Abstract
AIM
To compare the axial lengths (ALs) measured with Lenstar, IOLMaster and A-scan contact ultrasound (Ultrasound) in normal and high myopia (HM).
METHODS
Eighty-four normal eyes and 49 HM eyes were included. Three consecutive measurements were performed on each eye in the following order: Lenstar, IOLMaster, and Ultrasound. The repeatabilities of the AL measurements for each instrument were assessed by calculating the pooled coefficients of variation (CVs) of 18 eyes in each group. Comparisons between the HM and normal groups were made with independent sample t-tests. The inter-device agreements were evaluated with Bland-Altman analyses and paired two-tailed t-tests.
RESULTS
For normal group, the CVs of the AL measurements taken with the Lenstar, IOLMaster and Ultrasound were 0.001%, 0.01% and 0.14%, respectively. The corresponding CVs for the HM group were 0.005%, 0.02% and 0.15%, respectively. There was significant difference between the Lenstar and the IOLMaster in normal group (P=0.031) but not in HM group (P=0.100). In the two groups, the Lenstar and the IOLMaster produced higher values than did the Ultrasound (all P<0.001). All three instruments exhibited good agreement in terms of AL values. For the intraocular lens (IOL) power calculation using SRK II formula, the Lenstar and the IOLMaster showed 0.5 D higher than Ultrasound in both groups (all P<0.001). No significant difference existed between the Lenstar and the IOLMaster for the IOL power calculation in both normal (P=0.474) and HM group (P=0.103).
CONCLUSION
The three devices exhibited excellent intra-visit repeatabilities in the AL measurements. The AL and IOL power difference between partial coherence interferometry and ultrasound instruments should be noticed.
Keywords: axial length, biometry, repeatability, intraocular lens, high myopia
INTRODUCTION
The precise measurement of axial length (AL) is crucial for intraocular lens (IOL) power calculation in cataract surgery. High myopia (HM) is a major worldwide vision health problem. Patients with HM are at high risk for other ocular abnormalities, such as macular holes, retinal detachment, glaucoma and chorioretinal atrophy[1]. Compared to corneal curvature, anterior chamber depth (ACD), lens thickness (LT), and vitreous chamber depth, AL has received more attention because this measure provides a coordinated estimation of the overall ocular structure and changes in that structure in myopia and high myopia[2].
Currently, there are two types of biometry that are based on different working principles. The first is optical biometry, and the second is ultrasound biometry. Optical biometry was designed based on partial coherence interferometry (PCI)[3]. Optical biometry does not require contact and provides more information about ocular parameters, such as corneal thickness, LT, ACD and AL, with a single measurement[4]–[5]. A-scan contact ultrasound (Ultrasound) can routinely obtain ocular parameters, such as AL, LT and ACD, using 10-MHz ultrasonic waves[6]. As a contact biometry, inappropriate fixation target distances and corneal applanation during the measurements can may produce significant errors even in normal subjects[7].
The purpose of this study was to compare AL measurements made with Lenstar, IOLMaster and Ultrasound instruments in normal and HM subjects. We also investigated the repeatabilities and agreements of the AL measurements and its influence on IOL power calculation made with these three instruments.
SUBJECTS AND METHODS
This study was performed at the Sixth People's Hospital Affiliated to Shanghai Jiao Tong University (Shanghai, China). Ethics committee approval was obtained from the Shanghai Clinical Research Center. The formal research protocols were approved by the institutional review boards of the Sixth People's Hospital Affiliated to Shanghai Jiao Tong University (Shanghai, China) and performed in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from each subject after they were provided with an explanation of the nature of the study.
Subjects
A total of 133 subjects (133 eyes), which included 84 normal eyes and 49 HM eyes, were included finally. We chose Han Chinese subjects for this study to eliminate the possible influence of different ethnic groups. The inclusion criteria for the normal subjects included the following: a best-corrected visual acuity (BCVA) ≥16/20, a refractive error <5 D spheres, normal slit-lamp and fundoscopy examinations, an IOP <22 mm Hg, and no history of ocular or systemic corticosteroid use. The inclusion criteria for the HM patients were as follows: BCVA ≥20/40, a spherical refractive error more negative than -6 D, and central fixation that was sufficiently stable to perform image capture. Subjects with severe cataracts, glaucoma or posterior abnormalities, such as choroidal neovascularization, retinoschisis, retinal detachment or macular holes, were excluded. An automatic refractometer (Auto Refractometer, RM-8800; Topcon Ltd., Tokyo, Japan) examination was performed for all subjects to obtain a measurement of the refractive status without cycloplegia.
