Abstract
Mycoplasma pneumoniae causes community-acquired respiratory tract infections, particularly in school-aged children and young adults. These infections occur both endemically and epidemically worldwide. M. pneumoniae lacks cell wall and is subsequently resistant to beta-lactams and to all antimicrobials targeting the cell wall. This mycoplasma is intrinsically susceptible to macrolides and related antibiotics, to tetracyclines and to fluoroquinolones. Macrolides and related antibiotics are the first-line treatment of M. pneumoniae respiratory tract infections mainly because of their low MIC against the bacteria, their low toxicity and the absence of contraindication in young children. The newer macrolides are now the preferred agents with a 7-to-14 day course of oral clarithromycin or a 5-day course of oral azithromycin for treatment of community-acquired pneumonia due to M. pneumoniae, according to the different guidelines worldwide. However, macrolide resistance has been spreading for 15 years worldwide, with prevalence now ranging between 0 and 15% in Europe and the USA, approximately 30% in Israel and up to 90–100% in Asia. This resistance is associated with point mutations in the peptidyl-transferase loop of the 23S rRNA and leads to high-level resistance to macrolides. Macrolide resistance-associated mutations can be detected using several molecular methods applicable directly from respiratory specimens. Because this resistance has clinical outcomes such as longer duration of fever, cough and hospital stay, alternative antibiotic treatment can be required, including tetracyclines such as doxycycline and minocycline or fluoroquinolones, primarily levofloxacin, during 7–14 days, even though fluoroquinolones and tetracyclines are contraindicated in all children and in children < 8 year-old, respectively. Acquired resistance to tetracyclines and fluoroquinolones has never been reported in M. pneumoniae clinical isolates but reduced susceptibility was reported in in vitro selected mutants. This article focuses on M. pneumoniae antibiotic susceptibility and on the development and the evolution of acquired resistance. Molecular detection of resistant mutants and therapeutic options in case of macrolide resistance will also be assessed.
Keywords: Mycoplasma pneumoniae, macrolides, resistance, molecular detection, treatment
Introduction
Mycoplasma pneumoniae is responsible for community-acquired respiratory tract infections, such as tracheobronchitis and pneumonia, particularly in school-aged children and young adults. These infections occur both endemically and epidemically at 3-to-7-year intervals worldwide (Atkinson et al., 2008). Numerous extra-respiratory manifestations of variable severity have also been associated with M. pneumoniae infections including dermatological manifestations and neurological complications. Before 2000, M. pneumoniae infections were easily treated using macrolides because only rare cases of resistance to macrolides had been reported in clinical isolates. Since 2000, macrolide resistance rates have been rising up to 90–100% in Asia, hindering the efficacy of common antibiotic regimens.
This mini-review focuses on M. pneumoniae intrinsic resistance, antibiotic susceptibility and on the development and the evolution of acquired macrolide resistance worldwide since the last published review (Bébéar et al., 2011). Methods for molecular detection of macrolide resistance-associated mutations and therapeutic options in case of infections with macrolide-resistant M. pneumoniae strains are also assessed.
Active antibiotics and intrinsic resistance
Like all microorganisms that lack cell wall, M. pneumoniae is intrinsically resistant to beta-lactams and to all antimicrobials targeting the cell wall, such as glycopeptides and fosfomycin. M. pneumoniae is also resistant to polymixins, sulfonamides, trimethoprim, rifampicin and linezolid (Bébéar and Kempf, 2005; Bébéar et al., 2011). Antibiotics with potential activity against M. pneumoniae that are used in clinical practice include macrolides, lincosamides, streptogramin combinations and ketolides (MLSK), tetracyclines and fluoroquinolones. These drugs achieve high intracellular concentration in mammalian cells and are thereby able to reach intracellular mycoplasmas. The MICs of the main antibiotics belonging to the MLSK group are the lowest against M. pneumoniae compared with those of the two other classes, except MIC of lincomycin that is high (see MIC of the sensitive reference strain M129 (ATCC 29342) in Table 1; Bébéar et al., 2011). MICs of tetracyclines and fluoroquinolones are about 10 times higher than those of MLSK, but newer fluoroquinolones such as levofloxacin and moxifloxacin show an enhanced activity against M. pneumoniae. Only fluoroquinolones and ketolides have a potential bactericidal action. Other antibiotics such as aminoglycosides and chloramphenicol show some activity against M. pneumoniae (MICs 2–10 μg/ml for chloramphenicol and MIC 4 μg/ml for gentamicin, Bébéar et al., 2011) but are not recommended for M. pneumoniae infections.
Table 1.
MICs of MLSK, tetracycline and fluoroquinolone antibiotics for M. pneumoniae clinical isolates resistant to macrolides and genetically characterized.
| 14-membered macrolides | 15-membered macrolide | 16-membered macrolides | Lincosamides | S.C. | Ketolide | Tetracycline | Fluoroquinolones | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolates | ERYa | CLA | AZM | JOS | MDM | RKI | LIN | CLI | Q–D | TEL | MIN | CIP | LEV | MXF |
| Sensitive reference strains M129 (ATCC 29342) | 0.004–0.03 | 0.002–0.015 | 0.002–0.06 | 0.03–0.12 | 0.008–0.06 | 0.01–0.06 | 8 | 4 | 0.25 | 0.002 | 0.25 | 1 | 0.12–1 | 0.03–0.25 |
| CLINICAL STRAINS WITH MUTATION IN DOMAIN V OF 23S rRNA: | ||||||||||||||
| A2058Gb | 32–>256 | 32–>256 | 2–>64 | 0.06–64 | 2–>64 | 0.01–16 | >256 | 16–256 | 0.06–1 | 16–>64 | 0.016–1 | 0.125–2 | 0.125–2 | <0.008–0.03 |
| A2058C | >256 | >256 | 16 | 64 | 64 | 4 | 64 | 32 | 1 | NDc | ND | ND | ND | ND |
| A2058T | 32–64 | 16–64 | 0.064–0.25 | 16 | ND | 4 | ND | 256 | ND | ND | 0.25–1 | 0.5–1 | 0.25–1 | 0.032 |
| A2059G | >64–>256 | 16–>256 | 4–64 | >64–256 | >64–>256 | 8–32 | 64 | 32 | 0.06–0.25 | 1–16 | 0.03–1 | 0.5–1 | 0.25–1 | 0.06–0.12 |
| C2611G | 8 | 1 | 0.03 | 0.25 | 0.25 | 0.06 | 16 | 4 | 0.25 | ND | ND | ND | ND | ND |
| C2611A | 1 | 0.5 | 0.03 | 0.06 | ND | 0.03 | ND | ND | ND | 0.06 | 1 | ND | 1 | 0.125 |
Adapted from Bébéar et al. (2011), Cao et al. (2010), Zhao et al. (2013b), Matsuda et al. (2013), Yamazaki et al. (2007), Pereyre et al (2004b), Waites et al. (2011), Morozumi et al. (2010).
ERY, erythromycin; CLA, clarithromycin; AZM, azithromycin; JOS, josamycin; MDM, midecamycin; RKI, rokitamycin; LIN, lincomycin; CLI, clindamycin; S.C., Streptogramin combination, Q-D, quinupristin-dalfopristin; TEL, telithromycin; MIN, minocycline; CIP, ciprofloxacin; LEV, levofloxacin; MXF, moxifloxacin.
E. coli numbering.
ND, not determined.
The in vitro activity of a few new agents was recently reported. AZD0914, a spiropyrimidinetrione DNA gyrase inhibitor, showed a MIC90 of 1 μg/ml, comparable to that of levofloxacin (Waites et al., 2015). ACH-702, a novel isothiazoloquinolone, and BC-3781, a semi-synthetic pleuromutilin antibiotic, showed better MICs, comparable to those of MLSK, with MIC90 of 0.015 and 0.006 μg/ml, respectively (Pucci et al., 2011; Sader et al., 2012).
