Abstract
INTRODUCTION
Interferon-free hepatitis C treatment regimens are effective but very costly. The cost-effectiveness, budget and public health impacts of current Medicaid treatment policies restricting treatment to patients with advanced disease remain unknown.
METHODS
Using a Markov model, we compared two strategies for 45–55 year old Medicaid beneficiaries: (1) Current Practice - only advanced disease is treated before Medicare eligibility; and (2) Full Access – both early-stage and advanced disease are treated before Medicare eligibility. Patients could develop progressive fibrosis, cirrhosis or hepatocellular carcinoma, undergo transplantation, or die each year. Morbidity was reduced after successful treatment. We calculated the incremental cost-effectiveness ratio and compared the costs and public health effects of each strategy from the perspective of Medicare alone as well as the Centers for Medicare and Medicaid Services (CMS) perspective. We varied model inputs in one-way and probabilistic sensitivity analyses.
RESULTS
Full Access was less costly and more effective than Current Practice for all cohorts and perspectives, with differences in cost from $5,369–$11,960 and in effectiveness from 0.82–3.01 quality adjusted life-years). In a probabilistic sensitivity analysis, Full Access was cost saving in 93% of model iterations. Compared to Current Practice, Full Access averted 5,994 hepatocellular carcinoma cases and 121 liver transplants per 100,000 patients.
CONCLUSIONS
Current Medicaid policies restricting hepatitis C treatment to patients with advanced disease are more costly and less effective than unrestricted, full access strategies. Collaboration between state and federal payers may be needed to realize the full public health impact of recent innovations in hepatitis C treatment.
Keywords: Hepatitis C, interferon-free, cost-effectiveness, Medicaid, Medicare
INTRODUCTION
Hepatitis C affects over 3.2 million patients in the United States and is a common cause of chronic liver disease worldwide (1, 2). Most infected patients develop chronic disease that can remain asymptomatic for decades. However, left untreated, chronic hepatitis C causes progressive hepatic fibrosis, which can result in severe complications. After developing cirrhosis, patients are at risk for hepatocellular carcinoma, may require liver transplantation, and have an increased risk of early mortality (3–5). Successful treatment can reduce morbidity and improve patients’ quality of life (5–7). In fact, if recent advances in drug regimens are widely implemented, hepatitis C could become a rare disease as early as 2036 (8).
New hepatitis C treatments are highly effective and have few side effects, but high costs could limit access to these medications. The preceding generation of interferon-based treatment regimens were poorly tolerated by patients and required lengthy treatment durations, so many patients have remained untreated (9). Recently approved interferon-free drug regimens for patients with genotype 1 disease are more than 94% effective in as few as 8 weeks for many patient sub-groups, but can cost up to $190,000 per patient (10–12). Despite their high cost, interferon-free regimens have been demonstrated to be cost-effective at thresholds of $50,000–$100,000 per quality-adjusted life year (QALY) (13–15).
Resource-constrained government health insurance programs, including Medicaid and Medicare, cover a substantial proportion of US patients with hepatitis C and are heavily impacted by the high prices of these drugs. Most state Medicaid programs restrict treatment of hepatitis C to patients with advanced liver disease due to medication costs (16). Because hepatitis C is most prevalent in patients aged 45 and older, many Medicaid patients with early-stage disease may not develop advanced disease or complications until after becoming eligible for Medicare (17, 18).
Restrictive hepatitis C treatment policies are likely to reduce short-term costs to state Medicaid programs. However, it is unclear how these policies might shift the financial burden of hepatitis C management to the Medicare program or impact overall costs to the Centers for Medicare and Medicaid Services (CMS). In addition, the public health impact of delaying treatment for early-stage patients until after disease progression remains unknown. Thus, this study evaluates the cost-effectiveness of current Medicaid policies restricting hepatitis C treatment to patients with advanced disease compared to a strategy providing unrestricted access to hepatitis C treatment. We also assessed the budget and public health impact of each strategy and estimated the feasibility and long-term effects of increased access to treatment for hepatitis C patients.
METHODS
Model Structure and Perspective
Using a Markov state-transition model, we conducted cost-effectiveness, budget, and public health impact analyses from the perspectives of: (1) the Medicare program alone, which included costs and effects accrued after patients became eligible for Medicare benefits; and (2) CMS, which incorporated costs and effects accrued during the entire study period. We considered lifetime costs and outcomes, used 3% annual discounting (varied in sensitivity analysis), and adjusted all prices to 2015 US dollars using the Consumer Price Index.
Model Cohort
We modeled hypothetical cohorts of 45-, 50-, and 55-year-old treatment-naïve and treatment-experienced Medicaid patients diagnosed with genotype 1 hepatitis C. Our selected age groups comprise approximately 95% of the Medicaid hepatitis C population (19). Our cohorts excluded patients with any prior history of decompensated cirrhosis, liver transplantation, or HIV co-infection. Chronic hepatitis C disease severity is measured using the Meta-analysis of Histologic Data in Viral Hepatitis (METAVIR) score, which describes five stages of liver fibrosis: F0, no hepatic fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis with few septa; F3, many septa without cirrhosis; F4, cirrhosis (20). We estimated the baseline distribution of METAVIR scores using model-based predictions of the HCV-infected population in 2014 (Table 1) (8, 13).
Table 1.