Methods
Axial length measurement
The data capture procedures for the Lenstar LS 900 (ver. 2.1.1, Haag-Streit AG, Koeniz, Switzerland) and IOLMaster (ver. 5.4.4.0006, Carl Zeiss Meditec, Jena, Germany) were as follows: the subject's chin was placed on a chin rest, the subject's forehead was pressed against a forehead strap and the subject's eye was aligned to the visual axis via a central fixation light or target. During the examination, the patients were asked to fixate on the internal light or the target, and the device was focused based on the image of the eye on the monitor. The patients were asked to perform a complete blink to ensure an optically smooth tear film over the cornea before image capture. Measurements contaminated by blinking or unstable fixation were excluded, and only non-contaminated measurements were included in the final analyses. A handheld A-scan ultrasound biometry device (UltraScan, Alcon, USA) was used for the contact AL measurements. One drop of topical anesthetic (0.4% oxybuprocaine hydrochloride eye drops) was instilled into the eye 3min before ultrasound biometry was performed.
For each device, three consecutive measurements per eye were obtained. To avoid the potential influence of contact by the ultrasound contact probes on the measurements, we performed all examinations in the following sequence: Lenstar, IOLMaster, and Ultrasound. For each instrument, a single trained operator performed all of the examinations following the procedural guidelines.
Intraocular lens power calculation
Based on the SRK II formula, we assume that each eye would use the same A constant and average corneal refractive power to observe the potential effect of AL measurement on IOL power calculation[8].
Intra-visit repeatability
The intra-visit repeatabilities of the measurements of the three instruments were calculated based on data from three sets of consecutive measurements within a single visit for 18 eyes in each group. The pooled coefficients of variation (CVs) were calculated by comparing three consecutive measurements obtained by a single operator.
Statistical Analysis
The statistical analyses were performed with commercial software (SPSS ver. 13.0; SPSS Inc.) and MedCalc software (ver. 12.3.0.0; MedCalc Software, Mariakerke, Belgium). The repeatability of each instrument was assessed by calculating the pooled CV. Independent sample t-tests were used to compare the differences in the AL measurements between normal and HM eyes. The statistical significances of the interdevice differences in the AL measurements and IOL power calculations were evaluated with paired two-tailed t-tests. The interdevice agreements were evaluated using Bland-Altman analyses[9]. The interdevice differences were plotted against their means, and the 95% limits of agreement (LoAs) were determined using this method. The significance level for all of the tests was set at 0.05.
RESULTS
The mean ages of all enrolled subjects in normal and HM groups were 58±17 (range, 23-88)y and 50±20 (range, 25-85)y, respectively. The mean AL values for each device in each group are shown in Table 1. Significant differences in AL values between normal and HM groups were found. The AL values of the normal group as measured with each of the three devices were significantly shorter than those of the HM group (P<0.001 for all).
Table 1. Axial length of each device in the normal and high myopia groups.
| Devices | Axial length (mm) |
P | |
| Normal (n=84) | High myopia (n=49) | ||
| Lenstar | 23.17±0.78 | 26.74±2.04 | 0.000 |
| IOLMaster | 23.18±0.77 | 26.73±2.05 | 0.000 |
| Ultrasound | 22.94±0.75 | 26.49±1.98 | 0.000 |
P-values from independent sample t-tests.
Among the AL measurements from the three devices in normal group, the IOLMaster produced the highest values, and the Ultrasound produced the lowest values (Table 2). Regarding the AL measurements from HM group, there were no significant differences between the Lenstar and IOLMaster instruments (P=0.100), however, both the Lenstar and IOLMaster produced longer AL values than did the Ultrasound (P<0.001 for both).
Table 2. Mean interdevice differences in axial length measurements between each pair of devices.
| Pairs of devices | Axial length (mm) |
|||
| Normal (n=84) | P | High myopia (n=49) | P | |
| Ultrasound-Lenstar | -0.23±0.09 | 0.000 | -0.25±0.13 | 0.000 |
| Ultrasound-IOLMaster | -0.24±0.09 | 0.000 | -0.24±0.14 | 0.000 |
| IOLMaster-Lenstar | 0.01±0.04 | 0.031 | -0.01±0.04 | 0.100 |
P-values from paired t-tests.
