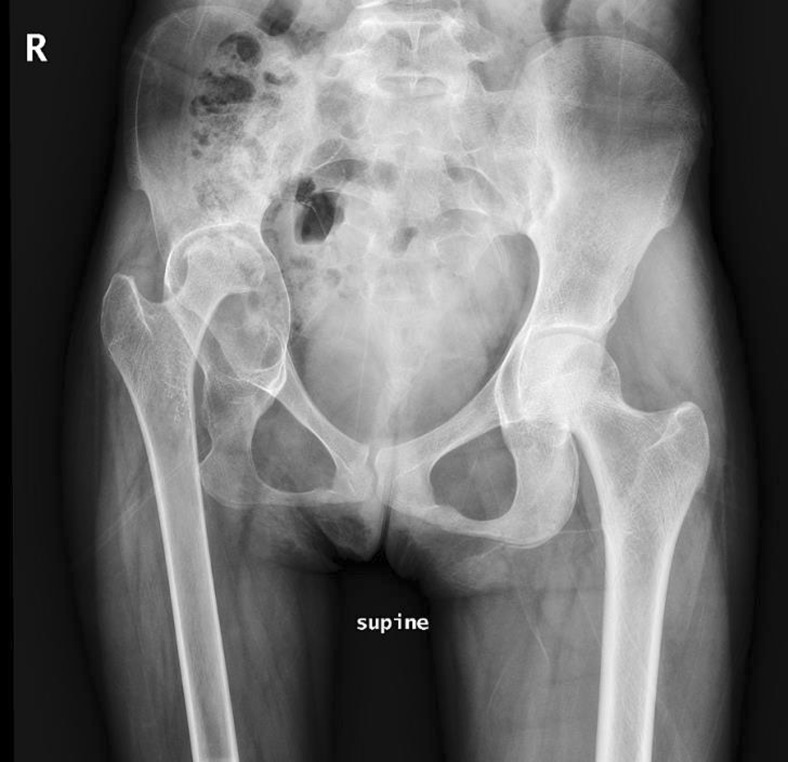
Fig. 2.
Radiograph of the pelvis demonstrating a severely arthritic right hip and severe acetabular protrusion with a markedly dilated acetabulum and a diminutive femoral head.
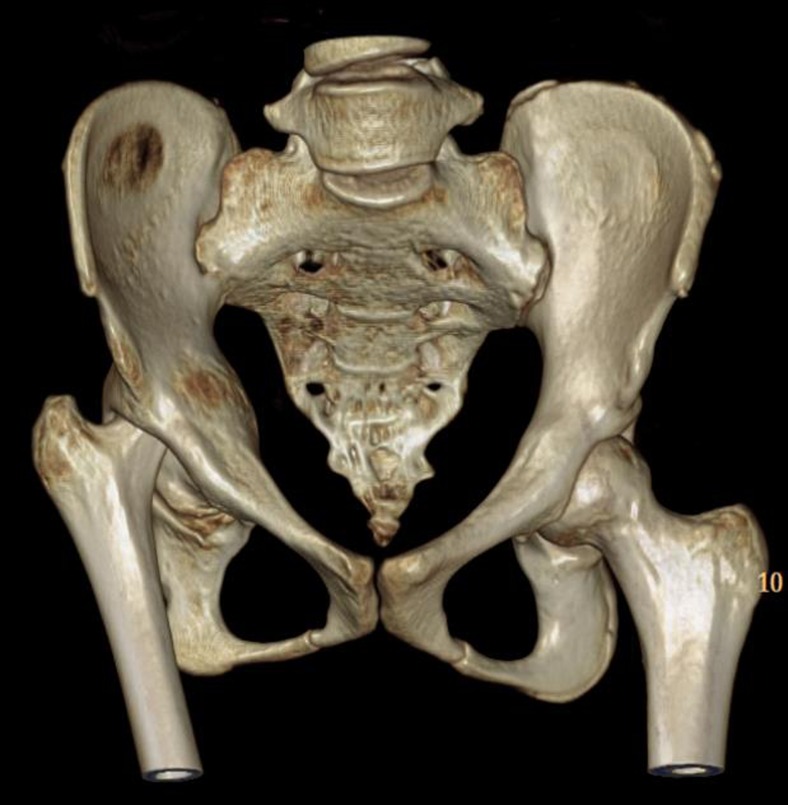
Fig. 3.
