Abstract
Background
Vitamin D appears to play an important role in bone and cartilage metabolism since its receptors are widely found in human articular chondrocytes. Thus, effects of variation of vitamin D may directly impact cartilage and bone biology.
Questions/Purposes
The aims of this study are to compare (1) articular cartilage structure and composition and (2) trabecular and cortical bone microstructure in rats with normal versus insufficient vitamin D levels.
Methods
Twenty-five mature, male Sprague-Dawley rats were allocated to two groups: (1) control arm (vitamin D replete—12 rats) and (2) an experimental arm (vitamin D deficient—13 rats). Vitamin D deficiency was induced using a vitamin D-deficient diet and UV light restriction. Rats were sacrificed after 4 weeks vitamin D deficiency was confirmed. The right knee was harvested for analysis of both the medial (MFC) and lateral femoral condyles (LFC). A region of interest was established on both condyles to correlate subchondral bone architecture and the overlying cartilage. Histological analysis was performed and graded using the modified Mankin score. Subchondral and cortical bony architecture was evaluated with micro-CT.
Results
After 4 weeks, the vitamin D-deficient group had statistically significant changes in cartilage structure in both the MFC and LFC [1.55 ± 0.6 vs. 4.23 ± 4.1 (p = 0.035) and 1.55 ± 0.6 vs. 3.53 ± 2.4 (p = 0.009), respectively]. Micro-CT analysis revealed no correlation between subchondral bone values and the overlying cartilage Mankin score (p = 0.460). No significant difference was evident between the subchondral bone of the control and study group.
Conclusions
Low levels of vitamin D have a deleterious effect on the cartilage.
Given the high prevalence of vitamin D deficiency in the general population, these findings raise important questions about the potential role of vitamin D in articular cartilage health.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-016-9492-x) contains supplementary material, which is available to authorized users.
Keywords: cartilage, subchondral bone, vitamin D, osteoarthritis, rat model
Introduction
Osteoarthritis (OA) is a major cause of reduced health-related quality of life [11, 14]. Over the next 25 years, the number of people affected and the social impact of diagnosed arthritis are projected to increase by 40% in the USA [22]. The etiology of osteoarthritis is multifactorial, showing strong associations with highly modifiable risk factors of mechanical overload, obesity, and joint injury. Currently, there is no cure for OA, and the development of innovative and cost-effective approaches to prevent the development and progression of OA is urgent and important.
Vitamin D appears to play a direct role in bone and cartilage metabolism [5]. Vitamin D receptors (VDRs) are found in human articular chondrocytes. Vitamin D or 1α-25(OH)2 D3 regulates the expression of metalloproteinase (MMP) and prostaglandin E2 (PGE2) in chondrocytes via VDRs [1]. MMPs are matrix-degrading enzymes that play a major role in OA. MMP-13 is thought to be the primary collagenase involved in OA pathology, with increased expression in OA [28]. Vitamin D also acts directly on the bone and could affect the ability of bone to respond optimally to pathophysiological processes in OA, and thus possibly prevent disease progression [10].
Considering these mechanisms, it is possible that vitamin D could play a role in the prevention and progression of OA [2, 13]. In this study, we hypothesized that low levels of vitamin D would affect the subchondral bone and induce subsequent degeneration of the articular cartilage.
The aims of this study were to (1) assess articular cartilage structure and composition using histological analysis of rats with normal versus insufficient vitamin D levels and (2) to compare trabecular and cortical bone microstructure using micro-CT scan in rats with normal versus insufficient vitamin D levels.
Materials and Methods
After approval from our Institutional Animal Care and Use Committee, 25 mature, male Sprague-Dawley rats (weight, 250–300 g) were allocated to two groups: (1) control arm (vitamin D replete—12 rats) and (2) an experimental arm (vitamin D deficient—13 rats). Vitamin D deficiency was induced in the experimental group using a specialized vitamin D-deficient diet (LabDiet; PMI Nutrition International, St. Louis, Missouri) and ultraviolet (UV) light restriction. Animals in the control arm (vitamin D replete) were provided with a regular diet and UV light exposure. The ultraviolet light restriction was achieved by using GAM UV filters (GAMPRODUCTS, INC., Los Angeles, California) that slide over the fluorescent lights. Animals in the control group underwent similar housing conditions with a regular diet and UV light exposure for the same time frame.
