Abstract
Background
Stiffness complaints after total knee arthroplasty (TKA) are frequent, yet poorly understood and can be challenging for surgeons to address. The WOMAC stiffness subscale is a widely used measure of stiffness and can serve as a simple screening tool for complaints.
Questions/Purposes
We aimed to identify a threshold for stiffness complaints on the WOMAC stiffness subscale and investigate its overlap with range of motion (ROM) in TKA patients.
Methods
TKA patients were enrolled preoperatively and followed for 6 months. At follow-up, patients reported their ROM, completed the WOMAC stiffness subscale (range 1–8 with 8 continuous stiffness) and indicated whether they experienced more stiffness than expected. To identify a threshold for complaints, we compared patients’ WOMAC stiffness scores to when they experienced more stiffness than expected, visually, and statistically. We also mapped ROM limitations at 6 months to WOMAC stiffness scores. Finally, we determined if baseline characteristics were associated with stiffness complaints.
Results
Two hundred and forty-six TKA patients were enrolled preoperatively with 82% follow-up rate at 6 months. Our results showed that patients with a WOMAC stiffness score = 3+ were significantly more likely to experience more stiffness than expected. Patients reporting full ROM (54%) reported a wide range of WOMAC stiffness subscale scores (1–6). Baseline WOMAC pain and function scores were the only factors associated with stiffness complaints.
Conclusions
ROM is a poor surrogate of patient-reported stiffness, and the patients’ perception of “stiffness” is clearly more complex than just ROM. We identified a WOMAC threshold that could potentially easily serve this purpose.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-016-9489-5) contains supplementary material, which is available to authorized users.
Keywords: stiffness, total knee arthroplasty, WOMAC
Introduction
Surgeons typically associate stiffness after total knee arthroplasty (TKA) with limitations in range of motion (ROM) [5]. Patients do not share this clarity regarding a definition of stiffness with their surgeons and cannot consistently distinguish between clinically defined stiffness and other factors such as pain. In the first year after TKA, stiffness is a common complaint that rarely indicates a problem that requires clinical intervention. However, a persistent perception of stiffness could adversely impact patients’ outlook of their ultimate outcome and affect their satisfaction [8]. Identifying these patients early will help address their perceptions and potentially improve their outcomes.
The WOMAC stiffness subscale is the most widely used measure of patient-reported stiffness in TKA patients. This subscale is composed of only two questions and can be easily completed by patients. As such, it could serve as a simple screening tool for patient concerns about stiffness. However, because it has a limited range of score values (0–8 on the raw score or 12.5 point increments on 0–100 converted score), its ability to detect granular changes in stiffness is limited. In fact, studies investigating the psychometric properties of the WOMAC function (17 questions), pain (five questions), and stiffness (two questions) subscales found that the WOMAC stiffness subscale has high internal consistency, similar to the two other subscales, yet, unlike the other two scales, had low reliability and responsiveness [9]. As a result, many TKA studies focus on the WOMAC function and pain subscales and do not report the WOMAC stiffness subscale score.
One alternative potential approach to interpreting the WOMAC stiffness subscale score is to identify a threshold score for patients’ concern with their stiffness. This dichotomy may provide a more meaningful interpretation of this subscale score and enhance its utility in clinical practice. In this paper, we aimed to determine a threshold score on the WOMAC stiffness subscale for a patient’s concern with stiffness. We also investigated the overlap between this threshold and reporting limitations in range of motion and determined the preoperative predictors of patient-reported stiffness.
Patients and Methods
This is a cohort study nested within our institutional TKA registry. We prospectively recruited 246 patients scheduled to undergo primary unilateral TKA after obtaining written informed consent. We included patients 18 years of age or older and excluded patients with cognitive deficits.