Three-dimensional CT reconstruction of the pelvis and femur documents the severe acetabular protrusion with markedly dilated acetabular cavity and diminutive and deformed femoral head.
Surgical Planning
Preoperative radiographs were used to plan the surgery (Fig. 4). Determining the center of the acetabulum was difficult given the marked deformity. The contralateral normal hip center was used to determine the center of rotation. Exam under anesthesia revealed flexion to 120°, internal rotation to 30°, and external rotation to 45° which was significantly different from the clinic exam.
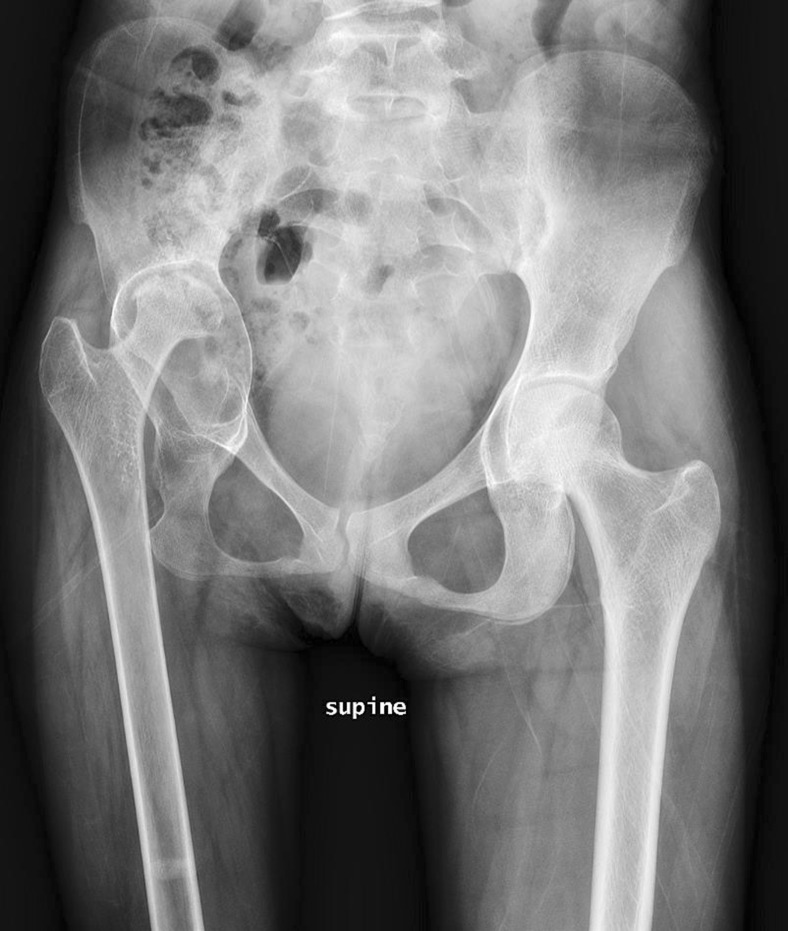
Fig. 4.
Preoperative AP pelvis radiograph.
A standard posterior approach was used to access the hip joint. An extensive soft tissue release was performed around the acetabular rim. The joint was unable to be safely dislocated due to the incarceration of the femoral head within the voluminous acetabulum; therefore, an in situ neck cut was made at which point the femoral head was removed. The diameter of the acetabular opening was 42 mm which then ballooned into a larger cavity. The rim of the acetabulum was reamed to 44 mm.
As the native femoral head was too small to fill the large acetabular defect, an allograft femoral head was placed into the cavernous acetabulum to return the hip center to a near normal position. Acetabular reamers were used to remove the cartilage from the femoral head and to size it appropriately to fit within the mouth of the acetabulum. Once the femoral head was appropriately sized, the very thin acetabulum was then gently reamed to expose cancellous bone. The allograft femoral head was then placed into the acetabulum. The femoral head was secured with two 4.5-mm cancellous screws; the first was placed in a lag technique from the acetabulum into the inferior femoral head, and a second anti-rotation, positional screw was placed from the edge of the acetabulum into the superior dome of the allograft femoral head and into the posterior column. The femoral head allograft was then reamed to form a hemispheric bone bed in which a Smith and Nephew (Memphis, TN) R3 cluster hole hemispherical cementless acetabular component was impacted. Two Smith and Nephew 6.5 mm Reflection screws were placed through the acetabular component and into the anterior column and ilium, respectively. A Smith and Nephew 0° XLPE highly cross-linked polyethylene liner was then placed into the cup.
