Abstract
AIM
To assess the feasibility of radial optic neurotomy (RON) in central retinal vein occlusion (CRVO) treatment with a Meta-analysis.
METHODS
Electronic databases were searched for comprehensive articles that compared efficacy of RON with that of other treatments in CRVO. Study quality was assessed and risk ratio (RR) and 95% confidence interval (CI) with fix- or random-effects model were calculated according to the heterogeneity.
RESULTS
A total of 200 eyes from 5 studies were included. The results indicated that no significant differences were found between groups with and without RON in improvement of visual acuity (VA) at 6mo follow-up (pooled RR 0.51, 95%CI 0.22 to 1.18, P=0.117) while improvement of VA showed significantly favourable in patients receiving RON treatment at 12mo follow-up (pooled RR 2.27, 95%CI 1.31 to 3.95, P=0.004). For complications, RON treatment was more effective in reducing neovascular glaucoma (pooled RR 0.45, 95%CI 0.21 to 0.97, P=0.042) but was comparable in retinal detachment (pooled RR 2.41, 95%CI 0.51 to 11.39, P=0.267) and vitreous hemorrhage (pooled RR 0.91, 95%CI 0.33 to 2.46, P=0.847).
CONCLUSION
Compared with some certain treatment modalities, RON might offer better VA at 12mo and decrease the rate of neovascular glaucoma without changing the rate of retinal detachment and vitreous hemorrhage. Further studies are required considering the limitation of the research.
Keywords: radial optic neurotomy, central retinal vein occlusion, Meta-analysis
INTRODUCTION
Retinal vein occlusion (RVO) is an important cause of vision loss and indeed a common retinal vascular disease secondary to diabetic retinopathy[1]–[2]. Central retinal vein occlusion (CRVO), which can block all venous outflow and result in severe complications, counts the most important in the realm of RVO. Treatment modalities of CRVO include close observation and other active treatments such as laser photocoagulation, intravitreal injection of anti-inflammatory agents and surgical approaches[2]–[3]. While controversies concerning the standard of care have remained for many years since no intervention had been proved to be absolutely effective and safe. Radial optic neurotomy (RON), which is based on the theory hypothesized by Opremcak et al[4] that a “compartment syndrome” occurs in CRVO with neurovascular compression within the optic nerve at the level of lamina cribrosa, arises as an exciting advancement in recent years. It is believed to exert its function by decompressing central retinal artery and vein and alleviating the potential syndrome finally. Given the outcomes of patients with CRVO who underwent RON as a method of treatment, RON was considered as a possible treatment of CRVO[4]–[7].
However, despite the previous favourable evidence, concerns were raised since part of subsequent studies didn't support the significance of RON in the treatment of CRVO, making RON treatment equivocal[8]–[11]. Besides that, few Meta-analysis was found in this field. We therefore conducted the Meta-analysis of the available evidence on efficacy of RON compared with other treatments in treating CRVO.
MATERIALS AND METHODS
Search Strategy
Medline, PubMed, Embase, Cochrane Library and Chinese database-Wanfang Database, Vip Database, China National Knowledge Internet were searched form inception until January 1, 2015. Language was restricted to English. We used mesh terms as “central retinal vein occlusion”, “radial optic neurotomy” and abbreviation of the keywords “CRVO”, “RON”. Additionally, the references lists of the identified articles were examined for extra eligible studies.
Inclusion Criteria
The goal of our study was to determine the efficacy in treating CRVO. Therefore, articles were considered eligible if they fulfilled the following criteria: 1) type of study: comparative studies including randomized controlled trials (RCTs), prospective and retrospective studies; 2) participates: patients diagnosed with CRVO with VA ≥0.3 logMAR (0.5 Snellen or less); 3) interventions and comparison: RON compared with other treatment; 4) outcomes: at least the outcome of visual acuity (VA) were included; 5) duration: the history of CRVO was not longer than 12mo. Exclusion criteria included animal studies, trial protocols, secondary studies and duplicate publications.
Data Extraction
Data extraction was performed by two independent reviewers (Chen ZN and Shao Y). Any disagreement was resolved by discussion and consensus during the extraction. Data extraction included: 1) general characteristics: first author, year and site of publication and number of eyes included in the study; 2) subjects: patient age, sex, disease duration and follow-up; 3) methodology: type and quality of study; 4) intervention (RON) and control group; 5) outcomes: outcome measurements include VA, proportion of eyes with a significant improvement. Complications such as retinal detachment, neovascular glaucoma, and vitreous hemorrhage were also included.
