Abstract
The risk of influenza A virus (IAV) is more likely caused by secondary bacterial infections. During the past decades, a great amount of studies have been conducted on increased morbidity from secondary bacterial infections following influenza and provide an increasing number of explanations for the mechanisms underlying the infections. In this paper, we first review the recent research progress that IAV infection increased susceptibility to bacterial infection. We then propose an assumption that autophagy and apoptosis manipulation are beneficial to antagonize post-IAV bacterial infection and discuss the clinical significance.
1. Introduction
(1) Influenza A Virus and Secondary Bacterial Infection. It was reported that a pandemic influenza killed over 40 million people in 1918 [1, 2]. With the development of modern medicine and improvement of hygiene habits, the probability of influenza pandemic outbreak has been greatly decreased. However, influenza A virus (IAV), a negative-sense RNA virus with 8 separate segments in its genome [3], is very easy to mutate, which results in novel hemagglutinin (HA) production [4]. These mutations render IAV to invade easily population without immunity [5]. Studies have shown that it is the synergy between the viruses and bacteria that presents a great threat to public health while the viral infection alone rarely causes severe consequence [6–8]. Outcomes of influenza infection vary with age. Secondary bacterial infection has been considered as a key factor responsible for IAV death [7, 9]. Among them, Streptococcus pneumoniae (SP) and Staphylococcus aureus (SA) are the most commonly seen bacterial types [10, 11]. Additionally, the cytokine burst is another main cause of IAV-related death. Cytokine burst causes severe immunopathological damage to body [12] and also increases susceptibility to secondary bacterial infection [13].
The timing recognition of IAV and bacterial infection can be dated back to 1918 “Spanish flu.” At that time, pneumonia-associated deaths closely followed the outbreak of influenza. A set of bacteria such as SP and SA were incubated from these autopsies [14, 15]. Clinical data have suggested that secondary bacterial infection usually occurs 1-2 weeks after IAV infection and frequently causes IAV-related death [16, 17]. In exploring the pathogenic mechanisms underlying the excess pneumonia mortality after influenza infections, various animals were used as in vivo models to investigate viral-bacterial interaction. Among them, a murine model established by McCullers et al. preferably manifested the clinical characterizations [18, 19]. In their study, IAV and SP were used to infect mice at various time sequence or pathway, which produced an intriguing mortality difference. The mortality was 60% in mice infected with IAV and SP simultaneously. It was up to 100% when SP was inoculated 7 days after IAV infection. On contrast, mice challenged with SP 7 days before IAV survived 100%. Mice infected with either IAV or SP alone had mortalities of 35% or 15%, respectively. Studies from other labs also supported that influenza infection followed by bacterial challenge rendered the most severe outcomes [20–24]. Thus, these results suggest IAV infection facilitates secondary SP infection.
(2) Prevailing Mechanisms of IAV Facilitating Hosts to Bacterial Pneumonia. Multiple factors are involved in virally bacterial pneumonia. Of them, respiratory epithelial damage is considered as the most classical one since the 1918 pandemic; that is, IAV incursion exposes the binding sites to bacteria [19, 25, 26]. This has been further proved by increasing number of pathological researches involving viral and bacterial infection [27–29]. In addition, the recognition of IAV by toll-like receptors (TLRs) increased interferons (IFNs) secretion. The latter suppressed the functions of macrophages and neutrophils and led to the failure of bacterial clearance [30, 31]. To observe the dynamics of timing and sequential infection between IAV and SP, Shrestha et al. developed a mathematical model to simulate these processes [32]. Consistent with results of McCullers and Rehg that pneumococcal challenge 7 days after IAV infection leads to the most severe disease state and rapid death [19], Shrestha et al. found that SP infection 4–6 days after influenza infection was the most efficient model to cause invasive pneumonia [32]. One main cause for the delayed SP infection is believed to be due to the inhibition of alveolar macrophages by IFN-γ, a product after IAV recognition by TLRs (Figure 1(a)) [33]. Other IAV-related factors promoting bacterial susceptibility include mucociliary dysfunction [34], bacterial receptors expressing on epithelial cells [35, 36], cytokines increasing vascular permeability (e.g., interleukin-16 (IL-6) and tumor necrosis factor-α (TNF-α)) [37], cytokines inhibiting early immune response (e.g., IL-35, IL-17, and IL-10) [30, 38, 39]. Investigations involving classical innate and adaptive immune responses on IAV infection and its sequelae have been well documented (as reviewed in [25, 40–42]). However, the role of apoptosis and autophagy is less reported, especially in IAV-related bacterial pneumonia.
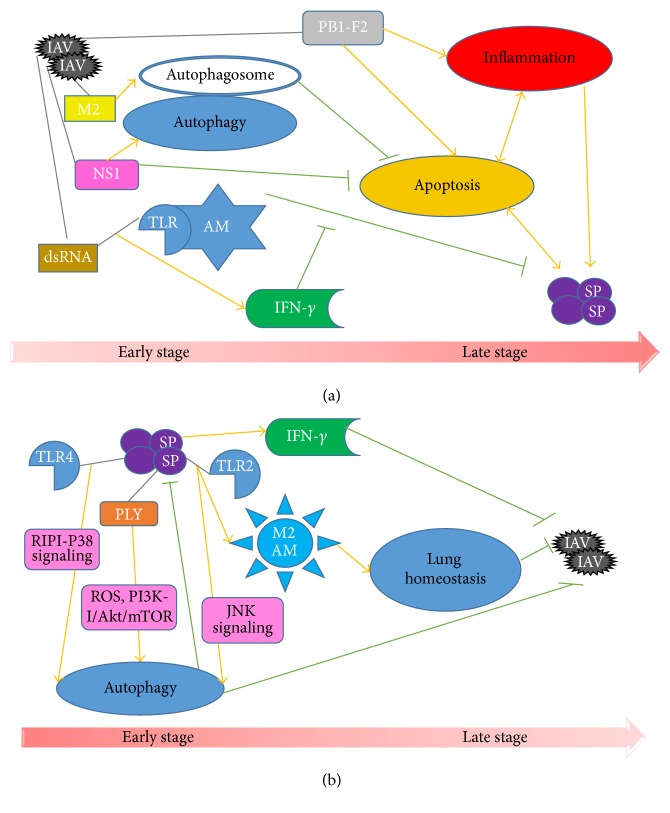
Figure 1.
Autophagy and apoptosis in the early and late stage of IAV-SP mixed infection. In the figure, the orange arrows represent links of stimulation, whereas the green bars correspond to inhibitory links. (a) Autophagy and apoptosis seem to act as sequential events after IAV infection. NS1 plays a critical role in regulating IAV-induced autophagy and apoptosis. M2 is necessary for the formation of autophagosomes. The latter delays the development of apoptosis. PB1-F2 induces inflammatory response, which has a mutual promotion with apoptosis. PB1-F2 of PR8 can cause apoptosis of monocytes. Both apoptosis and inflammation contribute to secondary bacterial infection. Recognition of viral dsRNA by TLR of alveolar macrophages promotes IFN-γ production, which prevents macrophages from clearing bacteria (such as SP). (b) SP triggers autophagy by interacting with TLR4 and TLR2 through RIPI-P38 signaling and JNK signaling, respectively. By the interaction of SP and TLR2, M2 alveolar macrophages (M2 AM) polarized from monocytes help maintain the lung homeostasis. PLY stimulate autophagy through ROS hypergeneration and PI3K-I/Akt/mTOR pathway. In addition, SP causes IFN-γ increase, together with autophagy and the maintenance of lung homeostasis, which alleviates subsequent IAV infection.
