Abstract
PLZF-expressing invariant natural killer T cells and CD4 T cells are unique subsets of innate T cells. Both are selected via thymocyte-thymocyte interaction, and they contribute to the generation of activated/memory-like CD4 and CD8 T cells in the thymus via the production of IL-4. Here, we investigated whether PLZF+ innate T cells also affect the development and function of Foxp3+ regulatory CD4 T cells. Flow cytometry analysis of the thymus and spleen from both CIITA transgenic C57BL/6 and wild-type BALB/c mice, which have abundant PLZF+ CD4 T cells and invariant natural killer T cells, respectively, revealed that Foxp3+ T cells in these mice exhibited a CD103+ activated/memory-like phenotype. The frequency of CD103+ regulatory T cells was considerably decreased in PLZF+ cell-deficient CIITATgPlzflu/lu and BALB/c.CD1d−/− mice as well as in an IL-4-deficient background, such as in CIITATgIL-4−/− and BALB/c.lL-4−/− mice, indicating that the acquisition of an activated/memory-like phenotype was dependent on PLZF+ innate T cells and IL-4. Using fetal thymic organ culture, we further demonstrated that IL-4 in concert with TGF-β enhanced the acquisition of the activated/memory-like phenotype of regulatory T cells. In functional aspects, the activated/memory-like phenotype of Treg cells was directly related to their suppressive function; regulatory T cells of CIITATgPIV−/− mice more efficiently suppressed ovalbumin-induced allergic airway inflammation compared with their counterparts from wild-type mice. All of these findings suggest that PLZF+ innate T cells also augmented the generation of activated/memory-like regulation via IL-4 production.
Keywords: IL-4, PLZF, regulatory T lymphocyte
INTRODUCTION
Several sophisticated regulatory mechanisms are used to maintain immune homeostasis, prevent autoimmunity and moderate the level of inflammation induced by pathogens and environmental insults (Vignali et al., 2008). The actions of CD4+ regulatory T (Treg) cells expressing forkhead box P3 (Foxp3) are now widely accepted as the primary mediators of peripheral tolerance (Vignali et al., 2008). Treg cells can be divided into the following two subsets based on their site of origin: naturally occurring Treg (nTreg) cells generated in the thymus and induced Treg (iTreg) cells that acquire Foxp3 expression and a suppressor function in the periphery (Gottschalk et al., 2010). Interleukin-2 (IL-2) and transforming growth factor-β (TGF-β) are necessary for the generation of nTreg cells and iTreg cells as well as for the maintenance and survival of both subsets in the periphery (Bayer et al., 2007; Bird, 2010; Burchill et al., 2007; Lio and Hsieh, 2008; Liu et al., 2008; Ouyang et al., 2010).
CD103 is the α chain of the integrin αEβ7, which provides tissue retention at sites that are enriched in E-cadherin, particularly at the epithelial lining of the gut, lungs and skin as well as at sites of inflammation (Karecla et al., 1995). In Treg cells, CD103 was identified as a marker of a highly potent Treg cell subset isolated from murine secondary lymphoid organs (Banz et al., 2003; Lehmann et al., 2002; McHugh et al., 2002). CD103− Treg cells circulate through lymphoid tissues, whereas CD103+ Treg cells display an activated/memory-like phenotype, expressing multiple adhesion molecules and receptors for inflammatory chemokines (Huehn et al., 2004). When these cells migrate into the inflamed site of an ongoing disease model, they efficiently suppress immune responses in situ (Banz et al., 2003; Huehn et al., 2004; Lehmann et al., 2002; Zhao et al., 2008). Although CD103+ activated/memory-like Tregs predominantly develop in the course of the in vitro (Rao et al., 2005) and in vivo (Siewert et al., 2008) generation of iTregs as well as the activation of nTregs when they encounter cognate antigens in the periphery (Siewert et al., 2008), a small number of CD103+ Treg cells still develop from the wild-type (WT) thymus with an activated/memory-like phenotype (Annacker et al., 2005; Stephens et al., 2007). However, the mechanisms by which Treg cells express CD103 molecules on their surface have not been thoroughly investigated.