Mechanisms of M. pneumoniae acquired resistance and resistance molecular detection methods
In M. pneumoniae, only antimicrobial target modifications by acquired mutations have been associated with antibiotic resistance (Bébéar and Pereyre, 2005). The high mutation rates and the small amount of genetic information dedicated to DNA repair in mycoplasmas (Rocha and Blanchard, 2002) may be associated with this single mode of antibiotic resistance. Resistance through mutation was reported in in vitro-selected mutants for all three classes of antibiotics used to treat M. pneumoniae whereas to date, resistance in clinical isolates was only reported for the MLSK antibiotic class.
Acquired resistance to macrolides and related antibiotics
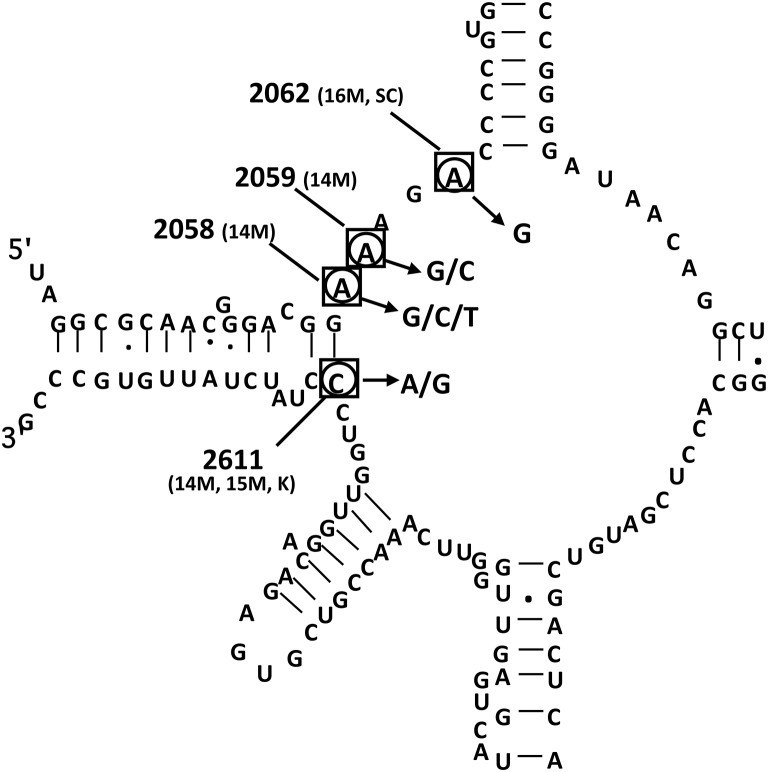
Macrolide resistance in the M. pneumoniae species, which harbors only one ribosomal operon, is defined by mutations in the ribosomal target of the antibiotic, i.e., the 23S rRNA and the ribosomal proteins L4 and L22 (Bébéar and Pereyre, 2005; Bébéar et al., 2011). The A2058G (Escherichia coli numbering) transition in the peptidyltransferase loop of domain V of 23S rRNA is the most common mutation that is associated with macrolide resistance (Figure 1, Table 2). Other substitutions have been reported at position 2058 (A2058C, A2058T), at position 2059 (A2059G, A2059C), at position 2062 (A2062G) and at position 2611 (C2611G, C2611A). No mutation has been detected in domain II of 23S rRNA. Mutations in conserved regions of ribosomal L4 and L22 proteins such as single amino acid change, insertion and deletion of amino acids have also been associated with low-level macrolide resistance in in vitro selected mutants (Pereyre et al., 2004a). Rare mutations have been reported in vivo in ribosomal proteins L4 and L22 but were not associated with significant increased MICs of macrolides (Cao et al., 2010). Comparison of sequencing results with antimicrobial susceptibility testing confirmed that mutations A2058G and A2059G led to a high level resistance to 14- and 15-membered macrolides and lincosamides (Xin et al., 2009; Cao et al., 2010; Akaike et al., 2012; Zhao et al., 2013b; Table 1). Whereas 16-membered macrolides were highly affected by the A2059G substitution, the A2058G mutation was associated with an intermediate level of resistance to these antibiotics. Mutations at position 2611 were associated with low-level of resistance to MLSK. Interestingly, the streptogramin combinations, quinupristin-dalfopristin and pristinamycin, and the ketolide solithromycin (CEM-101) retained activity on resistant mutants harboring mutations at position 2058, 2059, and 2611 (Pereyre et al., 2007; Waites et al., 2009; Table 1). However, an in vitro mutant selection study showed that the A2062G transition was associated with significant increased MICs of these two streptogramin combinations (Pereyre et al., 2004a).
Figure 1.
Peptidyltransferase loop of domain V of 23S rRNA of Mycoplasma pneumoniae (Escherichia coli numbering) with nucleotides found mutated in in vitro-selected strains and in clinical isolates of macrolide-resistant M. pneumoniae. Adapted from Bébéar et al. (2011). Squared nucleotides indicate positions mutated in in vitro-selected macrolide resistant mutants. Antibiotics used for in vitro selection are in parentheses (14M, 14-membered macrolides; 15M, 15-membered macrolides; 16M, 16-membered macrolides; SC, streptogramin combinations; K, ketolides). Circled nucleotides indicate positions mutated in clinical macrolide resistant isolates.
Table 2.
Prevalence of macrolide resistance in M. pneumoniae clinical isolates (continents and countries are presented in alphabetical order).
| Country | Year | % of macrolide resistance (number of resistant strains or M. pneumoniae-positive specimens/total strains or specimens tested) | 23S rRNA mutations (%) | References |
|---|---|---|---|---|
| AMERICA | ||||
| Canada (Ontario) | 2010–2012 | 12.1% (11/91) | A2058G (91%) | Eshaghi et al., 2013 |
| A2059G (18%) | ||||
| USA (14 states) | 2006–2013 | 10.8% (19/176) | ND | Diaz et al., 2015b |
| USA (St. Louis, Missouri) | 2010–2012 | 8.2% (4/49) | A2058G (100%) | Yamada et al., 2012 |
| USA (3 states) | 2010–2012 | 3.5% (7/202) | A2058G (85.7%) | Diaz et al., 2015a |
| A2059G (14.3%) | ||||
| USA (6 states) | 2012–2014 | 13.2% (12/91) | A2058G (100%) | Zheng et al., 2015 |
| ASIA | ||||
| China (Beijing) | 2003–2006 | 92% (46/50) | A2058G (86.9%) | Xin et al., 2009 |
| A2058C (2.2%) | ||||
| A2059G (10.9%) | ||||
| China (Shanghai) | 2005–2009 | 90.1% (137/152) | ND | Liu et al., 2012 |
| China (Beijing) | 2008–2009 | 69% (46/67) | A2058G (89.1%) | Cao et al., 2010 |
| A2059G (8.7%) | ||||
| A2058T (2.2%) | ||||
| China (Shanghai) | 2008–2009 | 90% (90/100) | A2058G (98%) | Liu et al., 2010 |
| A2058T (1%) | ||||
| A2059G (1%) | ||||
| China (Beijing) | 2008–2011 | 88.1% (177/201) | A2058G (96.6%) | Zhao et al., 2013a |
| A2059G (2.8%) | ||||
| A2059T (0.6%) | ||||
| China (Beijing) | 2008–2012 | 90.7% (280/309) | A2058G (97.1%) | Zhao et al., 2013b |
| A2059G (2.5%) | ||||
| A2058T (0.4%) | ||||
| China (Beijing) | 2009 | 91% (58/64) | A2058G (98.3%) | Lin et al., 2010 |
| A2058T (1.7%) | ||||
| China (Beijing) | 2010–2012 | 90.8% (59/65) | A2058G (100%) | Sun et al., 2013 |
| China (Beijing, Dongcheng, Xicheng) | 2011 | 95% (38/40) | A2058G (97%) | Zhao et al., 2011 |
| A2059G (3%) | ||||
| China (Zhejiang province) | 2012–2014 | 100% (71/71) | A2058G (100%) | Zhou et al., 2015 |
| China (Beijing) | 2013 | 98.5% (128/130) | A2058G (100%) | Yan et al., 2014 |
| Hong-Kong | 2011 | 13.6% (3/22) | A2058G (100%) | Ho et al., 2015 |
| 2012 | 30.7% (23/75) | |||
| 2013 | 36.6% (34/93) | |||
| 2014 | 47.1% (24/51) | |||
| Japan (65 institutions) | 2008 | 56% (9/16) | A2058G (95.9%)* | Kawai et al., 2013 |
| 2009 | 69% (9/13) | A2058T (3.2%) | ||
| 2010 | 71% (79/110) | A2059G (0.5%) | ||
| 2011 | 63% (176/281) | A2058C (0.2%) | ||
| 2012 | 82% (288/349) | C2611G (0.2%) | ||
| Japan (Fukuoka prefecture) | 2010–2011 | 89.2% (58/65) | A2058G (53%) | Matsuda et al., 2013 |
| A2058T (47%) | ||||
| Japan (5 institutions) | 2011 | 87.1% (176/202) | A2058G (90.9%) | Okada et al., 2012 |
| A2058T (6.2%) | ||||
| A2059G (2.3%) | ||||
| A2058C (0.6%) | ||||
| South Korea | 2003 | 2.9% (1/34) | A2058G (% ND) | Hong et al., 2013 |
| 2006 | 14.7% (10/68) | A2059G (% ND) | ||
| 2010 | 47.2% (25/53) | |||
| 2011 | 62.9% (44/70) | |||
| Taiwan | 2010–2011 | 23.3% (14/60) | A2058G (100%) | Wu et al., 2013 |
| EUROPE | ||||
| Denmark | 2010–2011 | 1.6% (6/365) | ND | Uldum et al., 2012 |
| England and Wales | 2010 | 0% (0/24) | - | Chalker et al., 2011 |