Assessment of Study Quality
The Downs and Black quality assessment method[12] and the Newcastle-Ottawa scale (NOS)[13] were used to assess the study quality also by two reviewers (Chen ZN and Shao Y) independently. The Downs and Black score was employed to evaluate RCTs and non-RCTs, while NOS only for non-RCT. The Downs and Black score system consisted of 27 questions, making total achievable scores from 0 to 32. These questions evaluated reporting, external validity, internal validity (bias and confounding) and power. NOS, which was only used to evaluate non-RCTs, included selection, comparability and exposure. Nine scores were the maximum score and studies ≥6 scores were considered to be with relatively higher quality.
Statistical Analysis
All statistical analyses were performed with State version 12.0. The primary outcome was proportion of eyes with a significant improvement defined as ≥3 lines of logMAR scale or any other methods corresponding to it. Improvement from light perception (LP) to hand movement (HM) or from HM to finger count (FC) were also included. The secondary outcomes were incidence of adverse event, such as retinal detachment, neovascular glaucoma and vitreous hemorrhage.
In the Meta-analysis, pooled risk ratio (RR) and 95% confidence intervals (CI) were measured. Statistically heterogeneity among studies was evaluated with an I2 test. A fixed-effect (Mantel-Haeszel) statistical model was employed when no heterogeneity was reported (P>0.10, I2<70%), otherwise the DerSimonian-Lairel random effects model was undertaken after exploring the cause of heterogeneity. We considered P<0.05 as statistically significant.
RESULTS
To explore the source of heterogeneity, we had also adopted a subgroup analysis. Potential publication bias was estimated with a funnel plot by examining visually the asymmetry and Egger's linear regression method.
Literature Search
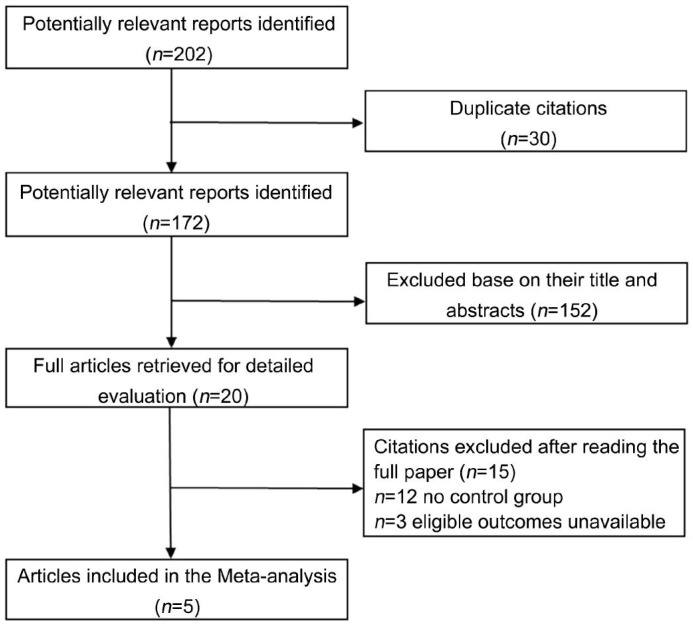
The number of studies identified through database search was 202. After checking the titles and abstracts of the articles, we reviewed 20 full texts and finally 5 were included in the Meta-analysis. Five articles were met by 2 RCTs[14]–[15], 2 prospective trials[16]–[17] and 1 retrospective ones[18] (Figure 1).
Figure 1. Flow diagram of trial selection.
Study Characteristics
Characteristics of articles included in Meta-analysis are presented in Tables 1 and 2. Geographic distributions were sporadic: 2 in Asia and 3 in Europe. Of the 5 eligible studies, intervention was RON surgery and controls were other available treatments. Three studies compared RON with intravenous injection of tissue plasminogen activator (tPA), retinal endovascular surgery (REVS) and panretinal photocoagulation (PRP) respectively[15]–[16],[18]. One study focused on the comparison with natural history[17]. Intravitreal triamcinolone (IVT) and natural history as controls were investigated in one study alone[14].