Unlike mouse thymocytes, human fetal thymocytes express major histocompatibility complex (MHC) class II molecules on their surface (Park et al., 1992). Research has suggested that CD4 T cells can be positively selected by interactions with other developing thymocytes expressing MHC class II molecules, which was referred to as thymocyte-thymocyte (T-T) interaction (Choi et al., 1997). This was confirmed in plck-CIITA transgenic (CIITATg) C57BL/6 mice, in which proximal lck promoter-driven expression of the human MHC class II transactivator (CIITA) transgene in developing thymocytes and mature T cells induced the expression of MHC class II molecules on the surface of these cells (Choi et al., 2005; Lee et al., 2010; Li et al., 2005). In these mice, thymocytes recognized MHC class II and self-peptide complex presented by other thymocytes, and this MHC class II-dependent T-T interaction interestingly allowed for the generation of innate CD4 T cells expressing promyelocytic leukemia zinc finger protein (PLZF) (Lee et al., 2010). This was a recapitulation of the previously reported developmental process of CD1d-restricted invariant natural killer T (iNKT) cells, another well-documented innate type of T cell: they are positively selected by the T-T interaction (restricted to CD1d molecules expressed on thymocytes) and express PLZF molecules (Treiner and Lantz, 2006). Importantly, the existence of human PLZF+ innate CD4 T cells was demonstrated in human fetal thymuses and spleens, signifying that the T-T interaction is a physiological event (Lee et al., 2009; 2010). Although PLZF+ innate CD4 T cells are somewhat different from iNKT cells in that they have a diverse TCR repertoire and are restricted by MHC class II molecules (Kang et al., 2015a; Lee et al., 2010), these two cell types share the following functional features: rapid production of both IL-4 and interferon-γ (IFN-γ) upon TCR stimulation and sole dependence on the signaling lymphocytic activation molecule (SLAM) and SLAM-associated protein (SAP) signal pathway in their generation (Alonzo and Sant’Angelo, 2011; Lee et al., 2009; Li et al., 2007). Recently, several groups reported the significant role of IL-4 produced by these two types of cell in the generation of activated/memory-like T cells in the thymus: eomesodermin-expressing innate CD8 (Min et al., 2011; Weinreich et al., 2010) and CD4 (Kang et al., 2015b; Prince et al., 2014a; 2014b) T cells. These studies imply that changes in the cytokine milieu can alter the properties of developing bystander thymocytes.
In the present study, we investigated whether PLZF+ innate T cells would also affect the development and function of Foxp3+ regulatory CD4 T cells via producing IL-4. To test this, we first dissected the thymus of CI ITATg and BALB/c mice and found that PLZF+ innate T cells augmented the generation of CD103+ activated/memory-like nTreg cells in the thymus of these mice. In terms of the mechanism controlling this event, the acquisition of the activated/memory-like phenotype of nTreg cells depended on TGF-β, and IL-4 synergistically enhanced the effect of this cytokine. Interestingly, the major sources of IL-4 were PLZF+ innate CD4 T cells in CIITATg mice and iNKT cells in WT BALB/c mice. These findings indicate that PLZF+ innate T cells allow both effector and regulatory T cells to be activated in the thymus prior to their exit to the periphery.
MATERIALS AND METHODS
Mice
As described previously, CIITATg mice were generated in our laboratory (Choi et al., 2005). In this transgenic mouse model, the expression of the human CIITA gene is under the control of the proximal lck promoter, which is first expressed early in thymocyte development at the double negative stage. C57BL/6 (wild type, CD45.1 congenic, IL-4−/−, and Plzf lu/lu) and BALB/c (wild type, IL-4−/−, and CD1d−/−) mice were purchased from Jackson Laboratory (USA). Mice carrying a deletion of the Mhc2ta promoter IV (PIV−/−) were kindly donated by H. Acha-Orbea (University of Lausanne, Switzerland). Positive selection of CD4 T cells is abrogated in this mouse model due to the absence of MHC class II expression in thymic epithelial cells. Backcrossing CIITATg mice to PIV−/− mice produced CIITATgPIV−/− mice, where positively selecting signals are only provided by MHC class II-expressing thymocytes due to the lack of MHC class II molecules on thymic epithelial cells. Foxp3-IRES-green fluorescent protein (GFP) knock-in mice were a generous gift from Dr. A. Rudensky (University of Washington, USA). Breeding CIITATg mice with Plzflu/lu or IL-4−/− mice resulted in CIITATgPlzflu/lu or CIITATgIL-4−/− mice, respectively. CIITATgPIV−/− mice were bred with Foxp3-GFP knock-in mice to obtain Foxp3-GFP knock-in CIITATgPIV−/− mice. All animals were bred or maintained under specific pathogen-free conditions in the animal facility at the Center for Animal Resource Development at Seoul National University College of Medicine. Experiments were performed after receiving approval from the Institutional Animal Care and Use Committee of the Institute of Laboratory Animal Resources at Seoul National University.