2. Role of Autophagy and Apoptosis in Viral-Bacterial Interaction
Interestingly, recent studies have shown that autophagy, an evolutionarily conserved cellular pathway existing ubiquitously in eukaryotes to degrade unwanted cytoplasmic materials such as long-lived proteins and organelles under stressed conditions like nutrition deprivation and hypoxia [43, 44] is involved in IAV infection [45, 46]. Autophagy was initially found to protect against microbial invasion. Some viruses, such as influenza viruses, have evolved to subvert this mechanism for their own benefit [47]. Zhou et al. first reported that autophagy was related to IAV replication and that virus yield was decreased by autophagy suppression [45]. They also reported that viral titer was decreased by enhanced autophagy at another study [48]. Additionally, autophagy has been considered as a new programmed cell death way. Evidences showed that cell death induced by H5N1 is predominantly autophagic rather than apoptotic. Autophagic cell death was considered as a main factor causing severe lung injury in H5N1-infected mice. This injury can be ameliorated by suppressing autophagy but not apoptosis [49]. These data suggest that autophagy, to a certain extent, is involved in H5N1-related cell death both in vitro and in vivo. Nevertheless, in some pathologic situation, excessive autophagy might also lead to cell death through apoptosis (as reviewed in [50]). Although autophagic cell death was more likely to be induced in highly pathogenic strains [49, 51], whether autophagy is a way of executing cell death or cell death is accompanied by autophagy remain controversial [50, 52].
Apoptosis, classified as type I programmed cell death, is generally characterized by nuclear fragmentation, chromatin condensation, cell shrinkage, plasma membrane blebbing, and intact cell membrane [53–55]. Relationship between IAV and apoptosis was early studied in vitro and in vivo [56–58]. Apoptosis was originally thought not to cause inflammation. Later studies showed that IAV induced caspase-1 and caspase-3, which proteolytically processed IL-1β and IL-18, and subsequently indirectly caused inflammatory responses [59], including in respiratory epithelial cells and leucocytes [60]. By recognizing viral RNA, members of nucleotide-binding domain and leucine-rich-repeat-containing (NLRs) family such as cryopyrin assemble inflammasomes to activate caspase-1 and then increase IL-1β and IL-18 secretion in macrophages [61, 62]. Thus, it is stimuli inducing apoptosis that determine whether apoptosis causes inflammatory response [63].
Many IAV proteins are involved in apoptosis, such as nucleoprotein (NP), matrix protein 1 (M1), matrix protein 2 (M2), nonstructural protein (NS1) [64], and PB1-F2 [65]. Fourteen years ago, in investigating an unknown antigenic peptide of IAV presented by CD8+ T lymphocytes, Chen et al. found a protein, PB1-F2, the eleventh viral protein encoded by the open reading frame (ORF) of PB1 gene. This strain specific protein is considered as one of the virulence factors contributing to the high pathogenicity of IAV, including the ability in promoting secondary bacterial infection by inducing cytokine storm [66, 67]. Thus, IAV tends to cause an inflammatory apoptosis.
2.1. Autophagy and Apoptosis Facilitates Secondary Bacterial Pneumonia after IAV Infection
2.1.1. IAV Induces Autophagy
Several IAV proteins are involved in progress of viral infection promoting bacterial superinfection via the regulation of autophagy and apoptosis, in which autophagy and apoptosis appear to be sequential events (Figure 1(a)). NS1 protein was expressed at the early stage of IAV infection [68]. It was reported that NS1 protein indirectly promoted autophagy at the early stage of IAV infection through upregulating the synthesis of HA and M2 [69] and downregulating apoptosis to facilitate viral replication [70]. NS1 protein also positively regulated PI3K-Akt pathway to inhibit apoptosis in the early stage of IAV infection, while in the late stage, NS1 induced p53 dependent or independent pathways to activate apoptosis [71]. Besides, NS1 may suppress apoptosis partly by antagonizing IFN. Apoptosis was enhanced and accelerated in IFN-competent cells infected by NS1-depeleted variant (delNS1) but was delayed in IFN-deficient cells infected by wild-type or delNS1 strains [70]. Furthermore, NS1 also exerted antiapoptosis effect by blocking the recognition of recognizing double-stranded RNA (dsRNA) dependent protein kinase (PKR) to viral dsRNA [72–75]. Notably, apoptosis suppressive effect of NS1 is strain specific. NS1 exhibited apoptosis suppression in some strains, like H1N1, H3N2, and H7N7 [70, 76]. Conversely, in some strains, like H5N9 and H5N1, NS1 promoted apoptosis [77, 78]. M2 protein acted as a proton channel for viral uncoating after fusion in endosomes [3]. M2 protein is also found to be necessary for autophagosome formation. Silencing M2 expression resulted in significantly reduced autophagosome accumulation during IAV infection. However, M2 blocked IAV-induced fusion of autophagosome and lysosome via binding to Beclin-1 with its first 60 amino acids [79]. As autophagy deficient cells exhibited enhanced apoptosis, M2-mediated autophagosome accumulation is likely to decrease apoptosis [79]. On the whole, influenza virus provides environment and time for viral replication by inhibiting apoptosis and triggering autophagy at the early stage of infection.
2.1.2. Autophagy in IAV Infection: Two Sides of a Coin
Autophagy is a doubled edge sword. On the one hand, autophagy is a prosurvival pathway in which it protects against various pathogenic invasions, including IAV [80]. The antiviral character of autophagy is mostly attributed to its function in adaptive immunity. One recent study showed that autophagy was essential for the maintenance of memory B cells against IAV infection in vivo [81]. In this study, Chen et al. demonstrated that mice with autophagy-related gene (Atg) 7 knockdown in B cells failed to produce secondary antibodies when they were infected with IAV again [81]. Similarly, autophagy displays a prosurvival role in effector CD8+ T cells during influenza infection. By using an inducible Atg5 knockout mouse system, Schlie et al. found that mice infected with IAV failed to recall a primary response peak, and the Atg5−/− CD8+ T cells exhibited feeble viability and upregulated P53 expression [82]. Additionally, a natural compound, pentagalloylglucose (PGG), which was reported to have anti-influenza activity [83], promoted autophagic flux via degradation of viral M2 protein, a protein that blocks the fusion of autophagosome and lysosome at enough high concentration, and subsequently caused the downregulation of several viral proteins, like NP, M1, HA, and M2 [84]. On the other hand, autophagy is beneficial to IAV replication and production. Zhou et al. reported in an in vitro experiment that autophagy was involved in IAV replication and autophagy suppression and decreased viral yield [45]. A large amount of studies showed that autophagic deficiency reduced IAV virulence [45, 51, 84–91]. It is noteworthy that IAV-induced autophagy is both strain and cell specific [46, 92]. In addition, autophagy is associated with influenza-induced inflammatory. For instance, TLR3 enhanced autophagy by dsRNA to promote the production of IFN and some other cytokines [93, 94]. Autophagy was also involved in the induction of IFN-α and CXCL10 in H9N2/G1 infected cells [46, 92]. Moreover, autophagy-mediated inflammatory response was also associated with nuclear factor-κB (NF-κB) and p38 mitogen-activated protein kinase (MAPK) signaling in H5N1 pseudovirus infection. This signaling in turn promoted the formation of autophagosomes, suggesting an important mechanism underlying H5N1-related hypercytokemia [95].
