Abstract
Phosphorylation of tyrosine residues in proteins, as well as their dephosphorylation, is closely related to various diseases. However, this phosphorylation is usually accompanied by more abundant phosphorylation of serine and threonine residues in the proteins and covers only 0.05% of the total phosphorylation. Accordingly, highly selective detection of phosphorylated tyrosine in proteins is an urgent subject. In this review, recent developments in this field are described. Monomeric and binuclear TbIII complexes, which emit notable luminescence only in the presence of phosphotyrosine (pTyr), have been developed. There, the benzene ring of pTyr functions as an antenna and transfers its photoexcitation energy to the TbIII ion as the emission center. Even in the coexistence of phosphoserine (pSer) and phosphothreonine (pThr), pTyr can be efficintly detected with high selectivity. Simply by adding these TbIII complexes to the solutions, phosphorylation of tyrosine in peptides by protein tyrosine kinases and dephosphorylation by protein tyrosine phosphatases can be successfully visualized in a real-time fashion. Furthermore, the activities of various inhibitors on these enzymes are quantitatively evaluated, indicating a strong potential of the method for efficient screening of eminent inhibitors from a number of candidates.
1. Introduction
In nature, enzymatic phosphorylation and dephosphorylation of proteins control many biological events. Cellular pathways regulated by these enzymatic modifications of proteins are so versatile. In the course of signal transduction in cells, for example, Ser, Thr, and Tyr, residues in proteins are reversibly phosphorylated and dephosphorylated, resulting in desired modulation of the activity of relevant enzymes [1, 2]. In terms of the importance of these enzymatic reactions, a number of elegant chemical sensors to detect them in proteins have been already reported. In most of these sensors, phosphate residue(s) of phosphoserine (pSer), phosphothreonine (pThr), and phosphotyrosine (pTyr) in proteins is selectively bound as the recognition target so that these three types of phosphorylations are detected at similar sensitivity without significant discrimination [3–11]. Valuable information on the roles of protein phosphorylations in biological systems has been obtained. The molecular designs of these sensors and their practical applications have been the subjects of many excellent reviews [12–21].
In contrast with these overall detections of phosphorylations of Ser, Thr, and Tyr in proteins, this review focuses on selective detection of phosphorylation of Tyr alone (Figure 1). This Tyr phosphorylation by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs) accounts for only 0.05% of the total phosphorylation in cells (the majority of phosphorylation occurs on Ser or Thr) but takes a crucial role in the regulation of highly important biological functions (differentiation, adhesion, cycle control, endocytosis, and many others) [22, 23]. In epidermal growth factor receptor (EGFR), its autophosphorylation of a Tyr residue triggers signal-cascade in cells [24, 25]. In the downstream, there work several Src family kinases, which are also controlled by their Tyr phosphorylations and in turn phosphorylate Tyr residues in other proteins [26–28]. If Tyr phosphorylation is excessive or insufficient, serious problems are induced to the living. Therefore, PTKs and PTPs are regarded as main targets in drug discovery [29–34]. For many years, a number of laboratories developed elegant optical sensors to evaluate the activities of these enzymes. In some of them, substrate peptide was conjugated (or fused) to a probe molecule (e.g., Tb(III) complexes [35–40], Mg(II) complexes [41–47], Ca(II) complex [48], Zn(II) complex [49], Cd(II) complex [50], peptide derivatives [51, 52], and others [53, 54]). The other sensors involve noncovalent interactions between a substrate and a probe (e.g., Tb(III) ion [55–62], Eu(III) complex [63, 64], platinum(II) complex [65], and Tb(III) complexes [66–69]).
Figure 1.
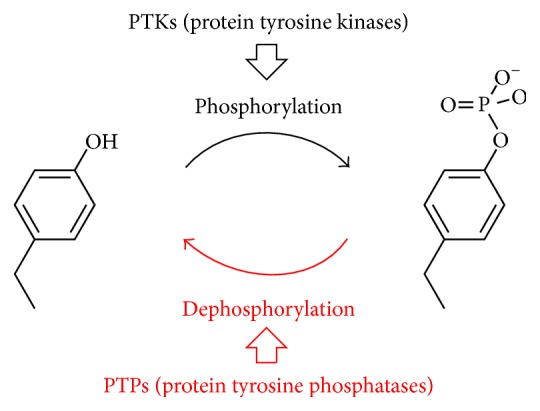
Phosphorylation of tyrosine residue by protein tyrosine kinases (PTKs) and its dephosphorylation by protein tyrosine phosphatases (PTPs) for the regulation of biological functions of proteins.
Among all the probes investigated, lanthanide ions and their complexes have been widely and successfully employed due to their unique light-emitting properties [70–77]. The photoluminescence from these ions has unusually long life-time (in the order of micro- to milliseconds), and thus the background signal can be minimized with the use of time-resolved spectroscopy. Alternatively, the kinase reactions were followed by the disappearance of ATP (source of the phosphate group for pTyr) [78, 79], whereas the phosphatase functions were monitored by the production of phosphoric acid [80]. However, these analytical methods are often complicated by the perturbation signals from other phosphate-containing solutes, ATP-dependent reactions, and/or phosphate-producing processes in the specimens. In addition to these chemical sensors, antibodies specific to pTyr are widely being used at present for practical applications, but their usage has been hampered by high costs, rather poor stability, and other factors. Accordingly, chemical probes that directly visualize PTK/PTP activity and produce unbiased signals are required for further developments of the field.
This paper reviews recent developments in optical methods to selectively detect pTyr in proteins. The primary concerns are high sensitivity of pTyr detection and its sufficient specificity (with respect to pSer and pThr, which exist much more abundantly in biological systems). As emission probes, lanthanide ions (especially TbIII ion) and their complexes are used. By combining unique properties of the emission from these metal ions with so-called “antenna effect,” the background signals are minimized, and only the signal from pTyr is selectively monitored [67]. The detection activity on pTyr is further promoted by forming binuclear TbIII complexes [68]. With the use of these chemical sensors, phosphorylation of peptides by PTKs and their dephosphorylation by PTPs are followed in a real-time fashion [38, 68, 69]. Applications of the methods to screening of efficient inhibitors on PTKs and PTPs are also presented.
2. Principle of Selective Detection of pTyr by Tb(III) Complexes
The emission from lanthanide ions is intrinsically weak, since the corresponding f-f transitions are Laporte-forbidden. However, this luminescence is enormously strengthened, when a chromophore (“antenna”) is placed near the lanthanide ions and transfers its photoexcitation energy to these emission centers [70–77, 81–91]. By combining lanthanide complexes with antenna molecules, elegant systems to detect various anionic guests have been already prepared. Sophisticated examples include the analysis of carboxylic acid derivatives [85–100], halide ions [95–98, 101–108], nitrate ions [96, 97, 101–105, 109], and hydrogen sulfate ion [110]. Furthermore, phosphate ion [95–99, 108–116], pyrophosphate [113–115], ATP [113–115, 117–121], and other molecules containing phosphate [120–122] were also detected by using lanthanide complexes. Upon the binding of the phosphate group(s) to the complexes, the chemical environments around the lanthanide(III) ions were altered, inducing a change in the luminescent property of the ions. With this strategy, however, highly selective detection of pTyr is rather difficult, since coexisting phosphate groups in solutions (e.g., pSer, pThr, ATP, and DNA) could show similar effects [123–125]. These factors are more critical when a TbIII ion (without any ligand) is used; note that nucleotides and nucleic acids are also eminent antenna (vide infra) [55, 56].
One successful solution to these problems (improvement of the selectivity of pTyr detection with respect to (i) nonphosphorylated Tyr, (ii) pSer and pThr, and (iii) other coexisting phosphate-containing biomolecules) is presented in Figure 2. This strategy, developed in our laboratory [67], is based on the fact that both the benzene ring and the phosphate group are definitely required for the efficient photoluminescence. First, the benzene ring of pTyr in the target peptides is used as an antenna to enhance the emission from the TbIII center. The irradiated light (Ex) is first absorbed by this benzene ring, and the excitation energy is then transferred to TbIII (ET). Finally, the metal center emits luminescence from its photoexcited state (Em). On the other hand, the phosphate is essential to bind to TbIII and places the benzene ring near the metal ion as the emission center. Among the coexisting solutes (Tyr, Ser, Thr, and their phosphorylated products), only pTyr possesses both notable antenna effect (benzene ring) and sufficient binding activity towards the TbIII complex (phosphate group). Thus, the selectivities (i) and (ii) are fulfilled. Furthermore, the selectivity (iii) to other coexisting phosphate-containing molecules is accomplished by using a bulky ligand which suppresses the access of these molecules to TbIII. Furthermore, nonspecific background signals can be removed by using time-resolved spectroscopy and analyzing only long life-time components of the luminescence emitted from TbIII.
Figure 2.
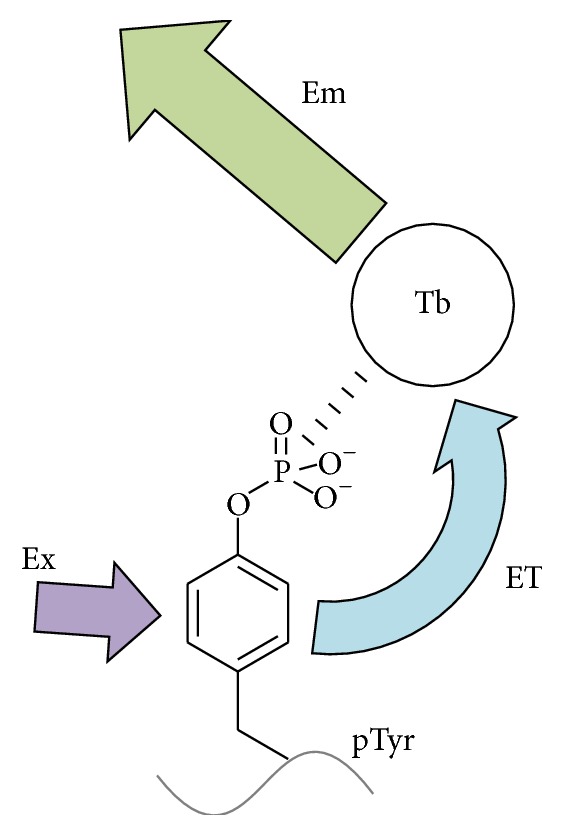
Mechanism of selective detection of enzymatic phosphorylation of Tyr by TbIII complex. The photoexcited energy (Ex) absorbed by the benzene ring (antenna) is transferred to the TbIII (ET), resulting in enormous promotion of the intensity of luminescence emitted from this metal ion (Em). Accordingly, the emission is evident only for pTyr which fulfills both of the two requirements for the mechanism (notable antenna effect and sufficient binding activity towards the TbIII).
