Abstract
Background: To assess progress in improving affordability of medicines since the introduction of mandatory health insurance in the Republic of Moldova.
Method: Using data from national health insurance, we estimate affordability of partially reimbursed medicines for the treatment of non-communicable diseases, and analyse which factors contributed to changes in affordability.
Results: Affordability of subsidized medicines improved over time. In 2013, it took a median of 0.84 days of income for the lowest income quintile (ranging from 0 to 3.32 days) to purchase 1 month of treatment for cardiovascular conditions in comparison to 1.85 days in 2006. This improvement however was mainly driven by higher incomes rather than deeper coverage through the reimbursement list.
Conclusion: If mandatory health insurance is to improve affordability of medicines for the Moldovan population, more funds need to be (re-)allocated to enable higher percentage coverage of essential medicines and efficiencies need to be generated within the health system. These should include a budget reallocation between secondary and primary care, strengthening primary care to manage chronic conditions and raise population awareness, implementation of evidence-based selection and quality use of medicines in both outpatient and inpatient settings, improving monitoring and regulation of prices and the supply chain; and alignment of national treatment guidelines and clinical practice with international best practices and evidence-based medicine.
Keywords: Affordability, medicines, mandatory health insurance, transition countries, Republic of Moldova
Key Messages
Affordability of partially compensated medicines for the treatment of NCDs has improved since the introduction of the first reimbursement list in 2006 for all income and expenditure quintiles.
This improvement, however, was mainly driven by higher incomes and expenditure rather than deeper coverage through the reimbursement list.
Simply increasing funding for medicines is likely not to deliver the expected results, nor to be a feasible or sustainable solution, if broader issues affecting use of medicines are not addressed.
Introduction
Medicines tend to represent the largest component of out-of-pocket payments expenditure on health, particularly in countries with incomplete health insurance (Ibrahimov et al. 2010; Khodjamurodov and Rechel 2010; Turcanu et al. 2012; Richardson et al. 2013). Assessing their affordability for the population is therefore very important and one of the complementary national indicators of the sustainable development goal agenda (3.31) (Sustainable Development Knowledge Platform 2015).
Affordability of medicines has been studied using both qualitative and quantitative methods and different indicators. Qualitative methods usually explore interviewees’ perceptions of affordability (Shafiq et al. 2011; Searles et al. 2013; Naci et al. 2014; Vialle-Valentin et al. 2015), whereas quantitative methods tend to benchmark the cost of a medicine against some threshold. The WHO/HAI method evaluates affordability of essential medicines by estimating the number of days of wage needed for the lowest paid public sector worker to purchase 1 month of treatment for a chronic condition or a course of treatment for an acute condition (WHO and HAI 2008). Medicines costing more than 1 day of work are considered unaffordable. The catastrophic expenditure and the impoverishment method both focus on the percentage of the population which would experience catastrophic health expenditure or impoverishment if they had to purchase the pharmaceutical treatment under analysis. Studies on affordability of medicines have defined expenditure (on medicines) as catastrophic if it exceeded 5% of daily income and a household to become impoverished if the residual income after purchasing medicines was less than US$1.25 or US$2 per day (Niëns et al. 2010, 2012). These two methods have been initially proposed to study affordability of health care as a whole (Wagstaff and van Doorslaer 2003) and applied in a number of studies on the subject using different thresholds (Xu et al. 2003; Su et al. 2006; Limwattananon et al. 2007; Somkotra and Lagrada 2008; Sun et al. 2009; van Doorslaer et al. 2006). Other studies compared unit costs of cancer medicines with months of income (Roy et al. 2012) and cost of treatment with monthly per capita expenditure (Yohana et al. 2011) .
In low- and middle-income countries, evidence on affordability of medicines is limited. Most available studies have either been cross-sectional or baseline and follow-up and they have often not considered that some medicines may be at least partially reimbursed. In Republic of Moldova, the only study on affordability of medicines was the 2011 WHO/HAI survey (WHO 2012). The country has introduced the first list of compensated medicines in 2005 as part of mandatory health insurance. However, despite spending on medicines is the largest component of private expenditure on health, very little analysis of what the introduction of mandatory health insurance has delivered in terms of increased affordability of NCD medicines for patients has been conducted. In an attempt to address this gap, we analysed changes in affordability of medicines following the introduction of mandatory health insurance in the Republic of Moldova.