| England and Wales | 2011–2012 | 0% (0/12) | - | Chalker et al., 2012 |
| England | 2014–2015 | 9.3 (4/43) | A2058G (100%) | Brown et al., 2015 |
| France | 2005–2007 | 9.8% (5/51) | A2058G (60%) | Peuchant et al., 2009 |
| A2059G (20%) | ||||
| C2611G (20%) | ||||
| France | 2007–2010 | 3.4% (1/29) | A2059G | Pereyre et al., 2012 |
| France | 2011 | 8.3% (6/72) | A2058G (67%) | Pereyre et al., 2013 |
| A2059G (16.5%) | ||||
| A2062G (16.5%) | ||||
| Germany | 2003–2008 | 1.2% (2/167) | A2058G | Dumke et al., 2010 |
| A2058C | ||||
| Germany | 2009–2012 | 3.6% (3/84) | A2058G (100%) | Dumke et al., 2013 |
| Germany | 2011–2012 | 3.1% (3/96) | A2058G (100%) | Dumke et al., 2015 |
| Italy | 2010 | 26% (11/43) | A2058G (63.6%) | Chironna et al., 2011 |
| A2059G (36.4%) | ||||
| Slovenia | 2006–2014 | 1% (7/783) | A2058G (100%) | Kogoj et al., 2015 |
| Switzerland | 2011–2013 | 2% (1/50) | A2058G | Meyer Sauteur et al., 2014 |
| MIDDLE EAST | ||||
| Israel | 2010 | 30% (9/30) | A2058G (100%) | Averbuch et al., 2011 |
| Israel | 2010 | 22% (9/41) | A2058G (100%) | Pereyre et al., 2012 |
| OCEANIA | ||||
| Australia (Sydney) | 2008–2012 | 3.3% (1/30) | A2059G | Xue et al., 2014 |
ND, not determined.
Percentages calculated among the 561 resistant isolates collected over the 5 years.
Cross-resistance was not observed between MLSK and other antibiotic families commonly used against M. pneumoniae because isolates with macrolide resistance-associated mutations remain susceptible to tetracyclines and fluoroquinolones (Table 1).
Several molecular methods applicable directly on respiratory specimens were developed to detect macrolide resistance and to circumvent the fastidious, insensitive and time-consuming isolation of M. pneumoniae from clinical samples. Apart from the conventional amplification and sequencing of the hot spots of the 23S rRNA gene, macrolide resistance determination was achieved by PCR-restriction fragment lengh polymorphism (Matsuoka et al., 2004), real-time PCR and melting curve analysis (Peuchant et al., 2009), pyrosequencing (Spuesens et al., 2010, 2012) and real-time PCR and high resolution melt (HRM) analysis (Wolff et al., 2008). A nested-PCR combined with single-strand conformation polymorphism and capillary electrophoresis (Lin et al., 2010) and a singe nucleotide polymorphism (SNP) PCR assay (Ji et al., 2014) were also developed to detect macrolide-resistant mutants directly from clinical specimens. Most of these in-house approaches allow resistance screening in M. pneumoniae-positive respiratory tract samples but the clinical sensitivity i.e., the proportion of M. pneumoniae-positive specimens capable of being resistance typed varies according to methods, ranging between 72.6 and 80.2% in the studies where it was calculated (Wolff et al., 2008; Peuchant et al., 2009; Spuesens et al., 2012). The need to perform such tests differs according to the prevalence of macrolide resistance in each country. In countries where the percentage of macrolide resistance is over 10%, it could be recommended that all M. pneumoniae detection be followed up with an assay capable of detecting macrolide resistance-associated mutations. This strategy would allow a non-macrolide treatment to be promptly started in the event that a macrolide-resistant genotype is detected in an individual patient. In contrast, in countries where macrolide resistance remains below 10%, this kind of test could be performed only in case of treatment failure.
Currently, this strategy is hampered by the lack of commercially available sensitive kits that detect macrolide resistance-associated mutations. However, such kits are currently in development and may soon become available. They will be useful for routine diagnostics in microbiology laboratories.
Acquired resistance to tetracyclines and fluoroquinolones
To date, no tetracycline or fluoroquinolone resistance has been reported in M. pneumoniae clinical isolates. However, resistant strains have been selected in vitro for both classes of drugs. Target mutations were identified in the 16S rRNA gene of tetracycline-resistant mutants selected with subinhibitory concentrations of doxycycline. Mutations were associated with reduced susceptibility to tetracycline, doxycycline and minocycline with MICs remaining below ≤ 2 μg/ml (Degrange et al., 2008). Mutations within conserved regions of the gyrA, gyrB, parC, and parE genes referred to as the quinolone resistance-determining regions were reported for fluoroquinolone-resistant mutants selected with different fluoroquinolones and were associated with MICs of ciprofloxacin, levofloxacin and moxifloxacin up to 32, 16, and 4 μg/ml, respectively (Gruson et al., 2005). Mutations rates were low for levofloxacin and moxifloxacin, ranging from 1.3 × 10−6 to 7 × 10−9 (Gruson et al., 2005).
Prevalence of macrolide resistance in M. pneumoniae
Recent rates of macrolide resistance in M. pneumoniae clinical isolates in countries in which publications have been released since the last review (Bébéar et al., 2011) are presented in Table 2. Prior to the year 2000, very few M. pneumoniae clinical isolates were resistant to macrolides. Rare strains resistant to erythromycin were reported in the literature between 1968 and 1999 in Japan, Israel, Finland, USA and France (Niitu et al., 1970; Stopler and Branski, 1986; Critchley et al., 2002; Pereyre et al., 2007). By contrast, several Japanese studies have reported a significant and constant increase in macrolide resistance rates since 2000, reaching 30% in 2006, around 60% in 2009 and up to 89% in 2010–2011 (Morozumi et al., 2008; Okada et al., 2012; Matsuda et al., 2013). However, regional differences in rates of macrolide-resistant M. pneumoniae were recently reported in Japan, for example in Hokkaido island, where rates ranged from 0 to 100% according to regions (Ishiguro et al., 2015). The situation is worse in China where a dozen of articles have reported a prevalence of macrolide resistance between 90 and 100% since 2003. Other Asian countries seem less affected with resistance rates of 62.9, 47.1, and 23.3% in South Korea, Hong-Kong and Taiwan, respectively (Table 2). It should be noted that most reports regarding macrolide resistance relate on hospitalized patients. It cannot be excluded that the macrolide resistant rate in M. pneumoniae may be higher in hospitalized patients in whom the resistant population may be concentrated than in outpatients. However, comprehensive studies on outpatients are not easily achievable because many M. pneumoniae infections such as mild tracheobronchitis are often undiagnosed.
