Abstract
We read with interest the article entitled “Circulating microRNAs characterizing patients with insufficient coronary collateral artery function” which was recently published in the PLOS ONE journal. It was demonstrated for the first time that specific circulating microRNAs (miRNAs) can distinguish patients with sufficient from those with insufficient coronary collateral circulation. Circulating miRNAs in the plasma of patients with stable CAD and chronic CTO could provide information with regard to the coronary collateral artery capacity. However, several aspects need to be taken into consideration before the use of miRNAs in the clinical practice. A risk model that would incorporate risk factors for cardiovascular disease and miRNAs could prove to be very useful. Although an association between the levels of miRNAs and the collateral artery capacity appears promising, it still does not confirm any causal role for miRNAs. Therefore, large clinical studies in populations with CTO are warranted to evaluate this finding.
Keywords: microRNAs (miRNAs), coronary artery collaterals, angiogenesis
Studies have shown that the presence of sufficient collateral circulation is beneficial for patients with stable CAD, as it improves the survival rates (1). A circulating biomarker that would provide us with the appropriate information regarding to the existence of collateral circulation in these patients would be very helpful in the clinical setting. microRNAs (miRNAs) actively participate in cardiovascular homeostasis and play an important role in the initiation and progression of cardiovascular disease (2). Recent data suggest that miRNAs contribute to the formation of vulnerable atherosclerotic plaques (3), while they enhance angiogenesis as well. Therefore, it has been speculated that circulating miRNAs can provide information about the collateral artery network of patients with chronic total occlusion (CTO). In the present article, Hakimzadeh et al. (4) showed for the first time that specific circulating miRNAs can distinguish patients with sufficient and patients with insufficient coronary collateral circulation.
miRNAs and angiogenesis
It was an interesting discovery that the enzymes involved in miRNAs maturation also participate in angiogenesis: Dicer inhibition resulted in a decrease in angiogenesis in vivo, while inhibition of Drosha resulted in an anti-angiogenic effect in vitro (5). Since then, studies have shown that several miRNAs upregulate angiogenesis, while others suppress angiogenic pathways (6) (Figure 1). However, the data were mainly derived from preclinical studies, until Nie et al. (7) showed that miR-126 levels, along with vascular endothelial growth factor (VEGF) levels, were higher in healthy people and in patients with well-developed collateral arteries compared to patients with under-developed collateral circulation. In addition, miR-126 levels could independently predict coronary collateral circulation formation. Nevertheless, Hakimzadeh et al. (4) provided further insight to this issue. Their study included patients undergoing successful percutaneous coronary intervention and had CTO of a coronary artery. The levels of miR 423-5p, miR-30d, miR-10b and miR-126 were increased in the setting of insufficient coronary collateral artery capacity. In comparison to healthy controls, though, only the levels of miR-30d and miR126 were found to be elevated. Indeed, there is evidence that gene modulation can discriminate between patients with well developed and patients with poorly developed collateral arteries. van der Laan (8) showed in patients with CTO that the mRNA expression of galectin-2 was increased in monocytes of patients with low collateral flow index (CFI). The rs7291467 polymorphism was associated with increased galectin-2 levels and a lower angiogenic response. Nevertheless, the role of miRNAs in angiogenesis was only recently examined.
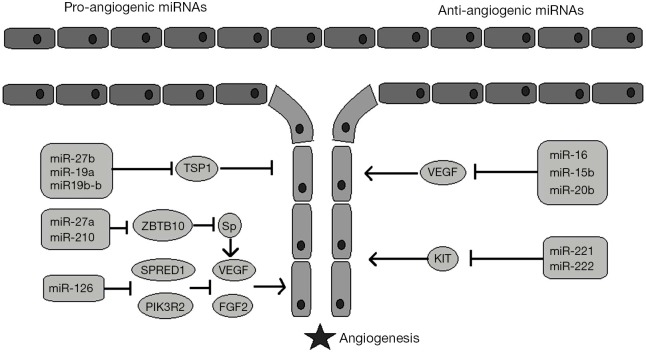
Figure 1.
Pro-angiogenic versus anti-angiogenic miRNAs. Some miRNAs upregulate angiogenesis by promoting angiogenic factors (i.e., VEGF) or by inhibiting anti-antiogenic mediators (i.e., TSP1), while others downregulate angiogenesis by inhibiting anigiogenic molecular pathways. VEGF, vascular endothelial growth factor; TSP1, thrombospondin 1; ZBTB10, zinc finger and BTB domain-containing protein 10; SPRED1, sprout-related EVH1 domain-containing protein 1; PIK3R2, phosphatidylinositol 3-kinase regulatory subunit beta; Sp, substance P; FGF2, fibroblast growth factor; KIT, receptor kinase protein/gene.