Orbital venipuncture was performed at time zero before starting the special diet and UV light restriction and 6 weeks later to confirm the induction of low levels of vitamin D. From a previous pilot study in Sprague-Dawley rats that were subjected to the same specialized vitamin D-deficient diet and UV light restriction, we demonstrated that it takes approximately 6 weeks for the rats to develop low levels of vitamin D (unpublished data).
After 6 weeks of special diet and light restriction, vitamin D deficiency was induced. After vitamin D deficiency was confirmed, animals were kept 4 more weeks before euthanasia was performed. After excluding surface fibrillation of the articular cartilage or other pathological changes, the right knee was harvested for analysis of both the medial femoral condyle (MFC) and lateral femoral condyle (LFC).
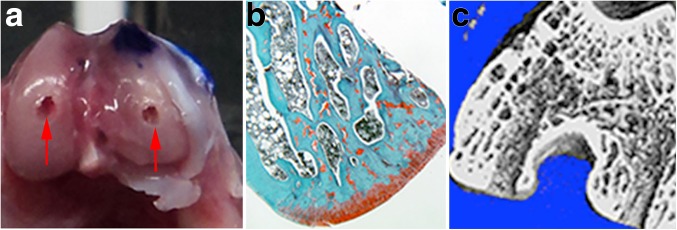
A region of interest corresponding to the weight-bearing zone was established on both condyles to correlate subchondral bone architecture and the overlying cartilage. The region of interest (ROI) was marked by drilling a 22-gauge needle through the cartilage and deep into bone in both MFC and LFC. A marking pen was also used to mark the LFC of the specimens (Fig. 1).
Fig. 1.
a Macroscopic picture illustrating the central hole (red arrows), drilled with a 22-mm needle, on both MCF (medial femoral condyle) and LFC (lateral femoral condyle). b Histological section showing the lateral femoral condyle next to the hole to determine the ROI (region of interest) of the cartilage to be analyzed. c Micro-CT showing the holes on both MFC and LFC corresponding to the histology and macroscopic pictures.
Histological Assessment
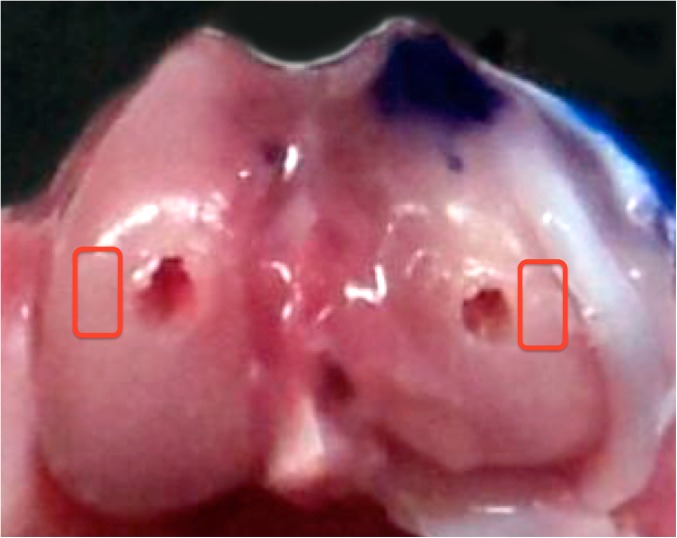
Specimens were decalcified in 10% EDTA, embedded in paraffin and sectioned at 4 μm, to correspond to the 3D micro-CT reconstructions. The tissue sections were stained with Safranin O. The sections were assessed by two blinded observers for degenerative changes using the modified Mankin grading system [20, 21, 29] (Table 1). The area analyzed in both the MFC and LFC was determined by the “marking hole.” The ROI was defined as the cartilage between the hole and lateral cortex for the LFC and the area between the hole and medial femoral cortex for the MFC (Fig. 2). The same ROI was determined with micro-CT for subchondral bone analysis. Correlations between the Mankin scores and subchondral bone measures were determined.
Table 1.