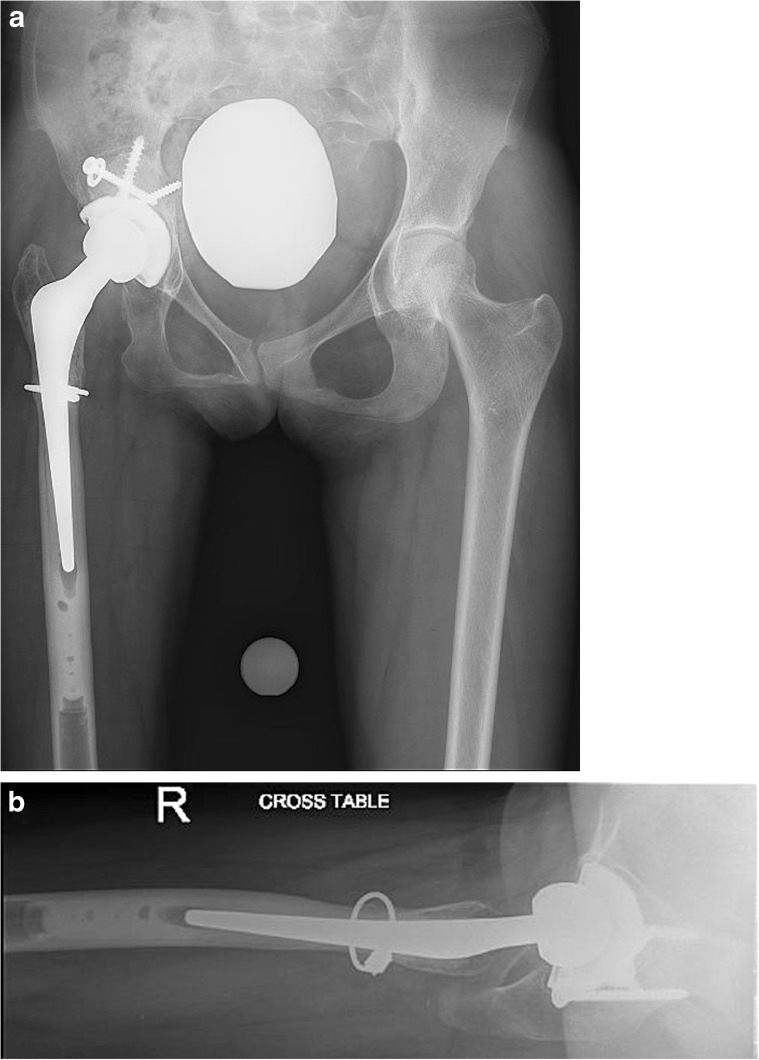
The acetabular retractors were removed, and attention was turned to the femur. The cut surface of the femoral neck was exceedingly small. A 5-mm Steinmann pin was used to instrument the canal and identify the center of the femur. The femur was broached and a Smith and Nephew extra-small cemented, collarless, polished stem (CPCS) was placed into the femur, and a trial reduction was performed. The anterior soft tissues were noted to be exceedingly tight at this point, and the decision was made to perform a subtrochanteric shortening osteotomy. A 2 cm shortening osteotomy was performed with a small sagittal saw to make two transverse cuts followed by a connecting longitudinal cut to split the intervening fragment. After the osteotomy fragment was split longitudinally, the femur shortened. The two halves of the osteotomy fragment were closed over the osteotomy site and secured with Stryker Dall-Miles 2 mm beaded cables. The CPCS stem was placed into the femur, and another trial reduction was performed now with acceptable soft tissue tension. A cement restrictor placed and using modern cement technique, the canal was filled and pressurized with cement with care to ensure no cement extruded from the osteotomy. The extra small CPCS stem was inserted with 10° of anteversion. A 28 mm, +0 Oxinium™ head was then placed and the joint was reduced. The soft tissues were repaired and the wound closed in layers. Aspirin was used for venous thromboembolism prophylaxis. The patient’s weight bearing was restricted for 6 weeks until radiographic evidence of osteotomy healing. The patient recovered uneventfully and had no perioperative complications. When the patient was seen 3 months following surgery, she had been weaned from all assistive devices, with excellent range of motion, and no pain. Radiographs at the 1-year follow up (Fig. 5a–b) revealed a well-healed subtrochanteric osteotomy, incorporation of the acetabular bone graft, and stable femoral and acetabular components with an unchanged cement mantle. At 1-year follow up, she had complete pain relief and no instability. She did not use any assistive devices and walked without a limp. The patient and her family were very satisfied with the results of the procedure.