The high macrolide resistance rates in these countries are certainly associated with antibiotic selective pressure because of extensive macrolide use. This is supported by the highest macrolide resistance rates being reported in countries with extensive macrolide use such as Japan (Okada et al., 2012). In addition, macrolide resistance was often associated with recent receipt of macrolides, suggesting that a resistant subpopulation may develop or expand during the course of macrolide therapy within an individual patient (Averbuch et al., 2011; Cardinale et al., 2011; Chironna et al., 2011; Hantz et al., 2012; Dumke et al., 2014). Acquisition of resistance has first been documented in patients receiving macrolides (Averbuch et al., 2011; Cardinale et al., 2011) then confirmed using typing methods such as adhesin P1 typing and multi-locus variable-number tandem-repeat analysis (MLVA) in patients receiving macrolides (Hantz et al., 2012; Dumke et al., 2014).
In North America, Europe, and Australia, rates of macrolide resistance dramatically contrast with those in reports from Asia. In the USA and Canada, rates have been recently reported between 3.5 and 13.2% (Table 2). In Europe, rates have remained below 10% except in Italy were a rate of 26% was observed on a small number of M. pneumoniae-positive specimens collected during an outbreak (Chironna et al., 2011).
All over the world, the A2058G transition largely predominates over the A2059G substitution and mutations at position 2611 and 2062 are rare (Table 2). Nevertheless, the rarely reported A2058T transversion was found in 47% of macrolide-resistant M. pneumoniae strains infecting children during an outbreak in Fukuoka, Japan (Matsuda et al., 2013). Despite the high proportion of the A2058G transition, no association was reported between MLVA types and macrolide resistance in several studies (Dégrange et al., 2009; Benitez et al., 2012; Liu et al., 2012; Zhao et al., 2013a,b; Dumke et al., 2015; Diaz et al., 2015a,b) indicating that macrolide resistance is a result of the spread of multiple resistant clones. A possible correlation was reported in Jerusalem, Israel, between the MLVA type Z (7-4-5-7-2) and the A2058G-associated macrolide resistance but the number of cases was limited (Pereyre et al., 2012). Recently, an association between macrolide resistant M. pneumoniae isolates and the MLVA type 4-5-7-2 was suggested in China and Hong-Kong (Ho et al., 2015; Yan et al., 2015). However the prevalence of this MLVA type was high in these countries and the deletion of the unstable MPN1 marker from the MLVA method (Chalker et al., 2015) led to a too weakly discriminant typing method to draw accurate conclusions.
Clinical relevance of M. pneumoniae macrolide resistance
Regarding clinical presentation, no difference was observed between patients infected by macrolide-resistant and macrolide-sensitive M. pneumoniae. Clinical symptoms, pneumonia severity, laboratory results, radiographic findings and prognostic factors were similar regardless of the M. pneumoniae susceptibility to macrolides (Matsubara et al., 2009; Cardinale et al., 2013; Miyashita et al., 2013; Wu et al., 2013; Diaz et al., 2015a). Most infections with macrolide-resistant M. pneumoniae have been reported in children because M. pneumoniae infections are more frequent in this population. Nevertheless, several adults have also been evaluated (Cao et al., 2010; Ferguson et al., 2013; Ho et al., 2015; Diaz et al., 2015a). To date, no difference has been found in disease manifestations between children and adults infected by macrolide-resistant M. pneumoniae.
As expected, the efficacy of macrolide treatment was shown to be lower in patients infected with macrolide-resistant isolates than in patients infected with macrolide-sensitive isolates. Despite macrolide administration, the duration of fever and cough, the duration of hospitalization and antibiotic administration were significantly longer in patients with macrolide-resistant M. pneumoniae infections. Moreover, the persistence of symptoms led to change of antibiotic prescription more often (Suzuki et al., 2006; Morozumi et al., 2008; Matsubara et al., 2009; Cardinale et al., 2013; Wu et al., 2013; Zhou et al., 2014). However, the clinical relevance of macrolide resistance in patients was usually limited to prolonging symptoms of the disease and not increasing the risk of complications. Only a single study has reported that the incidence of extrapulmonary complications was higher in children with macrolide-resistant isolates and that the radiological findings were more serious (Zhou et al., 2014).
Treatment of M. pneumoniae respiratory infections
Macrolides and related antibiotics are the first-line treatment of M. pneumoniae respiratory tract infections mainly because of their low MIC against the bacteria, their low toxicity and the absence of contraindication in young children. The agent of first choice differs from country to country according to different published guidelines and owing to the fact that not all agents are available in all countries (Mandell et al., 2007; Bradley et al., 2011; Harris et al., 2011; Woodhead et al., 2011; Waites and Bébéar, 2013). The newer macrolides are now often the preferred agents with a 7-to-14 day course of oral clarithromycin or a 5-day course of oral azithromycin for treatment of community-acquired pneumonia due to M. pneumoniae (Waites and Bébéar, 2013). An appropriate antimicrobial therapy usually shortens the symptomatic period of M. pneumoniae infections, and hastens radiological resolution and recovery. However, using real-time PCR, it has been shown that the median time for carriage of M. pneumoniae DNA was 7 weeks after disease onset and that an adequate antibiotic treatment did not shorten the period of persistence of M. pneumoniae DNA in patient specimens (Nilsson et al., 2008). No treatment recommendation is available for extrapulmonary manifestations. In a few published case reports, macrolides and fluoroquinolones, mainly levofloxacin, have successfully been used (Scapini et al., 2008; Atkinson et al., 2011; Esposito et al., 2011; Meyer Sauteur et al., 2012; Godron et al., 2013).
In cases of macrolide-resistant M. pneumoniae strains, alternative antibiotic treatment can be required, including tetracyclines such as doxycycline and minocycline, or fluoroquinolones, primarily levofloxacin, even though fluoroquinolones and tetracyclines are contraindicated in all children and in children < 8 year-old, respectively. Treatment lengths usually range between 7 and 14 days. As expected, fluoroquinolone and tetracycline regimens were shown to be more effective than macrolide regimens in patients infected by macrolide-resistant M. pneumoniae (Kawai et al., 2013; Miyashita et al., 2013). However, macrolides appear clinically effective in some patients infected by macrolide-resistant strains (Suzuki et al., 2006; Matsubara et al., 2009; Cardinale et al., 2013). This observation can be explained by the fact that M. pneumoniae infections are often self-limited diseases and that the anti-inflammatory effects of macrolides may improve clinical symptoms.
In Europe, Oceania, and America, where the prevalence of macrolide-resistant strains remains low, macrolides are the drug of choice in children with M. pneumoniae respiratory infections. Nevertheless, in these continents, clinicians should be vigilant for macrolide treatment failure and consider using alternative drugs if symptoms persist or if there are signs of clinical deteriorations. In countries in which the prevalence of macrolide-resistant strains is high, the replacement of macrolides as the first choice treatment by tetracyclines or fluoroquinolones was considered. However, surprisingly, in Japan, according to the 2013 recommendations of the Japanese Pediatric Society, macrolides remain the first-line treatment despite macrolide resistance rates over 80%. In this country, the efficacy of macrolides has to be evaluated by defervescence 48–72 h following the administration of these antimicrobials. In pneumonia cases in which the initial macrolide therapy resulted in failure, administration of alternative antimicrobial treatment, either respiratory fluoroquinolones or tetracyclines, must be considered. In contrast to Europe and to the United States, oral tosufloxacin, a fluoroquinolone antibiotic, was approved in Japan for pediatric use as a second line treatment in patients with community-acquired pneumonia. Indeed, in one study performed for the registration application of tosufloxacin in Japan, the occurrence of joint paint was only 0.85% (2/235) and there was no magnetic resonance imaging abnormal finding on joints (data given by Dr T. Oishi, Japan). Another study on 83 pediatric patients with M. pneumoniae pneumonia treated with tosufloxacin reported that side effects included mild diarrhea, but that no patients had joint symptoms (Sakata, 2012). Although, tosufloxacin was les effective than minocycline or doxycycline in achieving defervescence within 24 h and in decreasing the DNA load of M. pneumoniae (Okada et al., 2012; Kawai et al., 2013), its use is accepted in children under 8-year old. In countries where tosufloxacin is not available, other available respiratory fluoroquinolones might be chosen in severe cases despite contraindication. In children over 8-year old and adults, minocycline can be used as second-line treatment.