Modified Mankin score used for histological analysis
| I. Structure | Grade |
| Normal | 0 |
| Superficial layer disrupted | 1 |
| Superficial layer absent | 2 |
| Irregular surface | 3 |
| Fissures into the middle zone | 4 |
| Slight disorganization | 5 |
| Fissure into the deep zone | 6 |
| Chaotic disorganization | 7 |
| II. Cellular abnormalities | |
| Normal | 0 |
| Hypocellularity | 1 |
| Large clones | 2 |
| III. Matrix staining | |
| Normal | 0 |
| Staining reduced in middle layer | 1 |
| Staining reduced in territorial matrix of middle and deep layer | 2 |
| Staining present in interterritorial matrix and reduced in the territorial matrix | 3 |
| Staining reduced in both territorial and interterritorial matrices | 4 |
| Maximum score | 13 |
Fig. 2.
Macroscopic view of the ROI (region of interest—cartilage between the hole and the medial and lateral cortex, respectively). Note the lateral femoral condyle marked with a marking pen. Both squares show the ROI corresponding to LFC (lateral femoral condyle) and MFC (medial femoral condyle).
Micro-CT Assessment
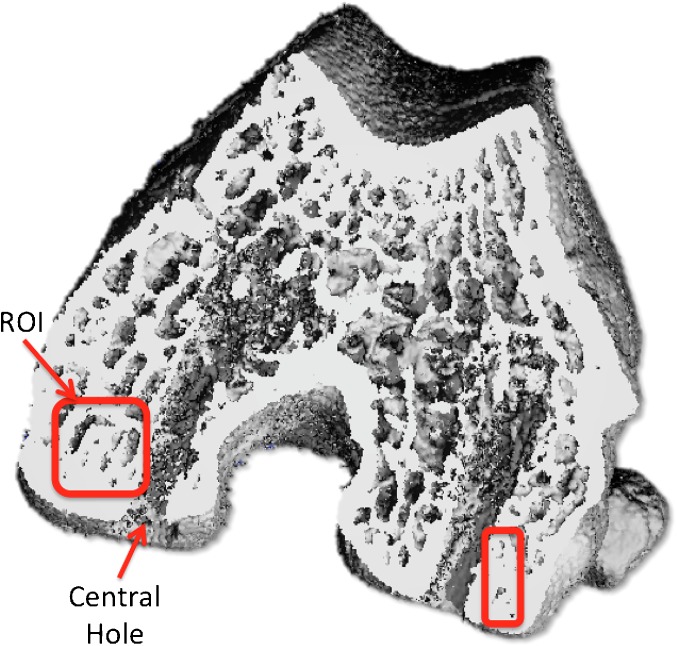
The distal ends of the rat femurs were used for trabecular and cortical bone microcomputed tomography (micro-CT) analysis using a Scanco μCT 35 (Scanco Medical, Brüttisellen, Switzerland) system prior to EDTA decalcification. The scan parameters were 15 μm voxel size, 55 kVp, 0.36° rotation step (180° angular range), and a 400-ms exposure per view, which were performed in DPBS 1X. The Scanco μCT software (HP, DECwindows Motif 1.6) was used for 3D reconstruction and viewing of images. After 3D reconstruction, half cylinder VOIs (volume of interest) with a diameter of 2.1 mm and height of 0.75 mm (with exclusion of the marking hole area) were created for trabecular analysis, while VOIs (volume of interest) around the holes were created for cortical analysis. VOIs were segmented using a global threshold of 4 g/c. ROI were identified considering the central hole that was drilled in the MFC and LFC. The trabecular bone corresponding to the subchondral bone next to the central hole was included for quantitative analysis (Fig. 3).
Fig. 3.
Micro-CT showing the ROI (square) corresponding to LFC and MFC.
Bone volume fraction (BV/TV), tissue mineral density (TMD), and thickness (Ct.Th) for the cortical bone were assessed. We also measured BV/TV, thickness (Tb.Th), number (Tb.N) and separation (Tb.Sp), TMD, and surface to volume ratio (BS/BV) for the trabecular bone.
Statistical Analysis
Independent samples t test was used to evaluate differences between the vitamin D-deficient group and the normal controls for the bone microstructural data and the Mankin scores. Correlations between the cartilage histological scores and the micro-CT quantitative data in both MFC and LFC were investigated using a Kendall’s Tau rank correlation analysis. Spearman’s rank was also used to correlate individually each micro-CT parameter with the corresponding histological Mankin score.