To recruit patients for the registry, patients scheduled for surgery were approached when they returned to the hospital for a required preoperative screening day which primarily involved blood tests and medical examinations. Patients who agreed to participate were enrolled at that time and subsequently completed a series of baseline assessments, including questions about demographics, the Knee Injury Osteoarthritis Outcomes Score (KOOS) [11, 12], the expectations survey [6, 7], the SF-36 [16–18], and the lower extremity activity scale [13]. The KOOS is a knee-specific patient-reported survey that has the Western Ontario and McMasters Universities Arthritis Index (WOMAC) [1] three subscales which are stiffness (range 0–8, 8 highest level of stiffness), function (0–68, 68 lowest level of function), and pain (0–20, 20 highest level of pain), in addition to symptom subscale, quality of life, and sports activity subscales [11, 12]. Expectations were captured using the validated Hospital for Special Surgery (HSS) 19-item TKA expectations survey, which was developed to evaluate expectations of the different aspects of the recovery including pain relief, walking, the ability to perform personal, recreational, and social activities of daily living and psychological well-being [7]. A patient expectation score ranges from 0 to 100 with 100 being the highest expectation of returning back to normal in all aspects [7]. The SF-36 is a general health status measure that has eight domains and two summary component scores, the physical component score and the mental component score. The lower extremity activity score (LEAS) is a validated activity scale for knee replacement patients.
At 6 months postoperatively, patients completed the KOOS and reported whether or not they had “more joint stiffness than they would like” in their replaced joint. Patients also reported whether they felt weakness and persistent pain in their operated knee. Using the KOOS, we calculated the 6 months WOMAC stiffness subscale score for each patient and derived information about range of motion. Active full range of motion was defined from the KOOS symptoms subscale as reporting being able to fully straighten and bend the replaced knee.
To determine a threshold on the WOMAC stiffness subscale at which patients start complaining about stiffness, we compared the WOMAC stiffness scores against an anchor question about whether patients had “more joint stiffness than they would like” in their replaced joint. The threshold was visually examined and statistically evaluated using the receiver operating characteristic (ROC) curve to evaluate the diagnostic ability of the WOMAC stiffness threshold in discriminating between those who expressed stiffness versus those who did not [4]. An area under the ROC curve of 0.5 indicates that the threshold is not better than chance in predicting complaints and an area of 1 indicates that the threshold perfectly predicts when a patient complains. We also calculated the sensitivity and specificity of the threshold. We then explored the association of this threshold with the WOMAC function and pain subscale scores as well as with perceptions of weakness and persistent pain to evaluate validity of the threshold. Second, we determined the concordance between achieving the threshold and reporting limitations in range of motion using the kappa statistic. Third, we used baseline data to determine characteristics associated with patient-reported stiffness. We compared the two groups on age, sex, baseline SF-36 PCS and MCS scores, baseline WOMAC stiffness, function, and pain scores, LEAS scores, and patient expectations. We also compared them on their Charlson comorbidity index score [3] and body mass index calculated from height and weight. Information about comorbidity and height and weight were abstracted from patients’ charts. ANOVA and chi-squared tests were used to determine differences among groups. We conducted analyses separately for patients who had ROM limitations and those who had full ROM to determine if ROM limitations affect our results. All analyses were conducted in SPSS 19. The study was approved by the Institutional Review Board at our institution.
Results
The mean age of the 246 recruited patients was 66.9 ± 9.5 years (range, 27–91 years) and women represented 65.4% of the study cohort. The mean BMI for the cohort was 29.9 ± 5.7 and approximately a quarter of patients (23.6%) lived alone. Two hundred and one patients (82%) completed questionnaires at 6 months.