2.1.3. IAV-Induced Apoptosis: More of a Foe Than a Friend
Apoptosis has dual characters as well as autophagy. Several proapoptotic factors definitely play an antiviral role. Recently, Chang et al. found that several avian influenza viruses induced early apoptosis in porcine alveolar macrophages, which inhibited viral replication and mitigated inflammation [96]. IL-24 was found to decrease IAV titer by activating TLR3 dependent apoptosis [97]. Although initial findings show that apoptosis is a host defensive mechanism against IAV infection [98], generally speaking, apoptosis is beneficial to viral replication, dissemination, and host immune cells kill. As such, apoptosis may serve as a contributor for secondary bacterial infection following influenza virus infection.
Firstly, IAV indeed triggers apoptosis through various mechanisms to damage host immunity ability. Some viral components, such as NS1, M2, PB1-F2, M1, NA (neuraminidase), NP, and dsRNA, are associated with apoptosis regulation. As described above, viral NS1 plays an antiapoptotic role in host immune response [69–75]. Combined with M2, these viral components regulate autophagy and apoptosis as sequential events [79]. PB1-F2, a viral protein encoded by an open reading frame of IAV PB1, is shown to induce apoptosis at the late stage of IAV infection via mitochondrial permeabilization in strain dependent and cell specific manners [65]. It is notable that only PB1-F2 produced by influenza A/Puerto Rico/8/34 (H1N1) (hereafter referred as to PR8), but not other strains, induced alveolar macrophages death rather than epithelial cells in the lung [65, 99, 100]. As a result, PB1-F2 interferes in viral and bacterial clearance and antigen presentation at the early stage. McAuley et al. introduced PB1-F2 protein of 1918 influenza H1N1 virus into PR8, resulting in a higher susceptibility to secondary bacterial pneumonia than wild-type PR8. Nonetheless, they also observed that PB1-F2 knockout variant resulted in lower mortality when followed by SP infection as compared to wild-type PR8, which expresses PB1-F2 with 87 amino acids [101], although both had similar viral loads in lung [66]. Recently, Yoshizumi et al. found that full-length PB1-F2 of highly pathogenic IAVs translocated into mitochondria via Tom40 channels and then impaired innate immune and contributed to symptomatic deterioration, while truncated PB1-F2 (lacking C-terminal region responsible for translocating into mitochondria) from low pathogenic IAVs was less harmful due to disability in translocating into mitochondria [102]. These findings indicate that PB1-F2 exerts the pathogenicity on postinfluenza bacterial infection more likely through other mechanisms rather than apoptosis. One of the most direct causes is excessive inflammation [103–105]. An in vivo experiment with different viral strains discovered that PB1-F2 was related to inflammatory infiltration of macrophages and neutrophils, hypertrophy of epithelial cells, and fibrin deposition [67]. Additionally, PB1-F2 induced inflammatory response by activating inflammasome [106], regulating NF-κB and IKKβ activity [107] and forming aggregates [108]. M1 and NA protein induced apoptosis by interacting with caspase-8 [109, 110] or activating tumor growth factor-β (TGF-β) [77, 111]. Human Clusterin (CLU) prevented intrinsic apoptosis pathway through binding to Bax, which interfered with viral NP protein [112]. dsRNA virus-mediated apoptosis was reported to be related to caspase-dependent pathway [113], PKR, TLR, retinoic acid-inducible gene (RIG), and other forms of signaling [114–117].
Secondly, many reports have shown that apoptosis inhibition decreases IAV pathogenicity. Herold et al. reported that macrophages, when recruited from peripheral blood to the lungs during IAV infection, released tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to induce apoptosis of alveolar epithelial cells and increase lung leakage and mortality, which in turn were rescued by blocking TRAIL signaling [118]. Additionally, Liu et al. found that caspase inhibitors decreased viral replication and release of certain kinds of proinflammatory cytokines and chemokines in IAV infected mast cells [119]. Jaworska et al. discovered that the interaction of host NLRX1 and viral PB1-F2 protein suppressed mitochondria-related apoptosis and enhanced macrophage function, which, as a result, mitigated viral replication, lung function disorder, and mortality [120]. Tran et al. used human whole-genome screen method to search for cell death related genes in IAV infection. USP47, TNF superfamily (TNFSF) 13, and TNFSF12-13 were identified as important components. Their depletion produced host protective effects [121].
As mentioned above, SP significantly aggravates IAV infection. To explore the role of apoptosis in postinfluenza SP pneumonia, Kosai et al. infected mice with IAV or SP alone or IAV 48 hours followed by SP. They found that apoptosis occurred earlier and more severe in mice infected with combination of IAV and SP than IAV or SP alone [122].
2.2. Preceding SP Infection Alleviates Onsets of Subsequent IAV Invasion: Role of Autophagy
2.2.1. Preceding Bacterial Infection Protects Host from IAV Infection
A prior bacterial exposure may protect the host from adverse impacts of following IAV infection. This concern is mainly derived from study made by McCullers and Rehg. In their study, mice infected with IAV 7 days after SP had 0% mortality, while other groups suffered from mortality from 25% to 100% [19]. Deprivation of commensal bacteria (such as SP and SA) from respiratory tract exacerbated influenza-induced disorder [123–125]. Recently, an in vivo experiment showed that preceding SP infection protected mice from IAV-related detriment [126]. One of possible mechanisms underlying this virus antagonistic effect is that bacteria create an inflammatory environment [40]. Indeed, SP infection promoted IFN-γ production [127–131], which results in an antiviral state (Figure 1(b)). However, the role of autophagy and apoptosis in this phenomenon is paid less attention.