Results
Orbital blood draws confirmed the induction of vitamin D deficiency in the 13 animals at 6 weeks after initial exposure to the specialized diet and UV light restriction. The mean 25(OH) D3 level at time zero before starting the specialized diet and UV light restriction in the 13 animals included in the experimental group was 10.9 ± 1.46 ng/mL compared with a mean level of 6.5 ± 1.14 ng/mL (p < 0.001) 6 weeks later. Vitamin D3 was also measured in the 12 control animals at 6 weeks showing similar levels to those at time zero, confirming that low levels of vitamin D was established in the study group.
Histological Analysis
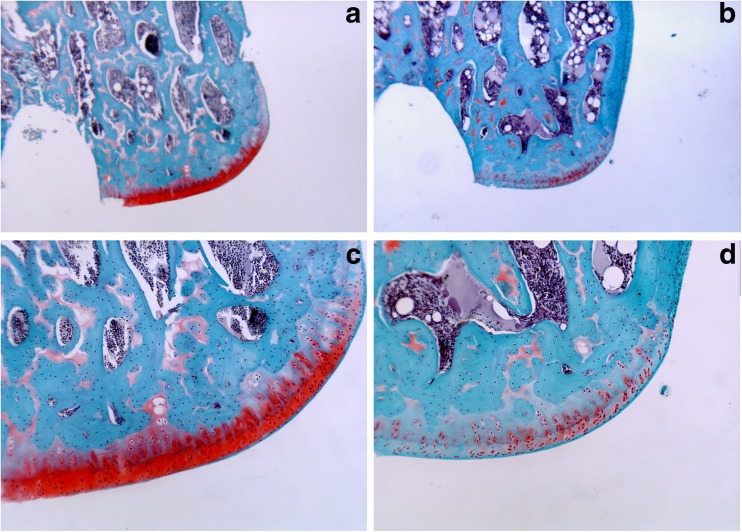
After 4 weeks of vitamin D deficiency, there were statistically significant changes in cartilage structure (Mankin scores) in both the MFC and LFC between control and study groups (Table 2). The most significant change visible on the histologic sections was decreased proteoglycan content as evidenced by the loss of the red matrix staining with the Safranin O. No difference was evident between the MFC and LFC (Fig. 4).
Table 2.
Histological analysis of articular cartilage
| Group | N | Mankin score | STD | p value | |
|---|---|---|---|---|---|
| LFC | Control | 12 | 1.55 | 0.6 | 0.009 |
| Vit D Def | 13 | 3.53 | 2.42 | ||
| MFC | Control | 12 | 1.55 | 0.6 | 0.035 |
| Vit D Def | 13 | 4.23 | 4.1 |
p value corresponds to comparison of control and study group in LFC and MFC, respectively
Vit D Def vitamin D deficiency group, LFC lateral femoral condyle, MFC medial femoral condyle
Fig. 4.
a Low magnification (×4) of control group. b Low magnification (×4) of vitamin D deficiency group. c Higher magnification (×10) of control group. Note the red Safranin O stain indicating proteoglycans in the cartilage matrix. d In this higher magnification (×10) of vitamin D deficiency group, the loss of proteoglycans is evident by the loss of red Safranin O staining.
Micro-CT Analysis
No statistically significant difference was found in trabecular thickness in the vitamin D-deficient group compared to control group (0.17 vs. 0.15 mm for MFC and 0.11 vs. 0.10 mm for LFC, p = 0.45). Trabecular spacing was similar between groups (Table 3).
Table 3.
Results of micro-CT in control and study (Vit D Depletion) group
| MFC | LFC | ||
|---|---|---|---|
| Trabecular spacing (mm) | Control | 0.15 (±0.02) | 0.148 (±0.016) |
| Vit D Depletion | 0.17 (±0.01) | 0.15 (±0.01) | |
| Bone volume | Control | 0.46 (±0.06) | 0.52 (±0.065) |
| Total volume (%) | Vit D Depletion | 0.55 (±0.03) | 0.60 (±0.05) |
| Trabecular thickness (mm) | Control | 0.15 (±0.02) | 0.10 (±0.01) |
| Vit D Depletion | 0.17 (±0.01) | 0.11 (±0.01) | |
No statistical difference was evident among control and Vit D Depletion group
Correlation Between Cartilage and Bone Changes for Each Group
Among groups, there was no correlation between the mean articular cartilage histologic scores and the mean micro-CT bone measurements (Tables 4 and 5).