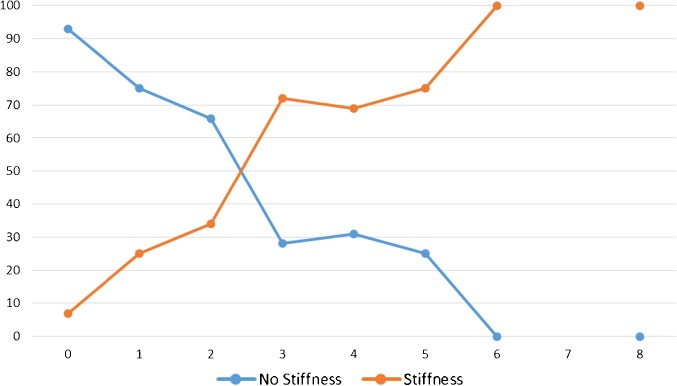
At follow-up, the mean WOMAC stiffness score was 2.1 ± 1.6 with a score range 0–8. 40.1% of the patients reported more stiffness than they would like. Figure 1 plots having more stiffness than one likes against the WOMAC stiffness subscale scores and shows that the likelihood of reporting more stiffness than one likes increases significantly at a threshold score of 3. The area under the ROC curve for this threshold was 0.74 [CI 0.67–0.81], P < 0.001, indicating fair predictive value. The threshold had sensitivity =84% and specificity = 61%. This threshold was associated with a significantly worse WOMAC pain and function scores (Table 1). In addition, this threshold was associated with a significantly increased likelihood of reporting muscle weakness and persistent pain.
Fig. 1.
Perception of stiffness more than expected vs. WOMAC stiffness subscale scores. Footnote: No patients reported a WOMAC stiffness score value of 7.
Table 1.
Differences at 6 months in pain, function, and weakness between TKR patients with WOMAC stiffness subscale score = 3+ and those with score < 3
| Factor | WOMAC stiffness = 3+ | WOMAC stiffness < 3 | P value | |
|---|---|---|---|---|
| Effect sizea | ||||
| WOMAC pain (0–20), mean ± SD | 5.6 ± 3.1 | 1.7 ± 1.7 | 3.9 ± 0.3 | <0.001 |
| WOMAC function (0–68), mean ± SD | 18.2 ± 11.5 | 6.7 ± 6.3 | 11.5 ± 1.3 | <0.001 |
| Odds ratioa | ||||
| Weakness, N (%) | 30 (45.5%) | 21 (17.2%) | 4.0 [CI 2.0, 7.9] | <0.001 |
| Persistent pain, N (%) | 24 (37.5%) | 6 (4.9%) | 11.6 [CI 4.4, 30.4] | <0.001 |
aEffect sizes and odds ratios have been adjusted for age, sex, and the Charlson comorbidity index score
The overlap between ROM and our threshold was assessed. There were 42.6% who reported being able to always extend and flex their knee fully; however, there was little overlap between range of motion limitations and a WOMAC threshold = 3+. The kappa statistic for concordance between the two measures was 0.3, indicating fair agreement.
Baseline characteristics of patients who met the WOMAC stiffness threshold score = 3+ were compared to those with a score <3. The former group had statistically worse baseline WOMAC stiffness and pain scores compared to the latter group (Table 2). No other differences were observed. Similar results were observed when we conducted our analyses separately for patients who had ROM limitations and those who had full ROM.
Table 2.
Baseline characteristics of TKR patients with WOMAC stiffness subscale score = 3+ compared to those reporting score < 3
| Factor | WOMAC stiffness = 3+ | WOMAC stiffness < 3 | P value |
|---|---|---|---|
| Age (years), mean ± SD | 66 ± 8.6 | 67.4 ± 8.8 | 0.28 |
| Female sex, N (%) | 48 (69.6%) | 82 (65.6%) | 0.35 |
| Charlson comorbidity index score, mean ± SD | 0.5 ± 1.1 | 0.5 ± 1.0 | 0.89 |
| BMI (kg/m2), mean ± SD | 29.7 ± 5.5 | 29.6 ± 5.4 | 0.94 |
| LEAS score (1–18), mean ± SD | 9.2 ± 2.9 | 9.5 ± 3.0 | 0.45 |
| SF-36 MCS (0–100), mean ± SD | 50.3 ± 12.9 | 51.9 ± 11.3 | 0.38 |
| SF-36 PCS (0–100), mean ± SD | 33.4 ± 7.3 | 34.0 ± 8.4 | 0.61 |
| WOMAC pain (0–20), mean ± SD | 10.3 ± 2.8 | 8.8 ± 3.6 | 0.004 |
| WOMAC stiffness (0–8), mean ± SD | 4.8 ± 1.4 | 4.1 ± 1.5 | 0.001 |
| WOMAC function (0–68), mean ± SD | 33.3 ± 9.7 | 30.51 ± 1.6 | 0.09 |
| Patient expectations score (0–100), mean ± SD | 78.3 ± 17.4 | 78.3 ± 17.8 | 0.99 |
Discussion
Patient reported stiffness is a prevalent yet poorly understood phenomenon among TKA patients, in large part because of the paucity of stiffness measures and the patients’ perception of stiffness. In this study, we identified a threshold score on the widely used WOMAC stiffness subscale that can easily identify patients whose stiffness is associated with significantly more pain and functional disability. We demonstrated low concordance between patient-reported stiffness and the ROM limitations that surgeons use to identify stiffness. We also found that conventional preoperative factors were poor predictors of postoperative patient-reported stiffness.