We use a modified version of the WHO/HAI methodology which takes into account that part of the medicines cost is covered by mandatory health insurance. This study does not claim to provide a superior method to the existing methods described, but rather to contribute to the research area of medicines affordability with longitudinal data in a country which, like many others, is working towards achieving universal health coverage. As other authors have noted, due to the ambiguous nature of affordability, it is best to be analysed from different perspective and using different metrics (Cameron et al. 2009a). The catastrophic expenditure and impoverishment methods take into account that households need to cover basic needs like food which will decrease the amount of income available for medicines. Further, they take into account the income distribution of the population. The WHO/HAI method only considers the wage of the lowest paid public sector worker, which was acknowledged to be higher that what some groups of the population earn (Cameron et al. 2009b; Niëns and Brouwer 2009). In addition, it does not consider other basic expenses households experience. Its main strengths are the straightforward interpretation and a numerical value (days of wage) replacing the cost of medicine but allowing for comparison across medicines. For these last two reasons, and because we want to compare how much more affordable medicines have become over time, and not only the percentage of the population experiencing either catastrophic expenditure or impoverishment, we decided to use a modified version of the WHO/HAI metric. In our analysis, we use health insurance data which enable us to take reimbursement into account and estimate affordability for different income and expenditure quintiles.
National health policy context relevant to medicines
Mandatory health insurance was introduced in 2004 in the Republic of Moldova. A year later the first reimbursement list for outpatient medicines was introduced. Inpatient medicines are in principle covered at 100% for all insured patients. However, evidence from a national survey in 2011 showed that 16.9% of the respondents had to purchase their medicines while in hospitals and 29.6% received only some of the required medicines free of charge and had to purchase the remaining ones (PAS Centre 2011).
According to the estimates of the National Health Insurance Company, in 2013, 83.2% of the population was covered (CNAM 2012). However, these estimates should be taken with caution because of difficulties in estimating the denominator due to migration. Using a different methodology and including only people aged 18–69 years (i.e. excluding the children and the elderly who are insured by the Government), the 2013 WHO STEPS survey estimated that only 66.4% (95% CI: 64.1–68.8) of all respondents aged 18–69 years were insured.
Improving access to medicines and financial protection are high on the Moldovan Government’s agenda. Moldova has a health care system development strategy for 2008–17 which contains a specific objective related to access to medicines, namely to increase budget allocation for compensated medicines and to improve resource allocation mechanisms (Government of the Republic of Moldova 2007). This will be monitored by the actual increase in budget though no concrete target was set. The National Health Insurance Company has also been putting emphasis on improving resource allocation for medicines in its institutional development strategies and has also set specific targets including the gradual increase in budget allocation for medicines to 10% by 2017 and a reduction in the proportion of private spending on medicines from 71.5% in 2014 to 65% by 2017 (CNAM 2012, 2013, 2014).
In this study, we aim to answer the following research question: How has financial protection against out-of-pocket payments in the Republic of Moldova changed following the introduction of mandatory health insurance? As medicines tend to represent a major share of out-of-pocket payments on health, this question is relevant to a number of countries currently trying to increase financial protection through the introduction of mandatory health insurance.
Methods
Medicines sample selection and data sources
The affordability analysis focused on cardiovascular and respiratory medicines (the latter only for adults as children are covered at 100%) dispensed in outpatient settings because these medicines are included in the reimbursement list but subject to co-payments. Cancer medicines, which are dispensed at the hospital, and diabetes medicines are reimbursed at 100% (initially diabetes medicines were reimbursed at 90%) and were therefore not included in the affordability analysis. Claims data on compensated medicines between 2006 and 2013 were obtained upon request from National Health Insurance Company. This included information on the number of units (tablets, inhalers, etc.), total expenditure and total reimbursement for different medicines–formulations included in the reimbursement list.
Estimation of affordability
We applied a modified version of the WHO/HAI methodology to measure affordability. Instead of estimating only how affordable medicines are for the lowest paid public sector worker, we estimated affordability for different income quintiles. To do this we used annual data on total spending and total reimbursement for NCD medicines included in the outpatient reimbursement list, but subject to co-payments. With this information, we calculated how many days of monthly disposable income (cash and in-kind) and expenditure, as estimated by household budget survey (NBS 2015), were necessary to purchase 1 month of treatment for different income quintiles.
More specifically, we used data on the number of units (tablets, inhalers, etc.) to estimate the total milligrams (mg) of active ingredient reimbursed per International Non-Proprietary Name (INN). This total was then divided by the WHO defined daily dose (DDD) for that INN (WHO Collaborating Centre for Drug Statistics Methodology 2013) to estimate the number of DDDs that were at least partially reimbursed and the average amount paid out-of-pocket per month. We then divided the amount paid out-of-pocket by the daily disposable income of different income quintiles. To test the sensitivity of the results to possible underreporting of income due to remissions, informal economy, etc., we estimated affordability also by expenditure quintiles using household budget survey data.
Steps
Patient cost per month: Patient cost per DDD*30.4 daysPatient cost per DDD = Total patient co-payment for a particular medicine/Number of DDDs dispensed of this medicinesNumber of DDD dispensed for a particular medicine = Medicine strength (mg)*Number of units dispensed/WHO DDD for this medicine30.4 = average number of days in a month (365 days in a year/12 month).
Days of wage needed to purchase 1 month of treatment = Patient cost per month/Daily income or expenditure for different quintiles.