Table 1. Characteristics of articles included in the Meta-analysis.
| First author, year | Country | No.of eyes (RON: control) | Mean age |
Sex (male:female) |
Disease duration |
Control group | Follow-up (mo) | |||
| RON | Control | RON | Control | RON | control | |||||
| Aggermann T, 2012[14]a | Austria | 63 (38:25) | NA | NA | NA | NA | <12mo | <12mo | IVT | 12 |
| Aggermann T, 2012[14]b | Austria | 58 (38:20) | NA | NA | NA | NA | <12mo | <12mo | Placobo | 12 |
| Crama N, 2010[16] | France | 6 (3:3) | 79 (58-85) | 45 (41-58) | 2:1 | 3:0 | 14 (4-18)wk | 24 (13-36)wk | REVS | 6 |
| Yamamoto T, 2009[15] | Japan | 21 (11:10) | 67.0±6.5 | 63.3±7.2 | 6:5 | 7:3 | 14.7±8.7wk | 10.6±6.4wk | tPA | 6 and 12 |
| Callizo J, 2009[17] | Germany | 63 (28:35) | 67.4±9.1 | 65.5±14.5 | 16:12 | 19:16 | ≤3mo | ≤3mo | Natural history | 12 |
| Kim TW, 2005[18] | Korea | 27 (11:16) | 52 (23-69) | 55 (23-77) | 5:6 | 5:11 | 2.13 (0.5-5)mo | 2.25 (0.5-6)mo | PRP | 6 and 12 |
NA: Not available; RON: Radial optic neurotomy; REVS: Retinal endovascular surgery; tPA: tissue plasminogen activato; PRP: Panretinal photocoagulation. IVT: Intravitreal triamcinolone. aA control group as a single dose of 4 mg IVT; bA control group who received a sham injection (placebo).
n=5
Table 2. Characteristics of articles included in the Meta-analysis.
| First author, year | VA |
Proportion of eyes with a significantly improvementc |
Complications (RON/control) |
||||
| Preoperative (RON/Control) | Outcome (RON/Control) | RON | Control | Retinal detachment | Neovascular glaucoma | Vitreous hemorrhage | |
| Aggermann T, 2012[14]a | 1.46 (0.9-1.36)/1.02 (0.75-2) | 0.75 (0.46-1.22)/0.86 (0.51-1.78) | 18/38 | 2/25 | 1:38/0:25 | 2:38/3:25 | 1:38/0:25 |
| Aggermann T, 2012[14]b | 1.46 (0.9-1.36)/1.02 (0.9-1.36) | 0.75 (0.46-1.22)/1.02 (0.85-3) | 18/38 | 5/20 | 1:38/0:20 | 2:38/3:20 | 1:38/2:20 |
| Crama N, 2010[16] | HM,14,14/17,50,35d | CF,CF,5/62,70,78d | 1/3 | 3/3 | NA | 1:3/0:3 | NA |
| Yamamoto T, 2009[15] | (16/200±20/51)/(10/200±20/69) | (20/182±20/43)/(20/154±20/65) (20/167±20/43)/(20/200±20/111) | 2/11 5/11 | 6/10 5/10 | 1:11/0:10 | 2:11/4:10 | 2:11/1:10 |
| Callizo J, 2009[17] | 0.10±0.087/0.23±0.18 | 0.23±0.20/0.28±0.19 | NA | NA | 1:28/0:35 | 1:28/4:35 | 1:28/3:35 |
| Kim TW, 2005[18] | (1.365±0.78)/(1.104±0.77) | NA | 1/11 1/11 | 0/16 0/16 | NA | 0:11/2:16 | 1:11/0:16 |
NA: Not available; HM: Hand movement; FC: Finger count. aA control group as a single dose of 4 mg IVT; bA control group who received a sham injection (placebo); cProportion of eyes with a significantly improvement defined as an improvement of 3 lines of logMAR scale, a 3-line change on the ETDRS chart, improvement from LP to HM or from HM to FC; dDescribed in ETDRS.
n=5
Quality Assessment
For the Downs and Black score, all studies were assessed from 5 different aspects and no study reached the limit of the maximum of 24 points. The lowest score was 17 points with only 6 patients included. Scores were on average 19.6 points (SD=2.7). Only 2 studies[14]–[15] made attempts to blind the subjects and assessor by means of a sealed-envelope system. Of 3 non-RCTs assessed by the NOS, only one was with high quality of 6 scores (Table 3).