Although, no tetracycline or fluoroquinolone resistance has been reported in clinical isolates to date, resistant strains have been selected in vitro for both classes of drugs with target mutations identified in mutants (Gruson et al., 2005; Degrange et al., 2008). Thus, the risk of emergence of resistance in clinical isolates exists, especially for fluoroquinolones, if these antibiotics are inappropriately used. It should be noted that clinical resistance to fluoroquinolones has already been reported already in Mycoplasma genitalium, a urogenital mycoplasma phylogenetically close to M. pneumoniae, in which macrolide resistance mechanisms are similar to that of M. pneumoniae (Couldwell et al., 2013; Bissessor et al., 2015).
Consequently to macrolide resistance in M. pneumoniae, reevaluation of existing classes using and investigation of new classes of antimicrobials may be required to get additional treatment alternative beyond tetracyclines and fluoroquinolones, especially in children under 8 year-old. Randomized therapeutic trials will be necessary to establish guidelines regarding the most appropriate molecule, dose and length of treatment to use against the resistant strains. In the future, it will also be interesting to evaluate the activity of streptogramin combinations, such as oral pristinamycin, which has been shown to retain activity against 23S rRNA M. pneumoniae in in vitro mutants and in a few clinical isolates (Pereyre et al., 2004a, 2007). Indeed, pristinamycin was reported to be active on a few cases of genital infections by macrolide-resistant fluoroquinolone-resistant M. genitalium isolates (Bissessor et al., 2015). Although, additional studies on a large number of strains are required, pristinamycin could become an alternative antibiotic treatment in countries where this antibiotic is available (Bebear, 2012).
Conclusion
Nowadays, M. pneumoniae macrolide resistance rates are extremely high in Asia and remain moderate in Europe and North America. Macrolide resistance detection using accurate molecular methods should be considered in all M. pneumoniae-positive specimens since it has both a direct application in clinical practice and an epidemiological surveillance interest. At the individual level, a rapid detection of resistance-associated mutations would enable the prompt prescription of an alternative antimicrobial regimen, especially in case of persistent or recurrent M. pneumoniae infection. At the community level, the high prevalence of macrolide-resistant M. pneumoniae isolates in Asia underscore the potential for rapid emergence of macrolide resistance within M. pneumoniae in other parts of the world. Thus, further epidemiological studies are needed in Europe and the USA to monitor macrolide resistance rates. Moreover, macrolide stewardship may be needed for restricting the use of these antibiotics, reduce unnecessary antibiotic prescribing, especially in countries with remaining low macrolide resistant rates. In Asia, the epidemiological surveillance of antibiotic resistance would also be of interest to early detect potential selections of fluoroquinolone- and tetracycline-resistant clinical isolates associated with the increasing use of these classes of antibiotics.
Author contributions
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Tomohiro Oishi (Department of Pediatrics, Kawasaki Medical School, Okayama, Japan) for helpful discussions.
References
- Akaike H., Miyashita N., Kubo M., Kawai Y., Tanaka T., Ogita S., et al. (2012). In vitro activities of 11 antimicrobial agents against macrolide-resistant Mycoplasma pneumoniae isolates from pediatric patients: results from a multicenter surveillance study. Jpn. J. Infect. Dis. 65, 535–538. 10.7883/yoken.65.535 [DOI] [PubMed] [Google Scholar]
- Atkinson T. P., Balish M. F., Waites K. B. (2008). Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol. Rev. 32, 956–973. 10.1111/j.1574-6976.2008.00129.x [DOI] [PubMed] [Google Scholar]
- Atkinson T. P., Boppana S., Theos A., Clements L. S., Xiao L., Waites K. (2011). Stevens-Johnson syndrome in a boy with macrolide-resistant Mycoplasma pneumoniae pneumonia. Pediatrics 127, e1605–e1609. 10.1542/peds.2010-2624 [DOI] [PubMed] [Google Scholar]
- Averbuch D., Hidalgo-Grass C., Moses A. E., Engelhard D., Nir-Paz R. (2011). Macrolide resistance in Mycoplasma pneumoniae, Israel, 2010. Emerging Infect. Dis. 17, 1079–1082. 10.3201/eid/1706.101558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebear C. (2012). Editorial commentary: infections due to macrolide-resistant Mycoplasma pneumoniae: now what? Clin. Infect. Dis. 55, 1650–1651. 10.1093/cid/cis791 [DOI] [PubMed] [Google Scholar]
- Bébéar C. M., Kempf I. (2005). Antimicrobial therapy and antimicrobial resistance, in Mycoplasmas Molecular Biology Pathogenicity and Strategies for Control, eds Blanchard A., Browning G. F. (Norfolk, VA: Horizon bioscience; ), 535–568. [Google Scholar]
- Bébéar C. M., Pereyre S. (2005). Mechanisms of drug resistance in Mycoplasma pneumoniae. Curr. Drug Targets Infect. Disord. 5, 263–271. 10.2174/1568005054880109 [DOI] [PubMed] [Google Scholar]
- Bébéar C., Pereyre S., Peuchant O. (2011). Mycoplasma pneumoniae: susceptibility and resistance to antibiotics. Future Microbiol. 6, 423–431. 10.2217/fmb.11.18 [DOI] [PubMed] [Google Scholar]
- Benitez A. J., Diaz M. H., Wolff B. J., Pimentel G., Njenga M. K., Estevez A., et al. (2012). Multilocus variable-number tandem-repeat analysis of Mycoplasma pneumoniae clinical isolates from 1962 to the present: a retrospective study. J. Clin. Microbiol. 50, 3620–3626. 10.1128/JCM.01755-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissessor M., Tabrizi S. N., Twin J., Abdo H., Fairley C. K., Chen M. Y., et al. (2015). Macrolide resistance and azithromycin failure in a Mycoplasma genitalium-infected cohort, and response of azithromycin failures to alternative antibiotic regimens. Clin. Infect. Dis. 60, 1228–1236. 10.1093/cid/ciu1162 [DOI] [PubMed] [Google Scholar]
- Bradley J. S., Byington C. L., Shah S. S., Alverson B., Carter E. R., Harrison C., et al. (2011). The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53, e25–e76. 10.1093/cid/cir531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. J., Macfarlane-Smith L., Phillips S., Chalker V. J. (2015). Detection of macrolide resistant Mycoplasma pneumoniae in England, September 2014 to September 2015. Euro. Surveill. 20:30078. 10.2807/1560-7917.ES.2015.20.48.30078 [DOI] [PubMed] [Google Scholar]
- Cao B., Zhao C. J., Yin Y. D., Zhao F., Song S. F., Bai L., et al. (2010). High prevalence of macrolide resistance in Mycoplasma pneumoniae isolates from adult and adolescent patients with respiratory tract infection in China. Clin. Infect. Dis. 51, 189–194. 10.1086/653535 [DOI] [PubMed] [Google Scholar]
- Cardinale F., Chironna M., Chinellato I., Principi N., Esposito S. (2013). Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J. Clin. Microbiol. 51, 723–724. 10.1128/JCM.02840-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale F., Chironna M., Dumke R., Binetti A., Daleno C., Sallustio A., et al. (2011). Macrolide-resistant Mycoplasma pneumoniae in paediatric pneumonia. Eur. Respir. J. 37, 1522–1524. 10.1183/09031936.00172510 [DOI] [PubMed] [Google Scholar]