Hepatitis C Cohort Characteristics, Natural History, Costs, and Utilities
| Description | Base Case | Low | High | Distribution | Source |
|---|---|---|---|---|---|
| Cohort Characteristics (%) | |||||
| F0–2 | 0.51 | 0.38 | 0.64 | Dirichlet | (8, 13) |
| F3 | 0.21 | 0.16 | 0.26 | Dirichlet | (8, 13) |
| F4 | 0.28 | 0.21 | 0.35 | Dirichlet | (8, 13) |
| Treatment-Naive | 0.61 | 0.46 | 0.76 | Beta | (8, 13) |
| Risk of Disease Progression (%) | |||||
| F0–2 to F3 | 0.12 | 0.11 | 0.13 | Beta | (65) |
| F3 to F4 | 0.12 | 0.09 | 0.14 | Beta | (65) |
| F3 to HCC | 0.01 | 0 | 0.03 | Beta | (66) |
| F4 to DC | 0.04 | 0.01 | 0.04 | Beta | (66, 67) |
| F4 to HCC | 0.03 | 0.01 | 0.08 | Beta | (66, 68) |
| DC to HCC | 0.07 | 0.03 | 0.08 | Beta | (69) |
| DC to Transplant | 0.03 | 0.02 | 0.06 | Beta | (70, 71) |
| HCC to Transplant | 0.04 | 0 | 0.14 | Beta | (72, 73) |
| Progression After SVR (%) | |||||
| F3 to HCC | 0.007 | 0.006 | 0.008 | Beta | (5, 74) |
| F4 to DC | 0.005 | 0.002 | 0.096 | Beta | (34, 74) |
| F4 to HCC | 0.007 | 0 | 0.019 | Beta | (34, 74, 75) |
Note: DC - decompensated cirrhosis, F0–2, F3, F4 - METAVIR stages of hepatic fibrosis, HCC - hepatocellular carcinoma, RR - relative risk, SVR - sustained virologic response,
- compared to all-cause mortality,
- compared to F0–2,
- compared to pre-treatment state.
Natural History Model
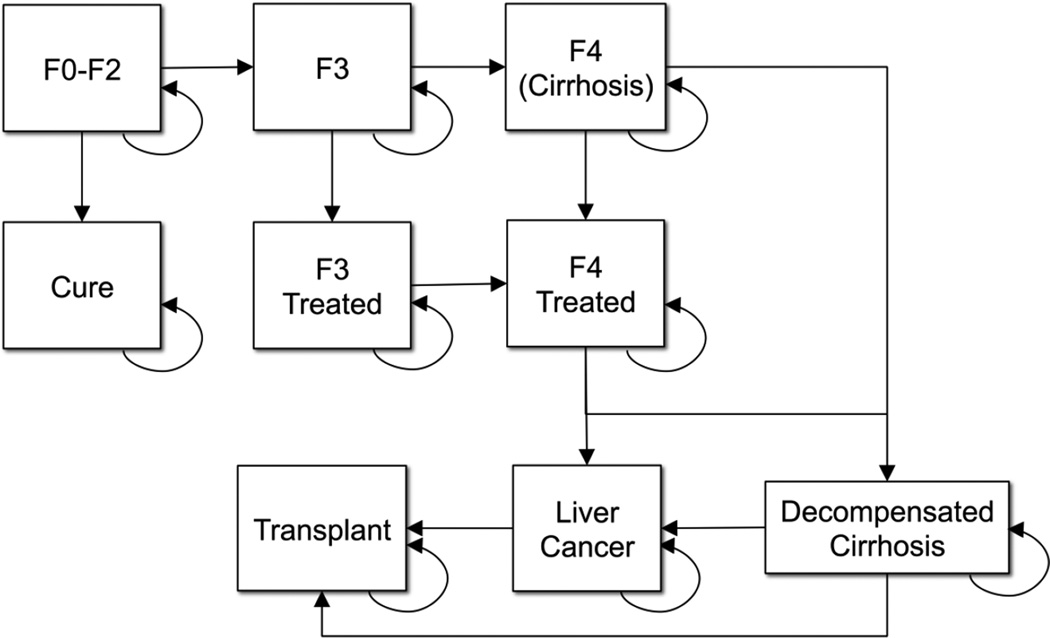
We created a Markov model to simulate the natural history and epidemiology of hepatitis C infection (Figure 1). Patients accrued liver-related treatment and follow-up costs as well as quality-adjusted life years (QALYs) for their Markov state at the end of each one-year cycle. Patients could make one state transition each year. Mortality was possible during each model stage; we estimated age-specific, annual all-cause mortality rates using US life tables (21). Disease progression and excess liver-related mortality occurred according to stage-specific transition probabilities and relative risks of mortality established in prior studies (Table 1).
Figure 1.
Markov State Transition Model Simulating the Natural History of Hepatitis C
*Note: Transition probabilities derived from recent population-based studies. F0–2, F3 and F4 represent METAVIR stages of hepatic fibrosis. F3 and F4 treated states involve reduced risks of liver-related morbidity and mortality compared to untreated states.
We grouped patients into three stages of baseline disease severity: early-stage disease (METAVIR F0–F2), advanced fibrosis (METAVIR F3), and compensated cirrhosis (METAVIR F4). Patients with compensated cirrhosis could later develop complications including decompensated cirrhosis, liver transplantation, and hepatocellular carcinoma. Patients with early-stage disease, advanced fibrosis, or compensated cirrhosis could receive hepatitis C treatment. We assumed that after successful treatment, patients with early-stage disease would return to full health and accrue no further hepatitis C infection-related costs. In contrast, patients with advanced fibrosis or cirrhosis would have markedly reduced risks of disease progression, complications, and mortality, but no reduction in follow-up costs after successful treatment (Table 1).
Treatment
We assumed that all patients would be treated with one of two currently available interferon-free hepatitis C drug regimens: a single dose two-drug combination of sofosbuvir/ledipasvir (SOF/LDV) or a multi-dose three-drug combination of ombitasvir, paritaprevir, and ritonavir with dasabuvir (3D). At the time of analysis, the American Association for the Study of Liver Diseases recommended both of these treatments for patients with genotype 1 hepatitis C (Supplementary Table 1). Because utility data were not available for the 3D regimen at the time of our analysis, we performed our primary analysis using data for SOF/LDV (Table 2) and used estimates for 3D in sensitivity analyses. We estimated the efficacy of each treatment regimen using data from recently published clinical trials (22–30). In patient subgroups for which several alternative treatment options have demonstrated similar effectiveness, we chose the least costly drug regimen.
Table 2.