Fig. 5.
a–b These AP and Lateral radiographs obtained 1 year following surgery demonstrate a well-healed subtrochanteric osteotomy, incorporation of the acetabular bone graft, and stable fixation of the femoral and acetabular components.
Discussion
MCTO is a disorder of progressive bone resorption predominantly though not exclusively of the carpal and tarsal bones, which often leads to crippling joint deformities. It usually begins in early childhood with joint pain and swelling, which may lead to a misdiagnosis of JIA. MCTO may subside spontaneously after puberty [14]. Skeletal trauma or surgery has been reported to incite osteolysis [15, 16]. Case reports in the literature of synovial tissue biopsy usually show no evidence of an inflammatory process with relatively normal histology of the bone and cartilage [7, 8, 11, 16].
Despite genetic homogeneity, there is considerable clinical variability, suggesting the possibility of yet unidentified modifier gene(s) [6, 11]. Affected individuals can have subtle craniofacial abnormalities such as in our patient and commonly develop nephropathy which may progress to end stage renal failure. Reported renal biopsies have shown focal and segmental glomerulosclerosis [3, 5, 14]. The presence of Arnold-Chiari malformation in a patient with the MAFB mutation in MCTO has been described in one other case [6]. Both inherited and sporadic cases of MCTO have been reported.
A heterozygous missense genetic mutation in a single exon gene MAFB was discovered as the cause for MCTO in 2012 by Zankl et al using exome sequencing [18]. MAFB codes for a member of the V-MAF musculoaponeurotic fibrosarcoma oncogene family of transcription factors. It is known to play a critical role in both osteoclast differentiation and activation [10] and podocyte differentiation and renal tubule survival [12]. MAFB negatively regulates RANKL-induced osteoclastogenesis, and therefore, reduction of MAFB expression enhances osteoclastogenesis leading to bone resorption, and RANKL further downregulates expression of MAFB [10, 13]. In case reports, bisphosphonates have not been shown to stop the progression of osteolysis but appear to slow progression and allow better mineralization of the adjacent bones [17]. Since the development of denosumab, an anti-RANKL antibody therapy, its use in this condition has been hypothesized but has not been reported in literature.
In summary, orthopedic surgeons or rheumatologists may be the first to recognize MCTO in a patient with joint pain or deformity. Recognition of typical osteolysis on radiographs in the setting of negative rheumatologic workup and early referral to a clinical geneticist are keys to making a timely diagnosis. Options for medical therapy are limited; however, the need for appropriate screening and monitoring of comorbidities, particularly renal disease, must be recognized and accounted for in the perioperative management of these patients. Surgical interventions are challenging due to the altered anatomy, but are often necessary to restore function, which underscores the need for appropriate referral for surgical care. Our patient underwent right total hip replacement, and early follow up visits demonstrate successful healing without complications, incorporating bone graft, and no evidence of increased osteolysis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Compliance with Ethical Standards
Conflict of Interest
Kai Sun, MD, Brian Barlow, MD, Fardina Malik, MD, Allan Inglis, MD, Mark Figgie, MD and Susan Goodman, MD have declared that they have no conflict of interest.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was waived from all patients for being included in the study.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Work was performed at Hospital for Special Surgery.