Several studies have proved the angiogenic potential of miR-126. A study that included patients with right ventricular heart failure and pulmonary hypertension showed that lower levels of miR-126 were expressed in right ventricular tissues of patients with decompensated heart failure. Of note, this was associated with decreased capillary density. The in vivo upregulation of miR-126 improved vascular density in an experimental animal model of pulmonary artery hypertension (9). More recently, the administration of miR-126 through ultrasound-targeted microbubble destruction resulted in increased vascular density in an animal model of chronic ischemia, as it enhanced VEGF and promoted angiopoietin-1 signaling (10).
In turn miRNA-10b has been shown to regulate angiogenesis in glioblastoma multiforme (11) and it was found to be implicated in vascular smooth muscle cell proliferation which is associated with atherosclerosis progression (12). miR-423-5p has been recently recognized as a novel biomarker for congestive heart failure and correlates with pro-brain natriuretic peptide (pro-BNP) levels. As opposed to miR-30d, though, miR-423-5p has been linked to increased cardiomyocyte apoptosis (13).
miRNAs as circulating biomarkers for coronary collateral circulation: challenges to meet
A circulating biomarker that would determine patients with poorly developed collateral coronary artery network would be of great clinical significance, since the invasive procedure of coronary angiography could potentially be avoided. In addition, coronary angiography can disclose arteries of >100 μm (14), thus cannot provide insight into microcirculation. It appears that the findings of Hakimzadeh et al. (4) are promising. However, up to date, no equivalent circulating biomarker exists (Table 1). That makes it difficult to compare the discriminatory capacity of miR 423-5p, miR-30d, miR-10b and miR-126 with the discriminatory capacity of an established circulating biomarker. It should be mentioned that a generally accepted definition of low collateral capacity is necessary, so that the results of the future studies will be comparable. For example, Nie et al. (7) used Rentrop grades to assess coronary collateral circulation, while other studies (1) considered collateral circulation capacity to be insufficient when CFI was <0.25. Hakimzadeh et al. (4) defined insufficient collateral circulation as the one with CFI<0.39 and this was in agreement with the study of van der Hoeven et al. (19). It should be stressed out that the intracoronary assessment of CFI in healthy individuals is not feasible, thereby limiting the information about the collateral network in this population. Despite this limitation, the levels of miR-30d and miR-126 were found to be lower in healthy individuals compared to patients with CTO (4).
Table 1. Selected studies investigating predictors for poor coronary collateral capacity.
| Studies | Year | Population | N | Collateral flow stratification | Result |
|---|---|---|---|---|---|
| Hakimzadeh et al. (4) | 2015 | CTO patients undergoing coronary angiography | 41 | Poor CCC: CFI <0.39 Good CCC: CFI >0.39 |
(+) miRNA-126, miRNA-10b, miRNA-30d and miRNA-423-5p in patients with poor CCC |
| Nie et al. (7) | 2014 | CAD patients with ≥95% stenosis in a coronary artery | 120 | Poor CCC: grade 0 and grade 1 Rentrop Good CCC: grade 1 and grade 2 Rentrop |
miR-126 and VEGF levels independently predicted CCC development |
| Ýleri et al. (15) | 2016 | Patients with NSTEMI | 224 | Poor CCC: grade 0 and grade 1 Rentrop Good CCC: grade 1 and grade 2 Rentrop |
DM, WBC, neutrophil counts and NLR independently predicted low CCC; age negatively predicted poor CCC |
| Kalkan et al. (16) | 2014 | Patients with CTO | 274 | Poor CCC: grade 0 and grade 1 Rentrop Good CCC: grade 1 and grade 2 Rentrop |
NLR, hs –CRP, WBC independently predicted poor CCC; |
| Baykan et al. (17) | 2015 | Patients with CTO | 163 | Poor CCC: grade 0 and grade 1 Rentrop Good CCC: grade 1 and grade 2 Rentrop |
(+) AIx, PWV, fasting glucose, creatine, uric acid, neutrophil count and NLR in patients with low CCC |
| Yetkin et al. (18) | 2015 | Patients with at least one coronary stenosis of ≥95% that underwent coronary angiography | 502 (228 with CTO) | Poor CCC: grade 0 and grade 1 Rentrop Good CCC: grade 1 and grade 2 Rentrop |
DM and female gender predicted poor CCC; monocyte count was independent of CCC |
| van der Hoeven et al. (19) | 2013 | Patients with CTO | 295 | Poor CCC: CFI <0.39 Good CCC: CFI >0.39 |
Beta blockers, hypertension and angina pectoris were positively associated with CFI; WBC, prior MI and high DBP were negatively associated with CFI |
| van der Laan et al. (8) | 2012 | Patients with CTO | 50 | Dichotomized according to CFI | (+) mRNA expression of galectin-2 in monocytes of patients with poor CCC; (+) polymorphism rs7291467 CC genotype in patients with poor CCC |
(+), increased. CCC, coronary collateral circulation; CTO, chronic total occlusion; CFI, collateral flow index; CAD, coronary artery disease; VEGF, vascular endothelial growth factor; NSTEMI, non ST-elevation myocardial infarction; WBC, white blood cell; NLR, neutrophil to monocyte ratio; hs-CRP, high sensitivity CRP; AIx, augmentation index; PWV, pulse wave velocity; MI, myocardial infarction; DBP, diastolic blood pressure.