Hepatitis C Treatment Parameters
| Parameters | Base Case | Low | High | Distribution | Source |
|---|---|---|---|---|---|
| Treatment Efficacy | |||||
| SOF/LDV × 8 weeks | 0.94 | 0.90 | 0.97 | Beta | (22) |
| SOF/LDV × 12 weeks (Naïve) | 0.96 | 0.92 | 1.00 | Beta | (22, 23) |
| SOF/LDV × 12 weeks (Naïve F4) | 0.97 | 0.84 | 1.00 | Beta | (23) |
| SOF/LDV × 12 weeks (Experienced) | 0.95 | 0.89 | 0.99 | Beta | (24) |
| SOF/LDV/RBV × 12 weeks (F4) | 0.88 | 0.72 | 0.92 | Beta | (24, 25) |
| Treatment Disutilities | |||||
| SOF/LDV × 8 weeks | 0.03 | −0.19 | 0.25 | Normal | (31) |
| SOF/LDV × 12 weeks | 0.04 | −0.20 | 0.28 | Normal | (31) |
| SOF/LDV/RBV × 12 weeks | −0.02 | −0.30 | 0.26 | Normal | (31) |
| Drug Costs (weekly) | |||||
| SOF/LDV | $5,874 | $2,500 | $7,875 | Gamma | NADAC |
| Ribavirin | $152.78 | $114.59 | $190.98 | Gamma | (34) |
| Medical Monitoring Costs (each, ±25%) | |||||
| Office visits (CPT 99213) | $72.94 | $51.13 | $79.69 | Gamma | MPFS |
| Complete blood count | $10.58 | $8.81 | $14.30 | Gamma | MPFS |
| Complete metabolic panel | $14.37 | $11.51 | $19.43 | Gamma | MPFS |
| Quantitative HCV PCR | $58.29 | $38.61 | $78.77 | Gamma | MPFS |
Note: AWP – Average wholesale price, HCV – hepatitis C, 3D – ombitasvir, ritonavir, and paritaprevir with dasabuvir, MPFS – Medicare Physician Fee Schedule 2015, NADAC – National Average Drug Acquisition Cost, PCR – polymerase chain reaction test, SOF/LDV – sofosbuvir/ledipasvir
We determined SOF/LDV treatment disutility using data from a quality-of-life study conducted alongside recent clinical trials (31). Because utility data for the 3D and 3D with ribavirin regimens were not available, we used treatment disutility data for the SOF/LDV and SOF/LDV with ribavirin regimens, respectively, in our sensitivity analysis (Table 2).
Costs and Effectiveness
We estimated treatment and follow-up costs for patients with hepatitis C (Table 1). In the base case, we included a 23.1% discount from the national average drug acquisition price for each drug regimen, which is required as part of the Medicaid drug rebate program; we varied drug prices in sensitivity analysis. We used the Medicare physician fee schedule to calculate the costs of on-treatment medical monitoring (32), including a single pre-treatment office visit, complete blood count, complete metabolic panel, and viral load measurement; monthly office visits, viral load measurements and metabolic panels during treatment; and a single post-treatment office visit, viral load measurement, and metabolic panel. We assumed that patients using ribavirin-containing regimens were monitored more frequently, with twice-monthly office visits and complete blood counts (Table 2).
From the Medicare perspective, costs and QALYs began to accrue upon Medicare eligibility at age 65 (or earlier for the share eligible due to disability). From the CMS perspective, costs and QALYs accrued throughout the study period. Because Medicare Part D can involve substantial cost-sharing for seniors not receiving low income subsidies, we subtracted expected patient out-of-pocket costs estimated using current Part D coverage rules (33), but assumed that the prescription drug coverage gap (i.e. “donut hole”) would not be in place by the time the oldest cohort becomes eligible for Medicare benefits.
We determined annual follow-up costs for each health state using recent estimates for Medicare and managed care patients (34–37), and used age-specific median utility values for healthy patients (38). We estimated utility weights for each hepatitis C-related health state based on recent comprehensive reviews of the literature (34, 39, 40). Finally, we varied all parameters over feasible ranges in sensitivity analyses (Table 1).
Strategies
We compared two strategies for managing hepatitis C infection in Medicaid beneficiaries: (1) Current Practice – only patients with advanced fibrosis or cirrhosis are treated for hepatitis C before becoming eligible for Medicare, treatment for patients with early-stage disease is deferred until disease progression or Medicare eligibility; and (2) Full Access – patients with early-stage disease, advanced fibrosis, and cirrhosis are treated before becoming eligible for Medicare benefits (Figure 2). Because some Medicare Advantage plans are adopting more restrictive treatment strategies, we assumed in the base case that 50% of patients with early stage disease would be treated upon Medicare eligibility (varied 0–100% in sensitivity analysis).