Table 4.
Mankin score and micro-CT values among study and control group for medial and lateral femoral condyles
| MFC | LFC | |
|---|---|---|
| Mankin score | ||
| Control (n = 12) | 1.55 (±0.6) | 1.55 (±0.6) |
| Vit D Depletion (n = 13) | 4.23 (±4.1)* | 3.53 (±2.42)* |
| Trabecular spacing (mm) | ||
| Control (n = 12) | 0.15 (±0.02) | 0.148 (±0.016) |
| Vit D Depletion (n = 13) | 0.17 (±0.01) | 0.15 (±0.01) |
| Bone volume/total volume (%) | ||
| Control (n = 12) | 0.46 (±0.06) | 0.52 (±0.065) |
| Vit D Depletion (n = 13) | 0.55 (±0.03) | 0.60 (±0.05) |
| Trabecular thickness (mm) | ||
| Control (n = 12) | 0.15 (±0.02) | 0.10 (±0.01) |
| Vit D Depletion (n = 13) | 0.17 (±0.01) | 0.11 (±0.01) |
MFC medial femoral condyle, LFC lateral femoral condyle
*p < 0.05
Table 5.
Overall correlation between histological scores and micro-CT trabecular bone values for medial and lateral femoral condyles
| Trabecular LFC | Bone volume/trabecular volume | Tissue mineral density | Trabecular number | Trabecular thickness | Trabecular spacing | Bone surface/bone volume | |
| Trabecular data Mankin | Correlation coefficient | −0.139 | −0.077 | 0.185 | −0.309 | 0.085 | 0.201 |
| p value | 0.460 | 0.682 | 0.325 | 0.101 | 0.652 | 0.286 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 | |
| Trabecular MFC | Bone volume/trabecular volume | Tissue mineral density | Trabecular number | Trabecular thickness | Trabecular spacing | Bone surface/bone volume | |
| Trabecular data Mankin | Correlation coefficient | 0.132 | −0.231 | 0.082 | 0.082 | 0.016 | −0.066 |
| p value | 0.491 | 0.228 | 0.667 | 0.667 | 0.931 | 0.730 | |
| N | 25 | 25 | 25 | 25 | 25 | 25 |
MFC medial femoral condyle, LFC lateral femoral condyle
Overall Correlation Between Cartilage and Bone Changes
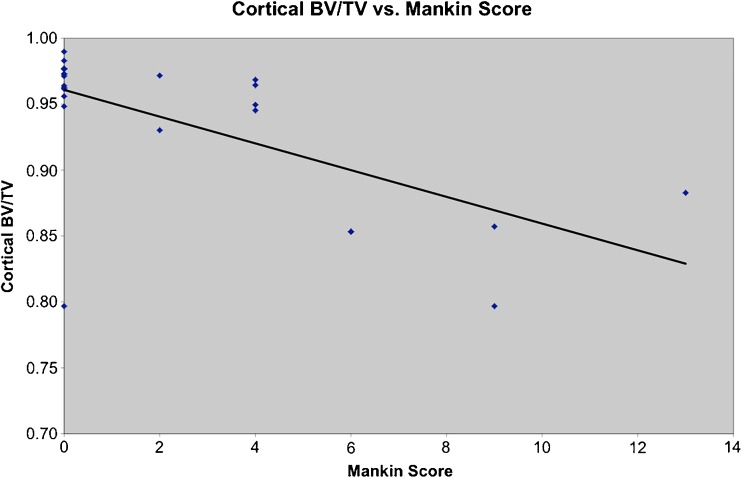
Overall, a negative statistical correlation for each individual sample between Mankin score and cortical bone was evident in both lateral and medial femoral condyle (Table 6 and Fig. 5).
Table 6.