Table 3. Evaluation of the articles included in the Meta-analysis.
| First author, year | Study design | Downs and Black sore | NOS |
|||
| Selection | Comparability | Expose/outcome | Total Score | |||
| Aggermann T, 2012[14] | RCT | 23 | - | - | - | - |
| Crama N, 2010[16] | Prospective | 17 | 3 | 1 | 1 | 5 |
| Yamamoto T, 2009[15] | RCT | 22 | - | - | - | - |
| Callizo J, 2009[17] | Prospective | 18 | 4 | 0 | 1 | 5 |
| Kim TW, 2005[18] | Retrospective | 18 | 3 | 1 | 2 | 6 |
-: No data provided; RCT: Randomized-controlled trials; NOS: Newcastle-Ottawa Scale. aThe Study quality is evaluated by Downs and NOS. Downs and Black Score for both RCT and non-RCT while NOS for only non-RCT.
Postoperative Visual Acuity Improvement
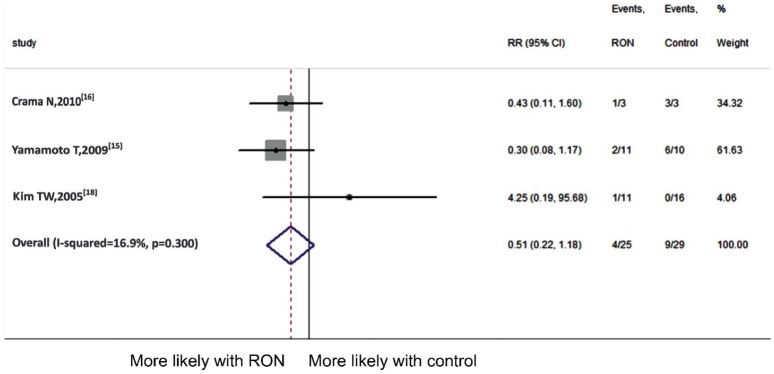
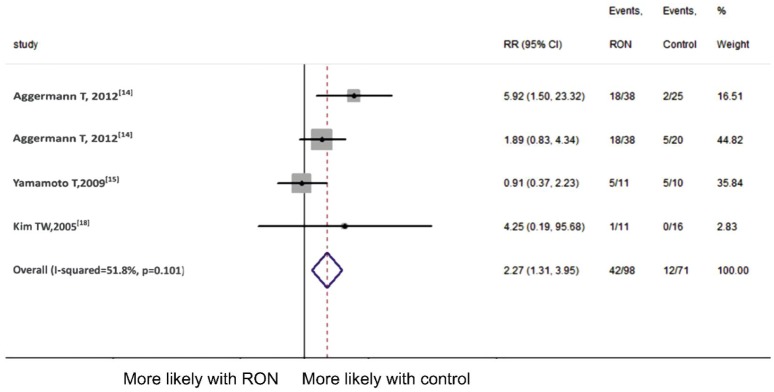
To evaluate the efficacy of RON, we used proportion of eyes with a significant improvement in RON and control group at 6 and 12mo follow-up. We included 3 articles[15]–[16],[18] at 6mo follow-up (Figure 2). Since no significant heterogeneity (P =0.300, I2=16.9%) was detected, we combined the results using fixed-effect model. The result demonstrated that dramatic postoperative VA was not significantly different at 6mo follow-up in two groups (pooled RR 0.51, 95%CI 0.22 to 1.18, P =0.117). While as is shown in Figure 3 which included 4 comparisons[14]–[15],[18], the result demonstrated that compared to those receiving other treatments, patients in RON group had significant better postoperative VA improvement at 12mo follow-up (pooled RR 2.27, 95%CI 1.31 to 3.95, P =0.004). Fixed-effect model was employed for the Meta-analysis (P =0.101, I2=51.8%).
Figure 2. Meta-analysis of postoperative VA at 6mo follow-up.
Figure 3. Meta-analysis of postoperative VA at 12mo follow-up.