3. Selective Detection of Enzymatic Phosphorylation of Tyr by TbIII Complex-Based Sensors
Based on the strategy depicted in Figure 2, enzymatic phosphorylations and dephosphorylations of Tyr were monitored by using TbIII complexes. In the first part of Section 3.1, a monomeric TbIII complex was prepared primarily to show the validity of the working hypothesis. In Section 3.2, the sensitivity of pTyr detection has been greatly enhanced by forming binuclear TbIII complexes. As a result, useful tools to monitor enzymatic phosphorylation of Tyr (and its dephosphorylation) have been obtained and used for practical applications in the following sections. Among lanthanide ions, TbIII has been most widely employed, together with Eu(III), for biological applications, emitting the most intensive line at around 545 nm.
3.1. Monomeric TbIII Complex Showing Sufficient Selectivity for pTyr Detection [67]
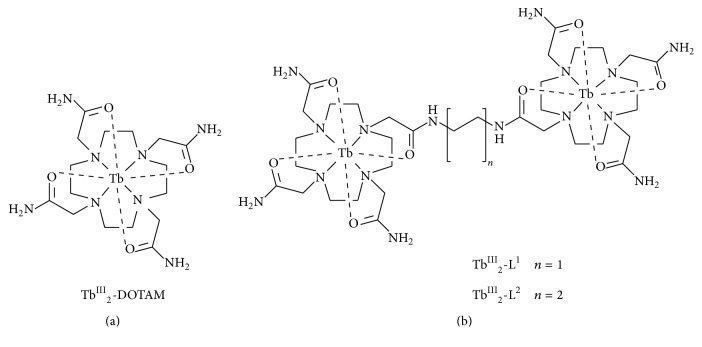
The sample solutions used for the sensing of pTyr always contain many other biological molecules, and some of them show notable antenna effects to induce the emission from lanthanide ions. Among them, nucleobases and nucleic acids especially deserve attention. For example, guanosine 5′-monophosphate (GMP) enormously enhances the luminescence through the binding to TbIII ion by multicoordination of both the phosphate and the guanine (N7 and O6). Thus, TbIII ion itself is not directly applicable to the sensing. In order to suppress the emission due to these coexisting molecules and accomplish efficient sensing of Tyr phosphorylation, an appropriate ligand is necessary to prevent the access of these molecules to TbIII ion. For this purpose, DOTAM (2,2′,2′′,2′′′-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetamide) was used (Figure 3(a)). This well-known ligand for lanthanide ions has no aromatic ring to work as antenna [126–129], and its TbIII complexes have +3 net charges which are favourable to bind negatively charged pTyr. According to the design, the bulkiness of DOTAM should sterically interfere with the interactions between bulky nucleobases (or nucleic acids) and TbIII. On the other hand, the effect of DOTAM on the binding of the phosphate in pTyr to the TbIII is little (or in much smaller magnitude) because of its smaller size.
Figure 3.
Structures of mononuclear DOTAM-TbIII complex (a) and binuclear complexes TbIII 2-L1 and TbIII 2-L2 (b) used for selective detection of pTyr.
Exactly as designed, pTyr notably increased the intensity of luminescence from TbIII-DOTAM complex (blue bars in Figure 4). Apparently, the phosphate residue of pTyr satisfactorily interacted with the complex despite the bulkiness of DOTAM, and the excitation energy of the benzene ring was efficiently transferred to the emission metal center. In contrast, GMP, as well as other nucleotides and nucleic acids, hardly promoted the luminescence from TbIII-DOTAM. Furthermore, nonphosphorylated Tyr, pSer, and pThr induced only marginal increase, as expected from the mechanism in Figure 2. The effect of either phenylalanine or tryptophan was negligible. Thus, TbIII-DOTAM complex is sufficiently effective in detecting pTyr selectively even in the coexistence of various analytes which otherwise produce undesirable noises of nonnegligible intensity. Using this complex, the tyrosine phosphorylation in a nonapeptide (Ac-Glu-Glu-Glu-Ile-Tyr-Glu-Glu-Phe-Asp-CONH2; P1 peptide [130]) was successfully monitored with a high signal-to-noise ratio. The mode of interaction between the metal center in TbIII-DOTAM and the phosphate group of pTyr was investigated by using phenyl phosphate (PhOP) as a model compound of pTyr (note that it also notably increased the luminescence from the DOTAM complex in Figure 4). In the presence or the absence of PhOP, the q value (the number of coordinated water molecules on TbIII) was determined by luminescence life-time measurements. Interestingly and importantly, q value was always around 1, whether or not PhOP was binding to the TbIII-DOTAM complex. In other words, one water molecule was originally coordinated to TbIII in the TbIII-DOTAM complex, and this water molecule was never removed from TbIII when the TbIII complex interacted with PhOP. Thus, it has been concluded that the interaction between TbIII-DOTAM and PhOP (and thus pTyr also) is an ion-pairing rather than direct coordination of the phosphate to TbIII. Nevertheless, the benzene ring of pTyr is placed in a sufficient proximity of TbIII and satisfactorily works as antenna.
Figure 4.
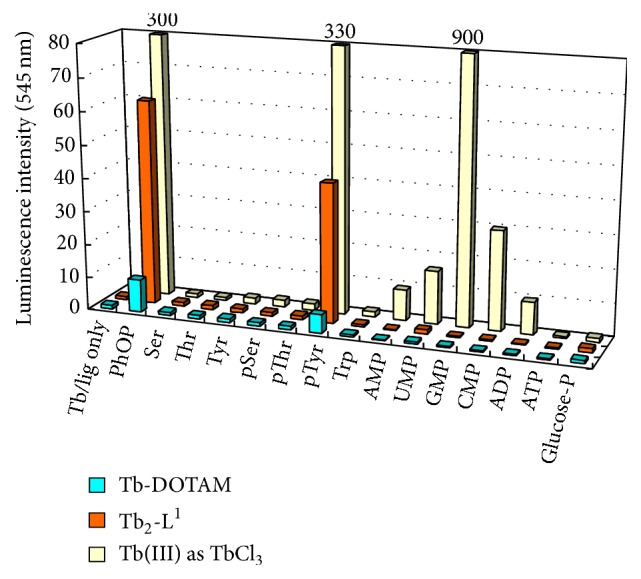
The luminescence intensity at 545 nm of TbIII-DOTAM (blue bars) and TbIII 2-L1 (red bars) in the presence of various phosphorylated and nonphosphorylated amino acids, nucleoside derivatives, and PhOP (a model compound of pTyr). Conditions: [TbIII complex] = [additive] = 100 μM, pH 7.0 (10 mM HEPES buffer), λ ex = 262.5 nm. For the purpose of comparison, the results using TbIII ion without ligand are also presented (yellow bars). Note that nucleotides (UMP, GMP, CMP, and ADP) showed notable signals and thus selective detection of pTyr was unsuccessful.
3.2. Binuclear TbIII Complexes for Promoted Detection Sensitivity on pTyr [68]
As shown in the previous section, mononuclear TbIII complex has eminent selectivity for pTyr detection. However, the sensitivity is still rather limited, primarily because of its poor binding of phosphate group. In order to further increase the detection sensitivity of TbIII-DOTAM on pTyr, its binuclear complexes (TbIII 2-L1 and TbIII 2-L2) were developed (Figure 3(b)). In the ligands used, two DOTAM groups were connected by appropriate linkers of different length. These complexes, as well as TbIII-DOTAM, show intrinsically minimal luminescence in the absence of pTyr (no antenna moiety is available). Importantly, the luminescence from these binuclear TbIII complexes was greatly enhanced when pTyr was added to the solution (red bars in Figure 4). Moreover, the pTyr-induced enhancements of luminescence from these binuclear complexes were far greater than pTyr-induced enhancement of luminescence from the mononuclear complex TbIII-DOTAM (compare the red bar with the blue bar in Figure 4). The origins of remarkable enhancements for the binuclear TbIII complexes were investigated in detail using a model compound PhOP in place of pTyr. When [TbIII complex] = [PhOP] = 100 μM, the luminescence from TbIII 2-L1 is stronger than that from TbIII-DOTAM by more than 10-fold. By analyzing the relationship between the luminescence intensity and the concentration of TbIII complex in terms of Michaelis-Menten type equation, the dissociation constant of TbIII 2-L1/PhOP complex was determined to be 29 μM. This value was 110 times smaller than the corresponding value for TbIII-DOTAM. Thus, the binuclear TbIII complexes have superior photoemission activity, mainly because they bind PhOP (and thus pTyr also) more efficiently. Apparently, the doubled positive charges of these binuclear complexes (+6) are responsible for the tighter interactions with the negatively charged phosphate of pTyr (note that the electrostatic interactions are primarily responsible for the binding; vide ante). Furthermore, the TbIII center and the benzene ring of pTyr are in sufficient proximity for energy-transfer to occur smoothly. In addition to these enhancements in fluorescence intensity, the selectivity of pTyr detection of the binuclear TbIII complexes, with respect to other cosolutes in solutions, is kept sufficiently high and comparable with that of the mononuclear TbIII-DOTAM. By using these binuclear TbIII complexes, Tyr-phosphorylated nonapeptide (P1-pY) was clearly distinguished at pH 7 from nonphosphorylated nonapeptide (P1).
4. Real-Time Monitoring of Enzymatic Tyrosine Phosphorylation and Dephosphorylation [68, 69]
By using TbIII 2-L1, the time-course of Tyr phosphorylation of peptides by PTKs can be straightforwardly monitored in real-time. For example, phosphorylation of the tyrosine residue in the center of a nonapeptide P1 by Src kinase was analyzed in Figure 5. To the solution containing TbIII 2-L1 and P1, as well as ATP and MnCl2 (essential factors in this enzymatic reaction), Src tyrosine kinase was added and then the luminescence at 545 nm (5D4 → 7F5 transition) was measured. The luminescence intensity increased time-dependently, reflecting the Tyr phosphorylation. The magnitude of increase in luminescence intensity is exactly consistent with the difference in the concentration of P1. Without the substrate peptide, the luminescence was never enhanced. When Tyr-phosphorylated P1 was used as the substrate, the luminescence was strong from the beginning and not enhanced even after the addition of Src kinase. In order to confirm the validity of the method furthermore, the rate of this enzymatic phosphorylation was independently determined using TAMRA-labeled P1. There, the reaction was stopped at several reaction times and the products were analyzed by polyacrylamide gel-electrophoresis. The results of these two methods fairly agreed with each other, as expected.
