Survival of patients with adult T-cell leukemia (ATL), which is caused by human T-cell lymphotropic virus type-1 (HTLV-1), has been improved by the introduction of anti-CCR4 monoclonal antibody and allogeneic hematopoietic stem cell transplantation. However, not all patients benefit from these modalities, necessitating a novel therapeutic strategy.1, 2 Recently, an adoptive T-cell immunotherapy with chimeric antigen receptor (CAR) is clinically promising for patients with refractory blood diseases.3, 4, 5, 6, 7, 8 Thus, CD38 is an attractive target of CAR therapy for lymphoid neoplasms because it is widely expressed on cells of B- and T-lymphoid malignancies. We previously demonstrated marked cytotoxicity of T cells engineered to express anti-CD38-CAR against B-lymphoma cells and myeloma cells expressing CD38.9, 10, 11 To expand anti-CD38-CAR applicability against ATL cells that usually express undetectable or low CD38 levels, we must induce CD38 on the ATL cell surface. Interestingly, all-trans retinoic acid (ATRA), which is clinically used to treat patients with acute promyelocytic leukemia (APL), enhances CD38 expression on HL60 cells.12 Moreover, the upstream sequence of the CD38 gene contains an interferon regulatory factor-1 (IRF-1)-binding site. Here we show the marked cytotoxicity of anti-CD38-CAR T cells in HTLV-1-transformed cell lines as well as in cells from patients with ATL through the induction of CD38 expression by treatment with both ATRA and interferon (IFN)-α.
HTLV-1-transformed cell lines MT-2, MT-4, S1T, Su9T and ED were from Miyazaki University. Hut102 cells were obtained from the Cell Research Center for Biomedical Research (Sendai, Japan). Cells were cultured in RPMI-1640 medium containing 10% fetal calf serum and l-glutamine (Sigma, St Louis, MO, USA). ATL cells (acute type) from bone marrow, accounting for over 65% of mononuclear cells and peripheral blood, were provided after obtaining informed consent. These ATL cells and donors' cells were examined for approval by the institutional review board of Hiroshima University. A retroviral vector consisting of green fluorescent protein (GFP), CD8α, and 4-1BB, CD3ζ and anti-CD38 scFv was previously developed.9, 10, 11 Peripheral blood mononuclear cells were stimulated for 48 h with 7 μg/ml PHA-M (Sigma) and 200 IU/ml interleukin-2 (PeproTech, London, UK). T cells were transduced in an RD114-pseudotyped retrovirus-containing medium with 4 μg/ml polybrene (Sigma) in a retronectin-coated tube (Takara-Bio, Otsu, Japan) by spinoculation. An anti-CD38 antibody (CPK-H; MBL, Nagoya, Japan) was added to protect transduced T cells from autolysis through cross-linkage of anti-CD38-CAR with intrinsic CD38, as described previously.10 To detect anti-CD38-CAR, cells were stained with a goat anti-mouse (Fab')2 antibody-biotin (Jackson ImmunoResearch, West Grove, PA, USA), followed by PerCP–streptavidin (BD, Franklin Lakes, NJ, USA). Antibody staining was detected using a FACSCalibur flow cytometer (BD).9, 10, 11 For lactate dehydrogenase (LDH)-releasing cytotoxicity assay, cells (1 × 105 cells per ml) were incubated with transduced T cells (1 × 105 cells per ml) for 18 h at 37 °C in Opti-MEM medium (Invitrogen, Carlsbad, CA, USA). Solution containing tetrazolium salt and diaphorase was added to the supernatant collected before measuring absorbance using the LDH Cytotoxicity Detection Kit (Takara-Bio). To evaluate cytotoxicity of anti-CD38-CAR T cells, co-cultured cells were collected and stained with an anti-CD38 antibody-APC (BD). The specific cytotoxicity of anti-CD38-CAR T cells against CD38+ ATL cells treated with ATRA (Sigma) and/or IFN-α (PeproTech) was evaluated by flow cytometry.9, 10, 11