2.2.2. Role of Autophagy in Postbacterial IAV Infection
Autophagy seems to play a critical role in improving host immunity in SP infection (Figure 1(b)). Guo et al. found that SP-induced autophagy was a defense mechanism against bacterial infection [132]. The mechanism may be partly attributed to recognition of LPS of SP by TLR4 to trigger autophagy through receptor-interacting protein 1 (RIP1-P38) signaling to promote SP clearance [133, 134]. Also, Li et al. reported that SP clearance was enhanced by autophagy; conversely bacterial clearance was reduced by autophagy suppression [135]. In addition, although preadministrated SP failed to produce any detectable effects on either cell morphology or IAV replication in epithelial cells [136], another in vivo study conducted by Wang et al. showed bacteria colonization, to some extent, indeed preventing viral infection [125]. Comparing to SA-free mice and wild-type mice, specific pathogen-free (SPF) mice were more susceptible to fatalness induced by IAV [125]. This is consistent with the results from McCullers and Rehg, although different cocci were used [19]. One of possible mechanisms underlying this result is that peripheral CCR2+CD11b+ monocytes were recruited into alveoli and then were polarized to M2 alveolar macrophages by the interaction between SA and TLR2. Therefore, SA colonization increases immunity ability. Nonetheless, as TLR2 is crucial for lung homeostasis rather than bacterial elimination, TLR2 deficiency failed to interfere in SA elimination [125]. TLR2 was also reported to trigger autophagy through JNK signaling [137]. In this situation, TLR2 could serve as a stimulus for the development of bacteria-induced autophagy. These may explain why Ouyang et al. did not detect the influence of SP pretreatment on IAV replication. Recently, Wolf et al. reported that pneumolysin, a bacterial virulence factor important in inducing immune responses, protected postpneumococcus IAV infection in an in vivo model [126]. Li et al. further supported that pneumolysin was a key factor in triggering autophagy through ROS hypergeneration and inhibition of PI3K-I/Akt/mTOR pathways in A549 cells [135]. In other words, SP might exert protective effects against IAV via autophagy mechanism. Taken together, it is clear that preceding SP infection produces systematic defense reactions, including autophagy to attenuate the followed IAV infection.
3. Role of Autophagy and Apoptosis in Viral-Bacterial Coinfection: A Potential Research Field
3.1. Present Treatment against IAV and Bacterial Infection
Present treatments against IAV and bacterial infection include viral and bacterial vaccines, antiflu drugs, and antibiotics. As reviewed by Christopoulou et al., application of viral and bacterial vaccines effectively decreases post-IAV bacterial infection [138]. Antiflu drugs are also shown to not only reduce IAV infection but also decrease clinical morbidity of secondary bacterial infection [139, 140]. Nonetheless, as IAV is an ssRNA virus with 8 segments, which contribute to low self-correcting ability during transcription, IAVs are easily to produce new mutants which are consequently resistant to antiviral drugs or fail to be neutralized by vaccines [42, 141, 142]. Additionally, multidrug resistant SA (MDRSA), especially methicillin-resistant SA (MRSA), have been widely disseminated in hospital and community [143, 144]. For these concerns, although vaccines and antibiotics are currently primary treatments for possible post-IAV bacterial infections, further mechanism exploration for more treatment targets, including autophagy and apoptosis, is still significantly important.
3.2. Autophagy and Apoptosis Regulation: A Potential Alternative Method to Fight against Increased Susceptibility to Post-IAV Bacterial Infection
Here, we propose an assumption that regulating autophagy at the early stage or suppressing apoptosis at the late stage may be a promising strategy to antagonize post-IAV SP pneumonia. Until now, there are a number of studies showing that autophagy suppression ameliorates the impact of IAV risk [45, 51, 84–91]. Although IAV has been proved to inhibit autophagy to facilitate its replication, decreased viral titer was also observed in presence of autophagy stimulator rapamycin in MDCK cells [48]. What is more, autophagy plays a protective role in adaptive immunity against virus-infected hosts [81, 82]. Some chemicals exert virus suppressive effect by triggering autophagy [83, 84]. These indicate that excessive or insufficient autophagy is detrimental for IAV.
What needs to be paid special attention is a moderate regulation of autophagy. Hahn et al. established a mouse model in which Atg5 gene was knockout in the distal respiratory epithelium to achieve different degrees of autophagic reduction [145]. They infected these mice with 50% autophagy ability with H3N2 virus. The results showed that viral replication was decreased, lung structure and function were improved, and morbidity and mortality were decreased. When mice with 10% autophagy ability were used, lung injury in elderly group was exacerbated with time, while alveolar septum was thickened in adult group. Therefore, an appropriate autophagic level is necessary to fight against IAV invasion. As aforementioned, inhibiting apoptosis presents an overall beneficial effect for hosts infected by IAV [118–121]. Thus, treating IAV infection with autophagy regulators and apoptosis inhibitors in in vivo models can be regarded as a potential researching field to explore a new breakthrough fighting against influenza and its sequelae.
The significance of this assumption is that the high conservativeness of autophagy and apoptosis in eukaryotes, to some extent, prevents the complexity of IAV mutation. The concept that autophagy and apoptosis are conserved among eukaryotes has been applied in drug screening and discovery, where cell-based assays were used for antiviral drug screening [86, 87, 89]. Further validation is necessary in in vivo models.
4. Conclusion
Collectively, IAV facilitates its host to suffer from bacterial pneumonia via various pathways. Among the underlying mechanisms, autophagy and apoptosis act as sequential events to regulate post-IAV bacterial pneumonia. Systemic analysis of autophagy and apoptosis may provide a new strategy for prophylactic and therapeutic treatment of influenza virus infection.
Acknowledgments
This work was financially supported by the National Science Foundation of China (Grant no. 30671964) and State Key Laboratory of Oral Diseases (Grant no. SKLOD2015OF08).
Competing Interests
The authors declared no competing interests.
References
- 1.Loo Y.-M., Gale M., Jr. Influenza: fatal immunity and the 1918 virus. Nature. 2007;445(7125):267–268. doi: 10.1038/445267a. [DOI] [PubMed] [Google Scholar]
- 2.Johnson N. P. A. S., Mueller J. Updating the accounts: global mortality of the 1918-1920 ‘Spanish’ influenza pandemic. Bulletin of the History of Medicine. 2002;76(1):105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 3.Palese P., Shaw M. Orthomyxoviridae: the viruses and their replication. In: Knipe D. M., Howley P. M., editors. Fields Virology. 4th. 2007. pp. 1647–1689. [Google Scholar]
- 4.Li K. S., Guan Y., Wang J., et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430(6996):209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- 5.Palese P. Influenza: old and new threats. Nature Medicine. 2004;10(12):S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien K. L., Walters M. I., Sellman J., et al. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clinical Infectious Diseases. 2000;30(5):784–789. doi: 10.1086/313772. [DOI] [PubMed] [Google Scholar]
- 7.Morens D. M., Taubenberger J. K., Fauci A. S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. Journal of Infectious Diseases. 2008;198(7):962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muscedere J., Ofner M., Kumar A., et al. The occurrence and impact of bacterial organisms complicating critical care illness associated with 2009 influenza A(H1N1) infection. Chest. 2013;144(1):39–47. doi: 10.1378/chest.12-1861. [DOI] [PubMed] [Google Scholar]
- 9.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17(1):S3–S10. doi: 10.1016/s0264-410x(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 10.Klugman K. P., Chien Y.-W., Madhi S. A. Pneumococcal pneumonia and influenza: a deadly combination. Vaccine. 2009;27(3):C9–C14. doi: 10.1016/j.vaccine.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Madhi S. A., Klugman K. P., Vaccine Trialist Group A role for Streptococcus pneumoniae in virus-associated pneumonia. Nature Medicine. 2004;10(8):811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zavitz C. C. J., Bauer C. M. T., Gaschler G. J., et al. Dysregulated macrophage-inflammatory protein-2 expression drives illness in bacterial superinfection of influenza. Journal of Immunology. 2010;184(4):2001–2013. doi: 10.4049/jimmunol.0903304. [DOI] [PubMed] [Google Scholar]
- 13.Mifsud E. J., Tan A. C., Short K. R., Brown L. E., Chua B. Y., Jackson D. C. Reducing the impact of influenza-associated secondary pneumococcal infections. Immunology and Cell Biology. 2016;94:101–108. doi: 10.1038/icb.2015.71. [DOI] [PubMed] [Google Scholar]
- 14.Soper G. A. The pandemic in the Army camps. Journal of the American Medical Association. 1918;71:1899–1909. [Google Scholar]
- 15.Spooner L. H., Scott J. M., Heath E. H. A bacteriologic study of the influenza epidemic at Camp Devens. Journal of the American Medical Association. 1919;72(3):155–159. doi: 10.1001/jama.1919.02610030001001. [DOI] [Google Scholar]
- 16.Prevention CDC. Influenza. CDC [homepage on the Internet], 2012, April 2016, http://www.cdc.gov/vaccines/pubs/pinkbook/flu.html.