Figure 5.
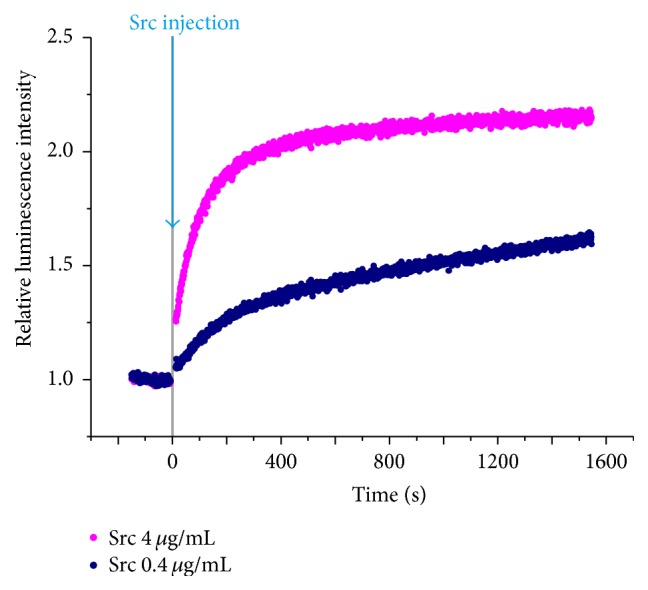
Time-dependent change of the luminescence intensity from TbIII 2-L1 for the phosphorylation of P1 by protein tyrosine kinase Src. At time = 0, Src kinase was added to the solution containing other species and the reaction was started. [Src] = 4 (pink) and 0.4 μg/mL (navy). [P1] = 5 μM, [TbIII 2-L1] = 100 μM, [ATP] = 5 μM, and [MnCl2] = 1 mM. The excitation at 262.5 nm and the emission at 545 nm. Reprinted with permission from [69]. Copyright 2014, Springer.
Similarly, the reverse reactions of the phosphorylations, dephosphorylations of a tyrosine-phosphorylated peptide by PTPs, were also visualized by TbIII 2-L1 in real-time (Figure 6). Here, the peptide as kinase substrate was simply substituted with the corresponding phosphorylated peptide. When Shp-1 tyrosine phosphatase was added to the solution containing both P1-pY and TbIII 2-L1, the luminescence intensity gradually decreased. The magnitude of luminescence change was exactly dependent on PTP concentration. The TbIII 2-L1 binds relatively weakly to the pTyr residue and does not much disrupt the phosphatase reactions. Still more complicated sequential reactions of PTK and PTP were also monitored in one-pot fashion (Figure 7). When nonphosphorylated P1 was first phosphorylated by Src kinase, the luminescence intensity gradually increased. Then (e.g., 1500 seconds later), Shp-1 phosphatase was added to the solution. The luminescence decreased due to the dephosphorylation of P1-pY. After 300 seconds, Src kinase was again added (at the same time, sodium orthovanadate Na3VO4 was added to the reaction mixture to deactivate Shp-1 phosphatase). The luminescence intensity increased again. Apparently, the second Src kinase reaction was successfully monitored, even when the mixture was so complicated and contained many components (the products of the foregoing phosphorylation and dephosphorylation reactions, the deactivated Shp-1, and other remaining reagents).
Figure 6.
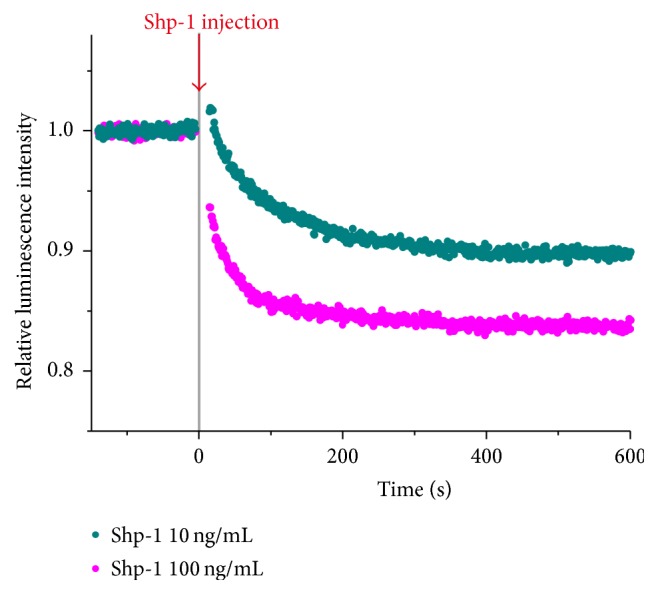
Time-dependent change of the luminescence intensity from TbIII 2-L1 for the dephosphorylation of P1-pY by Shp-1 tyrosine phosphatase. [Shp-1] = 100 (pink) and 10 ng/mL (cyan). [P1-pY] = 10 μM and [TbIII 2-L1] = 100 μM. The excitation at 262.5 nm and the emission at 545 nm. Reprinted with permission from [69]. Copyright 2014, Springer.
Figure 7.
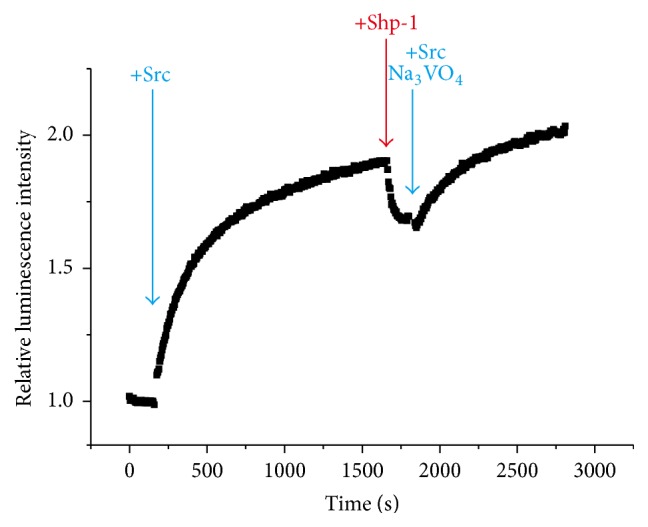
One-pot visualization by TbIII 2-L1 of the sequential reactions of a PTK (Src kinase) and a PTP (Shp-1 phosphatase). To the reaction solution, Src kinase, Shp-1, and Na3VO4 (inhibitor of Shp-1) were added at the timings shown by the arrows. Reprinted with permission from [69]. Copyright 2014, Springer.
Most of previous kinetic studies on these enzymatic reactions involved either radiolabeling of the peptide/ATP or chemical labeling of peptide with chromophores. The labeling procedures are time-consuming and still, more importantly, could cause nonnegligible perturbations on the enzymatic reactions. Compared with these methods, the present methods using the TbIII complexes are advantageous in that no labeling is required and kinetic information can be straightforwardly obtained. Simply by adding the TbIII complexes to the reaction mixture and monitoring the photoluminescence, the time-courses of reactions can be directly obtained in situ in real-time. Accordingly, detailed kinetic results are precisely obtained, even when the enzymatic systems are highly complicated and the reaction rates do not strictly obey simple Michaelis-Menten equation (e.g., allosteric control in the enzymatic systems and inhibition by other products).
5. Quantitative Evaluation of PTK and PTP Inhibitors Using TbIII-Based Chemical Sensor [69]
There are many kinds of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs) in our bodies. Each of them takes an important role in the corresponding reaction and is strongly related to various diseases. Thus, inhibitors on a predetermined enzyme among these PTKs/PTPs have been regarded as promising targets for drug discovery and the subject of growing interest. This section presents the application of binuclear TbIII complexes to screening of inhibitors from a pool of candidates. As described above, the binuclear TbIII complexes can visualize the enzymatic phosphorylation and dephosphorylation in a real-time fashion. Accordingly, the inhibition activity can be evaluated in terms of both kinetic aspects and static aspects, providing new kinds of information to these fields. In this section, the specificity and activity of well-known inhibitors on PTKs and PTPs were determined by using TbIII complexes and compared with the literature data primarily to confirm the validity of method.
5.1. Inhibitors on PTK
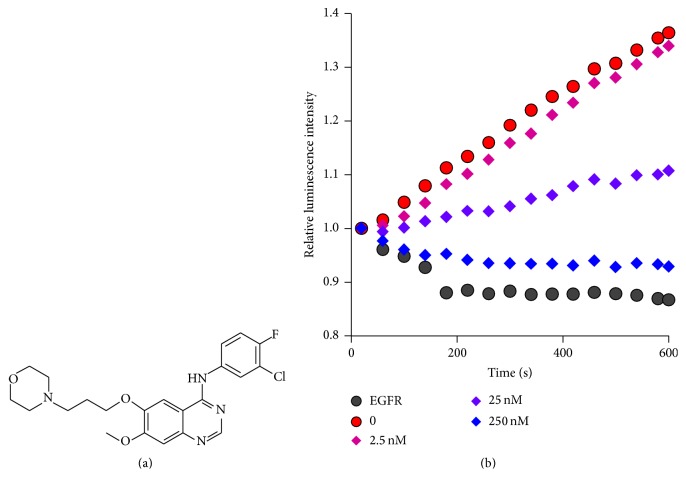
Using TbIII 2-L1 as a chemical sensor, the inhibitory effects of PTK inhibitors on three kinds of PTKs (Src, Fyn, and EGFR) were analyzed. Among the PTK inhibitors investigated, staurosporine is a general kinase inhibitor with minimum selectivity [131, 132]. On the other hand, gefitinib is effective only in inhibiting EGFR [133], and dasatinib strongly inhibits the other two PTKs [134]. Imatinib is inactive to all of them [135]. To the solution containing P1, MnCl2, TbIII 2-L1, and a PTK, gefitinib was added, and the reaction was started with the addition of ATP. The inhibitory effects were visually analyzed in terms of the real-time kinetics. Gefitinib drastically suppressed the enzymatic reaction of EGFR (its target PTK) depending on its concentration (Figure 8). When [gefitinib] = 25 nM, the activity of EGFR was reduced to approximately half. In contrast, this inhibitor was much less effective to the other two PTKs (Src and Fyn). Even with [gefitinib] = 25 μM (1000-fold larger than EGFR case), they showed sufficient activity. The inhibition specificity was completely identical with the known specificity.