- Chalker V. J., Pereyre S., Dumke R., Winchell J., Khosla P., Sun H., et al. (2015). International Mycoplasma pneumoniae typing study: interpretation of M. pneumoniae multilocus variable-number tandem-repeat analysis. New Microb. New Infect. 7, 37–40. 10.1016/j.nmni.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker V., Stocki T., Litt D., Bermingham A., Watson J., Fleming D., et al. (2012). Increased detection of Mycoplasma pneumoniae infection in children in England and Wales, October 2011 to January 2012. Euro. Surveill. 17:20081. [PubMed] [Google Scholar]
- Chalker V., Stocki T., Mentasti M., Fleming D., Harrison T. (2011). Increased incidence of Mycoplasma pneumoniae infection in England and Wales in 2010: multilocus variable number tandem repeat analysis typing and macrolide susceptibility. Euro Surveill. 16:19865. [PubMed] [Google Scholar]
- Chironna M., Sallustio A., Esposito S., Perulli M., Chinellato I., Di Bari C., et al. (2011). Emergence of macrolide-resistant strains during an outbreak of Mycoplasma pneumoniae infections in children. J. Antimicrob. Chemother. 66, 734–737. 10.1093/jac/dkr003 [DOI] [PubMed] [Google Scholar]
- Couldwell D. L., Tagg K. A., Jeoffreys N. J., Gilbert G. L. (2013). Failure of moxifloxacin treatment in Mycoplasma genitalium infections due to macrolide and fluoroquinolone resistance. Int. J. STD AIDS. 24, 822–828. 10.1177/0956462413502008 [DOI] [PubMed] [Google Scholar]
- Critchley I. A., Jones M. E., Heinze P. D., Hubbard D., Engler H. D., Evangelista A. T., et al. (2002). In vitro activity of levofloxacin against contemporary clinical isolates of Legionella pneumophila, Mycoplasma pneumoniae and Chlamydia pneumoniae from North America and Europe. Clin. Microbiol. Infect. 8, 214–221. 10.1046/j.1469-0691.2002.00392.x [DOI] [PubMed] [Google Scholar]
- Dégrange S., Cazanave C., Charron A., Renaudin H., Bébéar C., Bébéar C. M. (2009). Development of multiple-locus variable-number tandem-repeat analysis for molecular typing of Mycoplasma pneumoniae. J. Clin. Microbiol. 47, 914–923. 10.1128/JCM.01935-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrange S., Renaudin H., Charron A., Pereyre S., Bébéar C., Bébéar C. M. (2008). Reduced susceptibility to tetracyclines is associated in vitro with the presence of 16S rRNA mutations in Mycoplasma hominis and Mycoplasma pneumoniae. J. Antimicrob. Chemother. 61, 1390–1392. 10.1093/jac/dkn118 [DOI] [PubMed] [Google Scholar]
- Diaz M. H., Benitez A. J., Cross K. E., Hicks L. A., Kutty P., Bramley A. M., et al. (2015a). Molecular detection and characterization of Mycoplasma pneumoniae among patients hospitalized with community-acquired pneumonia in the United States. Open Forum Infect. Dis. 2:ofv106. 10.1093/ofid/ofv106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M. H., Benitez A. J., Winchell J. M. (2015b). Investigations of Mycoplasma pneumoniae infections in the United States: trends in molecular typing and macrolide resistance from 2006 to 2013. J. Clin. Microbiol. 53, 124–130. 10.1128/JCM.02597-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumke R., Luck C., Jacobs E. (2013). Low rate of macrolide resistance in Mycoplasma pneumoniae strains in Germany between 2009 and 2012. Antimicrob. Agents Chemother. 57, 3460. 10.1128/AAC.00706-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumke R., Schnee C., Pletz M. W., Rupp J., Jacobs E., Sachse K., et al. (2015). Mycoplasma pneumoniae and Chlamydia spp. infection in community-acquired pneumonia, Germany, 2011-2012. Emerg. Infect. Dis. 21, 426–434. 10.3201/eid2103.140927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumke R., Stolz S., Jacobs E., Juretzek T. (2014). Molecular characterization of macrolide resistance of a Mycoplasma pneumoniae strain that developed during therapy of a patient with pneumonia. Int. J. Infect. Dis. 29, 197–199. 10.1016/j.ijid.2014.07.014 [DOI] [PubMed] [Google Scholar]
- Dumke R., von Baum H., Luck P. C., Jacobs E. (2010). Occurrence of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Clin. Microbiol. Infect. 16, 613–616. 10.1111/j.1469-0691.2009.02968.x [DOI] [PubMed] [Google Scholar]
- Eshaghi A., Memari N., Tang P., Olsha R., Farrell D. J., Low D. E., et al. (2013). Macrolide-resistant Mycoplasma pneumoniae in humans, Ontario, Canada, 2010-2011. Emerg. Infect. Dis. 19, 1525–1527. 10.3201/eid1909.121466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S., Tagliabue C., Bosis S., Principi N. (2011). Levofloxacin for the treatment of Mycoplasma pneumoniae-associated meningoencephalitis in childhood. Int. J. Antimicrob. Agents. 37, 472–475. 10.1016/j.ijantimicag.2011.01.008 [DOI] [PubMed] [Google Scholar]
- Ferguson G. D., Gadsby N. J., Henderson S. S., Hardie A., Kalima P., Morris A. C., et al. (2013). Clinical outcomes and macrolide resistance in Mycoplasma pneumoniae infection in Scotland, UK. J. Med. Microbiol. 62, 1876–1882. 10.1099/jmm.0.066191-0 [DOI] [PubMed] [Google Scholar]
- Godron A., Pereyre S., Monet C., Llanas B., Harambat J. (2013). Hemolytic uremic syndrome complicating Mycoplasma pneumoniae infection. Pediatr. Nephrol. 28, 2057–2060. 10.1007/s00467-013-2541-5 [DOI] [PubMed] [Google Scholar]
- Gruson D., Pereyre S., Renaudin H., Charron A., Bébéar C., Bébéar C. M. (2005). In vitro development of resistance to six and four fluoroquinolones in Mycoplasma pneumoniae and Mycoplasma hominis, respectively. Antimicrob. Agents Chemother. 49, 1190–1193. 10.1128/AAC.49.3.1190-1193.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantz S., Garnier F., Peuchant O., Menetrey C., Charron A., Ploy M. C., et al. (2012). Multilocus variable-number tandem-repeat analysis-confirmed emergence of a macrolide resistance-associated mutation in Mycoplasma pneumoniae during macrolide therapy for interstitial pneumonia in an immunocompromised child. J. Clin. Microbiol. 50, 3402–3405. 10.1128/JCM.01248-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M., Clark J., Coote N., Fletcher P., Harnden A., McKean M., et al. (2011). British Thoracic Society guidelines for the management of community acquired pneumonia in children: update. Thorax. 66 (Suppl. 2), ii1–ii23. 10.1136/thoraxjnl-2011-200598 [DOI] [PubMed] [Google Scholar]
- Ho P. L., Law P. Y., Chan B. W., Wong C. W., To K. K., Chiu S. S., et al. (2015). Emergence of macrolide-resistant Mycoplasma pneumoniae in Hong Kong is linked to increasing macrolide resistance in multilocus variable-number tandem-repeat analysis type 4-5-7-2. J. Clin. Microbiol. 53, 3560–3564. 10.1128/JCM.01983-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K. B., Choi E. H., Lee H. J., Lee S. Y., Cho E. Y., Choi J. H., et al. (2013). Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000-2011. Emerg. Infect. Dis. 19, 1281–1284. 10.3201/eid1908.121455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro N., Koseki N., Kaiho M., Kikuta H., Togashi T., Oba K., et al. (2015). Regional differences in prevalence of macrolide-resistant Mycoplasma pneumoniae in Hokkaido, Japan. Jpn. J. Infect. Dis. 69, 186–190. 10.7883/yoken.JJID.2015.054 [DOI] [PubMed] [Google Scholar]