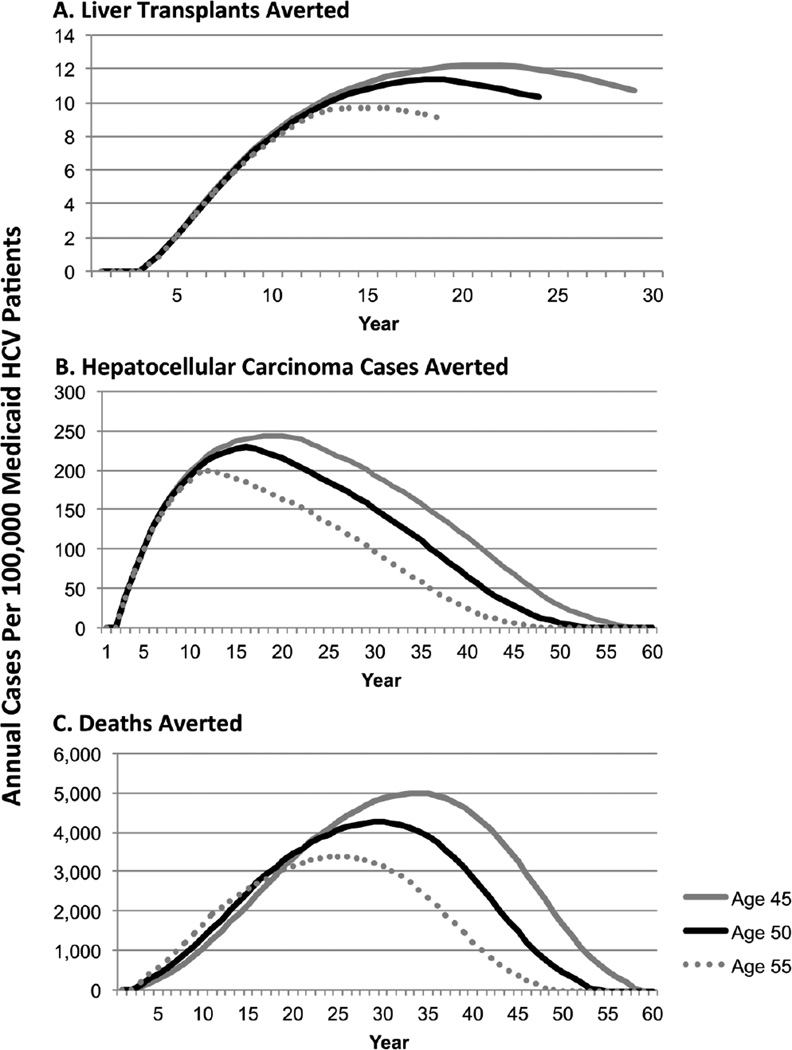
Figure 2.
Annual Public Health Impact of Unrestricted vs. Restricted Access to Hepatitis C Treatment Among Medicaid Beneficiaries
Assumptions
To perform this analysis, we made a number of simplifying assumptions to systematically bias the model against the Full Access strategy. We assumed that: (1) patients who failed treatment with sofosbuvir- or ombitasvir-based regimens would not be retreated because guidelines for retreatment had not yet been developed; (2) only patients 75 years of age or younger would undergo liver transplantation (41); (3) the Medicare and Medicaid programs would have similar follow-up and treatment costs; (4) patients would become eligible for full Medicare benefits at age 65, however to account for Medicare-Medicaid dual eligibility, we estimated that 14% of Medicaid recipients under age 65 would receive Medicare disability benefits while 14% of Medicare beneficiaries over 65 received Medicaid benefits (42, 43); and (5) the size of the Medicaid hepatitis C population would remain static over time. We accounted for a one-time Medicaid expansion in a sensitivity analysis.
Cost-Effectiveness Analyses
We completed the analyses separately for cohorts of 45-, 50-, 55-year-old Medicaid beneficiaries with hepatitis C. In the base case, we calculated the incremental cost-effectiveness ratio (ICER), which reflects the additional investment required to gain an additional QALY. While a $50,000/QALY threshold has classically been used in cost-effectiveness analyses, ICER thresholds of $100,000–$150,000/QALY may better reflect contemporary preferences (44, 45).
We also conducted sensitivity analyses to determine whether variations in model inputs would change the preferred strategy. First, we varied model inputs individually over a range of plausible values in one-way sensitivity analyses (Table 1). Then, we used Monte Carlo probabilistic sensitivity analyses, in which values are randomly sampled from each variable’s probability distribution and repeated over 5,000 iterations to determine the likelihood that each strategy is cost-effective (46). We performed all analyses using TreeAge Pro 2015 (TreeAge Software, Williamstown, MA).
Structural Sensitivity Analyses
Because it is not feasible to treat all Medicaid patients with HCV in a single year, we also conducted structural sensitivity analyses using staged treatment strategies, in which patients would be treated over time. We estimated that 450,000 patients with genotype 1 hepatitis C are currently receiving Medicaid benefits, and up to 600,000 may be enrolled if Medicaid expansion is widely adopted (17, 19, 47–50).
We also estimated treatment capacity for each strategy. Based on total Medicaid hepatitis C drug expenditures in 2014 and previous reports of treatment capacity, we estimated that approximately 30,000 Medicaid patients with hepatitis C could be treated in a given year (51, 52). Because more patients are likely to be treated each year under the Full Access strategy, we also modeled an expanded Full Access strategy with an annual treatment capacity of 40,000 patients. Recent developments suggest that increased treatment capacity is likely to be feasible because new drug regimens are now 24–36 weeks shorter in duration than interferon-based regimens, allowing more patients to be treated by the same number of physicians in any given year. In addition, a recent study demonstrated that primary care providers can effectively administer hepatitis C treatment in uncomplicated cases (53). If this practice were widely adopted in the U.S., then a much larger physician workforce would be available to treat early-stage patients with hepatitis C. To derive approximate annual treatment probabilities, we estimated that 13% of earlystage patients die or progress each year, while the number of patients with advanced-stage disease is reduced by approximately 1% each year, accounting for entry, progression, and death, based on data from our natural history model (Supplementary Table 2). In the Current Practice strategy, treatment would be offered to early-stage patients only after all patients with advanced fibrosis or cirrhosis have been treated. In the Full Access strategies, treatment would be equally allocated across fibrosis stages each year.
Budget & Public Health Impact Analyses
Finally, we compared the budget and public health impact of each treatment strategy. Using a Markov cohort analysis, which describes the costs and utilities associated with each Markov state during each model year, we estimated and compared cost estimates as well as adverse health outcomes for each strategy. We compared the annual and cumulative costs for both treatment strategies in our base case analysis. Next, we used the model to estimate the annual and cumulative number of cases of adverse health outcomes such as hepatocellular carcinoma, liver transplantation, and mortality, per 100,000 Medicaid recipients.
RESULTS
Base Case Analyses
In the base case, the Full Access strategy was cost saving and more effective compared to the Current Practice strategy for all age cohorts from the Medicare perspective (Table 3). For the 50-year-old cohort, which represented the average Medicaid patient with hepatitis C, the Current Practice strategy ($30,610, 5.47 QALYs) cost an additional $9,200 per patient and yielded 0.84 fewer QALYs compared to the Full Access strategy ($21,410, 6.31 QALYs). Cost savings for the Full Access strategy increased with cohort age.