Figure 8.
Real-time monitoring by TbIII 2-L1 on the gefitinib inhibition of P1 phosphorylation by protein tyrosine kinase EGFR. (a) The structure of the inhibitor gefitinib. In (b), gefitinib was added to the solution containing P1, MnCl2, EGFR kinase, and TbIII 2-L1, and the reaction was started with addition of ATP. Gray: control without EGFR; red: control without gefitinib. Reproduced with permission from [69]. Copyright 2014, Springer.
Still more quantitative assay of the activity of inhibitors in terms of the half maximal inhibitory concentration (IC50) was also successful (Figure 9). By the use of time-resolved luminescence spectroscopy, high signal-to-noise ratios were accomplished for various combinations of PTKs and their inhibitors, and clear sigmoidal dose-response relationships were obtained. From these curves, the IC50 values of these inhibitors to the corresponding enzyme were determined (see Table 1). The specificity of all the inhibitors investigated fairly agreed with that reported in the literature [131]. The present method should be very powerful and promising for various applications, especially when a number of substrates, enzymes, and/or inhibitors are analyzed to screen eminent inhibitors from a pool of candidates.
Figure 9.
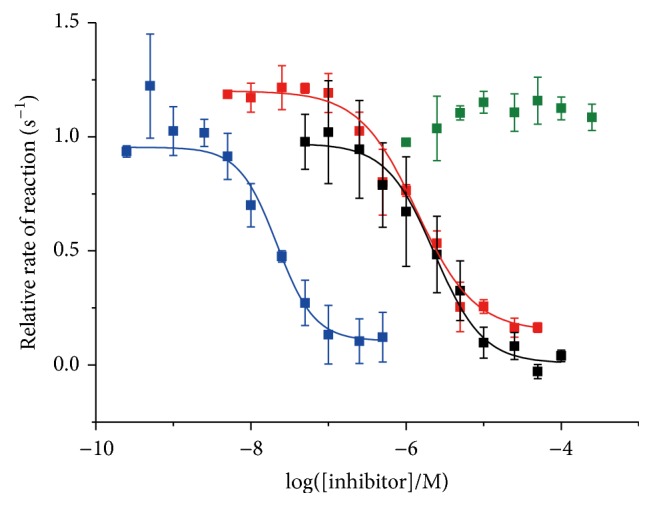
Plots of the rates of EGFR kinase-catalyzed phosphorylation of P1 versus the concentrations of various inhibitors. The rates of phosphorylation of P1 by the kinase were determined in the presence of dasatinib (red), gefitinib (blue), imatinib (green), and staurosporine (black) by the method in Figure 8. The IC50 values of the inhibitors, calculated by fitting these sigmoidal curves, are presented in Table 1, together with the values on Src and Fyn kinases. Reproduced with permission from [69]. Copyright 2014, Springer.
Table 1.
IC50 of PTK inhibitors.
| IC50 (nM) | Dasatinib | Gefitinib | Imatinib | Staurosporine |
|---|---|---|---|---|
| Src | 12 ± 0.96 | >5000a | —b | 310 ± 28 |
| Fyn | 26 ± 2.2 | >2000a | >10000a | 260 ± 19 |
| EGFR | 1400 ± 110 | 22 ± 2.4 | —b | 2300 ± 820 |
aNot determined due to poor curve fitting of weak inhibitors. bInhibition was not observed.
5.2. Inhibitors on PTP
The inhibitory effects of Na3VO4 [136] and α-bromo-4′-hydroxyacetophenone [137] on PTPs (Shp-1 and PTP1B) were investigated using TbIII 2-L1. The luminescence intensity gradually decreased as the enzymatic dephosphorylation proceeded and the concentration of pTyr decreased. Upon the addition of inhibitors, the magnitude of this decrease became smaller. Consistently, the change in luminescence intensity by PTP-induced dephosphorylation of P1-pY depended on the inhibitor concentration. The IC50 values, calculated from the sigmoidal curves, were completely consistent with known selectivity of these inhibitors.
6. Attempts to Improve the Detection Sensitivity: Conjugation of Binuclear TbIII Complexes to Substrate Peptides [38]
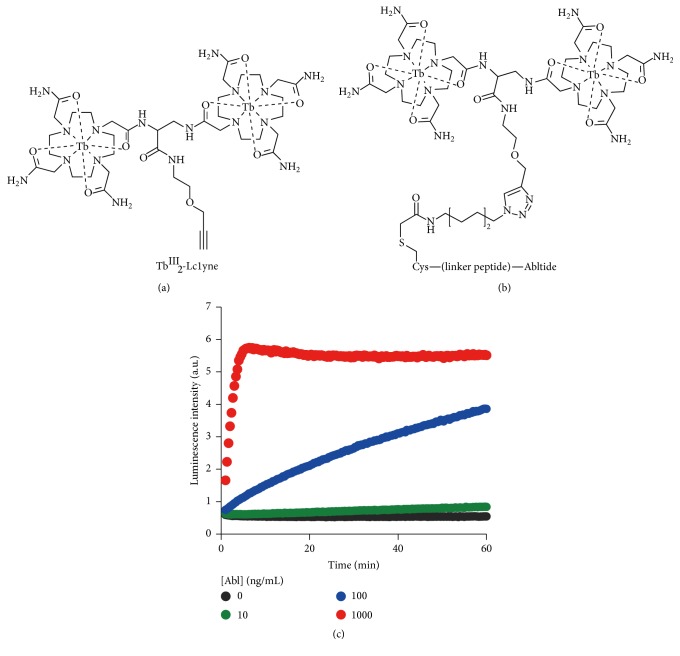
As described above, both phosphorylation of tyrosine by PTK and dephosphorylation by PTP are visually monitored simply by adding the binuclear TbIII complexes (TbIII 2-L1 and TbIII 2-L2) to the reaction mixtures. The method is simple, straightforward, and useful. However, the detection sensitivity is in some case rather small, especially when the peptide substrates are positively charged. There, the association of peptides with the positively charged TbIII complexes is suppressed by electrostatic repulsion, and thus the energy-transfer from pTyr to TbIII does not satisfactorily proceed (note that bindings are primarily due to electrostatic interactions). As a general strategy to overcome these drawbacks, substrate peptide and the TbIII complex were covalently connected, and the intermolecular association between them was converted to more efficient intramolecular one. In order to facilitate the synthesis of these conjugates, a new binuclear TbIII complex (TbIII 2-Lc1yne; Figure 10(a)) was prepared by attaching an alkynyl group to TbIII 2-L1. Separately, an azido group was bound through a linker to a cysteine residue which was additionally introduced to the peptide substrate. The conjugate was easily obtained by click reaction between the alkyne in TbIII 2-Lc1yne and the azido group in the peptide (Figure 10(b)). This strategy can be applied to various peptide substrates without significant limitation in the structures.
Figure 10.
(a) TbIII 2-Lc1yne and (b) its conjugate with Abltide peptide prepared by click reaction. In (c), phosphorylation of Abltide by tyrosine kinases Abl was monitored in real-time using the conjugate by measuring time-resolved luminescence at 545 nm. The concentrations of Abl were 1000 (red), 100 (blue), and 10 ng/mL (green). Conditions: [the conjugate] = 5 μM, [ATP] = 100 μM, [MgCl2] = 1 mM, and [NaCl] = 7.5 mM. Reproduced with permission from [38]. Copyright 2015, American Chemical Society.
The Tyr phosphorylation of Abltide (Glu-Ala-Ile-Tyr-Ala-Ala-Pro-Phe-Ala-Lys-Lys-Lys; a well-known substrate for Abl kinase) [130] by Abl kinase was monitored (Figure 10(c)). At the pH for the phosphorylation, this peptide bears net charge of +2 and the interaction with TbIII 2-L1 was too weak. All the attempts to monitor its phosphorylation using nonconjugated TbIII 2-L1 complex were unsuccessful. Accordingly, a cysteine residue was introduced to the N-terminus of Abltide, and an azido was bound thereto. The conjugation of this modified peptide with Tb2-Lc1yne by click chemistry proceeded smoothly. When the resultant conjugate was treated with Abl kinase in the presence of ATP, the luminescence intensity increased time-dependently due to the phosphorylation of Tyr. When the peptide concentration was 300 nM, the signal-to-noise ratio was 15.1, being sufficient for detailed quantitative analysis of the reaction. The signal for the Tyr phosphorylation was clearly observed even when the peptide concentration was decreased down to 50 nM. Similar results were obtained when Src was used as PTK and the Tyr phosphorylation of Abltide was successfully monitored. In addition to the N-terminus conjugation, the Tb2-Lc1yne can be also conjugated to the C-terminus of Abltide. The advantage of the present conjugation strategy is conclusive.
The effect of the distance between the TbIII complex and the pTyr on the monitoring activity of the conjugate was analyzed by changing the length of linker peptide between the N-terminus of Abltide and the cysteine residue (1 to 5 amino acids). The rate of phosphorylation was almost the same for all the five conjugates. The TbIII complex bound to the Abltide imposes minimal steric hindrance on the enzymatic reaction. Furthermore, the luminescence increased in almost the same magnitude (10-fold) upon the phosphorylation. Apparently, the structure of the complex formed between the Tb2-Lc1yne and the pTyr residue in the peptide is similar irrespective of the length of the linker peptide. This ensures the applicability of this strategy to various substrates without strict structural restriction.
7. Conclusions
The importance of phosphorylation of tyrosine residues in proteins and their dephosphorylation has been well recognized, and chemical sensors to monitor this phosphorylation selectively have been attracting interests. Recently, significant progress has been made in this field. A DOTAM complex of TbIII showing very high selectivity to pTyr has been developed. The photoemission from TbIII-DOTAM complex is notable only when pTyr exists in the solutions. There, the benzene ring of pTyr functions as an antenna and transfers its photoexcitation energy to the TbIII ion as the emission center. Accordingly, the emission is selective to pTyr, since nonphosphorylated tyrosine cannot efficiently bind to the TbIII complex, and neither phosphoserine nor phosphothreonine can satisfactorily provide an antenna effect. Furthermore, the binding of bulky cosolutes (e.g., nucleotides and nucleic acids) to TbIII is suppressed by the steric hindrance of DOTAM. By the use of time-resolved luminescence analysis, only the long life-time luminescence from TbIII is analyzed and high signal-to-noise ratios are accomplished.