Overall, a negative statistical correlation for each individual sample between Mankin score and cortical bone was evident in both lateral and medial femoral condyle
| Cortical bone volume/total volume | Cortical tissue mineral density | Cortical bone volume | Cortical total volume | Cortical thickness | ||
|---|---|---|---|---|---|---|
| Mankin LFC | Pearson correlation | −0.530 | 0.154 | −0.320 | −0.217 | −0.405 |
| p value | 0.006 | 0.461 | 0.119 | 0.298 | 0.045 | |
| N | 25 | 25 | 25 | 25 | 25 | |
| Mankin MFC | Pearson correlation | −0.631 | 0.178 | −0.322 | −0.194 | −0.361 |
| p value | 0.001 | 0.395 | 0.117 | 0.353 | 0.077 | |
| N | 25 | 25 | 25 | 25 | 25 |
LFC lateral femoral condyle, MFC medical femoral condyle
Fig. 5.
Overall, a negative correlation was evident between histological Mankin score and cortical bone.
Discussion
The present study provides the first report of the effects of low levels of vitamin D on articular cartilage in an animal model. The most important finding in this study was that low levels of vitamin D seem to have a deleterious effect on the cartilage.
The limitations of this study are use of a relatively small sample and the unknown correlation of these findings with human cartilage. However, to our knowledge, this study is the first to examine the effects of low vitamin D levels on articular cartilage histology and thus can provide a baseline for further study.
Histological differences were seen between groups after 4 weeks of a vitamin D-deficient diet and UV light restriction. At this time point, degeneration of the cartilage in the study group was observed. There was significant loss of proteoglycan staining in the vitamin D-deficient group on both the MFC and LFC. Significant microstructural changes in the subchondral bone could not be confirmed with micro-CT. Previous clinical studies have correlated severe vitamin D deficiency with decreased cartilage thickness [16]. In contrast to our study, this study was performed in human subjects and the cartilage was evaluated using US (ultrasound) to assess cartilage thickness. Also, there was no information about the duration of vitamin D deficiency in these patients.
Different surgically induced rat models of OA showed that early changes of OA can be detected as early as 3–6 weeks post-surgery and progress to total loss of cartilage after 12 months. Loss of proteoglycans has been suggested as one of the main early events in OA [9, 15, 24, 25]. In our study, the animals were treated with a specialized vitamin D-deficient diet and UV light restriction for 6 weeks. At that time point, serum tests confirmed low levels of vitamin D. Different rat models suggest that after 6 weeks of a specific vitamin D-deficient diet, animals develop vitamin D deficiency [26]. Our rats were kept for an additional 4 weeks after the deficiency was developed, and with this period of vitamin D deficiency, early loss of proteoglycan staining of the cartilage matrix was evident, simulating what normally happens in the early stages of OA. No other major histologic change was evident. This finding could be explained by the effect that vitamin D has on cartilage. Previous studies have shown an upregulation of VDR (vitamin D receptors) in chondrocytes within osteoarthritic cartilage [27]. Furthermore, MMPs (metalloproteinases) appear to play an important role in cartilage degeneration. Specifically, MMP 1, 3, and 13 have all been demonstrated in both intra- and extracellular locations in osteoarthritic cartilage. Correlation between VDR and MMPs expression has already been suggested [27]. Future studies evaluating MMP and VDR, in this same animal model, will be necessary to better understand the role of vitamin D on articular cartilage.
Different studies have shown subchondral bone changes in osteoarthritis [12, 19]. In our study, we did not observe significant differences in the subchondral bone between the groups. Interestingly, we noted a potential trend in that subchondral cancellous bone in the vitamin D-deficient group was thicker and denser as compared to the control group, with a slightly higher bone volume fraction and higher trabecular thickness. Again, these measurements were not significantly different but a trend was encountered. Further study is needed in order to better define the role of vitamin D in the trabecular thickness of the bone. A longer period of vitamin D deficiency may have resulted in this difference reaching statistical significance. Although vitamin D deficiency might be expected to result in decreased bone volume, we believe that an increase reflects the remodeling changes due to altered load transmission secondary to proteoglycan loss from the articular surface. This would be consistent with the sclerosis that is typically seen radiographically in OA. Previous rat OA model studies have already shown subchondral changes [18]. Similar to our study, in the early stage of OA, there was no statistically significant difference observed in trabecular thickness in any of the three knee compartments between the OA induced knee and control. However, at 4 weeks, there was a significant increase in trabecular thickness in the induced OA group.