Several co-existing parameters might affect the diagnostic ability of miRNAs in the general population. Diabetes mellitus interferes with the formation of collateral coronary artery network (15), while it has been found to down regulate the expression of several miRNAs, including miR-126 (20). A recent risk scoring model that predicts poor collateral coronary circulation showed that a combination of white blood cell count, age and history of myocardial infarction can predict poorly developed collaterals (15). Therefore it is necessary to stratify patients’ risk for poor collateral coronary circulation before attempting to evaluate the levels of a circulating biomarker. In the present article (4), patients with diabetes mellitus and a history of myocardial infarction were excluded from the study so that the expression patterns of miRNAs could be examined independently. Of note, the discriminatory power of miR-10b, miR-30d, miR-423-5p was evident only after multivariate analysis, which underscores the significance of taking all clinical parameters into consideration. Since leukocytes were found to be implicated in collateral artery growth (15), their assessment seems mandatory. Hakimzadeh et al. (4) found an association between miRNA-10b levels and monocyte/leukocyte count, suggesting a possible link between these parameters. Finally, aspirin administration has been found to decrease miR-126 levels, since platelets are a major pool of circulating miR-126 (21). Nevertheless, in the present article, aspirin administration did not blunt the discriminatory efficacy of miR-126 (4).
Conclusions
Circulating miRNAs in the plasma of patients with stable CAD and CTO have been shown to provide information about the coronary collateral artery capacity. This could possibly suggest an alternative diagnostic route to the invasive coronary angiography. However, several aspects need to be taken into consideration before the use of specific miRNAs could be applied into clinical practice. Co-existing parameters, such as diabetes mellitus and leukocyte count, affect angiogenesis and might interfere with the diagnostic efficacy of miRNAs; therefore, a risk model that would incorporate such parameters could be useful. It has become evident that an association between the levels of circulating miRNAs and the collateral artery capacity is not enough to confirm an underlying causative mechanism. Therefore, many more large clinical studies in populations with CTO are warranted to evaluate this finding.
Acknowledgements
None.