Binuclear TbIII complexes (TbIII 2-L1 and TbIII 2-L2), in which two TbIII-DOTAM complexes are connected through the linkers in the ligands, are far more effective in the detection of pTyr than the monomeric TbIII complex. The increase in the sensitivity is primarily ascribed to the stronger binding of pTyr to these complexes, due to enhanced electrostatic interactions between them. With the use of these complexes as sensors, phosphorylation of tyrosine by protein tyrosine kinases and dephosphorylation by protein tyrosine phosphatases are visualized in situ in a real-time fashion. Furthermore, the activities of various inhibitors on these enzymes are quantitatively evaluated by the TbIII complexes. This method should be useful in screening highly eminent inhibitors from a number of candidates. These enzymes take crucially important biological roles so that the information obtained by these studies should lead to development of new drugs for the therapy of relevant diseases. By immobilizing these TbIII complexes to some solid supports, the applications of the present methods should be further facilitated and widened.
Supplementary Material
By using binuclear TbIII complexes, phosphorylation of tyrosine in peptides by protein tyrosine kinases (PTKs) and dephosphorylation by protein tyrosine phosphatases (PTPs) can be successfully visualized in a real-time fashion. The activities of various inhibitors on these enzymes are quantitatively evaluated, indicating a strong potential of the method to efficient screening of eminent inhibitors from a number of candidates.
Acknowledgments
This work was partially supported by a Grant-in-Aid for Specially Promoted Scientific Research (22000007) and a Grant-in-Aid for Scientific Research (A) (15H02189) from Ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosure
Current address of Makoto Komiyama is World Premier International (WPI) Research Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Hunter T. Signaling-2000 and beyond. Cell. 2000;100(1):113–127. doi: 10.1016/s0092-8674(00)81688-8. [DOI] [PubMed] [Google Scholar]
- 2.Cohen P. The origins of protein phosphorylation. Nature Cell Biology. 2002;4(5):E127–E130. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto T., Ojida A., Hamachi I. Molecular recognition, fluorescence sensing, and biological assay of phosphate anion derivatives using artificial Zn(II)–Dpa complexes. Chemical Communications. 2009;(2):141–152. doi: 10.1039/b812374h. [DOI] [PubMed] [Google Scholar]
- 4.Ojida A., Mito-Oka Y., Inoue M.-A., Hamachi I. First artificial receptors and chemosensors toward phosphorylated peptide in aqueous solution. Journal of the American Chemical Society. 2002;124(22):6256–6258. doi: 10.1021/ja025761b. [DOI] [PubMed] [Google Scholar]
- 5.Ojida A., Mito-oka Y., Sada K., Hamachi I. Molecular recognition and fluorescence sensing of monophosphorylated peptides in aqueous solution by Bis(zinc(II)-dipicolylamine)-based artificial receptors. Journal of the American Chemical Society. 2004;126(8):2454–2463. doi: 10.1021/ja038277x. [DOI] [PubMed] [Google Scholar]
- 6.Kinoshita E., Takahashi M., Takeda H., Shiro M., Koike T. Recognition of phosphate monoester dianion by an alkoxide-bridged dinuclear zinc(II) complex. Dalton Transactions. 2004;(8):1189–1193. doi: 10.1039/b400269e. [DOI] [PubMed] [Google Scholar]
- 7.Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Molecular and Cellular Proteomics. 2006;5(4):749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Riechers A., Schmidt F., Stadlbauer S., König B. Detection of protein phosphorylation on SDS-PAGE using probes with a phosphate-sensitive emission response. Bioconjugate Chemistry. 2009;20(4):804–807. doi: 10.1021/bc9000307. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T., Edwards N. Y., Bonizzoni M., Anslyn E. V. The use of differential receptors to pattern peptide phosphorylation. Journal of the American Chemical Society. 2009;131(33):11976–11984. doi: 10.1021/ja9041675. [DOI] [PubMed] [Google Scholar]
- 10.Schulenberg B., Aggeler R., Beechem J. M., Capaldi R. A., Patton W. F. Analysis of steady-state protein phosphorylation in mitochondria using a novel fluorescent phosphosensor dye. Journal of Biological Chemistry. 2003;278(29):27251–27255. doi: 10.1074/jbc.C300189200. [DOI] [PubMed] [Google Scholar]
- 11.Schulenberg B., Goodman T. N., Aggeler R., Capaldi R. A., Patton W. F. Characterization of dynamic and steady-state protein phosphorylation using a fluorescent phosphoprotein gel stain and mass spectrometry. Electrophoresis. 2004;25(15):2526–2532. doi: 10.1002/elps.200406007. [DOI] [PubMed] [Google Scholar]
- 12.Pazos E., Vázquez M. E. Advances in lanthanide-based luminescent peptide probes for monitoring the activity of kinase and phosphatase. Biotechnology Journal. 2014;9(2):241–252. doi: 10.1002/biot.201300203. [DOI] [PubMed] [Google Scholar]
- 13.González-Vera J. A. Probing the kinome in real time with fluorescent peptides. Chemical Society Reviews. 2012;41(5):1652–1664. doi: 10.1039/c1cs15198c. [DOI] [PubMed] [Google Scholar]
- 14.Butler S. J., Parker D. Anion binding in water at lanthanide centres: from structure and selectivity to signalling and sensing. Chemical Society Reviews. 2013;42(4):1652–1666. doi: 10.1039/c2cs35144g. [DOI] [PubMed] [Google Scholar]
- 15.Haas K. L., Franz K. J. Application of metal coordination chemistry to explore and manipulate cell biology. Chemical Reviews. 2009;109(10):4921–4960. doi: 10.1021/cr900134a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris M. C. Fluorescent biosensors—probing protein kinase function in cancer and drug discovery. Biochimica et Biophysica Acta. 2013;34(7):1387–1395. doi: 10.1016/j.bbapap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q., Wang J., Boyd B. J. Peptide-based biosensors. Talanta. 2015;136:114–127. doi: 10.1016/j.talanta.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Zherdeva V. V., Savitsky A. P. Using lanthanide-based resonance energy transfer for in vitro and in vivo studies of biological processes. Biochemistry. 2012;77(13):1553–1574. doi: 10.1134/S0006297912130111. [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Li Y., Xu X., et al. Nanomaterial-based tools for protein kinase bioanalysis. TrAC—Trends in Analytical Chemistry. 2014;58:40–53. doi: 10.1016/j.trac.2014.01.009. [DOI] [Google Scholar]
- 20.Lawrence D. S., Wang Q. Seeing is believing: peptide-based fluorescent sensors of protein tyrosine kinase activity. ChemBioChem. 2007;8(4):373–378. doi: 10.1002/cbic.200600473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y. J., Xie W. H., Fang G. J. Fluorescence detection techniques for protein kinase assay. Analytical and Bioanalytical Chemistry. 2008;390(8):2049–2057. doi: 10.1007/s00216-008-1986-z. [DOI] [PubMed] [Google Scholar]
- 22.Hunter T. The Croonian Lecture 1997. The phosphorylation of proteins on tyrosine: its role in cell growth and disease. Philosophical Transactions of the Royal Society B: Biological Sciences. 1998;353(1368):583–605. doi: 10.1098/rstb.1998.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter T. Tyrosine phosphorylation: thirty years and counting. Current Opinion in Cell Biology. 2009;21(2):140–146. doi: 10.1016/j.ceb.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morandell S., Stasyk T., Skvortsov S., Ascher S., Huber L. A. Quantitative proteomics and phosphoproteomics reveal novel insights into complexity and dynamics of the EGFR signaling network. Proteomics. 2008;8(21):4383–4401. doi: 10.1002/pmic.200800204. [DOI] [PubMed] [Google Scholar]
- 25.Guo A., Villén J., Kornhauser J., et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(2):692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engen J. R., Wales T. E., Hochrein J. M., et al. Structure and dynamic regulation of Src-family kinases. Cellular and Molecular Life Sciences. 2008;65(19):3058–3073. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons S. J., Parsons J. T. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23(48):7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 28.Luo W., Slebos R. J., Hill S., et al. Global impact of oncogenic src on a phosphotyrosine proteome. Journal of Proteome Research. 2008;7(8):3447–3460. doi: 10.1021/pr800187n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitzki A. Protein tyrosine kinase inhibitors as novel therapeutic agents. Pharmacology and Therapeutics. 1999;82(2-3):231–239. doi: 10.1016/S0163-7258(98)00066-7. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J., Yang P. L., Gray N. S. Targeting cancer with small molecule kinase inhibitors. Nature Reviews Cancer. 2009;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 31.Krause D. S., Van Etten R. A. Tyrosine kinases as targets for cancer therapy. New England Journal of Medicine. 2005;353(2):172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Z.-X., Zhang Z.-Y. Targeting PTPs with small molecule inhibitors in cancer treatment. Cancer and Metastasis Reviews. 2008;27(2):263–272. doi: 10.1007/s10555-008-9113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott L. M., Lawrence H. R., Sebti S. M., Lawrence N. J., Wu J. Targeting protein tyrosine phosphatases for anticancer drug discovery. Current Pharmaceutical Design. 2010;16(16):1843–1862. doi: 10.2174/138161210791209027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bialy L., Waldmann H. Inhibitors of protein tyrosine phosphatases: next-generation drugs? Angewandte Chemie—International Edition. 2005;44(25):3814–3839. doi: 10.1002/anie.200461517. [DOI] [PubMed] [Google Scholar]