Compared to Ultrasound, significant about 0.5 D higher IOL power existed for the Lenstar and IOLMaster in the two groups. However, no significant difference was found between the Lenstar and IOLMaster in IOL power in both groups (Table 3). For normal group, the 95% confidence interval (CI) of the IOL power for the Ultrasound and Lenstar, Ultrasound and IOLMaster, and IOLMaster and Lenstar devices were (-0.57 D, -0.43 D), (-0.59 D, -0.44 D) and (-0.02 D, 0.04 D), respectively. Correspondingly, the 95% CI values for HM group were (-0.72 D, -0.53 D), (-0.70 D, -0.51 D) and (-0.05 D, 0.005 D), respectively.
Table 3. Mean interdevice differences in IOL power calculation based on SRK II formula between each pair of devices.
| Pairs of devices | IOL power (D) |
|||
| Normal (n=84) | P | High myopia (n=49) | P | |
| Ultrasound-Lenstar | -0.50±0.32 | 0.000 | -0.63±0.33 | 0.000 |
| Ultrasound-IOLMaster | -0.51±0.33 | 0.000 | -0.60±0.34 | 0.000 |
| IOLMaster-Lenstar | 0.01±0.13 | 0.474 | -0.02±0.10 | 0.103 |
P-values from paired t-tests.
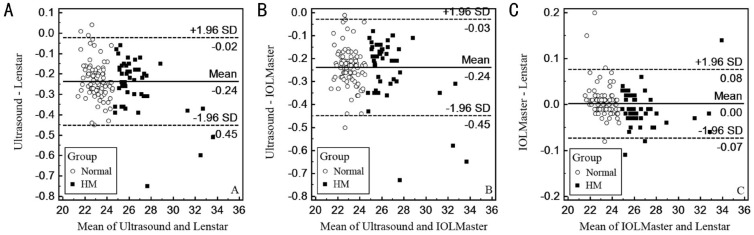
Bland-Altman plots were created to evaluate the differences in the individual measurement between each pair of instruments for each subject. Each pair of methods produced good agreement in the AL measurements (Figure 1). The interdevice 95% LoA ranges of the AL values for the Ultrasound and Lenstar, Ultrasound and IOLMaster, and IOLMaster and Lenstar devices were 0.43 mm, 0.42 mm and 0.15 mm, respectively. The differences between the AL values from the IOLMaster and Lenstar devices exhibited the smallest range of variation (Figure 1C).
Figure 1. Differences in the mean AL values between the Ultrasound and Lenstar (A), Ultrasound and IOLMaster (B), and IOLMaster and Lenstar (C) devices.
The means±SDs are indicated.
Eighteen normal and 18 HM eyes were scanned to assess the intra-visit repeatability of the measurements based on the pooled CVs. In the normal group, the CVs of the AL measurements taken with the Lenstar, IOLMaster and Ultrasound devices were 0.001%, 0.01% and 0.14%, respectively. The corresponding CVs for the HM group were 0.005%, 0.02% and 0.15%, respectively.
DISCUSSION
The accurate determination of AL is an important factor in intraocular lens power calculations for cataract surgery[8]. Ultrasound biometry has commonly been used for cataract patients for a long period of time, however, the requirement of contact and fluctuation in the patient's fixation make the acquisition of AL measurements more difficult and the resultant AL values more variable, particularly for pediatric patients[4]. Moreover, the topical anesthesia, corneal applanation and potential corneal abrasion associated with ultrasound biometry measurement might affect the AL values by inducing changes in corneal shape or thickness[10]–[11]. This also can be confirmed by the bigger CVs for Ultrasound in AL measurement in this study. Compared to the results of the study by Oliveira et al[12] that AL measurements from the normal eyes come from different races using ultrasound technology, our results were slightly lower, which might be attributable to different ages in this study and the potential negative correlation between age and AL[13]. Based on PCI technology, both Lenstar and IOLMaster can perform non-contact AL measurements. Similar to previous studies, the Lenstar and IOLMaster devices both produced significantly higher AL values compared to the Ultrasound values in normal eyes[4],[14]–[16]. We also found this different tendency in HM eyes. This significant difference in the AL values between optical biometry and ultrasound biometry might be attributable to two factors: 1) the contactless operation of optical biometry, which eliminate the confound of corneal applanation in AL measurements; and 2) optical biometry measures the distance from the tear film to the retinal pigment epithelium, which differs from the distance from the cornea to the vitreoretinal interface that is measured by ultrasound technology.