Complications
To evaluate the safety of RON, we also made Meta-analysis for complications between compared groups in each study. We focused our attention on retinal detachment, neovascular glaucoma and vitreous hemorrhage.
Retinal Detachment
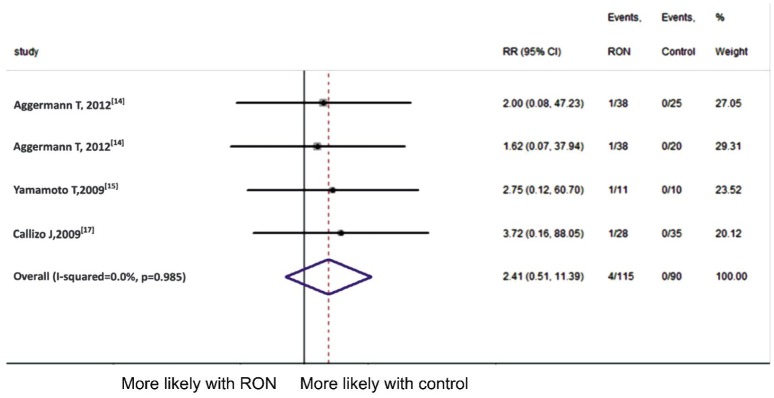
Retinal detachment was mentioned in three articles[14]–[15],[17]. There were 4 (3.5%) events of 115 patients in RON group while no retinal detachment was observed in control group. No signigicant heterogeneity (P =0.985, I2=0) was found in this analysis. Fixed-effect model was employed, showing that RON treatment was not associated with retinal detachment (pooled RR 2.41, 95%CI 0.51 to 11.39, P =0.985) (Figure 4).
Figure 4. Meta-analysis of retinal detachment.
Neovascular Glaucoma
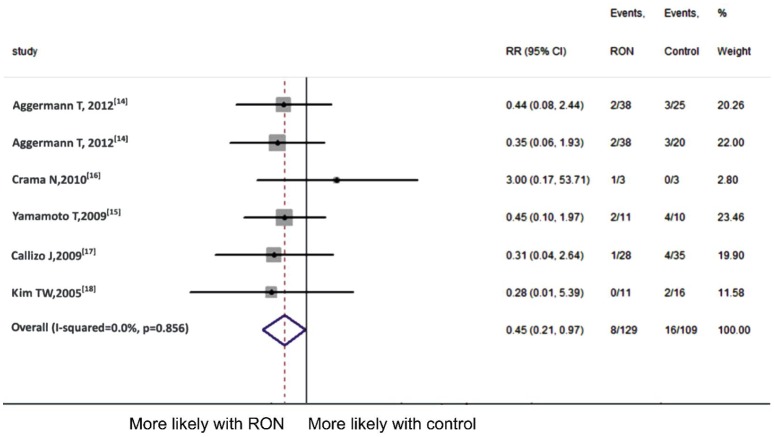
Five articles[14]–[18] were included in analysing the incidence of neovascular glaucoma. Eight (6.20%) of 129 CRVO patients in receiving RON experienced the complication compared with 16 (14.7%) of 109 patients in receiving control. Also no significant heterogeneity (P =0.856, I2=0.0%) was found and fixed-effect model was used. We found that the incidence of neovascular glaucoma in RON group was significantly less than that in control group (pooled RR 0.45, 95%CI 0.21 to 0.97, P =0.042) (Figure 5).
Figure 5. Meta-analysis of neovascular glaucoma.
Vitreous Hemorrhage
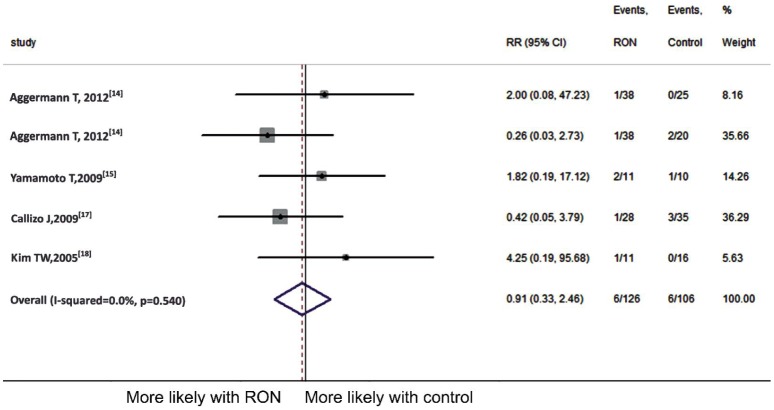
As to vitreous hemorrhage, 4 articles[14]–[15],[17]–[18] were included in the Meta-analysis. The complication ocurred in 6 (4.8%) of 126 patients in RON group as compared to 6 (5.7%) of 106 patients in the control. Since no significant heterogeneity (P=0.540, I2=0) was detected, we employed the fixed-effect model and found that RON was not associated with vitreous hemorrhage (pooled RR 0.91, 95%CI 0.33 to 2.46, P=0.847) (Figure 6).