Results
Affordability of medicines for the treatment of NCDs included in the reimbursement list
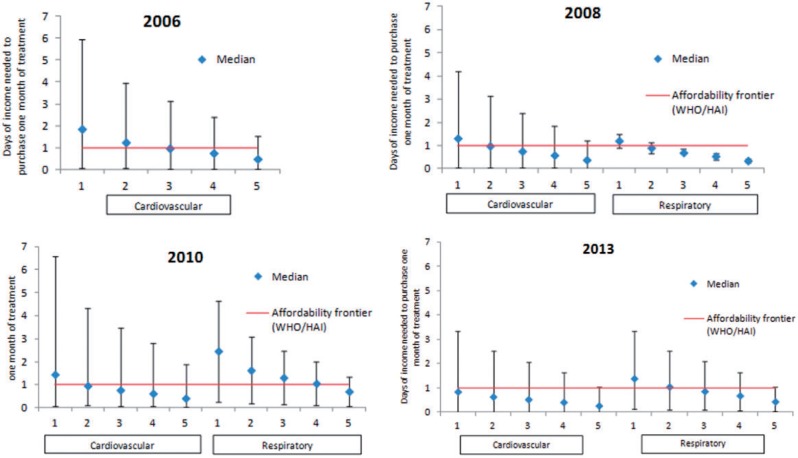
Median affordability for cardiovascular and respiratory medicines included in the reimbursement list but subject to co-payments improved between 2006 and 2013. In 2006, it took a median of 1.85 days of income for the lowest income quintile to purchase 1 month of treatment for cardiovascular conditions (Figure 1). For respiratory medicines, only aminophylline and salbutamol were reimbursed 100% for children 0–5 and salbutamol 50% for adults. However, according to expenditure and reimbursement data, 100% of salbutamol was reimbursed suggesting no partially compensated salbutamol was prescribed to adults.
Figure 1.
By income quintile: Affordability cardiovascular and respiratory medicines included in the reimbursement list but subject to co-payments.Notes: Diamonds represent the median value and the vertical bar shows the maximum and minimum values estimated. 1-5 represent wealth quintiles where 1 are the least wealthy and 5 the wealthiest. For comparison purposes, the minimum daily wage used in the WHO/HAI survey (World Health Organization, 2012) was 20 Moldovan Lei, which happens to correspond quite closely to one day of disposable income of the lowest income quintile (18 MDL in 2012).
In 2013, it took in median 0.84 days of income to purchase on month of treatment for cardiovascular and 1.13 days of income for respiratory conditions. Between 2008 and 2010 affordability of cardiovascular medicines decreased from 1.32 to 1.45 days of income and from 1.21 to 2.47 days for respiratory medicines.
Due to remittances from family members living abroad and a large informal economy, it is likely that the actual individual income is higher than the one reported in the household budget survey. To account for this, we estimated affordability using expenditure quintiles rather than income quintiles. Expenditure was lower than income for quintiles 1–2 but higher for quintiles 3–5 in both 2006 and 2013 hinting to under-reporting of income by relatively wealthier individuals. Although medicines become slightly more affordable for higher expenditure quintiles, the overall results do not change substantially (Supplementary Figure S1).
Drivers of medicines affordability
Medicines affordability (as defined in this study) depends on the income or expenditure distribution of the households, prices, of medicines, the percentage reimbursed and the maximum absolute level of reimbursement by the National Health Insurance Company for different medicines. In the following figures, we show changes in these variables over time.
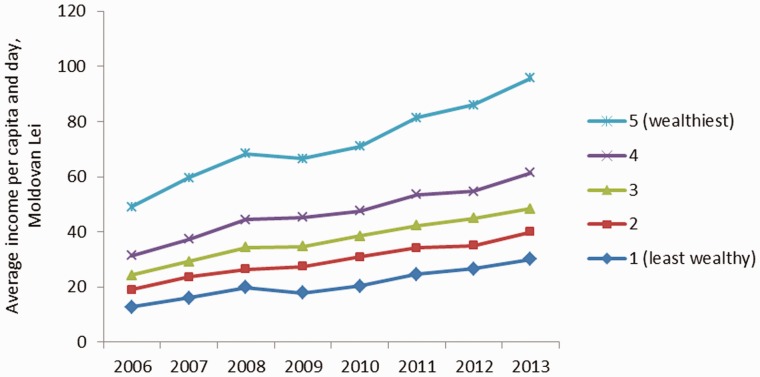
All quintiles experienced an increase in their level of income and expenditure (taking into account inflation). The daily disposable income for households has increased from Lei 13 in 2006 to Lei 30 in 2013 for the lowest income quintile and from Lei 49 in 2006 to Lei 96 for the highest income quintile (Figure 2). This corresponds to about a 140% increase for the least wealthy and a 100% increase for the wealthiest (at 2006 constant values). These findings reflect very closely the ones for expenditure quintiles, with the main difference that expenditure for the wealthiest increased only by 70% (Supplementary Figure S2).