- 35.Tremblay M. S., Zhu Q., Martí A. A., et al. Phosphorylation state-responsive lanthanide peptide conjugates: a luminescence switch based on reversible complex reorganization. Organic Letters. 2006;8(13):2723–2726. doi: 10.1021/ol060614u. [DOI] [PubMed] [Google Scholar]
- 36.Appelblom H., Sipponen A., Valanne A., Soukka T., Lövgren T., Niemelä P. Antibody-free lanthanide-based fluorescent probe for determination of protein tyrosine kinase and phosphatase activities. Microchimica Acta. 2011;172(1):25–29. doi: 10.1007/s00604-010-0450-x. [DOI] [Google Scholar]
- 37.Pazos E., Goličnik M., Mascareñas J. L., Vázquez M. E. Detection of phosphorylation states by intermolecular sensitization of lanthanide-peptide conjugates. Chemical Communications. 2012;48(76):9534–9536. doi: 10.1039/c2cc34958b. [DOI] [PubMed] [Google Scholar]
- 38.Akiba H., Sumaoka J., Tsumoto K., Komiyama M. Click conjugation of a binuclear terbium(III) complex for real-time detection of tyrosine phosphorylation. Analytical Chemistry. 2015;87(7):3834–3840. doi: 10.1021/ac5045466. [DOI] [PubMed] [Google Scholar]
- 39.Lipchik A. M., Perez M., Cui W., Parker L. L. Multicolored, Tb3+-based antibody-free detection of multiple tyrosine kinase activities. Analytical Chemistry. 2015;87(15):7555–7558. doi: 10.1021/acs.analchem.5b02233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tremblay M. S., Lee M., Sames D. A luminescent sensor for tyrosine phosphorylation. Organic Letters. 2008;10(1):5–8. doi: 10.1021/ol701920x. [DOI] [PubMed] [Google Scholar]
- 41.Shults M. D., Imperiali B. Versatile fluorescence probes of protein kinase activity. Journal of the American Chemical Society. 2003;125(47):14248–14249. doi: 10.1021/ja0380502. [DOI] [PubMed] [Google Scholar]
- 42.Shults M. D., Janes K. A., Lauffenburger D. A., Imperiali B. A multiplexed homogeneous fluorescence-based assay for protein kinase activity in cell lysates. Nature Methods. 2005;2(4):277–284. doi: 10.1038/nmeth747. [DOI] [PubMed] [Google Scholar]
- 43.Luković E., González-Vera J. A., Imperiali B. Recognition-domain focused chemosensors: versatile and efficient reporters of protein kinase activity. Journal of the American Chemical Society. 2008;130(38):12821–12827. doi: 10.1021/ja8046188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luković E., Taylor E. V., Imperiali B. Monitoring protein kinases in cellular media with highly selective chimeric reporters. Angewandte Chemie—International Edition. 2009;48(37):6828–6831. doi: 10.1002/anie.200902374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szalewski D. A., Beck J. R., Stains C. I. Design, synthesis, and evaluation of a selective chemosensor for leucine-rich repeat kinase 2. Bioorganic and Medicinal Chemistry Letters. 2014;24(24):5648–5651. doi: 10.1016/j.bmcl.2014.10.079. [DOI] [PubMed] [Google Scholar]
- 46.Beck J. R., Lawrence A., Tung A. S., Harris E. N., Stains C. I. Interrogating endogenous protein phosphatase activity with rationally designed chemosensors. ACS Chemical Biology. 2016;11(1):284–290. doi: 10.1021/acschembio.5b00506. [DOI] [PubMed] [Google Scholar]
- 47.Johnson W. H., Ozpolat B., Cho E. J., et al. High-throughput screens for eEF-2 kinase. Journal of Biomolecular Screening. 2014;19(3):445–452. doi: 10.1177/1087057113505204. [DOI] [PubMed] [Google Scholar]
- 48.Chen C.-A., Yeh R.-H., Lawrence D. S. Design and synthesis of a fluorescent reporter of protein kinase activity. Journal of the American Chemical Society. 2002;124(15):3840–3841. doi: 10.1021/ja017530v. [DOI] [PubMed] [Google Scholar]
- 49.Rhee H.-W., Lee S. H., Shin I.-S., et al. Detection of kinase activity using versatile fluorescence quencher probes. Angewandte Chemie—International Edition. 2010;49(29):4919–4923. doi: 10.1002/anie.201000879. [DOI] [PubMed] [Google Scholar]
- 50.Kikuchi K., Hashimoto S., Mizukami S., Nagano T. Anion sensor-based ratiometric peptide probe for protein kinase activity. Organic Letters. 2009;11(13):2732–2735. doi: 10.1021/ol9006508. [DOI] [PubMed] [Google Scholar]
- 51.Wakata A., Cahill S. M., Blumenstein M., et al. A mechanistic design principle for protein tyrosine kinase sensors: application to a validated cancer target. Organic Letters. 2008;10(2):301–304. doi: 10.1021/ol702675b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sahoo H., Hennig A., Florea M., Roth D., Enderle T., Nau W. M. Single-label kinase and phosphatase assays for tyrosine phosphorylation using nanosecond time-resolved fluorescence detection. Journal of the American Chemical Society. 2007;129(51):15927–15934. doi: 10.1021/ja074975w. [DOI] [PubMed] [Google Scholar]
- 53.Pritz S., Meder G., Doering K., et al. A fluorescence lifetime-based assay for Abelson kinase. Journal of Biomolecular Screening. 2011;16(1):65–72. doi: 10.1177/1087057110385817. [DOI] [PubMed] [Google Scholar]
- 54.Sculimbrene B. R., Imperiali B. Lanthanide-binding tags as luminescent probes for studying protein interactions. Journal of the American Chemical Society. 2006;128(22):7346–7352. doi: 10.1021/ja061188a. [DOI] [PubMed] [Google Scholar]
- 55.Ringer D. P., Etheredge J. L., Dalrymple B. L., Niedbalski J. S. Fluorescence of phosphotyrosine—terbium(III) complexes. Biochemical and Biophysical Research Communications. 1990;168(1):267–273. doi: 10.1016/0006-291x(90)91703-u. [DOI] [PubMed] [Google Scholar]
- 56.Galvan B., Christopoulos T. K. Fluorometric and time-resolved immunofluorometric assays for protein-tyrosine phosphatase activity. Clinical Biochemistry. 1996;29(2):125–131. doi: 10.1016/0009-9120(95)02026-8. [DOI] [PubMed] [Google Scholar]
- 57.Liu L. L., Franz K. J. Phosphorylation of an α-synuclein peptide fragment enhances metal binding. Journal of the American Chemical Society. 2005;127(27):9662–9663. doi: 10.1021/ja043247v. [DOI] [PubMed] [Google Scholar]
- 58.Zondlo S. C., Gao F., Zondlo N. J. Design of an encodable tyrosine kinase-inducible domain: detection of tyrosine kinase activity by terbium luminescence. Journal of the American Chemical Society. 2010;132(16):5619–5621. doi: 10.1021/ja100862u. [DOI] [PubMed] [Google Scholar]
- 59.Lipchik A. M., Parker L. L. Time-resolved luminescence detection of spleen tyrosine kinase activity through terbium sensitization. Analytical Chemistry. 2013;85(5):2582–2588. doi: 10.1021/ac3023422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui W., Parker L. L. A time-resolved luminescence biosensor assay for anaplastic lymphoma kinase (ALK) activity. Chemical Communications. 2015;51(2):362–365. doi: 10.1039/c4cc07453j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duodu E., Kraskouskaya D., Campbell J., Graca-Lima G., Gunning P. T. Selective detection of tyrosine-containing proximally phosphorylated motifs using an antenna-free Tb3+ luminescent sensor. Chemical Communications. 2015;51(30):6675–6677. doi: 10.1039/c5cc00679a. [DOI] [PubMed] [Google Scholar]
- 62.Lipchik A. M., Perez M., Bolton S., et al. KINATEST-ID: a pipeline to develop phosphorylation-dependent terbium sensitizing kinase assays. Journal of the American Chemical Society. 2015;137(7):2484–2494. doi: 10.1021/ja507164a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Atkinson P., Bretonniere Y., Parker D. Chemoselective signalling of selected phospho-anions using lanthanide luminescence. Chemical Communications. 2004;10(4):438–439. doi: 10.1039/b313496m. [DOI] [PubMed] [Google Scholar]
- 64.Atkinson P., Murray B. S., Parker D. A cationic lanthanide complex binds selectively to phosphorylated tyrosine sites, aiding NMR analysis of the phosphorylated insulin receptor peptide fragment. Organic and Biomolecular Chemistry. 2006;4(16):3166–3171. doi: 10.1039/b608528h. [DOI] [PubMed] [Google Scholar]
- 65.Yeung M. C.-L., Yam V. W.-W. Phosphate derivative-induced supramolecular assembly and NIR-emissive behaviour of alkynylplatinum(II) terpyridine complexes for real-time monitoring of enzymatic activities. Chemical Science. 2013;4(7):2928–2935. doi: 10.1039/c3sc50383f. [DOI] [Google Scholar]
- 66.Wang X., Yang T., Luo J., Yang L., Yao C. Site-selective recognition of peptide phosphorylation by a terbium(III) complex in aqueous solution. Chemical Communications. 2015;51(38):8185–8188. doi: 10.1039/c5cc01056j. [DOI] [PubMed] [Google Scholar]
- 67.Akiba H., Sumaoka J., Komiyama M. Selective detection of phosphotyrosine in the presence of various phosphate-containing biomolecules with the aid of a terbium (III) complex. ChemBioChem. 2009;10(11):1773–1776. doi: 10.1002/cbic.200900227. [DOI] [PubMed] [Google Scholar]
- 68.Akiba H., Sumaoka J., Komiyama M. Binuclear terbium(III) complex as a probe for tyrosine phosphorylation. Chemistry: A European Journal. 2010;16(17):5018–5025. doi: 10.1002/chem.200903379. [DOI] [PubMed] [Google Scholar]
- 69.Akiba H., Sumaoka J., Hamakubo T., Komiyama M. Conjugation-free, visual, and quantitative evaluation of inhibitors on protein tyrosine kinases and phosphatases with a luminescent Tb(III) complex. Analytical and Bioanalytical Chemistry. 2014;406(12):2957–2964. doi: 10.1007/s00216-014-7707-x. [DOI] [PubMed] [Google Scholar]