For the IOL power calculated using the SRK II formula, our study showed no statistical difference between the IOLMaster and Lenstar in normal and HM eyes, which is similar with the previous research of cataract patients[14]. Although we made the assumption of same A constant and average K readings for IOL power calculation, the disagreement of IOL power between PCI devices and Ultrasound was also found in our study[14].
Similar to previous tests of the repeatability of the ultrasound biometry method, which is the current gold standard for AL measurement, our study measured the intra-visit repeatabilities of all three devices by collecting three consecutive measurements from each patient in single visits. All three devices exhibited excellent repeatabilities and agreements in the AL measurements for both the normal and HM groups that were as high as those that have previously been reported[17]–[19].
The Bland-Altman plots revealed that the 95% LoA of the differences in the AL measurements between the Ultrasound and Lenstar ranged from -0.45 mm to -0.02 mm, which indicates that the Lenstar values could be as much as 0.43 mm longer than the Ultrasound values, and a similar difference was found between the IOLMaster and Ultrasound devices. These discrepancies are likely to be clinically significant. The Bland-Altman plots of the comparison of each pair of instruments revealed that the differences in the AL measurements varied with the actual AL measurements. Therefore, it might be possible to generate appropriate conversion formulae that will allow the readings to be converted between each pair of devices.
There are several limitations in this study. First, we performed all of the AL measurements with undilated pupils, which allowed the subjects to more easily fixate on the target during the examination. However, without the use of cycloplegia, the potential influences of accommodation on consecutive AL measurements cannot be excluded[20]–[21]. Second, compared to non-contact with the cornea using immersion A-scan biometry, we used applanation biometry, which requires the ultrasound probe be placed directly on the corneal surface. The applanation may unavoidably compress the cornea to make the AL measurements lower and more variable than those non-contact biometries[14],[22]. Moreover, the drift in the measurements among the devices, which might have been caused by device vibration during the examinations and signal instability, should be considered. Therefore, the routine recalibrations of each device are necessary in clinical practice[23].
In conclusion, this comparative study revealed good agreements between each pair of instruments in the evaluations of AL in both normal and HM eyes. The three devices exhibited excellent intra-visit repeatabilities in the AL measurements. However, the AL and IOL power difference between PCI and ultrasound instruments should be noticed.
Acknowledgments
Conflicts of Interest: Wang XG, None; Dong J, None; Pu YL, None; Liu HJ, None; Wu Q, None.
REFERENCES
- 1.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 2.Young TL, Metlapally R, Shay AE. Complex trait genetics of refractive error. Arch Ophthalmol. 2007;125(1):38–48. doi: 10.1001/archopht.125.1.38. [DOI] [PubMed] [Google Scholar]
- 3.Chae JB, Park HR, Yoon YH. Axial length measurement in silicone oil-filled eyes using laser Doppler interferometry. Retina. 2004;24(4):655–657. doi: 10.1097/00006982-200408000-00034. [DOI] [PubMed] [Google Scholar]
- 4.Gursoy H, Sahin A, Basmak H, Ozer A, Yildirim N, Colak E. Lenstar versus ultrasound for ocular biometry in a pediatric population. Optom Vis Sci. 2011;88(8):912–919. doi: 10.1097/OPX.0b013e31821cc4d6. [DOI] [PubMed] [Google Scholar]