Figure 2.
Income by quintiles, 2006–2013.
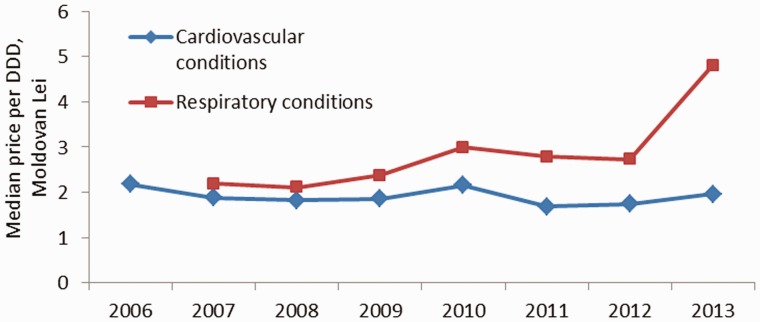
The median price (inflation adjusted) per DDD included in the reimbursement list remained quite stable between 2006 (MDL1 2.2 per DDD) and 2013 (1.97 Moldovan Lei per DDD) (Figure 3), despite some adjustments in the benefit package which have influenced the medicines basket composition (supplementary Table 1). There was a small increase in the median price per DDD of partially compensated respiratory medicines for adults in 2010, from MDL 2.38 to MDL 3.01, which was due at least in part to the introduction of aminophylline in the reimbursement list for adults (previously only children were covered at 100%). A more substantial increase in median price from MDL 2.73 to MDL 4.80 occurred in 2013 following the listing of two more respiratory medicines for adults (beclometasone and fluticasone).
Figure 3.
Median price per DDD of medicines included in the reimbursement list.
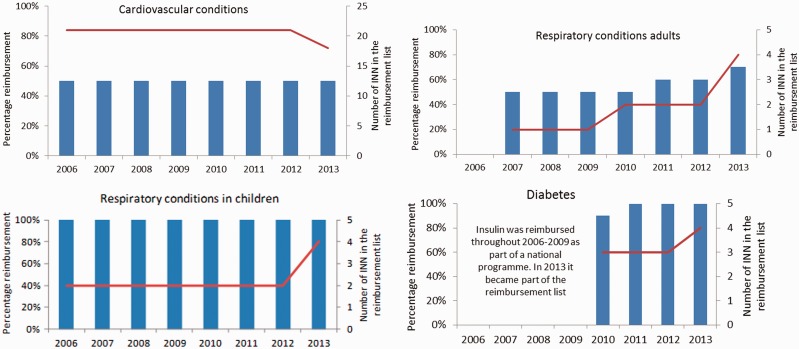
Despite an increase in the expected level of reimbursement for selected cardiovascular medicines in 2012, the median expected percentage covered remained stable at 50% between 2006 to 2013 (Figure 4). The number of cardiovascular medicines (different INNs) included in the reimbursement list remained stable at 21 until 2012, after which it dropped to 18. There was an increase in the expected level of median reimbursement for respiratory medicines from 50% to 60% in 2011 and to 70% in 2013. This was accompanied by an increase in the number of medicines reimbursed from 1 to 2 between 2009 and 2010 and from 2 to 4 between 2012 and 2013. Respiratory medicines in children were covered at 100% throughout 2006–13 and their number increased from 2 to 4 between 2012 and 2013. Diabetes medicines were initially reimbursed at 90% when they were first introduced in the reimbursement list in 2010 but were fully reimbursed from the following year onwards. The list of diabetes medicines initially included glibenclamide, glimepiride and metformin, from 2013 insulin was moved into the reimbursement list (already available free of charge before but vertically procured through a national programme with separate funding).
Figure 4.
Median percentage expected reimbursement by therapeutic group according to the reimbursement list, 2006–13.Note: This is the expected percentage reimbursement based on the maximum reimbursement sum. The actual percentage reimbursed depends on retail prices and may be higher or lower than the median expected percentage reimbursement
Only a certain absolute maximum amount is compensated per tablet. This means that the actual percentage covered may be higher or lower than the median expected 50% for cardiovascular medicines. Between 2006 and 2011 there was no noticeable change in the median maximum compensated sum which remained stable at MDL 0.79 for respiratory medicines and MDL 0.83–0.87 for cardiovascular medicines (Figure 5). This sum increased in 2012 for cardiovascular medicines to MDL 1.1 due to higher compensation temporarily granted to some medicines in this therapeutic group, but went down again to 0.89 in the following year when coverage was reduced to 50%. A smaller increase to MDL 0.88 also took place in 2012 for respiratory medicines due to higher reimbursement from 50% to 70% for salbutamol.
Figure 5.