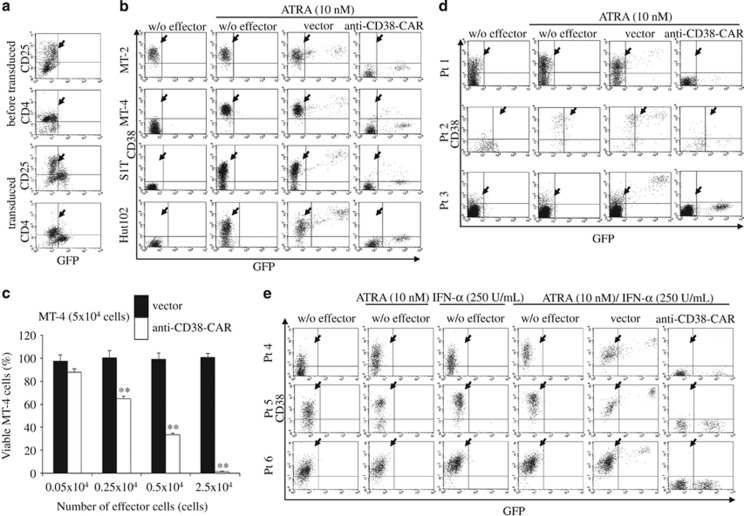
We first examined anti-CD38-CAR expression on retrovirally transduced human T cells from healthy donors using goat anti-mouse-IgG-PerCP, which cross-reacts with CAR. We confirmed that PerCP and GFP contained in the vector were co-expressed in transduced T cells (transduction efficiency: 61.26±10.66% (n=5)). Next, we investigated whether patients' ATL cells could be transduced with anti-CD38-CAR. GFP-positive T cells were negative for CD4 and CD25, indicating that ATL cells were not transduced with anti-CD38-CAR (Figure 1a). These results agreed with a previous observation that CD8+ T cells were markedly expanded and transduced with our methods.10 The transduction efficiency was 40.31±2.40% (n=4). Next, we evaluated the cytotoxicity of transduced T cells using the LDH releasing assay by co-incubating anti-CD38-CAR T cells with HTLV-1-transformed cell lines. As expected, MT-2 cells, with expression of CD38 being the highest among the six cell lines tested (97.06%), were efficiently abrogated by anti-CD38-CAR T cells (75.36±0.11% (n=3); Table 1). However, the other HTLV-I-transformed cell lines (MT-4, S1T, Hut102, Su9T and ED) lacking CD38 expression mostly survived after co-incubation with anti-CD38-CAR T cells (Table 1). Therefore, augmentation of CD38 expression was required to induce anti-CD38-CAR T-cell cytotoxicity against HTLV-1-transformed cell lines.
Figure 1.
Cytotoxic effects of T cells expressing anti-CD38-CAR against HTLV-1-transformed cells and primary ATL cells in the presence of ATRA and/or IFN-α. (a) Peripheral blood cells from a patient with ATL cells were transduced. Cells were stained with anti-CD25 antibody-PE after transduction with the retroviral vector and then analyzed by flow cytometry. The ATL cell population is indicated by the arrowhead. (b) Four HTLV-I-transformed cell lines (MT-2, MT-4, S1T and Hut102 cells) were co-cultured with T cells transduced with vector alone or anti-CD38-CAR in the presence of 10 nm of ATRA at an E:T ratio of 1:2 for 3 days, respectively. The cells collected from the co-culture wells were stained with an anti-CD38 antibody-APC. The viable CD38+ cell population is indicated by the arrowhead. (c) MT-4 cells were co-incubated with T cells bearing an empty vector or anti-CD38-CAR vector in ATRA presence at various E:T ratios for 3 days. MT-4 cells were stained with an anti-CD38 antibody-APC followed by flow cytometry. Asterisks denote a significant difference between two adjacent columns. (d) ATL cells from three patients (Pt 1, Pt 2 and Pt 3) were co-cultured with T cells transduced with an empty vector or anti-CD38-CAR vector in the presence of ATRA at an E:T ratio of 1:2 for 3 days. Cells were collected and stained with anti-CD38 antibody-APC. The arrowhead indicates CD38+ cell populations. (e) ATL cells obtained from three other patients (Pt 4, Pt 5 and Pt 6) were co-cultured with T cells transduced with or without an empty vector or a vector carrying anti-CD38-CAR in the presence of ATRA, IFN-α or both at an E:T ratio of 1:2 for 3 days. Viable CD38+ cell populations are indicated by the arrowhead.