Clinical studies of the effect of vitamin D on OA have been inconclusive. McAlindon et al. evaluated the relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham study [17]. In this study, they included 556 people who had been followed for more than 40 years. Patients were evaluated at years 18 and 22 of follow-up with a weight-bearing radiograph of the knee and classified using the Kellgren and Lawrence scale. Serum levels of vitamin D were collected through blood samples during this same examination. The study suggested that individuals with low vitamin D intake and low serum levels of vitamin D are approximately three times more likely to have progression of established osteoarthritis of the knee than those patients with high intake and high serum levels. In contrast, Felson et al. [7, 8] found no correlation between the vitamin D status and the risk of joint space or cartilage loss in knee OA. In this study, the investigators evaluated the results of two longitudinal studies. Patients were evaluated with a knee radiograph, an MRI, and levels of vitamin D. No association was found between vitamin D levels and progression of OA. Cao et al. [6] recently performed a systematic review on the association between serum levels of vitamin D and osteoarthritis. There was moderate evidence suggesting that low levels of vitamin D were associated with progression of knee OA [5]. A recent randomized controlled pilot study suggests that there is a statistically significant benefit to vitamin D treatment in patients with osteoarthritis [23]. Considering the high prevalence of vitamin D deficiency in elderly patients, the findings of this study could have importance for the prevention of OA. Randomized clinical trials will be necessary in the future to establish if supplementation of vitamin D could play a role in the prevention of OA. Further study is required to determine if vitamin D supplementation could impact OA prevalence, and to determine which patient population should be targeted and for how long. Also, side effects of long-term vitamin D intake should be considered [4]. Excessive vitamin D intake for a prolonged period of time may lead to vitamin D toxicity. Adverse events reported include hypercalcemia, nephrolithiasis, hypercalciuria, renal insufficiency, gastrointestinal disorders, cardiovascular disorders, psychiatric disorders, and skin disorders [3].
In summary, in this study, we demonstrate that a relatively brief period of vitamin D deficiency leads to adverse changes in hyaline cartilage in normal, healthy rats. Given the high prevalence of vitamin D deficiency in the general population, these findings raise important questions about the potential role of vitamin D in articular cartilage health.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Acknowledgment
The authors thank Liang Yang for her technical assistance.
Compliance with Ethical Standards
Conflict of Interest
Michael E. Angeline, MD; Jorge Chahla, MD; Xiang Hua Deng, MD; Cecilia Pascual-Garrido, MD; Cliff Voigt, MD; Richard Ma, MD; Joseph Nguyen, MPH; and Russell F. Warren, MD have declared that they have no conflict of interest. Scott A. Rodeo, MD reports personal fees and other from Rotation Medical and personal fees from Cayenne Medical, outside the work.
Human/Animal Rights:
All institutional and national guidelines for the care and use of laboratory animals were followed.
Informed Consent
N/A.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Investigation performed at the Laboratory for Soft Tissue Research, Hospital for Special Surgery, New York, NY.
References
- 1.Bahar-Shany K, Ravid A, Koren R. Upregulation of MMP-9 production by TNFalpha in keratinocytes and its attenuation by vitamin D. J Cell Physiol. 2010;222(3):729–737. doi: 10.1002/jcp.22004. [DOI] [PubMed] [Google Scholar]
- 2.Bergink AP, Uitterlinden AG, Van Leeuwen JP, et al. Vitamin D status, bone mineral density, and the development of radiographic osteoarthritis of the knee: The Rotterdam Study. J Clin Rheumatol. 2009;15(5):230–237. doi: 10.1097/RHU.0b013e3181b08f20. [DOI] [PubMed] [Google Scholar]
- 3.Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2011(7):Cd007470. doi: 10.1002/14651858.CD007470.pub2. [DOI] [PubMed]
- 4.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Winzenberg T, Nguo K, Lin J, Jones G, Ding C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: a systematic review. Rheumatology (Oxford) 2013;52(7):1323–1334. doi: 10.1093/rheumatology/ket132. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Winzenberg T, Nguo K, Lin J, Jones G, Ding C. Association between serum levels of 25-hydroxyvitamin D and osteoarthritis: a systematic review. Rheumatology. 2013;52(7):1323–1334. doi: 10.1093/rheumatology/ket132. [DOI] [PubMed] [Google Scholar]