Figure 6. Meta-analysis of vitreous hemorrhage.
Publication Bias
Publication bias was not calculated considering that just six studies were identified, making the calculation less informative.
DISCUSSION
This Meta-analysis compared RON with other treatment modalities in treating CRVO. Pooled data from two RCTs, two prospective and one retrospective study demonstrated that RON therapy was not associated with significant improvement on postoperative VA at 6mo follow-up and complications like retinal detachment and vitreous hemorrhage as compared with control. While for VA at 12mo follow-up and reduction of neovascular glaucoma, RON was shown to have better outcomes.
Since natural course of CRVO is always disappointing and no treatment modalities have been clarified to show certain effect, new therapy with high expectation is needed. When Opremcak et al[4] first proposed the use of RON in treating CRVO, RON has drawn more and more attention and also been compared with other treatments in various means. However, we found that conclusions from different studies were not consistent.
Although, we tried to conduct a thorough and convinced Meta-analysis from the existing literatures, there were still limitations that might reduce the reliability of evaluation. Firstly, although we searched in multiple databases, only published literatures were included in the Meta-analysis, which might cause publication bias. Secondly, the overall quality of included studies was not high. Given the numbers of articles comparing RON with other treatments were limited, criteria of inclusion was set regardless of the type of articles, type of CRVO and the number of patients. Considering that control group was consisted of different treatment modalities including observation, IVT, REVS, tPA and PRP and only one article was included in each comparison, we regarded the treatments as a whole rather than that in subgroups, which made the conclusion quite conservative. Thirdly, RON was always performed with pars plana vitrectomy (PPV) technically. Whether unspecific effects of the vitrectomy itself, like reduced oxygen consumption and removal of mediators achieved the beneficial effect of PPV was obscure[14],[18]–[20] and the mechanisms behind this effect objectively cannot be identified.
Despite these limitations listed above, our Meta-analysis showed that RON might serve as a useful tool in treating CRVO when other treatment modalities are not available. Further studies assessing efficacy and safety of RON are needed by selecting studies with large scale and patients with more matching factors. Furthermore, whether visual improvement is attributed to vitrectomy procedure itself is still a question. There is therefore an urgent need for further studies concerning prospective, randomized clinical trials to rest the debate over the efficacy of RON and PPV itself in the treatment of CRVO.
Acknowledgments
Foundation: Supported by National Science Foundation of Tianjin, China (No. 15JCQNJ11400).
Conflicts of Interest: Chen ZN, None; Shao Y, None; Li XR, None.