- 70.Richardson F. S. Terbium(III) and europium(III) ions as luminescent probes and stains for biomolecular systems. Chemical Reviews. 1982;82(5):541–552. doi: 10.1021/cr00051a004. [DOI] [Google Scholar]
- 71.Bünzli J.-C. G., Piguet C. Taking advantage of luminescent lanthanide ions. Chemical Society Reviews. 2005;34(12):1048–1077. doi: 10.1039/b406082m. [DOI] [PubMed] [Google Scholar]
- 72.Eliseeva S. V., Bünzli J.-C. G. Lanthanide luminescence for functional materials and bio-sciences. Chemical Society Reviews. 2010;39(1):189–227. doi: 10.1039/b905604c. [DOI] [PubMed] [Google Scholar]
- 73.Leonard J. P., Gunnlaugsson T. Luminescent Eu(III) and Tb(III) complexes: developing lanthanide luminescent-based devices. Journal of Fluorescence. 2005;15(4):585–595. doi: 10.1007/s10895-005-2831-9. [DOI] [PubMed] [Google Scholar]
- 74.dos Santos C. M. G., Harte A. J., Quinn S. J., Gunnlaugsson T. Recent developments in the field of supramolecular lanthanide luminescent sensors and self-assemblies. Coordination Chemistry Reviews. 2008;252(23-24):2512–2527. doi: 10.1016/j.ccr.2008.07.018. [DOI] [Google Scholar]
- 75.Wang X., Chang H., Xie J., et al. Recent developments in lanthanide-based luminescent probes. Coordination Chemistry Reviews. 2014;273-274:201–212. doi: 10.1016/j.ccr.2014.02.001. [DOI] [Google Scholar]
- 76.Heffern M. C., Matosziuk L. M., Meade T. J. Lanthanide probes for bioresponsive imaging. Chemical Reviews. 2014;114(8):4496–4539. doi: 10.1021/cr400477t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thibon A., Pierre V. C. Principles of responsive lanthanide-based luminescent probes for cellular imaging. Analytical and Bioanalytical Chemistry. 2009;394(1):107–120. doi: 10.1007/s00216-009-2683-2. [DOI] [PubMed] [Google Scholar]
- 78.Kurishita Y., Kohira T., Ojida A., Hamachi I. Rational design of FRET-based ratiometric chemosensors for in vitro and in cell fluorescence analyses of nucleoside polyphosphates. Journal of the American Chemical Society. 2010;132(38):13290–13299. doi: 10.1021/ja103615z. [DOI] [PubMed] [Google Scholar]
- 79.Schäferling M., Wolfbeis O. S. Europium tetracycline as a luminescent probe for nucleoside phosphates and its application to the determination of kinase activity. Chemistry. 2007;13(15):4342–4349. doi: 10.1002/chem.200601509. [DOI] [PubMed] [Google Scholar]
- 80.Geladopoulos T. P., Sotiroudis T. G., Evangelopoulos A. E. A malachite green colorimetric assay for protein phosphatase activity. Analytical Biochemistry. 1991;192(1):112–116. doi: 10.1016/0003-2697(91)90194-X. [DOI] [PubMed] [Google Scholar]
- 81.Moore E. G., Samuel A. P. S., Raymond K. N. From antenna to assay: lessons learned in lanthanide luminescence. Accounts of Chemical Research. 2009;42(4):542–552. doi: 10.1021/ar800211j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Armelao L., Quici S., Barigelletti F., et al. Design of luminescent lanthanide complexes: from molecules to highly efficient photo-emitting materials. Coordination Chemistry Reviews. 2010;254(5-6):487–505. doi: 10.1016/j.ccr.2009.07.025. [DOI] [Google Scholar]
- 83.Lis S., Elbanowski M., Makowska B., Hnatejko Z. Energy transfer in solution of lanthanide complexes. Journal of Photochemistry and Photobiology A: Chemistry. 2002;150(1–3):233–247. doi: 10.1016/s1010-6030(01)00637-2. [DOI] [Google Scholar]
- 84.Sabbatini N., Guardigli M., Lehn J.-M. Luminescent lanthanide complexes as photochemical supramolecular devices. Coordination Chemistry Reviews. 1993;123(1-2):201–228. doi: 10.1016/0010-8545(93)85056-A. [DOI] [Google Scholar]
- 85.Gunnlaugsson T., Harte A. J., Leonard J. P., Nieuwenhuyzen M. The formation of luminescent supramolecular ternary complexes in water: delayed luminescence sensing of aromatic carboxylates using coordinated unsaturated cationic heptadentate lanthanide ion complexes. Supramolecular Chemistry. 2003;15(7-8):505–519. doi: 10.1080/10610270310001605106. [DOI] [Google Scholar]
- 86.Gunnlaugsson T., Harte A. J., Leonard J. P., Nieuwenhuyzen M. Delayed lanthanide luminescence sensing of aromatic carboxylates using heptadentate triamide Tb(III) cyclen complexes: the recognition of salicylic acid in water. Chemical Communications. 2002;(18):2134–2135. doi: 10.1039/b204888d. [DOI] [PubMed] [Google Scholar]
- 87.Harte A. J., Jensen P., Plush S. E., Kruger P. E., Gunnlaugsson T. A dinuclear lanthanide complex for the recognition of bis(carboxylates): formation of terbium(III) luminescent self-assembly ternary complexes in aqueous solution. Inorganic Chemistry. 2006;45(23):9465–9474. doi: 10.1021/ic0614418. [DOI] [PubMed] [Google Scholar]
- 88.Leonard J. P., dos Santos C. M. G., Plush S. E., McCabe T., Gunnlaugsson T. pH driven self-assembly of a ternary lanthanide luminescence complex: the sensing of anions using a β-diketonate-Eu(III) displacement assay. Chemical Communications. 2007;(2):129–131. doi: 10.1039/b611487c. [DOI] [PubMed] [Google Scholar]
- 89.Plush S. E., Gunnlaugsson T. Luminescent sensing of dicarboxylates in water by a bismacrocyclic dinuclear Eu(III) conjugate. Organic Letters. 2007;9(10):1919–1922. doi: 10.1021/ol070339r. [DOI] [PubMed] [Google Scholar]
- 90.Plush S. E., Gunnlaugsson T. Solution studies of trimetallic lanthanide luminescent anion sensors: towards ratiometric sensing using an internal reference channel. Dalton Transactions. 2008;(29):3801–3804. doi: 10.1039/b805610b. [DOI] [PubMed] [Google Scholar]
- 91.Vaněk J., Lubal P., Hermann P., Anzenbacher P., Jr. Luminescent sensor for carbonate ion based on lanthanide(III) complexes of 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid (DO3A) Journal of Fluorescence. 2013;23(1):57–69. doi: 10.1007/s10895-012-1116-3. [DOI] [PubMed] [Google Scholar]
- 92.Parker D., Yu J. A pH-insensitive, ratiometric chemosensor for citrate using europium luminescence. Chemical Communications. 2005;(25):3141–3143. doi: 10.1039/b502553b. [DOI] [PubMed] [Google Scholar]
- 93.Wang L., Li B., Zhang L., Li P., Jiang H. An optical anion chemosensor based on a europium complex and its molecular logic behavior. Dyes and Pigments. 2013;97(1):26–31. doi: 10.1016/j.dyepig.2012.11.021. [DOI] [Google Scholar]
- 94.Butler S. J., McMahon B. K., Pal R., Parker D., Walton J. W. Bright mono-aqua europium complexes based on triazacyclononane that bind anions reversibly and permeate cells efficiently. Chemistry—A European Journal. 2013;19(29):9511–9517. doi: 10.1002/chem.201301273. [DOI] [PubMed] [Google Scholar]
- 95.Yang C., Xu J., Li J., Lu M., Li Y., Wang X. An efficiently colorimetric and fluorescent probe of fluoride, acetate and phosphate ions based on a novel trinuclear Eu-complex. Sensors and Actuators, B: Chemical. 2014;196:133–139. doi: 10.1016/j.snb.2014.01.123. [DOI] [Google Scholar]
- 96.Montalti M., Prodi L., Zaccheroni N., Charbonnière L., Douce L., Ziessel R. A luminescent anion sensor based on a europium hybrid complex. Journal of the American Chemical Society. 2001;123(50):12694–12695. doi: 10.1021/ja0118688. [DOI] [PubMed] [Google Scholar]
- 97.Charbonniére L. J., Ziessel R., Montalti M., et al. Luminescent lanthanide complexes of a bis-bipyridine-phosphine-oxide ligand as tools for anion detection. Journal of the American Chemical Society. 2002;124(26):7779–7788. doi: 10.1021/ja0200847. [DOI] [PubMed] [Google Scholar]
- 98.Zhang D., Shi M., Liu Z., Li F., Yi T., Huang C. Luminescence modulation of a terbium complex with anions and its application as a reagent. European Journal of Inorganic Chemistry. 2006;2006(11):2277–2284. doi: 10.1002/ejic.200501064. [DOI] [Google Scholar]
- 99.Hammell J., Buttarazzi L., Huang C.-H., Morrow J. R. Eu(III) complexes as anion-responsive luminescent sensors and paramagnetic chemical exchange saturation transfer agents. Inorganic Chemistry. 2011;50(11):4857–4867. doi: 10.1021/ic200075w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schäferling M., Ääritalo T., Soukka T. Multidentate europium chelates as luminoionophores for anion recognition: impact of ligand design on sensitivity and selectivity, and applicability to enzymatic assays. Chemistry—A European Journal. 2014;20(18):5298–5308. doi: 10.1002/chem.201304942. [DOI] [PubMed] [Google Scholar]
- 101.Yamada T., Shinoda S., Tsukube H. Anion sensing with luminescent lanthanide complexes of tris(2-pyridylmethyl)amines: pronounced effects of lanthanide center and ligand chirality on anion selectivity and sensitivity. Chemical Communications. 2002;(11):1218–1219. doi: 10.1039/b202822k. [DOI] [PubMed] [Google Scholar]
- 102.Mahajan R. K., Kaur I., Kaur R., et al. Anion receptor functions of lanthanide tris(β-diketonate) complexes: naked eye detection and ion-selective electrode determination of Cl− anion. Chemical Communications. 2003;9(17):2238–2239. doi: 10.1039/b306850a. [DOI] [PubMed] [Google Scholar]
- 103.Kataoka Y., Paul D., Miyake H., Shinoda S., Tsukube H. A Cl− anion-responsive luminescent Eu3+ complex with a chiral tripod: ligand substituent effects on ternary complex stoichiometry and anion sensing selectivity. Dalton Transactions. 2007;(26):2784–2791. doi: 10.1039/b703944a. [DOI] [PubMed] [Google Scholar]
- 104.Shinoda S. Dynamic cyclen-metal complexes for molecular sensing and chirality signaling. Chemical Society Reviews. 2013;42(4):1825–1835. doi: 10.1039/c2cs35295h. [DOI] [PubMed] [Google Scholar]