Table 1. Cytotoxicity of T cells expressing anti-CD38-CAR against HTLV-1-transformed cells and ATL cells.
| Cells | Percentage of CD38+ cells (%) | Overall cytotoxicity of anti-CD38-CAR T cells by LDH assay (%) | Percentage of CD38+ cells with ATRA (%) | Specific cytotoxicity of anti-CD38-CAR T cells against CD38+ cells with ATRA by FCM (%) | Overall cytotoxicity of anti-CD38-CAR T cells with ATRA by FCM (%) |
|---|---|---|---|---|---|
| MT-2 | 97.06±1.00 | 75.36±0.11 | 97.19±1.47 | 99.90±0.09 | 92.30±1.48 |
| MT-4 | 2.91±0.31 | 4.97±1.18 | 97.81±0.36 | 98.61±0.12 | 97.92±0.33 |
| S1T | 0.01±0.01 | 0.73±0.34 | 81.34±1.35 | 96.98±0.09 | 81.08±1.12 |
| Hut102 | 1.18±0.13 | 2.74±3.36 | 86.11±3.94 | 99.51±0.02 | 86.47±2.74 |
| Su9T | 0.05±0.04 | 1.61±1.49 | 0.04±0.03 | ND | ND |
| ED | 0.01±0.01 | 2.60±0.61 | 0.05±0.04 | ND | ND |
| Patient 1 | 29.21±0.88 | ND | 58.81±1.24 | 99.84±0.22 | 58.70±1.11 |
| Patient 2 | 5.24±0.89 | ND | 79.58±1.19 | 92.42±2.02 | 74.40±1.94 |
| Patient 3 | 0.01±0.02 | ND | 0.04±0.01 | ND | ND |
| Cells | Expression of CD38 (%) | Expression of CD38 with ATRA (%) | Expression of CD38 with IFN-α (%) | Expression of CD38 with ATRA and IFN-α (%) | Overall cytotoxicity of anti-CD38-CAR T cells with ATRA/IFN-α by FCM (%) |
|---|---|---|---|---|---|
| Patient 4 | 34.94±1.39 | 78.70±2.00 | 66.48±0.81 | 95.45±0.91 | 99.68±0.18 |
| Patient 5 | 50.36±1.30 | 70.36±1.21 | 74.03±0.44 | 93.41±1.04 | 95.98±0.17 |
| Patient 6 | 82.01±1.40 | 91.15±0.92 | 97.64±0.42 | 97.55±0.71 | 99.92±0.02 |
Abbreviations: ATL, adult T-cell leukemia; ATRA, all-trans retinoic acid; CAR, chimeric antigen receptor; FCM, flow cytometry; HTLV-1, human T-cell lymphotropic virus type-1; IFN-α, interferon-α LDH, lactate dehydrogenase; ND, not determined.
Results are the mean±s.d. for three experiments.
Specific cytotoxicity was evaluated by flow cytometry, following co-incubation of T cells bearing anti-CD38-CAR (E) with ATL cells (T) at an E:T ratio of 1:2 for 3 days.