REFERENCES
- 1.Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115(4):486–491. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- 2.MacDonald D. The ABCs of RVO: a review of retinal venous occlusion. Clin Exp Optom. 2014;97(4):311–323. doi: 10.1111/cxo.12120. [DOI] [PubMed] [Google Scholar]
- 3.Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86(3):245–252. doi: 10.1111/j.1755-3768.2007.01144.x. [DOI] [PubMed] [Google Scholar]
- 4.Opremcak EM, Bruce RA, Lomeo MD, Ridenour CD, Letson AD, Rehmar AJ. Radial optic neurotomy for central retinal vein occlusion: a retrospective pilot study of 11 consecutive cases. Retina. 2001;21(5):408–415. doi: 10.1097/00006982-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Stoffelns BM, Kramann C, Hoffmann E. Radial optic neurotomy (RON) for central retinal vein occlusion. Klin Monbl Augenheilkd. 2007;224(4):282–287. doi: 10.1055/s-2007-962895. [DOI] [PubMed] [Google Scholar]
- 6.Hasselbach H, Thale A, Bunse A, Paulsen F, Roider J. Ultrastructural analysis of the lamina cribrosa after radial optic neurotomy. Ann Anat. 2009;191(3):267–272. doi: 10.1016/j.aanat.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Verdaguer Agusti P, Nadal Reus J. Long-term clinical outcome of radial optic neurotomy. Arch Soc Esp Oftalmol. 2010;85(11):370–375. doi: 10.1016/j.oftal.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Beck AP, Ryan EA, Lou PL, Kroll AJ. Controversies regarding radial optic neurotomy for central retinal vein occlusion. Int Ophthalmol Clin. 2005;45(4):153–161. doi: 10.1097/01.iio.0000177429.82022.c3. [DOI] [PubMed] [Google Scholar]
- 9.Ramezani AR. Radial optic neurotomy for central retinal vein occlusion. J Ophthalmic Vis Res. 2009;4(2):115–121. [PMC free article] [PubMed] [Google Scholar]
- 10.Arevalo JF, Garcia RA, Arevalo JF, Garcia RA, Wu L, Rodriguez FJ, Dalma-Weiszhausz J, Quiroz-Mercado H, Morales-Canton V, Roca JA, Berrocal MH, Graue-Wiechers F, Robledo V, Pan-American Collaborative Retina Study Group Radial optic neurotomy for central retinal vein occlusion: results of the Pan-American Collaborative Retina Study Group (PACORES) Retina. 2008;28(8):1044–1052. doi: 10.1097/IAE.0b013e3181744153. [DOI] [PubMed] [Google Scholar]
- 11.Hayreh SS, Zimmerman MB, Podhajsky PA. Retinal vein occlusion and the optic disk. Retina. 2012;32(10):2108–2118. doi: 10.1097/IAE.0b013e31825620f2. [DOI] [PubMed] [Google Scholar]
- 12.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Aggermann T, Brunner S, Krebs I, Haas P, Womastek I, Brannath W, Binder S, Rovo Study Group A prospective, randomised, multicenter trial for surgical treatment of central retinal vein occlusion: results of the radial optic neurotomy for central vein occlusion (ROVO) study group. Graefes Arch Clin Exp Ophthalmol. 2013;251(4):1065–1072. doi: 10.1007/s00417-012-2134-1. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Kamei M, Sakaguchi H, Oshima Y, Ikuno Y, Gomi F, Ohji M, Tano Y. Comparison of surgical treatments for central retinal vein occlusion; RON vs cannulation of tissue plasminogen activator into the retinal vein. Retina. 2009;29(8):1167–1174. doi: 10.1097/IAE.0b013e3181a46a5e. [DOI] [PubMed] [Google Scholar]
- 16.Crama N, Gualino V, Restori M, Charteris DG. Central retinal vessel blood flow after surgical treatment for central retinal vein occlusion. Retina. 2010;30(10):1692–1697. doi: 10.1097/IAE.0b013e3181d8e7e8. [DOI] [PubMed] [Google Scholar]
- 17.Callizo J, Kroll P, Mennel S, Schmidt JC, Meyer CH. Radial optic neurotomy for central retinal vein occlusion: long-term retinal perfusion outcome. Ophthalmologica. 2009;223(5):313–319. doi: 10.1159/000217730. [DOI] [PubMed] [Google Scholar]
- 18.Kim TW, Lee SJ, Kim SD. Comparative evaluation of radial optic neurotomy and panretinal photocoagulation in the management of central retinal vein occlusion. Korean J Ophthalmol. 2005;19(4):269–274. doi: 10.3341/kjo.2005.19.4.269. [DOI] [PubMed] [Google Scholar]
- 19.Skevas C, Wagenfeld L, Feucht M, Galambos P, Richard G, Zeitz O. Radial optic neurotomy in central retinal vein occlusion does not influence ocular hemodynamics. Ophthalmologica. 2011;225(1):41–46. doi: 10.1159/000314716. [DOI] [PubMed] [Google Scholar]
- 20.McAllister IL. Central retinal vein occlusion: a review. Clin Experiment Ophthalmol. 2012;40(1):48–58. doi: 10.1111/j.1442-9071.2011.02713.x. [DOI] [PubMed] [Google Scholar]