- 5.Hill W, Angeles R, Otani T. Evaluation of a new IOLMaster algorithm to measure axial length. J Cataract Refract Surg. 2008;34(6):920–924. doi: 10.1016/j.jcrs.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Lara F, Fernandez-Sanchez V, Lopez-Gil N, Cervino A, Montes-Mico R. Comparison of partial coherence interferometry and ultrasound for anterior segment biometry. J Cataract Refract Surg. 2009;35(2):324–329. doi: 10.1016/j.jcrs.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 7.Cass K, Thompson CM, Tromans C, Wood IC. Evaluation of the validity and reliability of A-scan ultrasound biometry with a single use disposable cover. Br J Ophthalmol. 2002;86(3):344–349. doi: 10.1136/bjo.86.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stopyra W. The accuracy of IOL power calculation formulas for eyes of axial length exceeding 24.5 mm. Klin Oczna. 2013;115(2):93–95. [PubMed] [Google Scholar]
- 9.Hanneman SK. Design, analysis and interpretation of method-comparison studies. AACN Adv Crit Care. 2008;19(2):223–234. doi: 10.1097/01.AACN.0000318125.41512.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchis-Gimeno JA, Palanca-Sanfrancisco JM, Garcia-Lazaro S, Madrid-Costa D, Cervino A. The effect of anesthetic eye drop instillation on the distribution of corneal thickness. Cornea. 2013;32(5):e102–e105. doi: 10.1097/ICO.0b013e318275e7a6. [DOI] [PubMed] [Google Scholar]
- 11.Landers J, Goggin M. Comparison of refractive outcomes using immersion ultrasound biometry and IOLMaster biometry. Clin Experiment Ophthalmol. 2009;37(6):566–569. doi: 10.1111/j.1442-9071.2009.02091.x. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira C, Harizman N, Girkin CA, Xie A, Tello C, Liebmann JM, Ritch R. Axial length and optic disc size in normal eyes. Br J Ophthalmol. 2007;91(1):37–39. doi: 10.1136/bjo.2006.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuft SJ, Bunce C. Axial length and age at cataract surgery. J Cataract Refract Surg. 2004;30(5):1045–1048. doi: 10.1016/j.jcrs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 14.Jasvinder S, Khang TF, Sarinder KK, Loo VP, Subrayan V. Agreement analysis of LENSTAR with other techniques of biometry. Eye (Lond) 2011;25(6):717–724. doi: 10.1038/eye.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakhli FR. Comparison of optical biometry and applanation ultrasound measurements of the axial length of the eye. Saudi J Ophthalmol. 2014;28(4):287–291. doi: 10.1016/j.sjopt.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohrer K, Frueh BE, Walti R, Clemetson IA, Tappeiner C, Goldblum D. Comparison and evaluation of ocular biometry using a new noncontact optical low-coherence reflectometer. Ophthalmology. 2009;116(11):2087–2092. doi: 10.1016/j.ophtha.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Carkeet A, Saw SM, Gazzard G, Tang W, Tan DT. Repeatability of IOLMaster biometry in children. Optom Vis Sci. 2004;81(11):829–834. doi: 10.1097/01.opx.0000145020.33250.c0. [DOI] [PubMed] [Google Scholar]
- 18.Shen P, Zheng Y, Ding X, Liu B, Congdon N, Morgan I, He M. Biometric measurements in highly myopic eyes. J Cataract Refract Surg. 2013;39(2):180–187. doi: 10.1016/j.jcrs.2012.08.064. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Chen Z, Zhou Z, Ding L, Zhou X. Evaluation of the repeatability of the Lenstar and comparison with two other non-contact biometric devices in myopes. Clin Exp Optom. 2013;96(1):92–99. doi: 10.1111/j.1444-0938.2012.00793.x. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh A, Collins MJ, Read SA, Davis BA. Axial length changes with shifts of gaze direction in myopes and emmetropes. Invest Ophthalmol Vis Sci. 2012;53(10):6465–6471. doi: 10.1167/iovs.12-9973. [DOI] [PubMed] [Google Scholar]
- 21.Read SA, Collins MJ, Woodman EC, Cheong SH. Axial length changes during accommodation in myopes and emmetropes. Optom Vis Sci. 2010;87(9):656–662. doi: 10.1097/OPX.0b013e3181e87dd3. [DOI] [PubMed] [Google Scholar]
- 22.Yang QH, Chen B, Peng GH, Li ZH, Huang YF. Accuracy of axial length measurements from immersion B-scan ultrasonography in highly myopic eyes. Int J Ophthalmol. 2014;7(3):441–445. doi: 10.3980/j.issn.2222-3959.2014.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen T, Thorwest M. Calibration of axial length measurements with the Zeiss IOLMaster. J Cataract Refract Surg. 2005;31(7):1345–1350. doi: 10.1016/j.jcrs.2004.12.066. [DOI] [PubMed] [Google Scholar]