- 17.Brundage J. F. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infectious Diseases. 2006;6(5):303–312. doi: 10.1016/S1473-3099(06)70466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullers J. A., Webster R. G. A mouse model of dual infection with influenza virus and Streptococcus pneumoniae . International Congress Series. 2001;1219:601–607. doi: 10.1016/s0531-5131(01)00631-8. [DOI] [Google Scholar]
- 19.McCullers J. A., Rehg J. E. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. Journal of Infectious Diseases. 2002;186(3):341–350. doi: 10.1086/341462. [DOI] [PubMed] [Google Scholar]
- 20.Seki M., Higashiyama Y., Tomono K., et al. Acute infection with influenza virus enhances susceptibility to fatal pneumonia following Streptococcus pneumoniae infection in mice with chronic pulmonary colonization with Pseudomonas aeruginosa . Clinical and Experimental Immunology. 2004;137(1):35–40. doi: 10.1111/j.1365-2249.2004.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullers J. A., McAuley J. L., Browall S., Iverson A. R., Boyd K. L., Normark B. H. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. Journal of Infectious Diseases. 2010;202(8):1287–1295. doi: 10.1086/656333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNamee L. A., Harmsen A. G. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infection and Immunity. 2006;74(12):6707–6721. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis G. T., Davidson S., Crotta S., Branzk N., Papayannopoulos V., Wack A. TRAIL+ monocytes and monocyte-related cells cause lung damage and thereby increase susceptibility to influenza-Streptococcus pneumoniae coinfection. EMBO Reports. 2015;16(9):1203–1218. doi: 10.15252/embr.201540473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berg J., Hellwig K., Stoll D., et al. IVA induced IFNs facilitate development of secondary pneumococcal pneumonia in human lung tissue. Pneumologie. 2015;69—A1 doi: 10.1055/s-0035-1548631. [DOI] [Google Scholar]
- 25.McCullers J. A. Insights into the interaction between influenza virus and pneumococcus. Clinical Microbiology Reviews. 2006;19(3):571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plotkowski M.-C., Puchelle E., Beck G., Jacquot J., Hannoun C. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. American Review of Respiratory Disease. 1986;134(5):1040–1044. doi: 10.1164/arrd.1986.134.5.1040. [DOI] [PubMed] [Google Scholar]
- 27.Mauad T., Hajjar L. A., Callegari G. D., et al. Lung pathology in fatal novel human influenza A (H1N1) infection. American Journal of Respiratory and Critical Care Medicine. 2010;181(1):72–79. doi: 10.1164/rccm.200909-1420OC. [DOI] [PubMed] [Google Scholar]
- 28.Nickerson C. L., Jakab G. J. Pulmonary antibacterial defenses during mild and severe influenza virus infection. Infection and Immunity. 1990;58(9):2809–2814. doi: 10.1128/iai.58.9.2809-2814.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louie J., Jean C., Chen T. H., et al. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May–August 2009. Morbidity and Mortality Weekly Report. 2009;58(38):1071–1074. [PubMed] [Google Scholar]
- 30.Li W., Moltedo B., Moran T. M. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of γδ T cells. Journal of Virology. 2012;86(22):12304–12312. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee B., Robinson K. M., McHugh K. J., et al. Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2015;309(2):L158–L167. doi: 10.1152/ajplung.00338.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrestha S., Foxman B., Dawid S., et al. Time and dose-dependent risk of pneumococcal pneumonia following influenza: a model for within-host interaction between influenza and Streptococcus pneumoniae . Journal of the Royal Society Interface. 2013;10(86, article 0233) doi: 10.1098/rsif.2013.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun K., Metzger D. W. Inhibition of pulmonary antibacterial defense by interferon-γ during recovery from influenza infection. Nature Medicine. 2008;14(5):558–564. doi: 10.1038/nm1765. [DOI] [PubMed] [Google Scholar]
- 34.Pittet L. A., Hall-Stoodley L., Rutkowski M. R., Harmsen A. G. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. American Journal of Respiratory Cell and Molecular Biology. 2010;42(4):450–460. doi: 10.1165/rcmb.2007-0417OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avadhanula V., Rodriguez C. A., De Vincenzo J. P., et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. Journal of Virology. 2006;80(4):1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li N., Ren A., Wang X., et al. Influenza viral neuraminidase primes bacterial coinfection through TGF-β—Mediated expression of host cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(1):238–243. doi: 10.1073/pnas.1414422112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang S., Le T. Q., Kurihara N., et al. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. Journal of Infectious Diseases. 2010;202(7):991–1001. doi: 10.1086/656044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y., Wang C. J., Lin S. H., Zhang M., Li S. Y., Xu F. Interleukin-35 is upregulated in response to influenza virus infection and secondary bacterial pneumonia. Cytokine. 2016;81:23–27. doi: 10.1016/j.cyto.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 39.van der Sluijs K. F., Van Elden L. J. R., Nijhuis M., et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. The Journal of Immunology. 2004;172(12):7603–7609. doi: 10.4049/jimmunol.172.12.7603. [DOI] [PubMed] [Google Scholar]
- 40.McCullers J. A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nature Reviews Microbiology. 2014;12(4):252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 41.Short K. R., Habets M. N., Hermans P. W. M., Diavatopoulos D. A. Interactions between Streptococcus pneumoniae and influenza virus: a mutually beneficial relationship? Future Microbiology. 2012;7(5):609–624. doi: 10.2217/fmb.12.29. [DOI] [PubMed] [Google Scholar]
- 42.Smith A. M., McCullers J. A. Secondary bacterial infections in influenza virus infection pathogenesis. Current Topics in Microbiology and Immunology. 2014;385:327–356. doi: 10.1007/82_2014_394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Z., Klionsky D. J. Eaten alive: a history of macroautophagy. Nature Cell Biology. 2010;12(9):814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klionsky D. J., Emr S. D. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Z., Jiang X., Liu D., et al. Autophagy is involved in influenza A virus replication. Autophagy. 2009;5(3):321–328. doi: 10.4161/auto.5.3.7406. [DOI] [PubMed] [Google Scholar]
- 46.Law A. H. Y., Lee D. C. W., Lau A. S. Y. Role for autophagy in cellular response to influenza virus infection. Hong Kong Medical Journal. 2014;20(supplement 6):S20–S24. [PubMed] [Google Scholar]
- 47.Yordy B., Iwasaki A. Autophagy in the control and pathogenesis of viral infection. Current Opinion in Virology. 2011;1(3):196–203. doi: 10.1016/j.coviro.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z. Association of influenza a virus with autophagy [Ph.D. dissertation] National Digital Library of China; 2007 (Chinese) [Google Scholar]
- 49.Sun Y., Li C., Shu Y., et al. Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Science Signaling. 2012;5(212, article ra16) doi: 10.1126/scisignal.2001931. [DOI] [PubMed] [Google Scholar]