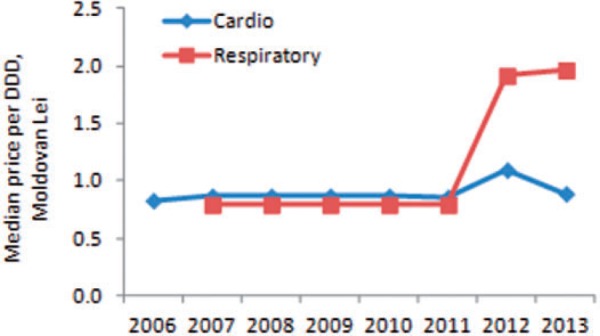
Median maximum reimbursed sum per DDD by therapeutic group, 2006–13.
The actual proportion of total cost reimbursed is determined by median expected percentage reimbursement set in the list and the maximum sum compensated. For cardiovascular medicines, it increased only modestly from 47% in 2006 to 52% in 2012 (Figure 6). The increase was greater for respiratory medicines in adults, where actual coverage increased from 58% in 2007 to 73% in 2013.
Figure 6.
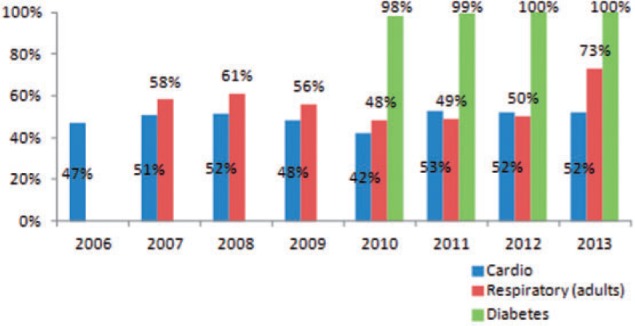
Actual proportion of total cost reimbursed.
Discussion
Our analysis of changes in affordability of partially compensated NCD medicines since the introduction of mandatory health insurance in the Republic of Moldova showed that increased affordability was driven by raising household income rather than deeper coverage through the reimbursement list. The results of the affordability analysis using national health insurance data show similar results for 2011 to the WHO/HAI survey (for medicines included in both studies) based on pharmacy retail prices in the same year (WHO 2012). The latter has to be interpreted with caution though because it did not take into account that some medicines are at least partially reimbursed by the National Health Insurance Company for insured patients.
There are several reasons why the reimbursement list has not achieved deeper coverage for cardiovascular and respiratory (for adults) medicines over time. For cardiovascular medicines, the expected percentage coverage for reimbursed medicines has largely remained unchanged at median 50% between 2006 and 2013 (coverage was only briefly increased to 70% and 90% for selected cardiovascular medicines in 2012) and the same applies for the maximum reimbursement sum per tablet. This meant that between 2006 and 2013, actual coverage for partially compensated cardiovascular medicines, only increased from 47% to 52%. The situation is better for respiratory medicines in adults. For these, median expected coverage in the list has increased from 50% to 60% in 2011 and from 60% to 70% in 2013. However, the median maximum reimbursement sum per table has remained stable at 0.79 from 2007 to 2011 and only increased to 0.88 in 2012. Despite this limitation, there was an increase in the actual proportion covered from 58% in 2007 to 73% in 2013.
The very limited increase in coverage for these medicines is multifaceted. One issue is funding constraints. In 2013, only 3.9% of the National Health Insurance Company’s budget was spent on medicines corresponding to MDL 55 (US$4.4) per insuree. For comparison purposes, Estonia, Romania and Scotland spent US$108, US$68 and US$317 per capita, respectively, on outpatient medicines in the same year (2014 in Romania). This corresponded to 15%, 22.6% and 11% of the total national health insurance expenditure in Estonia, Romania and Scotland, respectively [authors’ estimation based on IDS Scotland (2015), Estonian Health Insurance Fund (2015) and Alianta pentru sănătate din România (2014)]. However, simply increasing funding for medicines is likely not to deliver the expected results, nor to be a feasible or sustainable solution, if broader issues affecting the country’s health system and quality use of medicines are not addressed. First of all, resources need to be generated by reducing inefficiencies at secondary care level so that more funds can be allocated to primary care, including medicines. Once more funds are available, these need to be spent effectively. Medicines to be reimbursed need to be selected based cost-effectiveness criteria, prescribing needs to follow quality use of medicines principles and better value for money needs to be obtained through improved pricing as well as supply chain regulation as well as monitoring (Sautenkova et al. 2012). The case of salbutamol syrup illustrates some of these issues and their inter-linkages (Box 1).