Table 3.
Cost-Effectiveness of Restricted Access to Hepatitis C Treatment: Base Case Results
| Strategy | Medicare Perspective | CMS Perspective | ||||
|---|---|---|---|---|---|---|
| Costs | QALYs | ICER ($/QALY) | Costs | QALYs | ICER ($/QALY) | |
| 45-year-old cohort | ||||||
| Full Access | $20,196 | 5.31 | $93,151 | 17.14 | ||
| Current Practice | $27,707 | 4.50 | Dominated | $104,426 | 14.13 | Dominated |
| 50-year-old cohort | ||||||
| Full Access | $21,410 | 6.31 | $90,524 | 15.79 | ||
| Current Practice | $30,610 | 5.47 | Dominated | $98,527 | 13.06 | Dominated |
| 55-year-old cohort | ||||||
| Full Access | $22,778 | 7.58 | $87,543 | 14.36 | ||
| Current Practice | $34,738 | 6.76 | Dominated | $92,912 | 12.05 | Dominated |
Note: ICER - incremental cost-effectiveness ratio, QALY - quality-adjusted life-year
From the CMS perspective, the Full Access strategy was also cost saving for each age cohort, but to a lesser degree. Compared to the Full Access strategy ($90,524, 15.79 QALYs), the Current Practice strategy cost an additional $8,003 per patient and yielded 2.73 fewer QALYs ($98,527, 13.06 QALYS) for the 50-year-old cohort (Table 3). The Full Access strategy was more cost saving for younger cohorts from the CMS perspective.
Sensitivity Analyses
In one-way sensitivity analyses from the Medicare perspective, the Full Access strategy was cost saving for all age cohorts regardless of variations in any individual model input. From the CMS perspective, variations in the follow-up costs for patients with early-stage disease and in the discount rate impacted the ICER differently in each age cohort. The Full Access strategy remained cost saving as long as the cost of follow-up for early-stage patients was more than approximately $200 per year in the 45-year-old cohort, $350 per year in the 50-year-old cohort, and $600 per year in the 55-year-old cohort. In addition, the Full Access strategy was cost saving for discount rates below 5–6%, depending on the age of the cohort. The Full Access strategy was cost saving over the range of plausible values for all other model inputs.
In probabilistic sensitivity analysis, the Full Access strategy was cost-effective in 100% of iterations from the Medicare perspective at all willingness-to-pay thresholds. From the CMS perspective, the Full Access strategy was cost-effective in 93% of iterations at the cost saving threshold of $0/QALY and in 100% of iterations at $4,500/QALY. Including the three-drug regimen instead of SOF/LDV did not change the preferred strategy from either perspective. In our structural sensitivity analysis, the staged Full Access strategy was cost saving compared to the staged Current Practice strategy for all age cohorts, regardless of annual treatment capacity or the size of the Medicaid hepatitis C population (Table 4).
Table 4.
Cost-Effectiveness of Staged Treatment Strategies for Medicaid Patients with Hepatitis C, by Number of Medicaid Beneficiaries with Hepatitis C
| 450,000 HCV Patients | 600,000 HCV Patients | |||
|---|---|---|---|---|
| Strategy | Costs | QALYs | Costs | QALYs |
| 45-year-old cohort | ||||
| Expanded Full Access | $97, 138 | 14. 73 | $97, 462 | 14. 20 |
| Full Access | $97,462 | 14.20 | $97,541 | 13.64 |
| Current Practice | $100,608 | 14.08 | $100,833 | 13.55 |
| 50-year-old cohort | ||||
| Full Access | $92, 657 | 12. 98 | $92, 220 | 12. 45 |
| Expanded Full Access | $92,775 | 13.48 | $92,657 | 12.98 |
| Current Practice | $95,861 | 12.86 | $95,601 | 12.36 |
| 55-year-old cohort | ||||
| Full Access | $86, 841 | 11. 68 | $85, 542 | 11. 18 |
| Expanded Full Access | $87,778 | 12.15 | $86,841 | 11.68 |
| Current Practice | $90,344 | 11.56 | $89,190 | 11.09 |
Note: Population size estimates reflect current Medicaid HCV population & potential increase in prevalence due to Medicaid expansion. Current Practice & Full Access – 30,000 patients treated per year, Expanded Full Access – 40,00 patients treated per year, QALYs-quality-adjusted life years.
Budget and Public Health Impact Analyses
Our budget impact analyses revealed that, from the CMS perspective, the Full Access strategy became cost saving compared to the Current Practice strategy after 13–16 years, depending on cohort age. By the end of the study period, the Full Access strategy saved $10,340 per patient for the 45-year-old cohort, $8,148 for 50-year-olds, and $5,695 for 55-year-old patients. With staged treatment strategies, Full Access became cost saving after 9 years for each age cohort. In the worst-case scenario, with 600,000 hepatitis C patients and 30,000 treated per year, the Full Access strategy saved $3,197–$3,568 per patient by the end of the study period, depending on the age of the cohort. For a cohort of 450,000 50-year-old Medicaid patients with hepatitis C, treating 30,000 patients per year using interferon-free regimens would cost Medicaid programs an average of $4,746 per beneficiary annually with the Current Practice strategy and $4,568 per beneficiary annually with the Full Access strategy. If a total of 600,000 patients required treatment, costs would decrease to $4,640 per beneficiary with Current Practice and $4,428 per beneficiary with Full Access. By comparison, no treatment would cost $2,309 per beneficiary.
The public health impact analysis demonstrated that for every 100,000 50-year-old Medicaid beneficiaries, the Full Access strategy could avert approximately 5,994 cases of hepatocellular carcinoma and 121 liver transplants compared to the Current Practice strategy. The number of cases averted varied over time for each age cohort (Figure 2).