The WOMAC stiffness subscale score is widely used in orthopedic outcomes research to assess knee stiffness. We identified a threshold on the WOMAC stiffness subscale that was associated with significant discomfort to the patient; this threshold could potentially enhance interpretability of the score and increase its utility. A WOMAC stiffness score = 3+ was associated with significantly and clinically worse WOMAC pain and function scores. This often-ignored subscale is easy to administer to patients, because it has only two questions, and thus can serve as a simple screening tool for identifying potentially disabling patient-perceived stiffness. Our proposed threshold may provide new insights into how patient-reported stiffness is analyzed in future studies; however, further validation is still needed in large more representative sample of patients, and over a longer period of follow-up to confirm our results.
This study has a number of limitations. First, range of motion was self-reported in this study using the KOOS symptoms subscale and was not clinically assessed. However, a recent study showed that there were statistically significant associations between self-reported ROM and clinically observed ROM for flexion and extension [2]. Second, we relied on one anchor question to determine the threshold for stiffness. Other measures such as a measure of tightness may be considered in the future to validate our threshold. Third, this is a one-center study. Our findings may not be generalizable to patients in other orthopedic settings such as community hospitals.
In our cohort of patients, both patient-perceived stiffness and ROM limitations were prevalent, yet there was little concordance between the two measures. This discordance highlights additional aspects of the recovery process that should be addressed. To do so, a better understanding of this phenomenon is warranted. According to our study, patients’ stiffness complaints were also associated with persistent pain and muscle weakness. Patients reporting muscle weakness may be deriving suboptimal benefit from outpatient rehabilitation post TKA, either due to poor compliance (35%) or due to non-responsiveness to prescribed rehabilitation [14, 15]. Persistent pain is less understood. Puolakka et al. [10] examined 648 primary TKA patients and found that the risk for developing persistent pain increased three to ten times when the patient reported moderate to intolerable pain during the first postoperative week.
A multi-faceted intervention may help reduce this phenomenon. TKA is designed to be functional, not normal. Emphasizing this fact to patients through their pre-operative education, and that this may result in stiffness postoperatively, may help reduce complaints of stiffness and increase patient satisfaction after TKA. Targeting the subset of patients who preoperatively have higher WOMAC pain and stiffness scores may be especially beneficial. The higher preoperative baseline WOMAC stiffness scores was associated with our threshold. In addition, based on Poulakka et al.’s findings, managing pain in the first week postoperatively, may help reduce persistent pain, and thus these complaints. Finally, surgeons should communicate preoperatively to all patients the need to be compliant with postoperative outpatient rehabilitation to address muscle weakness.
In conclusion, stiffness complaints after TKA could adversely impact patient outcomes and there is a need to better understand this phenomenon. In this paper, we have defined a threshold for assessing patient-reported stiffness using an existing widely used scale for measuring stiffness and investigated potential contributing factors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 510 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1225 kb)
(PDF 1355 kb)
Compliance with Ethical Standards
Conflict of Interest
Christina Herrero, Alejandro Gonzalez Della Valle, MD and Geoffrey H. Westrich, MD have declared that they have no conflict of interest. Hassan M.K. Ghomrawi, PhD, MPH reports institutional grants from the National Institute of Child and Human Development (National Institutes of Health career development grant [R00 HD060686]) and the Agency of Health Research and Quality through a Center for Education and Research on Therapeutics grant (CERTs; Agency of Healthcare Research and Quality RFA-HS-05-14) during the conduct of the study. Carol A. Mancuso, MD reports grants from Hospital for Special Surgery, during the conduct of the study; grants from NHLBI, AHRQ, NIA—through internal Cornell award, outside the work.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5).