Box 1. The case of salbutamol syrup for children
Since 2008 salbutamol syrup (2 mg/5 ml in 150 ml) has been included in the list of reimbursed trade names and formulations of salbutamol. In 2013, the price per DDD of this formulation was MDL 8.4, the highest among the four formulations reimbursed for children. The lowest cost formulation (100 mcg per 200 doses) cost only MDL 0.9 per DDD and, contrary to syrup, is included in the WHO model list of essential medicines for children (World Health Organization 2015b). The price of salbutamol syrup in the Republic of Moldova seems to be even higher than in the United Kingdom (the same trade name from a UK manufacturer was compared which, in 2013, was the only trade name of salbutamol syrup reimbursed in the Republic of Moldova). In fact, the ex-factory price registered as of September 2015 in Moldova was MDL 30.24 per unit while the NHS net price per unit in the British National Formulary was GBP 0.73 which corresponds to approximately MDL 22. But the high price is not the only issue. According to a 2011 review of the literature, oral salbutamol is ineffective in the treatment of paediatric asthma and is associated with an increased incidence of adverse events compared with inhaled formulations. Paediatric masks and spacers can facilitate administration of inhaled salbutamol to all patients; therefore, there is no role for oral salbutamol (Herd 2011). Instead, clinical protocols for family physicians in the Republic of Moldova, recommend oral salbutamol (by trade name, not generic name) as first line emergency treatment in children (Ministerul Sanatatii al Republicii Moldova 2010). In 2013, syrup accounted for 32% of all DDDs reimbursed for salbutamol in children.
Concrete steps have already been taken to address some of these issues but many more will be necessary to achieve long-lasting changes. Guidelines for selecting medicines for reimbursement according to evidence-based medicine and cost-effectiveness criteria have been developed in collaboration with the WHO and approved by the Ministry of Health in August 2015. A technical Secretariat has been established within the National Health Insurance Company who will be responsible to conduct budget impact analyses and assess manufacturer submissions to the outpatient reimbursement list.
After conducting country’s first STEPS survey in 2013, which showed that less than half of the Moldovan population with hypertension is on regular treatment (WHO Regional Office for Europe 2014), and in line with the targets of the Global NCD monitoring framework (World Health Organization 2015a), the Government of the Republic of Moldova has agreed with the World Bank to monitor its spending performance against, among other indicators, the percentage of patients with hypertension who are controlled. With this target in mind, the Government has increased compensation for medicines for the treatment of hypertension from 50% to 70%.
Finally, while concrete actions are yet to follow, the need to reduce inefficiencies in the hospital sector has been highlighted National Health Care System Development Strategy for 2008–17 (Government of the Republic of Moldova 2007). Further, there was agreement during a high level meeting of Government officials and head of health institutions in August 2015 that the resources freed by addressing inefficiencies at hospital level could be used to strengthen primary care and access to medicines.
Despite its limitations, it is important to recognize that the outpatient reimbursement list covers, either fully or partially, a number of essential medicines. Cardiovascular and respiratory medicines included in the list but subject to co-payments have become more affordable over time and diabetes medicines are fully covered. Evidence from a study comparing the proportion of the population foregoing medicines in 2001 and 2010 in selected Former Soviet Union countries seems to support the positive impact the reimbursement list has had on access over time. The study found the greatest decline in forgoing medicines in the Republic of Moldova [rate ratio (RR) = 0.67 (0.63; 0.71)] and Kyrgyzstan [RR = 0.63 (0.60; 0.67)] (Footman et al. 2014). It also highlighted that improvements were greatest in countries with more progressive pharmaceutical policies, despite lower incomes (Footman et al. 2014).
Finally, compared with other Former Soviet Union countries with similar income levels, the Republic of Moldova spends a much higher share of gross domestic product on health, 11.7% in 2012 which was the highest among Former Soviet Union countries (World Health Organization 2014).
Limitations
The income and expenditure data used in this study were taken from national household budget surveys. These surveys have well-known limitations. Although the samples of the household budget survey used in this study were random, they may not necessarily be representative of the Moldovan population. Data are based on self-reporting and no verification takes place. This could for example affect both reported income and expenditure downwards.
Further, although various definitions of affordability and methods to estimate it are available, we only used one method. In future studies, it would be interesting to apply different methods and compare their results.
Conclusion
Affordability of partially compensated medicines for the treatment of NCDs has improved since the introduction of the first reimbursement list in 2006 for all income and expenditure quintiles. This improvement, however, was mainly driven by higher incomes and expenditure rather than deeper coverage through the reimbursement list. If the list is to improve affordability of medicines for the Moldovan population, more funds need to be (re-)allocated to enable higher percentage coverage of essential medicines.
Following approval by the Parliament to increase funds for medicines, in November 2015, compensation of hypertensive medicines was increased from 50% to 70%. This is a welcome step in the right direction, however, for it to be sustainable in the long term, efficiencies need to be generated within the health system including a budget reallocation between secondary and primary care, strengthening primary care to manage chronic conditions and raise population awareness about NCD risk factors and disease management, implementation of evidence-based selection and quality use of medicines in both outpatient and inpatient settings, improve monitoring and regulation of prices and the supply chain; and alignment of national treatment guidelines and clinical practice with international best practices and evidence-based medicine.
Supplementary data
Supplementary data are available at HEAPOL online.