CONCLUSIONS
This cost-effectiveness analysis revealed that for current Medicaid beneficiaries, unrestricted access to hepatitis C treatment is cost saving compared to the current policy restricting treatment to only patients with advanced liver disease. The increased short-term costs of unrestricted access to care can be offset by savings from reduced complications in 9–16 years, depending on the treatment strategy and age of the cohort. Furthermore, increased access to treatment could avert numerous future cases of hepatocellular carcinoma, reduce the need for liver transplantation and prevent early mortality.
We demonstrated that the Full Access strategy led to long-term cost savings compared to the more restrictive Current Practice strategy. In fact, under ideal circumstances, the total savings could exceed $3.5 billion for the 450,000 Medicaid beneficiaries with hepatitis C. This is because under both strategies, all patients will ultimately be treated unless they decompensate or die before becoming eligible. An open access strategy would lead to treatment earlier in the natural history of the disease, substantially reducing follow-up costs for patients with early-stage disease. This interpretation is supported by the results of our sensitivity analysis, which demonstrated that the full access strategy is cost saving only if annual follow-up costs for early-stage patients exceed $600, meaning that it is economically advantageous to avert these costs. In addition, open access to treatment would reduce the number of early-stage patients who progress to advanced fibrosis or cirrhosis before being treated. This is important because even after successful treatment, patients with advanced disease still have high follow-up costs and a small risk of developing costly complications, while successfully treated early-stage patients have similar outcomes to their uninfected age-matched peers. Although the overall budget impact is considerable, we demonstrate that the cost to Medicaid of managing hepatitis C with interferon-free regimens is less than $5,000 per capita annually regardless of treatment strategy. This is significantly less than the costs to Medicaid of managing many other major diseases, from respiratory illnesses ($8,100 per capita annually) to diabetes ($13,500 per capita annually)(54) (54, 55).
Our results were robust to variations in most model inputs. In sensitivity analyses, the Full Access strategy was no longer cost saving for very high discount rates (≥5%) or very low follow-up costs for early-stage patients (<$600), both of which are unlikely. Cost-effectiveness guidelines suggest that a 3% discount rate is likely to be appropriate as the Office of Management and Budget recently suggested that a 3.4% nominal 30-year interest rate should be used for cost-effectiveness analyses (56, 57). Similarly, most studies suggest that costs of follow-up for early-stage patients with hepatitis C are much higher than $600. Recently, the rate of hospitalizations for patients with early-stage and advanced hepatitis C has increased, which suggests that the costs of managing these patients are likely to be increasing as well (58).
Because the assumptions made were generally biased against the Full Access strategy, our estimates are likely to be conservative. The Full Access strategy was cost saving even if there was no associated increase in treatment capacity. In reality, doubling the pool of eligible patients is likely to increase the absolute number of patients seeking treatment, bounded only by physician availability, patients’ knowledge of their disease status, and medical eligibility for treatment. Finally, we assumed that drug prices would be similar for Medicare and Medicaid. However, many state Medicaid programs are negotiating dramatic price discounts for hepatitis C treatment regimens, which could reduce the total cost of the Full Access strategy (59). Meanwhile, because the Medicare program cannot negotiate drug prices, the costs of waiting to treat patients after Medicare eligibility are likely to be higher than our estimates, which were based on Medicaid prices.
Our results are consistent with those of recent studies evaluating the impact of novel interferon-free treatment regimens demonstrating that novel interferon-free drug regimens are cost-effective for many patient subgroups (13, 15). One study in particular demonstrated that the SOF/LDV regimen could be cost saving compared to the previous standard of care if treatment was substantially discounted, but did not evaluate the effects of restrictive vs. inclusive treatment strategies (15). In addition, the results of our public health impact analysis are consistent with findings from Kabiri et al (8), who also demonstrated that increased access to hepatitis C treatment could result in substantial long-term reductions in morbidity and mortality. This study addresses the dilemma of determining which patients with hepatitis C should be treated first, which has been highlighted in numerous recent editorials (16, 60, 61). Here, we offer empiric evidence to inform this debate and demonstrate that, from a government payer perspective, allowing access to treatment for early-stage patients may be the less costly and more effective long-term strategy.
Our analysis is interesting in light of current events in public health. For example, the US Preventive Services Task Force recently recommended birth cohort screening for hepatitis C for adults born between 1945 and 1965 (62). The analysis demonstrating that screening is cost-effective assumed that patients would be treated after disease was identified, albeit with older drug regimens (63). It will become important to consider the ethical and economic implications if positive birth cohort screens are not paired with treatment initiation. In addition, although the prevalence is highest among patients aged 45 and older, the incidence of hepatitis C has recently been rising at an alarming rate among younger injection drug users (64). Although our analysis focused on older cohorts, we demonstrate that Full Access is increasingly cost saving for younger patients, who would live with the disease for a longer period of time. Treating these patients while they have early-stage disease would reduce the high costs of disease management and potential complications that are likely to occur if treatment is deferred until disease progression. Treating younger patients may also curb the spread of the disease and reduce the duration of the epidemic.
This study has some limitations that must be acknowledged. First, analytic methods that directly account for resource constraints may provide more precise estimates. However, because our assumptions biased the model against the full access strategy, the conclusions are likely to be similar. Second, we estimated treatment efficacy using clinical trial data, which may overestimate real-word effectiveness. Third, some Medicaid managed care plans may receive smaller drug discounts than those mandated by the Medicaid drug rebate program. To account for this, we varied drug prices widely in sensitivity analysis. Finally, our model only included liver-related costs and did not account for the potential increases in cumulative healthcare costs associated with reduced early mortality. This is beyond the scope of this analysis but is an interesting topic for future study.
In conclusion, using cost-effectiveness analyses, we found that current Medicaid policies restricting hepatitis C treatment to patients with advanced disease are more costly and less effective than strategies with unrestricted access to treatment for patients with early-stage and advanced disease. Although our results provide empiric support for providing open access to treatment for hepatitis C, additional factors, including the size of the physician workforce and budgetary limitations, must also be considered. This study also highlights that, in a multi-payer healthcare system, efforts to minimize costs for individual payers can result in cost-shifting and economic efficiency for the system as a whole. In light of this, collaborative efforts between state and federal payers may be needed to realize the full public health impact of recent advances in hepatitis C therapy.