Footnotes
Provenance: This is a Guest Editorial commissioned by Section Editor Zhijun Han, MD (Department of Laboratory Medicine, Wuxi Second Hospital, Nanjing Medical University, Wuxi, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Meier P, Gloekler S, Zbinden R, et al. Beneficial effect of recruitable collaterals: a 10-year follow-up study in patients with stable coronary artery disease undergoing quantitative collateral measurements. Circulation 2007;116:975-83. 10.1161/CIRCULATIONAHA.107.703959 [DOI] [PubMed] [Google Scholar]
- 2.Papageorgiou N, Tousoulis D, Androulakis E, et al. The role of microRNAs in the initiation and progression of stable atheromatous plaque. Curr Pharm Des 2013;19:1651-7. [PubMed] [Google Scholar]
- 3.Haver VG, Slart RH, Zeebregts CJ, et al. Rupture of vulnerable atherosclerotic plaques: microRNAs conducting the orchestra? Trends Cardiovasc Med 2010;20:65-71. 10.1016/j.tcm.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 4.Hakimzadeh N, Nossent AY, van der Laan AM, et al. Circulating MicroRNAs Characterizing Patients with Insufficient Coronary Collateral Artery Function. PLoS One 2015;10:e0137035. 10.1371/journal.pone.0137035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuehbacher A, Urbich C, Zeiher AM, et al. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res 2007;101:59-68. 10.1161/CIRCRESAHA.107.153916 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Banda M, Speyer CL, et al. Regulation of the expression and activity of the antiangiogenic homeobox gene GAX/MEOX2 by ZEB2 and microRNA-221. Mol Cell Biol 2010;30:3902-13. 10.1128/MCB.01237-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie X, Su L, Zhou Y, et al. Association between plasma levels of microRNA-126 and coronary collaterals in patients with coronary artery disease. Zhonghua Xin Xue Guan Bing Za Zhi 2014;42:561-5. [PubMed] [Google Scholar]
- 8.van der Laan AM, Schirmer SH, de Vries MR, et al. Galectin-2 expression is dependent on the rs7291467 polymorphism and acts as an inhibitor of arteriogenesis. Eur Heart J 2012;33:1076-84. 10.1093/eurheartj/ehr220 [DOI] [PubMed] [Google Scholar]
- 9.Potus F, Ruffenach G, Dahou A, et al. Downregulation of MicroRNA-126 Contributes to the Failing Right Ventricle in Pulmonary Arterial Hypertension. Circulation 2015;132:932-43. 10.1161/CIRCULATIONAHA.115.016382 [DOI] [PubMed] [Google Scholar]
- 10.Cao WJ, Rosenblat JD, Roth NC, et al. Therapeutic Angiogenesis by Ultrasound-Mediated MicroRNA-126-3p Delivery. Arterioscler Thromb Vasc Biol 2015;35:2401-11. 10.1161/ATVBAHA.115.306506 [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Teo S, Lam DH, et al. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis 2012;3:e398. 10.1038/cddis.2012.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X, Li Z, Chen G, et al. MicroRNA-10b Induces Vascular Muscle Cell Proliferation Through Akt Pathway by Targeting TIP30. Curr Vasc Pharmacol 2015;13:679-86. 10.2174/1570161113666150123112751 [DOI] [PubMed] [Google Scholar]
- 13.Luo P, He T, Jiang R, et al. MicroRNA-423-5p targets O-GlcNAc transferase to induce apoptosis in cardiomyocytes. Mol Med Rep 2015;12:1163-8. [DOI] [PubMed] [Google Scholar]
- 14.Kolibash AJ, Bush CA, Wepsic RA, et al. Coronary collateral vessels: spectrum of physiologic capabilities with respect to providing rest and stress myocardial perfusion, maintenance of left ventricular function and protection against infarction. Am J Cardiol 1982;50:230-8. 10.1016/0002-9149(82)90171-0 [DOI] [PubMed] [Google Scholar]
- 15.İleri M, Güray Ü, Yetkin E, et al. A new risk scoring model for prediction of poor coronary collateral circulation in acute non-ST-elevation myocardial infarction. Cardiol J 2016;23:107-13. 10.5603/CJ.a2015.0064 [DOI] [PubMed] [Google Scholar]
- 16.Kalkan M, Sahin M, Kalkan A, et al. The relationship between the neutrophil-lymphocyte ratio and the coronary collateral circulation in patients with chronic total occlusion. Perfusion 2014;29:360-6. 10.1177/0267659114521102 [DOI] [PubMed] [Google Scholar]
- 17.Baykan AO, Gür M, Acele A, et al. Coronary collateral development and arterial stiffness in patients with chronic coronary total occlusions. Scand Cardiovasc J 2015;49:228-34. 10.3109/14017431.2015.1062130 [DOI] [PubMed] [Google Scholar]
- 18.Yetkin E, Topal E, Erguzel N, et al. Diabetes mellitus and female gender are the strongest predictors of poor collateral vessel development in patients with severe coronary artery stenosis. Angiogenesis 2015;18:201-7. 10.1007/s10456-015-9460-y [DOI] [PubMed] [Google Scholar]
- 19.van der Hoeven NW, Teunissen PF, Werner GS, et al. Clinical parameters associated with collateral development in patients with chronic total coronary occlusion. Heart 2013;99:1100-5. 10.1136/heartjnl-2013-304006 [DOI] [PubMed] [Google Scholar]
- 20.Jansen F, Yang X, Hoelscher M, et al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation 2013;128:2026-38. 10.1161/CIRCULATIONAHA.113.001720 [DOI] [PubMed] [Google Scholar]
- 21.de Boer HC, van Solingen C, Prins J, et al. Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur Heart J 2013;34:3451-7. 10.1093/eurheartj/eht007 [DOI] [PubMed] [Google Scholar]