- 50.Kroemer G., Levine B. Autophagic cell death: the story of a misnomer. Nature Reviews Molecular Cell Biology. 2008;9(12):1004–1010. doi: 10.1038/nrm2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma J., Sun Q., Mi R., Zhang H. Avian influenza A virus H5N1 causes autophagy-mediated cell death through suppression of mTOR signaling. Journal of Genetics and Genomics. 2011;38(11):533–537. doi: 10.1016/j.jgg.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Debnath J., Baehrecke E. H., Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005;1(2):66–74. doi: 10.4161/auto.1.2.1738. [DOI] [PubMed] [Google Scholar]
- 53.Elmore S. Apoptosis: a review of programmed cell death. Toxicologic Pathology. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwartzman R. A., Cidlowski J. A. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocrine Reviews. 1993;14(2):133–151. doi: 10.1210/er.14.2.133. [DOI] [PubMed] [Google Scholar]
- 55.Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takizawa T., Matsukawa S., Higuchi Y., Nakamura S., Nakanishi Y., Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. Journal of General Virology. 1993;74(11):2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- 57.Hinshaw V. S., Olsen C. W., Dybdahl-Sissoko N., Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. Journal of Virology. 1994;68(6):3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori I., Komatsu T., Takeuchi K., Nakakuki K., Sudo M., Kimura Y. In vivo induction of apoptosis by influenza virus. Journal of General Virology. 1995;76(11):2869–2873. doi: 10.1099/0022-1317-76-11-2869. [DOI] [PubMed] [Google Scholar]
- 59.Brydon E. W. A., Morris S. J., Sweet C. Role of apoptosis and cytokines in influenza virus morbidity. FEMS Microbiology Reviews. 2005;29(4):837–850. doi: 10.1016/j.femsre.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Julkunen I., Melén K., Nyqvist M., Pirhonen J., Sareneva T., Matikainen S. Inflammatory responses in influenza A virus infection. Vaccine. 2000;19(1):S32–S37. doi: 10.1016/S0264-410X(00)00275-9. [DOI] [PubMed] [Google Scholar]
- 61.Allen I. C., Scull M. A., Moore C. B., et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas P. G., Dash P., Aldridge J. R., Jr., et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30(4):566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Restifo N. P. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Current Opinion in Immunology. 2000;12(5):597–603. doi: 10.1016/s0952-7915(00)00148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hament J.-M., Kimpen J. L. L., Fleer A., Wolfs T. F. W. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunology and Medical Microbiology. 1999;26(3-4):189–195. doi: 10.1016/s0928-8244(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 65.Chen W., Calvo P. A., Malide D., et al. A novel influenza A virus mitochondrial protein that induces cell death. Nature Medicine. 2001;7(12):1306–1312. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- 66.McAuley J. L., Hornung F., Boyd K. L., et al. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host and Microbe. 2007;2(4):240–249. doi: 10.1016/j.chom.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McAuley J. L., Chipuk J. E., Boyd K. L., Van De Velde N., Green D. R., Jonathan A. M. PB1-F2 proteins from H5N1 and 20th century pandemic influenza viruses cause immunopathology. PLoS Pathogens. 2010;6(7):1–12. doi: 10.1371/journal.ppat.1001014.e1001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krug R. Unique Functions of the NS1 Protein. Oxford, UK: Textbook of Inffuenza Blackwell Science; 1998. [Google Scholar]
- 69.Zhirnov O. P., Klenk H. D. Influenza a virus proteins NS1 and hemagglutinin along with M2 are involved in stimulation of autophagy in infected cells. Journal of Virology. 2013;87(24):13107–13114. doi: 10.1128/JVI.02148-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhirnov O. P., Konakova T. E., Wolff T., Klenk H.-D. NS1 protein of influenza A virus down-regulates apoptosis. Journal of Virology. 2002;76(4):1617–1625. doi: 10.1128/JVI.76.4.1617-1625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhirnov O. P., Klenk H.-D. Control of apoptosis in influenza virus-infected cells by up-regulation of Akt and p53 signaling. Apoptosis. 2007;12(8):1419–1432. doi: 10.1007/s10495-007-0071-y. [DOI] [PubMed] [Google Scholar]
- 72.Hatada E., Saito S., Fukuda R. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. Journal of Virology. 1999;73(3):2425–2433. doi: 10.1128/jvi.73.3.2425-2433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu Y., Wambach M., Katze M. G., Krug R. M. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the eIF-2 translation initiation factor. Virology. 1995;214(1):222–228. doi: 10.1006/viro.1995.9937. [DOI] [PubMed] [Google Scholar]
- 74.Tan S.-L., Katze M. G. Biochemical and genetic evidence for complex formation between the influenza a virus NS1 protein and the interferon-induced PKR protein kinase. Journal of Interferon & Cytokine Research. 1998;18(9):757–766. doi: 10.1089/jir.1998.18.757. [DOI] [PubMed] [Google Scholar]
- 75.Ludwig S., Wang X., Ehrhardt C., et al. The influenza A virus NS1 protein inhibits activation of Jun N-terminal kinase and AP-1 transcription factors. Journal of Virology. 2002;76(21):11166–11171. doi: 10.1128/jvi.76.21.11166-11171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morris S. J., Nightingale K., Smith H., Sweet C. Influenza A virus-induced apoptosis is a multifactorial process: exploiting reverse genetics to elucidate the role of influenza A virus proteins in virus-induced apoptosis. Virology. 2005;335(2):198–211. doi: 10.1016/j.virol.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 77.Schultz-Cherry S., Hinshaw V. S. Influenza virus neuraminidase activates latent transforming growth factor beta. Journal of Virology. 1996;70(12):8624–8629. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Daidoji T., Koma T., Du A., et al. H5N1 avian influenza virus induces apoptotic cell death in mammalian airway epithelial cells. Journal of Virology. 2008;82(22):11294–11307. doi: 10.1128/JVI.01192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gannagé M., Dormann D., Albrecht R., et al. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host and Microbe. 2009;6(4):367–380. doi: 10.1016/j.chom.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shintani T., Klionsky D. J. Autophagy in health and disease: a double-edged sword. Science. 2004;306(5698):990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen M., Hong M. J., Sun H., et al. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nature Medicine. 2014;20(5):503–510. doi: 10.1038/nm.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schlie K., Westerback A., DeVorkin L., et al. Survival of effector CD8+ T cells during influenza infection is dependent on autophagy. Journal of Immunology. 2015;194(9):4277–4286. doi: 10.4049/jimmunol.1402571. [DOI] [PubMed] [Google Scholar]
- 83.Liu G., Xiong S., Xiang Y.-F., et al. Antiviral activity and possible mechanisms of action of pentagalloylglucose (PGG) against influenza A virus. Archives of Virology. 2011;156(8):1359–1369. doi: 10.1007/s00705-011-0989-9. [DOI] [PubMed] [Google Scholar]