- 7.Felson DT. CORR Insights®: Does Vitamin D Improve Osteoarthritis of the Knee: A Randomized Controlled Pilot Trial. Clin Orthop Relat Res. 2013;471(11):3563–3564. doi: 10.1007/s11999-013-3269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felson DT, Niu J, Clancy M, et al. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. 2007;56(1):129–136. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 9.Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24–34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 10.Goula T, Kouskoukis A, Drosos G, et al. Vitamin D status in patients with knee or hip osteoarthritis in a Mediterranean country. J Orthop Traumatol. 2015;16(1):35–39. doi: 10.1007/s10195-014-0322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 12.Lacourt M, Gao C, Li A, et al. Relationship between cartilage and subchondral bone lesions in repetitive impact trauma-induced equine osteoarthritis. Osteoarthritis Cartilage. 2012;20(6):572–583. doi: 10.1016/j.joca.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Lane NE, Gore LR, Cummings SR, et al. Serum vitamin D levels and incident changes of radiographic hip osteoarthritis: a longitudinal study. Study of Osteoporotic Fractures Research Group. Arthritis Rheum. 1999;42(5):854–860. doi: 10.1002/1529-0131(199905)42:5<854::AID-ANR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenzo P, Bayliss MT, Heinegard D. Altered patterns and synthesis of extracellular matrix macromolecules in early osteoarthritis. Matrix Biol. 2004;23(6):381–391. doi: 10.1016/j.matbio.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Malas FU, Kara M, Aktekin L, Ersoz M, Ozcakar L. Does vitamin D affect femoral cartilage thickness? An ultrasonographic study. Clin Rheumatol. 2014;33(9):1331–1334. doi: 10.1007/s10067-013-2432-y. [DOI] [PubMed] [Google Scholar]
- 17.McAlindon TE, Felson DT, Zhang Y, et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125(5):353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 18.Mohan G, Perilli E, Kuliwaba JS, Humphries JM, Parkinson IH, Fazzalari NL. Application of in vivo micro-computed tomography in the temporal characterisation of subchondral bone architecture in a rat model of low-dose monosodium iodoacetate-induced osteoarthritis. Arthritis Res Ther. 2011;13(6):R210. doi: 10.1186/ar3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan J, Wang B, Li W, et al. Elevated cross-talk between subchondral bone and cartilage in osteoarthritic joints. Bone. 2012;51(2):212–217. doi: 10.1016/j.bone.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual Garrido C, Hakimiyan AA, Rappoport L, Oegema TR, Wimmer MA, Chubinskaya S. Anti-apoptotic treatments prevent cartilage degradation after acute trauma to human ankle cartilage. Osteoarthritis Cartilage. 2009;17(9):1244–1251. doi: 10.1016/j.joca.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearson RG, Kurien T, Shu KS, Scammell BE. Histopathology grading systems for characterisation of human knee osteoarthritis—reproducibility, variability, reliability, correlation, and validity. Osteoarthritis Cartilage. 2011;19(3):324–331. doi: 10.1016/j.joca.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Sacks JJ, Luo Y-H, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res. 2010;62(4):460–464. doi: 10.1002/acr.20041. [DOI] [PubMed] [Google Scholar]
- 23.Sanghi D, Mishra A, Sharma AC, et al. Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clin Orthop Relat Res. 2013;471(11):3556–3562. doi: 10.1007/s11999-013-3201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthritis Rheum. 2003;48(5):1261–1270. doi: 10.1002/art.10976. [DOI] [PubMed] [Google Scholar]
- 25.Stubendorff JJ, Lammentausta E, Struglics A, Lindberg L, Heinegard D, Dahlberg LE. Is cartilage sGAG content related to early changes in cartilage disease? Implications for interpretation of dGEMRIC. Osteoarthritis Cartilage. 2012;20(5):396–404. doi: 10.1016/j.joca.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Tare M, Emmett SJ, Coleman HA, et al. Vitamin D insufficiency is associated with impaired vascular endothelial and smooth muscle function and hypertension in young rats. J Physiol. 2011;589(Pt 19):4777–4786. doi: 10.1113/jphysiol.2011.214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tetlow LC, Woolley DE. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthritis Cartilage. 2001;9(5):423–431. doi: 10.1053/joca.2000.0408. [DOI] [PubMed] [Google Scholar]
- 28.Troeberg L, Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 2012;1824(1):133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Sluijs JA, Geesink RG, van der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992;10(1):58–61. doi: 10.1002/jor.1100100107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)