- Ji M., Lee N. S., Oh J. M., Jo J. Y., Choi E. H., Yoo S. J., et al. (2014). Single-nucleotide polymorphism PCR for the detection of Mycoplasma pneumoniae and determination of macrolide resistance in respiratory samples. J. Microbiol. Methods. 102, 32–36. 10.1016/j.mimet.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Kawai Y., Miyashita N., Kubo M., Akaike H., Kato A., Nishizawa Y., et al. (2013). Nationwide surveillance of macrolide-resistant Mycoplasma pneumoniae infection in pediatric patients. Antimicrob. Agents Chemother. 57, 4046–4049. 10.1128/AAC.00663-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogoj R., Mrvic T., Praprotnik M., Kese D. (2015). Prevalence, genotyping and macrolide resistance of Mycoplasma pneumoniae among isolates of patients with respiratory tract infections, Central Slovenia, 2006 to 2014. Euro Surveill. 20:30018. 10.2807/1560-7917.ES.2015.20.37.30018 [DOI] [PubMed] [Google Scholar]
- Lin C., Li S., Sun H., Zhao H., Feng Y., Cao L., et al. (2010). Nested PCR-linked capillary electrophoresis and single-strand conformation polymorphisms for detection of macrolide-resistant Mycoplasma pneumoniae in Beijing, China. J. Clin. Microbiol. 48, 4567–4572. 10.1128/JCM.00400-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ye X., Zhang H., Xu X., Li W., Zhu D., et al. (2010). Characterization of macrolide resistance in Mycoplasma pneumoniae isolated from children in Shanghai, China. Diagn. Microbiol. Infect. Dis. 67, 355–358. 10.1016/j.diagmicrobio.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Liu Y., Ye X., Zhang H., Xu X., Wang M. (2012). Multiclonal origin of macrolide-resistant Mycoplasma pneumoniae isolates as determined by multilocus variable-number tandem-repeat analysis. J. Clin. Microbiol. 50, 2793–2795. 10.1128/JCM.00678-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell L. A., Wunderink R. G., Anzueto A., Bartlett J. G., Campbell G. D., Dean N. C., et al. (2007). Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 44 (Suppl. 2), S27–S72. 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K., Morozumi M., Okada T., Matsushima T., Komiyama O., Shoji M., et al. (2009). A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J. Infect. Chemother. 15, 380–383. 10.1007/s10156-009-0715-7 [DOI] [PubMed] [Google Scholar]
- Matsuda K., Narita M., Sera N., Maeda E., Yoshitomi H., Ohya H., et al. (2013). Gene and cytokine profile analysis of macrolide-resistant Mycoplasma pneumoniae infection in Fukuoka, Japan. BMC Infect. Dis. 13:591. 10.1186/1471-2334-13-591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M., Narita M., Okazaki N., Ohya H., Yamazaki T., Ouchi K., et al. (2004). Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob. Agents Chemother. 48, 4624–4630. 10.1128/AAC.48.12.4624-4630.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Sauteur P. M., Bleisch B., Voit A., Maurer F. P., Relly C., Berger C., et al. (2014). Survey of macrolide-resistant Mycoplasma pneumoniae in children with community-acquired pneumonia in Switzerland. Swiss Med. Wkly. 144:w14041. 10.4414/smw.2014.14041 [DOI] [PubMed] [Google Scholar]
- Meyer Sauteur P. M., Huber B. M., Goetschel P. (2012). Neuroinvasive Mycoplasma pneumoniae infection without intrathecal antibody response. Pediatr. Infect. Dis. J. 31, 1199–1200. 10.1097/INF.0b013e318266abff [DOI] [PubMed] [Google Scholar]
- Miyashita N., Akaike H., Teranishi H., Ouchi K., Okimoto N. (2013). Macrolide-resistant Mycoplasma pneumoniae pneumonia in adolescents and adults: clinical findings, drug susceptibility, and therapeutic efficacy. Antimicrob. Agents Chemother. 57, 5181–5185. 10.1128/AAC.00737-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozumi M., Iwata S., Hasegawa K., Chiba N., Takayanagi R., Matsubara K., et al. (2008). Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob. Agents Chemother. 52, 348–350. 10.1128/AAC.00779-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozumi M., Takahashi T., Ubukata K. (2010). Macrolide-resistant Mycoplasma pneumoniae: characteristics of isolates and clinical aspects of community-acquired pneumonia. J. Infect. Chemother. 16, 78–86. 10.1007/s10156-009-0021-4 [DOI] [PubMed] [Google Scholar]
- Niitu Y., Hasegawa S., Suetake T., Kubota H., Komatsu S., Horikawa M. (1970). Resistance of Mycoplasma pneumoniae to erythromycin and other antibiotics. J. Pediatr. 76, 438–443. 10.1016/S0022-3476(70)80485-1 [DOI] [PubMed] [Google Scholar]
- Nilsson A. C., Bjorkman P., Persson K. (2008). Polymerase chain reaction is superior to serology for the diagnosis of acute Mycoplasma pneumoniae infection and reveals a high rate of persistent infection. BMC Microbiol. 8:93. 10.1186/1471-2180-8-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T., Morozumi M., Tajima T., Hasegawa M., Sakata H., Ohnari S., et al. (2012). Rapid effectiveness of minocycline or doxyxycline against macrolide-resistant Mycoplasma pneumoniae infection in a outbreak among japanese children. Clin. Infect. Dis. 55, 1642–1649. 10.1093/cid/cis784 [DOI] [PubMed] [Google Scholar]
- Pereyre S., Charron A., Hidalgo-Grass C., Touati A., Moses A. E., Nir-Paz R., et al. (2012). The spread of Mycoplasma pneumoniae is polyclonal in both an endemic setting in France and in an epidemic setting in Israel. PLoS ONE 7:e38585. 10.1371/journal.pone.0038585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyre S., Charron A., Renaudin H., Bébéar C., Bébéar C. M. (2007). First report of macrolide-resistant strains and description of a novel nucleotide sequence variation in the P1 adhesin gene in Mycoplasma pneumoniae clinical strains isolated in France over 12 years. J. Clin. Microbiol. 45, 3534–3539. 10.1128/JCM.01345-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyre S., Guyot C., Renaudin H., Charron A., Bébéar C., Bébéar C. M. (2004a). In vitro selection and characterization of resistance to macrolides and related antibiotics in Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 48, 460–465. 10.1128/AAC.48.2.460-465.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyre S., Renaudin H., Bébéar C., Bébéar C. M. (2004b). In vitro activities of the newer quinolones garenoxacin, gatifloxacin, and gemifloxacin against human mycoplasmas. Antimicrob. Agents Chemother. 48, 3165–3168. 10.1128/AAC.48.8.3165-3168.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyre S., Touati A., Petitjean-Lecherbonnier J., Charron A., Vabret A., Bébéar C. (2013). The increased incidence of Mycoplasma pneumoniae in France in 2011 was polyclonal, mainly involving M. pneumoniae type 1 strains. Clin. Microbiol. Infect. 19, E212–E217. 10.1111/1469-0691.12107 [DOI] [PubMed] [Google Scholar]
- Peuchant O., Menard A., Renaudin H., Morozumi M., Ubukata K., Bébéar C. M., et al. (2009). Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by real-time PCR and melting curve analysis. J. Antimicrob. Chemother. 64, 52–58. 10.1093/jac/dkp160 [DOI] [PubMed] [Google Scholar]
- Pucci M. J., Podos S. D., Thanassi J. A., Leggio M. J., Bradbury B. J., Deshpande M. (2011). In vitro and in vivo profiles of ACH-702, an isothiazoloquinolone, against bacterial pathogens. Antimicrob. Agents Chemother. 55, 2860–2871. 10.1128/AAC.01666-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha E. P. C., Blanchard A. (2002). Genomic repeats, genome plasticity and the dynamics of Mycoplasma evolution. Nucleic Acids Res. 30, 2031–2042. 10.1093/nar/30.9.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sader H. S., Paukner S., Ivezic-Schoenfeld Z., Biedenbach D. J., Schmitz F. J., Jones R. N. (2012). Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs). J. Antimicrob. Chemother. 67, 1170–1175. 10.1093/jac/dks001 [DOI] [PubMed] [Google Scholar]