We investigated whether ATRA enhanced cytotoxicity of anti-CD38-CAR T cells by inducing CD38 expression on HTLV-1-transformed cell lines. As little as 10 nm of ATRA compared with an effective blood concentration for treating patients with APL, increased CD38 expression by over 80% in MT-4, S1T and Hut102 cells, but not in Su9T and ED cells (Figure 1b; Supplementary Figure 1a; Table 1). Three-day co-incubation of anti-CD38-CAR T cells with these cell lines at an effector (E): target (T) ratio of 1:2 in ATRA presence resulted in efficient elimination of MT-4, S1T and Hut102 cells according to the increased levels of CD38 expression (Figure 1b; Table 1). Cytotoxicity against cell lines was dependent on the number of T cells with anti-CD38-CAR in ATRA presence (Supplementary Figures 1b and c). Alternatively, ATRA withdrawal led to the basal level of CD38 expression of MT-4 cells before ATRA administration for 10 days (data not shown). However, CD38 induction by ATRA was insufficient to completely eliminate HTLV-1-transformed cells because 10–20% of S1T and Hut102 cells did not express CD38 in ATRA presence. To further improve the killing of HTLV-1-transformed cells and primary ATL cells by anti-CD38-CAR T cells through induced CD38 expression, we examined whether IFN-α and/or IFN-γ could enhance expression of the CD38 gene, whose upstream contains binding sites for IRF-1. IFN-α and IFN-γ efficiently enhanced CD38 expression in MT-4 cells even at a concentration below the therapeutic level, but not in Su9T, ED or S1T cells (Supplementary Figures 1a and c). As low as 2.5 U/ml of IFN-α induced CD38 expression in MT-4 cells for 18 h (CD38 expression: >95%). CD38 induction was more efficient with IFN-α than IFN-γ.
We then investigated whether ATL cells from patients were killed by anti-CD38-CAR T cells. Expression levels of CD38 in ATL cells from three patients varied (0.01–29.21%). Interestingly, 3-day treatment with 10 nm ATRA markedly enhanced CD38 expression in ATL cells from two of three patients (CD38 expression: 58.81 and 79.58%). Most importantly, anti-CD38-CAR T cells exerted marked cytotoxicity against ATL cells with CD38 expression enhanced by ATRA compared with T cells transduced with the vector control (Figure 1d; Table 1). However, CD38 induction by ATRA alone was much lower in patients' cells compared with that in cell lines. Thus, we examined whether the combination of ATRA with IFN-α enhanced surface CD38 expression. Notably, combined treatment with 10 nm ATRA and IFN-α synergistically enhanced CD38 expression in ATL cells from patients (CD38 expression: >90% Figure 1e; Table 1). ATRA and IFN-α did not reflect ATL cell numbers, because these reagents were used at extremely low concentrations (data not shown). Three-day co-culture of ATL cells from three patients with anti-CD38-CAR T cells in the presence of both ATRA and IFN-α at an E:T ratio of 1:2 resulted in eradication of >95% of ATL cells, demonstrating that they can be efficiently eliminated by anti-CD38-CAR T cells with both ATRA and IFN-α (Figure 1e; Table 1; Supplementary Figure 1d).
Treatment of ATL cells with both ATRA and IFN-α markedly enhanced the cytotoxicity of anti-CD38-CAR T cells against ATL cells through augmented CD38 expression. IFN-α partially suppressed ATL cell viability in vitro, suggesting an additional therapeutic benefit of IFN-α when used in combination with anti-CD38-CAR T cells.13, 14 CD38 is expressed in peripheral blood cells and restricted lineage-committed precursors in the bone marrow, as well as in the thymus and prostate. Thus, untoward toxicities in these organs may occur in anti-CD38-CAR T-cell therapy. Interestingly, an anti-CD38 antibody, daratumumab, has successfully been used to treat myeloma, indicating that therapeutic targeting of CD38 is a clinically feasible strategy. It has recently been reported that ATRA enhances the efficacy of daratumumab in myeloma patients whose myeloma cells expressed low levels of CD38 with fewer adverse effects.15 These findings suggest that ATRA may safely be used in combination with a CD38-targeting therapy. Further clinical studies are required to establish the safety and eligibility of ATL patients regarding the clinical use of anti-CD38-CAR T cells in combination with ATRA and/or IFN-α.