Supplementary Material
Acknowledgments
FUNDING: AC was supported by the National Institutes of Health (TL1TR000145).
SR receives research funding for unrelated projects from Gilead Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
POTENTIAL CONFLICTS OF INTEREST: All additional authors report no potential conflicts of interest.
REFERENCES
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Chak E, Talal AH, Sherman KE, et al. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver international : official journal of the International Association for the Study of the Liver. 2011;31:1090–1101. doi: 10.1111/j.1478-3231.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic Steatohepatitis is the Second Leading Etiology of Liver Disease Among Adults Awaiting Liver Transplantation in the U.S. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Davis GL, Albright JE, Cook SF, et al. Projecting future complications of chronic hepatitis C in the United States. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 5.Butt AA, Wang X, Moore CG. Effect of hepatitis C virus and its treatment on survival. Hepatology. 2009;50:387–392. doi: 10.1002/hep.23000. [DOI] [PubMed] [Google Scholar]
- 6.Dieperink E, Pocha C, Thuras P, et al. All-cause mortality and liver-related outcomes following successful antiviral treatment for chronic hepatitis C. Digestive diseases and sciences. 2014;59:872–880. doi: 10.1007/s10620-014-3050-5. [DOI] [PubMed] [Google Scholar]
- 7.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA : the journal of the American Medical Association. 2012;308:2584–2593. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 8.Kabiri M, Jazwinski AB, Roberts MS, et al. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Annals of internal medicine. 2014;161:170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holmberg SD, Spradling PR, Moorman AC, et al. Hepatitis C in the United States. The New England journal of medicine. 2013;368:1859–1861. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang TJ, Ghany MG. Therapy of Hepatitis C — Back to the Future. New England Journal of Medicine. 2014;370:2043–2047. doi: 10.1056/NEJMe1403619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A 4-drug combination (Viekira Pak) for hepatitis C. The Medical letter on drugs and therapeutics. 2015;57:15–17. [PubMed] [Google Scholar]
- 12.A combination of ledipasvir and sofosbuvir (Harvoni) for hepatitis C. The Medical letter on drugs and therapeutics. 2014;56:111–112. [PubMed] [Google Scholar]
- 13.Chhatwal J, Kanwal F, Roberts MS, et al. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Annals of internal medicine. 2015;162:397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chidi AP, Rogal S, Bryce CL, et al. Cost-Effectiveness of New Antiviral Regimens for Treatment-Naive US Veterans with Hepatitis C. Hepatology. 2015 doi: 10.1002/hep.28327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Annals of internal medicine. 2015;162:407–419. doi: 10.7326/M14-1152. [DOI] [PubMed] [Google Scholar]
- 16.Haque M, Zariat A. Treatment Strategy for Hepatitis C: A Dilemma for the Payers and the Providers. The American journal of gastroenterology. 2014;109:1953–1954. doi: 10.1038/ajg.2014.320. [DOI] [PubMed] [Google Scholar]
- 17.Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic Hepatitis C Virus Infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Annals of internal medicine. 2014;160:293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuman T, Hoadley J, Cubanski J. The Cost Of A Cure: Medicare’s Role In Treating Hepatitis C. Health Affairs Blog [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Data. In: Centers for Disease Control and Prevention, editor. U.S. Department of Health and Human Services. Hyattsville, MD: 2012. [Google Scholar]
- 20.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 21.Arias E. National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2014. United States Life Tables, 2009. [PubMed] [Google Scholar]
- 22.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. The New England journal of medicine. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 23.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. The New England journal of medicine. 2014;370:1889–1898. doi: 10.1056/NEJMoa1402454. [DOI] [PubMed] [Google Scholar]
- 24.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. The New England journal of medicine. 2014;370:1483–1493. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 25.Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 26.Zeuzem S, Jacobson IM, Baykal T, et al. Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. The New England journal of medicine. 2014;370:1604–1614. doi: 10.1056/NEJMoa1401561. [DOI] [PubMed] [Google Scholar]
- 27.Andreone P, Colombo MG, Enejosa JV, et al. ABT-450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment-experienced patients with HCV genotype 1b infection. Gastroenterology. 2014;147:359–365. e1. doi: 10.1053/j.gastro.2014.04.045. [DOI] [PubMed] [Google Scholar]
- 28.Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. The New England journal of medicine. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 29.Ferenci P, Bernstein D, Lalezari J, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. The New England journal of medicine. 2014;370:1983–1992. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 30.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. The New England journal of medicine. 2014;370:1973–1982. doi: 10.1056/NEJMoa1402869. [DOI] [PubMed] [Google Scholar]
- 31.Younossi ZM, Stepanova M, Marcellin P, et al. Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: Results from the Ion-1, 2 and 3 clinical trials. Hepatology. 2015 doi: 10.1002/hep.27724. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Medicare and Medicaid Services (CMS) Medicare Physician Fee Schedule. 2015 [Google Scholar]
- 33.Centers for Medicare and Medicaid Services. Closing the Coverage Gap—Medicare Prescription Drugs Are Becoming More Affordable. 2015 [Google Scholar]
- 34.Coffin PO, Scott JD, Golden MR, et al. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:1259–1271. doi: 10.1093/cid/cis011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAdam-Marx C, McGarry LJ, Hane CA, et al. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. Journal of managed care pharmacy : JMCP. 2011;17:531–546. doi: 10.18553/jmcp.2011.17.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younossi ZM, Singer ME, Mir HM, et al. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. Journal of hepatology. 2014;60:530–537. doi: 10.1016/j.jhep.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 37.Schnitzler MA, Skeans MA, Axelrod DA, et al. OPTN/SRTR 2013 Annual Data Report: economics. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(Suppl 2):1–24. doi: 10.1111/ajt.13201. [DOI] [PubMed] [Google Scholar]