- 105.Shinoda S., Tsukube H. Luminescent lanthanide complexes as analytical tools in anion sensing, pH indication and protein recognition. Analyst. 2011;136(3):431–435. doi: 10.1039/c0an00808g. [DOI] [PubMed] [Google Scholar]
- 106.Wang Q., Tamiaki H. Highly efficient and selective turn-off quenching of ligand-sensitized luminescence from europium imidazo[4,5-f]-1,10-phenanthroline complex by fluoride ion. Journal of Photochemistry and Photobiology A: Chemistry. 2009;206(2-3):124–128. doi: 10.1016/j.jphotochem.2009.05.024. [DOI] [Google Scholar]
- 107.Butler S. J. Quantitative determination of fluoride in pure water using luminescent europium complexes. Chemical Communications. 2015;51(54):10879–10882. doi: 10.1039/c5cc03428k. [DOI] [PubMed] [Google Scholar]
- 108.Tan C., Wang Q. Two novel europium (III) centered anion receptors and their naked eye detections. Synthetic Metals. 2012;162(15-16):1416–1420. doi: 10.1016/j.synthmet.2012.06.007. [DOI] [Google Scholar]
- 109.Caffrey D. F., Gunnlaugsson T. Displacement assay detection by a dimeric lanthanide luminescent ternary Tb(iii)-cyclen complex: high selectivity for phosphate and nitrate anions. Dalton Transactions. 2014;43(48):17964–17970. doi: 10.1039/c4dt02341b. [DOI] [PubMed] [Google Scholar]
- 110.Xu W., Zhou Y., Huang D., et al. Luminescent sensing profiles based on anion-responsive lanthanide(III) quinolinecarboxylate materials: solid-state structures, photophysical properties, and anionic species recognition. Journal of Materials Chemistry C. 2015;3(9):2003–2015. doi: 10.1039/c4tc02369b. [DOI] [Google Scholar]
- 111.dos Santos C. M. G., Fernández P. B., Plush S. E., Leonard J. P., Gunnlaugsson T. Lanthanide luminescent anion sensing: evidence of multiple anion recognition through hydrogen bonding and metal ion coordination. Chemical Communications. 2007;(32):3389–3391. doi: 10.1039/b705560a. [DOI] [PubMed] [Google Scholar]
- 112.Dos Santos C. M. G., Gunnlaugsson T. The recognition of anions using delayed lanthanide luminescence: the use of Tb(III) based urea functionalised cyclen complexes. Dalton Transactions. 2009;(24):4712–4721. doi: 10.1039/b902955a. [DOI] [PubMed] [Google Scholar]
- 113.Li S.-H., Yu C.-W., Yuan W.-T., Xu J.-G. A lanthanide hybrid cluster as a selective optical chemosensor for phosphate-containing anions in aqueous solution. Analytical Sciences. 2004;20(10):1375–1377. doi: 10.2116/analsci.20.1375. [DOI] [PubMed] [Google Scholar]
- 114.Wang Y.-W., Liu S.-B., Yang Y.-L., Wang P.-Z., Zhang A.-J., Peng Y. A terbium(III)-complex-based on–off fluorescent chemosensor for phosphate anions in aqueous solution and its application in molecular logic gates. ACS Applied Materials and Interfaces. 2015;7(7):4415–4422. doi: 10.1021/am5089346. [DOI] [PubMed] [Google Scholar]
- 115.Nadella S., Sahoo J., Subramanian P. S., Sahu A., Mishra S., Albrecht M. Sensing of phosphates by using luminescent EuIII and TbIII complexes: application to the microalgal cell Chlorella vulgaris . Chemistry—A European Journal. 2014;20(20):6047–6053. doi: 10.1002/chem.201304664. [DOI] [PubMed] [Google Scholar]
- 116.Andolina C. M., Morrow J. R. Luminescence resonance energy transfer in heterodinuclear LnIII complexes for sensing biologically relevant anions. European Journal of Inorganic Chemistry. 2011;2011(1):154–164. doi: 10.1002/ejic.201000779. [DOI] [Google Scholar]
- 117.Banerjee S., Bhuyan M., König B. Tb(III) functionalized vesicles for phosphate sensing: membrane fluidity controls the sensitivity. Chemical Communications. 2013;49(50):5681–5683. doi: 10.1039/c3cc42132e. [DOI] [PubMed] [Google Scholar]
- 118.Shao N., Jin J., Wang G., Zhang Y., Yang R., Yuan J. Europium(III) complex-based luminescent sensing probes for multi-phosphate anions: modulating selectivity by ligand choice. Chemical Communications. 2008;(9):1127–1129. doi: 10.1039/b715719c. [DOI] [PubMed] [Google Scholar]
- 119.Mameri S., Charbonnière L. J., Ziessel R. F. Lanthanide/ATP interaction in water mediated by Luminescent hemispherical-shaped complexes. Inorganic Chemistry. 2004;43(6):1819–1821. doi: 10.1021/ic030295s. [DOI] [PubMed] [Google Scholar]
- 120.Weitz E. A., Chang J. Y., Rosenfield A. H., Pierre V. C. A selective luminescent probe for the direct time-gated detection of adenosine triphosphate. Journal of the American Chemical Society. 2012;134(39):16099–16102. doi: 10.1021/ja304373u. [DOI] [PubMed] [Google Scholar]
- 121.Weitz E. A., Chang J. Y., Rosenfield A. H., Morrow E. A., Pierre V. C. The basis for the molecular recognition and the selective time-gated luminescence detection of ATP and GTP by a lanthanide complex. Chemical Science. 2013;4(10):4052–4060. doi: 10.1039/c3sc51583d. [DOI] [Google Scholar]
- 122.Best M. D., Anslyn E. V. A fluorescent sensor for 2,3-bisphosphoglycerate using a europium tetra-N-oxide bis-bipyridine complex for both binding and signaling purposes. Chemistry—A European Journal. 2003;9(1):51–57. doi: 10.1002/chem.200390003. [DOI] [PubMed] [Google Scholar]
- 123.Fu P. K.-L., Turro C. Energy transfer from nucleic acids to Tb(III): selective emission enhancement by single DNA mismatches. Journal of the American Chemical Society. 1999;121(1):1–7. doi: 10.1021/ja9826082. [DOI] [Google Scholar]
- 124.Ringer D. P., Burchett S., Kizer D. E. Use of terbium(III) fluorescence enhancement to selectively monitor DNA and RNA guanine residues and their alteration by chemical modification. Biochemistry. 1978;17(22):4818–4825. doi: 10.1021/bi00615a032. [DOI] [PubMed] [Google Scholar]
- 125.Topal M. D., Fresco J. R. Fluorescence of terbium ion-nucleic acid complexes: a sensitive specific probe for unpaired residues in nucleic acids. Biochemistry. 1980;19(24):5531–5537. doi: 10.1021/bi00565a011. [DOI] [PubMed] [Google Scholar]
- 126.Amin S., Morrow J. R., Lake C. H., Churchill M. R. Lanthanide(III) tetraamide macrocyclic complexes as synthetic ribonucleases: structure and catalytic properties of [La(tcmc)(CF3SO3)(EtOH)](CF3SO3)2 . Angewandte Chemie—International Edition. 1994;33(7):773–775. doi: 10.1002/anie.199407731. [DOI] [Google Scholar]
- 127.Amin S., Voss D. A., Jr., Horrocks W. D., Jr., Lake C. H., Churchill M. R., Morrow J. R. Laser-induced luminescence studies and crystal structure of the europium(III) complex of 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane. The link between phosphate diester binding and catalysis by lanthanide(III) macrocyclic complexes. Inorganic Chemistry. 1995;34(12):3294–3300. doi: 10.1021/ic00116a023. [DOI] [Google Scholar]
- 128.Rodríguez-Rodríguez A., Esteban-Gómez D., de Blas A., et al. Solution structure of Ln(III) complexes with macrocyclic ligands through theoretical evaluation of 1H NMR contact shifts. Inorganic Chemistry. 2012;51(24):13419–13429. doi: 10.1021/ic302322r. [DOI] [PubMed] [Google Scholar]
- 129.Baranyai Z., Bányai I., Brücher E., Király R., Terreno E. Kinetics of the formation of [Ln(DOTAM)]3+ complexes. European Journal of Inorganic Chemistry. 2007;2007(23):3639–3645. doi: 10.1002/ejic.200700178. [DOI] [Google Scholar]
- 130.Songyang Z., Carraway K. L., III, Eck M. J., et al. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995;373(6514):536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- 131.Karaman M. W., Herrgard S., Treiber D. K., et al. A quantitative analysis of kinase inhibitor selectivity. Nature Biotechnology. 2008;26(1):127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 132.Anastassiadis T., Deacon S. W., Devarajan K., Ma H., Peterson J. R. Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nature Biotechnology. 2011;29(11):1039–1045. doi: 10.1038/nbt.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ciardiello F., Tortora G. EGFR antagonists in cancer treatment. The New England Journal of Medicine. 2008;358(11):1160–1174. doi: 10.1056/nejmra0707704. [DOI] [PubMed] [Google Scholar]
- 134.Montero J. C., Seoane S., Ocaña A., Pandiella A. Inhibition of Src family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clinical Cancer Research. 2011;17(17):5546–5552. doi: 10.1158/1078-0432.ccr-10-2616. [DOI] [PubMed] [Google Scholar]
- 135.Capdeville R., Buchdunger E., Zimmermann J., Matter A. Glivec (ST1571, imatinib), a rationally developed, targeted anticancer drug. Nature Reviews Drug Discovery. 2002;1(7):493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- 136.Gordon J. A. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods in Enzymology. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- 137.Arabaci G., Guo X.-C., Beebe K. D., Coggeshall K. M., Pei D. α-Haloacetophenone derivatives as photoreversible covalent inhibitors of protein tyrosine phosphatases. Journal of the American Chemical Society. 1999;121(21):5085–5086. doi: 10.1021/ja9906756. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
By using binuclear TbIII complexes, phosphorylation of tyrosine in peptides by protein tyrosine kinases (PTKs) and dephosphorylation by protein tyrosine phosphatases (PTPs) can be successfully visualized in a real-time fashion. The activities of various inhibitors on these enzymes are quantitatively evaluated, indicating a strong potential of the method to efficient screening of eminent inhibitors from a number of candidates.