Patients receiving immunotherapy with anti-CD19-CAR T cells, which has a significant cytotoxicity against B-cell neoplasms, suffer from a prolonged B-cell aplasia and have to be periodically injected with γ-globulin. Furthermore, CAR therapy reportedly causes cytokine storm that can be lethal. Therefore, minimizing the cytotoxic activity of CAR T cells on normal cells, as well as augmenting expression of surface molecule on target cells, would be crucial in developing an effective therapy. Addition of a death domain to CAR may enable manipulation of CAR T cells to prevent the unwanted side effects before they occur. We envision that a novel immunotherapeutic strategy involving T cells carrying anti-CD38-CAR in combination with ATRA and IFN-α in the treatment of ATL may serve as a basis for the development of future CAR therapies.
Acknowledgments
We thank Dr Martin Andreansky (University of Miami) for providing his expertize on cloning and experiments. This study was supported in part by grants from the Ministry of Health, Labour and Welfare of Japan.
Author contributions
KM designed and performed the experiments, analyzed the data and wrote the manuscript; TY performed the experiments; YT, AK, KS and KM analyzed the data; YT, SI and TI helped write the manuscript. All authors contributed to interpretation of the results.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Tsukasaki K, Tobinai K. Biology and treatment of HTLV-1 associated T-cell lymphomas. Best Pract Res Clin Haematol 2013; 26: 3–14. [DOI] [PubMed] [Google Scholar]
- Utsunomiya A, Miyazaki Y, Takatsuka Y, Hanada S, Uozumi K, Yashiki S et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 2001; 27: 15–20. [DOI] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012; 119: 2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011; 118: 4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365: 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 2013; 122: 2965–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K, Bhattacharyya J, Kitanaka A, Yanagihara K, Kubo T, Takei Y et al. T-cell immunotherapy with a chimeric receptor against CD38 is effective in eliminating myeloma cells. Leukemia 2012; 26: 365–367. [DOI] [PubMed] [Google Scholar]
- Mihara K, Yanagihara K, Takigahira M, Imai C, Kitanaka A, Takihara Y et al. Activated T-cell-mediated immunotherapy with a chimeric receptor against CD38 in B-cell non-Hodgkin lymphoma. J Immunother 2009; 32: 737–743. [DOI] [PubMed] [Google Scholar]
- Mihara K, Yanagihara K, Takigahira M, Kitanaka A, Imai C, Bhattacharyya J et al. Synergistic and persistent effect of T-cell immunotherapy with anti-CD19 or anti-CD38 chimeric receptor in conjunction with rituximab on B-cell non-Hodgkin lymphoma. Br J Haematol 2010; 151: 37–46. [DOI] [PubMed] [Google Scholar]
- Drach J, Zhao S, Malavasi F, Mehta K. Rapid induction of CD38 antigen on myeloid leukemia cells by all trans-retinoic acid. Biochem Biophys Res Commun 1993; 195: 545–550. [DOI] [PubMed] [Google Scholar]
- Sugata K, Satou Y, Yasunaga J, Hara H, Ohshima K, Utsunomiya A et al. HTLV-1 bZIP factor impairs cell-mediated immunity by suppressing production of Th1 cytokines. Blood 2012; 119: 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, Taylor G et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol 2010; 28: 4177–4183. [DOI] [PubMed] [Google Scholar]
- Nijhof IS, Groen RW, Lokhorst HM, van Kessel B, Bloem AC, van Velzen J et al. Upregulation of CD38 expression on multiple myeloma cells by all-trans retinoic acid improves the efficacy of daratumumab. Leukemia 2015; 29: 2039–2049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.