- Sakata H. (2012). Clinical efficacy of tosufloxacin in children with pneumonia due to Mycoplasma pneumoniae. Jpn. J. Antibiot. 65, 173–179. [PubMed] [Google Scholar]
- Scapini J. P., Flynn L. P., Sciacaluga S., Morales L., Cadario M. E. (2008). Confirmed Mycoplasma pneumoniae endocarditis. Emerging Infect. Dis. 14, 1664–1665. 10.3201/eid1410.080157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spuesens E. B., Hoogenboezem T., Sluijter M., Hartwig N. G., van Rossum A. M., Vink C. (2010). Macrolide resistance determination and molecular typing of Mycoplasma pneumoniae by pyrosequencing. J. Microbiol. Methods. 82, 214–222. 10.1016/j.mimet.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Spuesens E. B., Meijer A., Bierschenk D., Hoogenboezem T., Donker G. A., Hartwig N. G., et al. (2012). Macrolide-resistance determination and molecular typing of Mycoplasma pneumoniae in respiratory specimens collected between 1997 and 2008 in The Netherlands. J. Clin. Microbiol. 50, 1999–2004. 10.1128/JCM.00400-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopler T., Branski D. (1986). Resistance of Mycoplasma pneumoniae to macrolides, lincomycin and streptogramin B. J. Antimicrob. Chemother. 18, 359–364. 10.1093/jac/18.3.359 [DOI] [PubMed] [Google Scholar]
- Sun H., Xue G., Yan C., Li S., Cao L., Yuan Y., et al. (2013). Multiple-locus variable-number tandem-repeat analysis of Mycoplasma pneumoniae clinical specimens and proposal for amendment of MLVA nomenclature. PLoS ONE. 8:e64607. 10.1371/journal.pone.0064607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Yamazaki T., Narita M., Okazaki N., Suzuki I., Andoh T., et al. (2006). Clinical Evaluation of Macrolide-Resistant Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 50, 709–712. 10.1128/AAC.50.2.709-712.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldum S. A., Bangsborg J. M., Gahrn-Hansen B., Ljung R., Molvadgaard M., Fons Petersen R., et al. (2012). Epidemic of Mycoplasma pneumoniae infection in Denmark, 2010 and 2011. Euro. Surveill. 17:20073. [DOI] [PubMed] [Google Scholar]
- Waites K. B., Bade D. J., Bébéar C., Brown S. D., Davidson M., Duffy L. B., et al. (2011). Methods for Antimicrobial Susceptibility Testing for Human Mycoplasmas : Approved Guideline. Wayne, PA: Clinical and Laboratory Standards Institute, Document M43–P. [PubMed] [Google Scholar]
- Waites K. B., Bébéar C. M. (2013). Chemotherapy of Mycoplasma and Ureaplasma infections, in Encyclopedia of Pharmaceutical Microbiology, eds Gahan C., Nightingale C. H., Charles H. (Springer; ). [Google Scholar]
- Waites K. B., Crabb D. M., Duffy L. B. (2009). Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob. Agents Chemother. 53, 2139–2141. 10.1128/AAC.00090-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites K. B., Crabb D. M., Duffy L. B., Huband M. D. (2015). In vitro antibacterial activity of AZD0914 against human Mycoplasmas and Ureaplasmas. Antimicrob. Agents Chemother. 59, 3627–3629. 10.1128/AAC.04945-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff B. J., Thacker W. L., Schwartz S. B., Winchell J. M. (2008). Detection of macrolide resistance in Mycoplasma pneumoniae by real-time PCR and high-resolution melt analysis. Antimicrob. Agents Chemother. 52, 3542–3549. 10.1128/AAC.00582-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhead M., Blasi F., Ewig S., Garau J., Huchon G., Ieven M., et al. (2011). Guidelines for the management of adult lower respiratory tract infections–full version. Clin. Microbiol. Infect. 17 (Suppl. 6), E1–E59. 10.1111/j.1469-0691.2011.03672.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. S., Chang L. Y., Lin H. C., Chi H., Hsieh Y. C., Huang Y. C., et al. (2013). Epidemiology and clinical manifestations of children with macrolide-resistant Mycoplasma pneumoniae pneumonia in Taiwan. Pediatr. Pulmonol. 48, 904–911. 10.1002/ppul.22706 [DOI] [PubMed] [Google Scholar]
- Xin D., Mi Z., Han X., Qin L., Li J., Wei T., et al. (2009). Molecular mechanisms of macrolide resistance in clinical isolates of Mycoplasma pneumoniae from China. Antimicrob. Agents Chemother. 53, 2158–2159. 10.1128/AAC.01563-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G., Wang Q., Yan C., Jeoffreys N., Wang L., Li S., et al. (2014). Molecular characterizations of PCR-positive Mycoplasma pneumoniae specimens collected from Australia and China. J. Clin. Microbiol. 52, 1478–1482. 10.1128/JCM.03366-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Buller R., Bledsoe S., Storch G. A. (2012). Rising rates of macrolide-resistant Mycoplasma pneumoniae in the central United States. Pediatr. Infect. Dis. J. 31, 409–400. 10.1097/INF.0b013e318247f3e0 [DOI] [PubMed] [Google Scholar]
- Yamazaki T., Sasaki T., Takahata M. (2007). Activity of garenoxacin against macrolide-susceptible and -resistant Mycoplasma pneumoniae. Antimicrob. Agents Chemother. 51, 2278–2279. 10.1128/AAC.01561-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Sun H., Lee S., Selvarangan R., Qin X., Tang Y. W., et al. (2015). Comparison of molecular characteristics of Mycoplasma pneumoniae collected from, U.S. and China. J. Clin. Microbiol. 53, 3891–3893. 10.1128/JCM.02468-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Sun H., Xue G., Zhao H., Wang L., Feng Y., et al. (2014). A single-tube multiple-locus variable-number tandem-repeat analysis of Mycoplasma pneumoniae clinical specimens by use of multiplex PCR-capillary electrophoresis. J. Clin. Microbiol. 52, 4168–4171. 10.1128/JCM.02178-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Cao B., Li J., Song S., Tao X., Yin Y., et al. (2011). Sequence analysis of the P1 adhesin gene of Mycoplasma pneumoniae in clinical isolates collected in Beijing in 2008 to 2009. J. Clin. Microbiol. 49, 3000–3003. 10.1128/JCM.00105-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Liu G., Cao B., Wu J., Gu Y., He L., et al. (2013a). Multiple-locus variable-number tandem-repeat analysis of 201 Mycoplasma pneumoniae isolates from Beijing, China, from 2008 to 2011. J. Clin. Microbiol. 51, 636–639. 10.1128/JCM.02567-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Liu G., Wu J., Cao B., Tao X., He L., et al. (2013b). Surveillance of macrolide-resistant Mycoplasma pneumoniae in Beijing, China, from 2008 to 2012. Antimicrob. Agents Chemother. 57, 1521–1523. 10.1128/AAC.02060-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Lee S., Selvarangan R., Qin X., Tang Y. W., Stiles J., et al. (2015). Macrolide-resistant Mycoplasma pneumoniae, United States. Emerg. Infect. Dis. 21, 1470–1472. 10.3201/eid2108.150273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zhang Y., Sheng Y., Zhang L., Shen Z., Chen Z. (2014). More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother. 58, 1034–1038. 10.1128/AAC.01806-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Li X., Chen X., Luo F., Pan C., Zheng X., et al. (2015). Macrolide-resistant Mycoplasma pneumoniae in adults in Zhejiang, China. Antimicrob. Agents Chemother. 59, 1048–1051. 10.1128/AAC.04308-14 [DOI] [PMC free article] [PubMed] [Google Scholar]