- 84.Liu G., Zhong M., Guo C., et al. Autophagy is involved in regulating influenza A virus RNA and protein synthesis associated with both modulation of Hsp90 induction and mTOR/p70S6K signaling pathway. The International Journal of Biochemistry & Cell Biology. 2016;72:100–108. doi: 10.1016/j.biocel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 85.Matarrese P., Nencioni L., Checconi P., et al. Pepstatin A alters host cell autophagic machinery and leads to a decrease in influenza A virus production. Journal of Cellular Physiology. 2011;226(12):3368–3377. doi: 10.1002/jcp.22696. [DOI] [PubMed] [Google Scholar]
- 86.Dai J.-P., Li W.-Z., Zhao X.-F., et al. A drug screening method based on the autophagy pathway and studies of the mechanism of evodiamine against influenza A virus. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0042706.e42706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dai J., Wang G., Li W., et al. High-throughput screening for anti-influenza A virus drugs and study of the mechanism of procyanidin on influenza A virus-induced autophagy. Journal of Biomolecular Screening. 2012;17(5):605–617. doi: 10.1177/1087057111435236. [DOI] [PubMed] [Google Scholar]
- 88.Yan Y., Zou Z., Sun Y., et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Research. 2013;23(2):300–302. doi: 10.1038/cr.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dai J.-P., Zhao X.-F., Zeng J., et al. Drug screening for autophagy inhibitors based on the dissociation of Beclin1-Bcl2 complex using bifc technique and mechanism of eugenol on anti-influenza A virus activity. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061026.e61026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu H.-Y., Han L., Shi X.-L., et al. Baicalin inhibits autophagy induced by influenza A virus H3N2. Antiviral Research. 2015;113:62–70. doi: 10.1016/j.antiviral.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 91.Yeganeh B., Ghavami S., Kroeker A. L., et al. Suppression of influenza A virus replication in human lung epithelial cells by noncytotoxic concentrations bafilomycin A1. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2015;308(3):L270–L286. doi: 10.1152/ajplung.00011.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Law A. H.-Y., Lee D. C.-W., Yuen K.-Y., Peiris M., Lau A. S.-Y. Cellular response to influenza virus infection: a potential role for autophagy in CXCL10 and interferon-alpha induction. Cellular and Molecular Immunology. 2010;7(4):263–270. doi: 10.1038/cmi.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kawai T., Akira S. Innate immune recognition of viral infection. Nature Immunology. 2006;7(2):131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 94.Shi C.-S., Kehrl J. H. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. The Journal of Biological Chemistry. 2008;283(48):33175–33182. doi: 10.1074/jbc.m804478200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pan H., Zhang Y., Luo Z., et al. Autophagy mediates avian influenza H5N1 pseudotyped particle-induced lung inflammation through NF-κB and p38 MAPK signaling pathways. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2014;306(2):L183–L195. doi: 10.1152/ajplung.00147.2013. [DOI] [PubMed] [Google Scholar]
- 96.Chang P., Kuchipudi S. V., Mellits K. H., et al. Early apoptosis of porcine alveolar macrophages limits avian influenza virus replication and pro-inflammatory dysregulation. Scientific Reports. 2015;5 doi: 10.1038/srep17999.17999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Weiss R., Laengle J., Sachet M., et al. Interleukin-24 inhibits influenza A virus replication in vitro through induction of toll-like receptor 3 dependent apoptosis. Antiviral Research. 2015;123:93–104. doi: 10.1016/j.antiviral.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 98.Everett H., McFadden G. Apoptosis: an innate immune response to virus infection. Trends in Microbiology. 1999;7(4):160–165. doi: 10.1016/s0966-842x(99)01487-0. [DOI] [PubMed] [Google Scholar]
- 99.McAuley J. L., McCullers J. A. Pro-inflammatory effects of H5N1 and 20th century pandemic influenza virus PB1-F2 proteins are mediated by macrophages. Influenza and other Respiratory Viruses. 2011;5:280–283. [Google Scholar]
- 100.Zamarin D., García-Sastre A., Xiao X., Wang R., Palese P. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS Pathogens. 2005;1(1, article e4) doi: 10.1371/journal.ppat.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen G.-W., Yang C.-C., Tsao K.-C., et al. Influenza A virus PB1-F2 gene in recent taiwanese isolates. Emerging Infectious Diseases. 2004;10(4):630–636. doi: 10.3201/eid1004.030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshizumi T., Ichinohe T., Sasaki O., et al. Influenza A virus protein PB1-F2 translocates into mitochondria via Tom40 channels and impairs innate immunity. Nature Communications. 2014;5, article 4713 doi: 10.1038/ncomms5713. [DOI] [PubMed] [Google Scholar]
- 103.Perrone L. A., Plowden J. K., García-Sastre A., Katz J. M., Tumpey T. M. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathogens. 2008;4(8) doi: 10.1371/journal.ppat.1000115.e1000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baskin C. R., Bielefeldt-Ohmann H., Tumpey T. M., et al. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3455–3460. doi: 10.1073/pnas.0813234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.de Jong M. D., Simmons C. P., Thanh T. T., et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nature Medicine. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McAuley J. L., Tate M. D., MacKenzie-Kludas C. J., et al. Activation of the NLRP3 inflammasome by IAV Virulence protein PB1-F2 contributes to severe pathophysiology and disease. PLoS Pathogens. 2013;9(5) doi: 10.1371/journal.ppat.1003392.e1003392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reis A. L., McCauley J. W. The influenza virus protein PB1-F2 interacts with IKKβ and modulates NF-κB signalling. PLoS ONE. 2013;8(5, article e63852) doi: 10.1371/journal.pone.0063852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chevalier C., Al Bazzal A., Vidic J., et al. PB1-F2 influenza A virus protein adopts a β-sheet conformation and forms amyloid fibers in membrane environments. The Journal of Biological Chemistry. 2010;285(17):13233–13243. doi: 10.1074/jbc.m109.067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Timofeeva T. A., Klenk H. D., Zhirnov O. P. Identification of the protease-binding domain in the N-terminal region of influenza virus A matrix protein M1. Molecular Biology. 2001;35(3):411–416. doi: 10.1023/a:1010435114543. [DOI] [PubMed] [Google Scholar]
- 110.Zhirnov O. P., Ksenofontov A. L., Kuzmina S. G., Klenk H. D. Interaction of influenza A virus M1 matrix protein with caspases. Biochemistry. 2002;67(5):534–539. doi: 10.1023/a:1015542110798. [DOI] [PubMed] [Google Scholar]
- 111.Morris S. J., Price G. E., Barnett J. M., Hiscox S. A., Smith H., Sweet C. Role of neuraminidase in influenza virus-induced apoptosis. Journal of General Virology. 1999;80(1):137–146. doi: 10.1099/0022-1317-80-1-137. [DOI] [PubMed] [Google Scholar]
- 112.Tripathi S., Batra J., Cao W., et al. Influenza A virus nucleoprotein induces apoptosis in human airway epithelial cells: implications of a novel interaction between nucleoprotein and host protein clusterin. Cell Death and Disease. 2013;4(3, article e562) doi: 10.1038/cddis.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ying H., Zaks T. Z., Wang R.-F., et al. Cancer therapy using a self-replicating RNA vaccine. Nature Medicine. 1999;5(7):823–827. doi: 10.1038/10548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Balachandran S., Kim C. N., Yeh W.-C., Mak T. W., Bhalla K., Barber G. N. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. The EMBO Journal. 1998;17(23):6888–6902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uetani K., Der S. D., Zamanian-Daryoush M., et al. Central role of double-stranded RNA-activated protein kinase in microbial induction of nitric oxide synthase. The Journal of Immunology. 2000;165(2):988–996. doi: 10.4049/jimmunol.165.2.988. [DOI] [PubMed] [Google Scholar]