References
- 1.Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5(3):98–104. doi: 10.1007/s11914-007-0024-y. [DOI] [PubMed] [Google Scholar]
- 2.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael KD, Launikitis RA, Kalia A. The orthopedic and renal manifestations of idiopathic carpal tarsal osteolysis. J Pediatr Orthop B. 2007;16(6):451–454. doi: 10.1097/BPB.0b013e3282e1c667. [DOI] [PubMed] [Google Scholar]
- 4.Chalidis BE, Dimitriou CG. Intercalary bone grafting for the reconstruction of phalangeal osteolysis in disappearing bone disease: case report. J Hand Surg. 2008;33(10):1873–1877. doi: 10.1016/j.jhsa.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Connor A, Highton J, Hung NA, et al. Multicentric carpal-tarsal osteolysis with nephropathy treated successfully with cyclosporine A: a case report and literature review. Am J Kidney Dis. 2007;50(4):649–654. doi: 10.1053/j.ajkd.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Dworschak GC, Draaken M, Hilger A, et al. An incompletely penetrant novel MAFB (p.Ser56Phe) variant in autosomal dominant multicentric carpotarsal osteolysis syndrome. Int J Mol Med. 2013;32(1):174–178. doi: 10.3892/ijmm.2013.1373. [DOI] [PubMed] [Google Scholar]
- 7.Faber MR, Verlaak R, Fiselier TJ, et al. Inherited multicentric osteolysis with carpal-tarsal localisation mimicking juvenile idiopathic arthritis. Eur J Pediatr. 2004;163(10):612–618. doi: 10.1007/s00431-004-1502-1. [DOI] [PubMed] [Google Scholar]
- 8.Gluck J, Miller JJ., 3rd Familial osteolysis of the carpal and tarsal bones. J Pediatr. 1972;81(3):506–510. doi: 10.1016/S0022-3476(72)80177-X. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb CA, Steffen JA, Whyte MP. Idiopathic multicentric osteolysis: upper extremity manifestations and surgical considerations during childhood. J Hand Surg. 2012;37(8):1677–1683. doi: 10.1016/j.jhsa.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Kim K, Kim JH, Lee J, et al. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109(8):3253–3259. doi: 10.1182/blood-2006-09-048249. [DOI] [PubMed] [Google Scholar]
- 11.Mehawej C, Courcet JB, Baujat G, et al. The identification of MAFB mutations in eight patients with multicentric carpo-tarsal osteolysis supports genetic homogeneity but clinical variability. Am J Med Genet A. 2013;161A(12):3023–3029. doi: 10.1002/ajmg.a.36151. [DOI] [PubMed] [Google Scholar]
- 12.Moriguchi T, Hamada M, Morito N, et al. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol. 2006;26(15):5715–5727. doi: 10.1128/MCB.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mumm S, Huskey M, Duan S, et al. Multicentric carpotarsal osteolysis syndrome is caused by only a few domain-specific mutations in MAFB, a negative regulator of RANKL-induced osteoclastogenesis. Am J Med Genet A. 2014;164A(9):2287–2293. doi: 10.1002/ajmg.a.36641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muyshondt I, Lateur L, Van Roost G, Maes B. Osteolysis induced by AV-fistula in idiopathic carpotarsal osteolysis. Nephrol Dial Transplant. 2003;18(10):2185–2188. doi: 10.1093/ndt/gfg331. [DOI] [PubMed] [Google Scholar]
- 15.Urlus M, Roosen P, Lammens J, et al. Carpo-tarsal osteolysis. Case report and review of the literature. Genet Couns. 1993;4(1):25–36. [PubMed] [Google Scholar]
- 16.Vichi GF, Falcini F, Pierattelli M, Jenuso R, Ceruso M. Case report 401: idiopathic carpal/tarsal osteolysis (ICTO) associated with nephropathy. Skelet Radiol. 1986;15(8):665–671. doi: 10.1007/BF00349866. [DOI] [PubMed] [Google Scholar]
- 17.Wenkert D, Mumm S, Wiegand SM, McAlister WH, Whyte MP. Absence of MMP2 mutation in idiopathic multicentric osteolysis with nephropathy. Clin Orthop Relat Res. 2007;462:80–86. doi: 10.1097/BLO.0b013e3180d09db8. [DOI] [PubMed] [Google Scholar]
- 18.Zankl A, Duncan EL, Leo PJ, et al. Multicentric carpotarsal osteolysis is caused by mutations clustering in the amino-terminal transcriptional activation domain of MAFB. Am J Hum Genet. 2012;90(3):494–501. doi: 10.1016/j.ajhg.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)