- 38.Gold MR, Franks P, McCoy KI, et al. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Medical care. 1998;36:778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Thein HH, Krahn M, Kaldor JM, et al. Estimation of utilities for chronic hepatitis C from SF-36 scores. The American journal of gastroenterology. 2005;100:643–651. doi: 10.1111/j.1572-0241.2005.40976.x. [DOI] [PubMed] [Google Scholar]
- 40.Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals. Hepatology. 2014 doi: 10.1002/hep.27151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim WR, Stock PG, Smith JM, et al. OPTN/SRTR 2011 Annual Data Report: liver. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(Suppl 1):73–102. doi: 10.1111/ajt.12021. [DOI] [PubMed] [Google Scholar]
- 42.Medicare-Medicaid Coordination Office. Medicare-Medicaid Dual Enrollment from 2006 through 2013 [Google Scholar]
- 43.Department of Health & Human Services. Actuarial Report on the Financial Outlook For Medicaid. 2013 [Google Scholar]
- 44.Braithwaite RS, Meltzer DO, King JT, Jr, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Medical care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 45.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. The New England journal of medicine. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 46.Doubilet P, Begg CB, Weinstein MC, et al. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Medical decision making : an international journal of the Society for Medical Decision Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 47.Stepanova M, Younossi ZM. Interferon-Free Regimens for Chronic Hepatitis C: Barriers Due to Treatment Candidacy and Insurance Coverage. Digestive diseases and sciences. 2015 doi: 10.1007/s10620-015-3709-6. [DOI] [PubMed] [Google Scholar]
- 48.Kaiser Family Foundation. The Cost of Not Expanding Medicaid. 2013 [Google Scholar]
- 49.Kaiser Family Foundation. Medicaid expansion under the affordable care act. JAMA : the journal of the American Medical Association. 2013;309:1219–1219. [Google Scholar]
- 50.Buettgens M, Holahan J, Recht H. Medicaid Expansion, Health Coverage, and Spending: An Update for the 21 States That Have Not Expanded Eligibility. Kaiser Family Foundation. 2015 [Google Scholar]
- 51.Volk ML, Tocco R, Saini S, et al. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750–1755. doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Medicare and Medicaid Services. 2014 National Summary State Drug Utilitization. 2014 [Google Scholar]
- 53.Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. The New England journal of medicine. 2011;364:2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaiser Family Foundation. The Role of Medicaid for People with Respiratory Disease. 2012 https://kaiserfamilyfoundation.files.wordpress.com/2013/01/8383_rd.pdf. [Google Scholar]
- 55.Kaiser Family Foundation. The Role of Medicaid for People with Diabetes. 2012 https://kaiserfamilyfoundation.files.wordpress.com/2013/01/8383_d.pdf. [Google Scholar]
- 56.Office of Management and Budget. Appendix C. Discount Rates for Cost-Effectiveness, Lease Purchase, and Related Analyses. Revised December 2014 ed. [Google Scholar]
- 57.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 58.Xu F, Tong X, Leidner AJ. Hospitalizations and costs associated with hepatitis C and advanced liver disease continue to increase. Health affairs (Project Hope) 2014;33:1728–1735. doi: 10.1377/hlthaff.2014.0096. [DOI] [PubMed] [Google Scholar]
- 59.Loftus P. States Work to Strike Deals for Hep C Drug Discounts. Wall Street Journal [Google Scholar]
- 60.Etzion O, Ghany MG. A Cure for the High Cost of Hepatitis C Virus Treatment A Cure for the High Cost of Hepatitis C Virus Treatment. Annals of internal medicine. 2015;162:660–661. doi: 10.7326/M15-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghany MG. The ongoing debate of who to treat for chronic hepatitis C virus. JAMA internal medicine. 2015;175:169–170. doi: 10.1001/jamainternmed.2014.4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.U.S. Preventive Services Taskforce. Final Recommendation Statement: Hepatitis C: Screening. 2015 May [Google Scholar]
- 63.Rein DB, Smith BD, Wittenborn JS, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Annals of internal medicine. 2012;156:263–270. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. Increases in Hepatitis C Virus Infection Related to Injection Drug Use Among Persons Aged ≤30 Years — Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. Morbidity and Mortality Weekly Report. 2015 [PMC free article] [PubMed] [Google Scholar]
- 65.Thein HH, Yi Q, Dore GJ, et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 66.Dienstag JL, Ghany MG, Morgan TR, et al. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54:396–405. doi: 10.1002/hep.24370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 68.Alazawi W, Cunningham M, Dearden J, et al. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Alimentary pharmacology & therapeutics. 2010;32:344–355. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 69.Planas R, Balleste B, Alvarez MA, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. Journal of hepatology. 2004;40:823–830. doi: 10.1016/j.jhep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 70.Thuluvath PJ, Guidinger MK, Fung JJ, et al. Liver transplantation in the United States, 1999–2008. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:1003–1019. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 71.Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. 21 e1–21 e6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 72.Lang K, Danchenko N, Gondek K, et al. The burden of illness associated with hepatocellular carcinoma in the United States. Journal of hepatology. 2009;50:89–99. doi: 10.1016/j.jhep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 73.Saab S, Hunt DR, Stone MA, et al. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: a decision analysis model. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2010;16:748–759. doi: 10.1002/lt.22072. [DOI] [PubMed] [Google Scholar]
- 74.Morgan TR, Ghany MG, Kim HY, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–844. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Centers for Disease Control and Prevention. Recommendations for the Identification of Chronic Hepatitis C Virus Infection Among Persons Born During 1945–1965. MMWR. 2012 [PubMed] [Google Scholar]
- 76.El-Kamary SS, Jhaveri R, Shardell MD. All-cause, liver-related, and non-liver-related mortality among HCV-infected individuals in the general US population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:150–157. doi: 10.1093/cid/cir306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jain A, Reyes J, Kashyap R, et al. Long-term survival after liver transplantation in 4,000 consecutive patients at a single center. Annals of surgery. 2000;232:490–500. doi: 10.1097/00000658-200010000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolfe RA, Roys EC, Merion RM. Trends in organ donation and transplantation in the United States, 1999–2008. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10:961–972. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.