Acknowledgements
The authors would like to thank the Compania Nationala de Asigurari în Medicina (CNAM) for providing data for this study. They would also like to thank Andrei Matei, Ala Negruta and Stefan Savin for their advice on data matters. Some of the data for this article were collected while A.F. was employed as a consultant for WHO. Most of the research work and writing was carried out in the authors’ own free time. The study was partly funded by the European Union/WHO joint initiative to support policy dialogue and universal health coverage. The views expressed in the article are those of the authors alone and do not necessarily reflect the decision or stated policy of the World Health Organization.
Conflict of interest statement: None declared.
1. MDL: Moldovan Lei (1 MDL = 0.053 USD, Treasury Reporting Rates of Exchange as of 30 June 2015, http://www.fiscal.treasury.gov).
Footnotes
1MDL: Moldovan Lei (1 MDL = 0.053 USD, Treasury Reporting Rates of Exchange as of 30 June 2015, http://www.fiscal.treasury.gov).
References
- Alianta pentru sănătate din România. 2014. România: Starea de fapt în asigurările de sănătate. Alianta pentru sănătate din România; Bucharest, Romania: http://www.medicalmanager.ro/docs/raportul_apsr_-_sistemul_de_asigurari_de_sanatate_din_romania_2014.pdf, accessed 15 September 2015. [Google Scholar]
- Cameron A, Ewen M, Auton M, Laing R. 2009a. Authors’ reply: Better measures of affordability required. Lancet 373: 1081–2. [DOI] [PubMed] [Google Scholar]
- Cameron A, Ewen M, Ross-Degnan D, Ball D, Laing R. 2009b. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet 373: 240–9. [DOI] [PubMed] [Google Scholar]
- CNAM. 2012. CNAM Institutional Development Strategy 2013-2017. Chisinau: National Health Insurance Company (CNAM; ). [Google Scholar]
- CNAM. 2013. 2014-2018 Institutional Development Strategy of the National Health Insurance Company. Chisinau: National Health Insurance Company (CNAM; ). [Google Scholar]
- CNAM. 2014. STRATEGIA de Dezvoltare Institutională A Companiei Nationale De Asigurări În Medicină Pentru Anii 2015-2019. Chisinau, Republic of Moldova. [Google Scholar]
- Estonian Health Insurance Fund. 2015. Estonian Health Insurance Fund Yearbook 2014. Tallin, Estonia: Estonian Health Insurance Fund. [Google Scholar]
- Footman K, Richardson E, Roberts B. et al. 2014. Foregoing medicines in the former Soviet Union: changes between 2001 and 2010. Health Policy 118: 184–92. [DOI] [PubMed] [Google Scholar]
- Government of the Republic of Moldova, M. o. H. 2007. Health Care System Development Strategy for the Period 2008-2017. Chisinau. [Google Scholar]
- Herd D. 2011. Oral Versus Inhaled Salbutamol for Acute Paediatric Asthma Best evidence topics http://www.bestbets.org/bets/bet.php?id=2283, accessed 20 September 2015.
- Ibrahimov F, Ibrahimova A, Kehler J, Richardson E. 2010. Azerbaijan: Health System Review. Health Systems in Transition 12(3): 1–117. [PubMed] [Google Scholar]
- IDS Scotland. 2015. Prescription Cost Analysis http://www.isdscotland.org/Health-Topics/Prescribing-and-Medicines/Community-Dispensing/Prescription-Cost-Analysis/, accessed 17 September 2015.
- Khodjamurodov G, Rechel B. 2010. Tajikistan: Health System Review. Health Systems in Transition 12(2): 1–154. [PubMed] [Google Scholar]
- Limwattananon S, Tangcharoensathienb V, Prakongsai P. 2007. Catastrophic and poverty impacts of health payments: results from national household surveys in Thailand. Bulletin of the World Health Organization 85: 600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerul Sanatatii al Republicii Moldova. 2010. Astmul Bronsic la Copil. Protocol Clinic Standardizat Pentru Medicii de Familie. Chisinau, Republic of Moldova. [Google Scholar]
- Naci H, Soumerai S, Ross-Degnan D. et al. 2014. Medication affordability gains following Medicare Part D are eroding among elderly with multiple chronic conditions. Health Affairs (Millwood) 33: 1435–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NBS. 2015. Website of the National Bureau of Statistics of the Republic of Moldova http://www.statistica.md, accessed 9 March 2015.