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Funding
The study was partially funded by the generous donation of Mr. Glenn Bergenfield and The Sidney Milton and Leoma Simon Foundation.
Previous Presentation
Parts of this work were presented at the 2013 Knee Society closed meeting.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level I, prognostic study.
This work was performed at Hospital for Special Surgery and Weill Cornell Medical College, New York, NY, USA.
References
- 1.Bellamy N. WOMAC osteoarthritis index: a user’s guide. London: Ontario; 1995. [Google Scholar]
- 2.Collins JE, Rome BN, Daigle ME, Lerner V, Katz JN, Losina E. A Comparison of Patient-Reported and Measured Range of Motion in a Cohort of Total Knee Arthroplasty Patients. J Arthroplasty. 2014. [DOI] [PMC free article] [PubMed]
- 3.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 4.Florkowski CM. Sensitivity, specificity, receiver-operating characteristic (ROC) curves and likelihood ratios: communicating the performance of diagnostic tests. The Clinical biochemist Reviews/Australian Association of Clinical Biochemists. 2008;29(Suppl 1):S83–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Lane J. Knee joint stiffness and function following total knee arthroplasty. Edinburgh: The University of Edinburgh; 2010. [Google Scholar]
- 6.Mancuso CA, Sculco TP, Wickiewicz TL, et al. Patients’ expectations of knee surgery. The Journal of bone and joint surgery American volume. 2001;83-A(7):1005–12. doi: 10.2106/00004623-200107000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Mancuso CA, Graziano S, Briskie LM, et al. Randomized trials to modify patients’ preoperative expectations of hip and knee arthroplasties. Clinical orthopaedics and related research. 2008;466(2):424–31. doi: 10.1007/s11999-007-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manrique J, Gomez MM, Parvizi J. Stiffness after Total Knee Arthroplasty. The journal of knee surgery. 2014. doi:10.1055/s-0034-1396079. [DOI] [PubMed]
- 9.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis and rheumatism. 2001;45(5):453–61. doi: 10.1002/1529-0131(200110)45:5<453::AID-ART365>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Puolakka PA, Rorarius MG, Roviola M, et al. Persistent pain following knee arthroplasty. Eur J Anaesthesiol. 2010;27(5):455–60. doi: 10.1097/EJA.0b013e328335b31c. [DOI] [PubMed] [Google Scholar]
- 11.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos EM, Toksvig-Larsen S. Knee injury and Osteoarthritis Outcome Score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. doi: 10.1186/1477-7525-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saleh KJ, Mulhall KJ, Bershadsky B, et al. Development and validation of a lower-extremity activity scale. Use for patients treated with revision total knee arthroplasty. The Journal of bone and joint surgery American volume. 2005;87(9):1985–94. doi: 10.2106/JBJS.D.02564. [DOI] [PubMed] [Google Scholar]
- 14.Sluijs EM, Kok GJ, van der Zee J. Correlates of exercise compliance in physical therapy. Phys Ther. 1993;73(11):771–82. doi: 10.1093/ptj/73.11.771. [DOI] [PubMed] [Google Scholar]
- 15.Ulrich SD, Bhave A, Marker DR, et al. Focused rehabilitation treatment of poorly functioning total knee arthroplasties. Clin Orthop Relat Res. 2007;464:138–45. [PubMed] [Google Scholar]
- 16.Ware JE, Jr.; Kosinski, M.; Gandek, B. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: Qualitymetric Incorporated,1993, 2000,; 2005.
- 17.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Gandek B, Kosinski M, et al. The equivalence of SF-36 summary health scores estimated using standard and country-specific algorithms in 10 countries: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1167–70. doi: 10.1016/S0895-4356(98)00108-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)
(PDF 1224 kb)
(PDF 1225 kb)
(PDF 1225 kb)
(PDF 1355 kb)