- 116.Kato H., Takeuchi O., Sato S., et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(1):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 117.Wurzer W. J., Ehrhardt C., Pleschka S., et al. NF-κB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. The Journal of Biological Chemistry. 2004;279(30):30931–30937. doi: 10.1074/jbc.m403258200. [DOI] [PubMed] [Google Scholar]
- 118.Herold S., Steinmueller M., von Wulffen W., et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. The Journal of Experimental Medicine. 2008;205(13):3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu B., Meng D., Wei T., Zhang S., Hu Y., Wang M. Apoptosis and pro-inflammatory cytokine response of mast cells induced by influenza A viruses. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0100109.e100109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jaworska J., Coulombe F., Downey J., et al. NLRX1 prevents mitochondrial induced apoptosis and enhances macrophage antiviral immunity by interacting with influenza virus PB1-F2 protein. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(20):E2110–E2119. doi: 10.1073/pnas.1322118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tran A. T., Rahim M. N., Ranadheera C., et al. Knockdown of specific host factors protects against influenza virus-induced cell death. Cell Death and Disease. 2013;4(8, article e769) doi: 10.1038/cddis.2013.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kosai K., Seki M., Tanaka A., et al. Increase of apoptosis in a murine model for severe pneumococcal pneumonia during influenza A virus infection. Japanese Journal of Infectious Diseases. 2011;64(6):451–457. [PubMed] [Google Scholar]
- 123.Abt M. C., Osborne L. C., Monticelli L. A., et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ichinohe T., Pang I. K., Kumamoto Y., et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang J., Li F., Sun R., et al. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nature Communications. 2013;4, article 2106 doi: 10.1038/ncomms3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wolf A. I., Strauman M. C., Mozdzanowska K., et al. Pneumolysin expression by Streptococcus pneumoniae protects colonized mice from influenza virus-induced disease. Virology. 2014;462-463(1):254–265. doi: 10.1016/j.virol.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miettinen M., Matikainen S., Vuopio-Varkila J., et al. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infection and Immunity. 1998;66(12):6058–6062. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakamatsu M., Yamamoto N., Hatta M., et al. Role of interferon-γ in Vα14+ natural killer T cell-mediated host defense against Streptococcus pneumoniae infection in murine lungs. Microbes and Infection. 2007;9(3):364–374. doi: 10.1016/j.micinf.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 129.Koppe U., Högner K., Doehn J.-M., et al. Streptococcus pneumoniae stimulates a STING- and IFN regulatory factor 3-dependent type I IFN production in macrophages, which regulates RANTES production in macrophages, cocultured alveolar epithelial cells, and mouse lungs. Journal of Immunology. 2012;188(2):811–817. doi: 10.4049/jimmunol.1004143. [DOI] [PubMed] [Google Scholar]
- 130.McCool T. L., Cate T. R., Moy G., Weiser J. N. The immune response to pneumococcal proteins during experimental human carriage. The Journal of Experimental Medicine. 2002;195(3):359–365. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Joyce E. A., Popper S. J., Falkow S. Streptococcus pneumoniae nasopharyngeal colonization induces type I interferons and interferon-induced gene expression. BMC Genomics. 2009;10, article 404 doi: 10.1186/1471-2164-10-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo X. G., Zhou S., Xia Y. Respirology. Hoboken, NJ, USA: Wiley-Blackwell; 2013. Autophagy is a defense mechanism in the infection of lung epithelial celss for streptococcus pneumonia; p. p. 165. [Google Scholar]
- 133.Xu Y., Jagannath C., Liu X.-D., Sharafkhaneh A., Kolodziejska K. E., Eissa N. T. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27(1):135–144. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Amano A., Nakagawa I., Yoshimori T. Autophagy in innate immunity against intracellular bacteria. Journal of Biochemistry. 2006;140(2):161–166. doi: 10.1093/jb/mvj162. [DOI] [PubMed] [Google Scholar]
- 135.Li P., Shi J., He Q., et al. Streptococcus pneumoniae induces autophagy through the inhibition of the PI3K-I/Akt/mTOR pathway and ROS hypergeneration in A549 cells. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0122753.e0122753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ouyang K., Woodiga S. A., Dwivedi V., et al. Pretreatment of epithelial cells with live Streptococcus pneumoniae has no detectable effect on influenza A virus replication In vitro . PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0090066.e90066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fang L., Wu H.-M., Ding P.-S., Liu R.-Y. TLR2 mediates phagocytosis and autophagy through JNK signaling pathway in Staphylococcus aureus-stimulated RAW264.7 cells. Cellular Signalling. 2014;26(4):806–814. doi: 10.1016/j.cellsig.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 138.Christopoulou I., Roose K., Ibañez L. I., Saelens X. Influenza vaccines to control influenza-associated bacterial infection: where do we stand? Expert Review of Vaccines. 2014;14(1):55–67. doi: 10.1586/14760584.2015.957191. [DOI] [PubMed] [Google Scholar]
- 139.Michiels B., van Puyenbroeck K., Verhoeven V., Vermeire E., Coenen S. The value of neuraminidase inhibitors for the prevention and treatment of seasonal influenza: a systematic review of systematic reviews. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0060348.e60348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Treanor J. J., Hayden F. G., Vrooman P. S., et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. Journal of the American Medical Association. 2000;283(8):1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 141.McCullers J. A. Textbook of Clinical Pediatrics. 2012. Influenza; pp. 1199–1208. [Google Scholar]
- 142.Carrat F., Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007;25(39-40):6852–6862. doi: 10.1016/j.vaccine.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 143.Boucher H. W., Corey G. R. Epidemiology of methicillin-resistant Staphylococcus aureus . Clinical Infectious Diseases. 2008;46(5):S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 144.Giersing B. K., Dastgheyb S. S., Modjarrad K., Moorthy V. Status of vaccine research and development of vaccines for Staphylococcus aureus . Vaccine. 2016 doi: 10.1016/j.vaccine.2016.03.110. [DOI] [PubMed] [Google Scholar]
- 145.Hahn D. R., Na C.-L., Weaver T. E. Reserve autophagic capacity in alveolar epithelia provides a replicative niche for influenza A virus. American Journal of Respiratory Cell and Molecular Biology. 2014;51(3):400–412. doi: 10.1165/rcmb.2013-0437OC. [DOI] [PMC free article] [PubMed] [Google Scholar]