- Niëns L, Brouwer W. 2009. Better measures of affordability required. Lancet 373: 1081. [DOI] [PubMed] [Google Scholar]
- Niëns L, Cameron A, Van de Poel E. et al. 2010. Quantifying the impoverishing effects of purchasing medicines: a cross-country comparison of the affordability of medicines in the developing world. PLoS Medicine 7(8): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niëns L, Van de Poel E, Cameron A. et al. 2012. Practical measurement of affordability: an application to medicines. Bulletin of the World Health Organisation 90: 219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAS Centre. 2011. Health Monitor: Access and Quality of Hospital Services in the Perception of the Population from the Republic of Moldova, Results of the National Poll, 2011. Chişinău. [Google Scholar]
- Richardson E, Malakhova I, Novik I, Famenka A. 2013. Belarus: Health System Review. Health Systems in Transition 15(5): 1–118. [PubMed] [Google Scholar]
- Roy V, Gupta U, Agarwal A. 2012. Cost of medicines & their affordability in private pharmacies in Delhi (India). The Indian Journal of Medical Research 136: 827–35. [PMC free article] [PubMed] [Google Scholar]
- Sautenkova N, Ferrario A, Bolokhovets G, Kanavos P. 2012. Availability and Affordability Of Medicines and Assessment of Quality Systems for Prescription of Medicines in the Republic of Moldova. World Health Organization Regional Office for Europe. [Google Scholar]
- Searles A, Doran E, Faunce T, Henry D. 2013. The affordability of prescription medicines in Australia: are copayments and safety net thresholds too high? Australian Health Review 37: 32–40. [DOI] [PubMed] [Google Scholar]
- Shafiq Y, Shaikh B, Kumar R. 2011. Availability and affordability of essential medicines: exploring the health seeking behaviours and health service utilisation for children under-5 years living in squatter settlement of Karachi, Pakistan. Journal of Ayub Medical College Abbottabad 23: 132–8. [PubMed] [Google Scholar]
- Somkotra T, Lagrada L. 2008. Payments for health care and its effect on catastrophe and impoverishment: experience from the transition to Universal Coverage in Thailand. Social Science & Medicine 67: 2027–35. [DOI] [PubMed] [Google Scholar]
- Su T, Kouyaté B, Flessa S. 2006. Catastrophic household expenditure for health care in a low-income society: a study from Nouna District, Burkina Faso. Bulletin of the World Health Organization 84: 21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Jackson S, Carmichael G, Sleigh A. 2009. Catastrophic medical payment and financial protection in rural China: evidence from the New Cooperative Medical Scheme in Shandong Province. Health Economics 18: 103–19. [DOI] [PubMed] [Google Scholar]
- Sustainable Development Knowledge Platform. 2015. Transforming Our World: The 2030 Agenda for Sustainable Development. Finalised text for adoption (1 August).
- Turcanu G, Domente S, Buga M, Richardson E. 2012. Health System Review for the Republic of Moldova. Health Systems in Transition 14(7): 1–151. [PubMed] [Google Scholar]
- van Doorslaer E, O’Donnell O, Rannan-Eliya R. et al. 2006. Effect of payments for health care on poverty estimates in 11 countries in Asia: an analysis of household survey data. Lancet 368: 1357–64. [DOI] [PubMed] [Google Scholar]
- Vialle-Valentin C, Serumaga B, Wagner A, Ross-Degnan D. 2015. Evidence on access to medicines for chronic diseases from household surveys in five low- and middle-income countries. Health Policy and Planning 30(8): 1044–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff A, van Doorslaer E. 2003. Catastrophe and impoverishment in paying for health care: with applications to Vietnam 1993-1998. Health Economics 12: 921–34. [DOI] [PubMed] [Google Scholar]
- WHO. 2012. Medicines Prices, Availability, and Affordability and Price Components in the Republic of Moldova. World Health Organization. [Google Scholar]
- WHO, HAI. 2008. Measuring Medicine Prices, Availability, Affordability and Price Components. World Health Organization and Health Action International. [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology. 2013. ATC/DDD Index 2013 http://www.whocc.no/atc_ddd_index/, accessed May–June 2013.
- WHO Regional Office for Europe. 2014. Prevalence of Noncommunicable Diseases Risk Factors in the Republic of Moldova. Copenhagen. [Google Scholar]
- World Health Organization. 2014. Global Health Expenditure Database http://apps.who.int/nha/database/DataExplorerRegime.aspx, accessed 19 September 2014.
- World Health Organization. 2015a. NCD Global Monitoring Framework. Ensuring Progress on Noncommunicable Diseases in Countries http://www.who.int/nmh/global_monitoring_framework/en/, accessed 20 September 2015.
- World Health Organization. 2015b. WHO Model List of Essential Medicines for Children.
- World Health Organization & The World Bank. 2013. Monitoring Progress towards Universal Health Coverage at Country and Global Levels. Framework, Measures and Targets. Geneva. [Google Scholar]
- Xu K, Evans DB, Kawabata K, Zeramdini R, Klavus J, Murray CJ. 2003. Household catastrophic health expenditure: a multicountry analysis. Lancet 362: 111–7. [DOI] [PubMed] [Google Scholar]
- Yohana E, Kamuhabwa A, Mujinja P. 2011. Availability and affordability of anticancer medicines at the Ocean Road Cancer Institute in Dar es Salaam, Tanzania. East African Journal of Public Health 8: 52–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.