Antibodies and flow cytometric analysis
The following fluorochrome- or biotin-labeled antibodies were purchased from BD Bioscience (USA) or eBioscience (USA): anti-CD4 (RM4-5), anti-CD8 (53–6.7), anti-CD11c (HL3), anti-CD25 (PC61), anti-TCRβ (H57-597), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD39 (24DMS1), anti-CD44 (IM7), anti-CD54 (3E2), anti-CD62L (MEL-14), anti-CD73 (TY/23), anti-CD103 (M290), anti-CD127 (7R34), anti-CTLA4 (UC10-4F10-11), anti-CXCR3 (CXCR3-173), anti-CCR4 (2G12), anti-CCR5 (HM-CCR5(7A4)), anti-CCR7 (4B12), anti-I-Ab (AF6-120.1), anti-Gr-1 (RB6-8C5), anti-Siglec F (E50-2440), and anti-Foxp3 (FJK-16s) antibodies. Cell surface staining was performed in fluorescence-activated cell sorting (FACS) buffer [1× phosphate-buffered saline (PBS) with 0.1% bovine serum albumin and 0.1% sodium azide] with different combinations of antibodies for 30 min at 4°C. Intracellular staining of Foxp3 was performed in accordance with the instructions of the Foxp3 staining kit (eBiosciences, USA). Samples were analyzed using a BD LSRFortessa™ (Becton-Dickinson, USA), and the acquired data were further processed with FlowJo software (Becton Dickinson, USA).
In vitro Treg suppression assays
To determine the suppressive ability of Treg cells, CD4+GFP+ Treg cells were isolated from the spleens of CD45.1+ Foxp3-IRES-GFP knock-in mice and CD45.1+ Foxp3-IRES-GFP knock-in CIITATgPIV−/− mice using a BD FACSAria™ III Cell Sorter. The responder CD4+GFP− T cell population was collected from the spleens of CD45.2+ Foxp3-IRES-GFP knock-in mice by the same procedure as described above; the purity was usually greater than 95%. Sorted CD4+GFP− responder T cells (5.0 × 104) were labeled with carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes, USA) (0.5 μM) and cultured with Dynabeads® Mouse T-Activator CD3/CD28 (Gibco, USA) at a 1:1 ratio (beads-to-cells) in a 96-well round-bottomed culture plate. CD4+GFP+ Treg cells were added to culture wells at various ratios (1:1, 1:3, and 1:9). The experiments were performed in triplicate. After 72 h of co-culture, cells were harvested and stained with allophycocyanin (APC)-conjugated CD45.1 and then analyzed using BD FACSCalibur™ (Beckton Dickinson, USA).
Bone marrow chimeras
Recipient CIITATg PIV−/− mice were exposed to total body irradiation of 900 rad from a [137Cs] source in two split doses that were given 4 h apart. The mice were then rested for 24 h before being administered bone marrow cells. Total bone marrow cells were extracted from the femurs and tibiae of donor mice, and T cells were depleted by the magnetic sorting method (Miltenyi Biotech, USA). Each recipient mouse received 3 × 106 T-cell-depleted bone marrow cells in a volume of 300 ml of PBS via lateral tail vein injection, and the thymus and spleen were analyzed 8 weeks later.
Fetal thymic organ culture
On embryonic day 15.5 (E15.5), fetal thymuses from C57BL/6 mice were removed and cultured on polycarbonate filters (pore size, 0.8 mm; Millipore, USA) in RPMI 1640 medium supplemented with 10% fetal bovine calf serum (HyClone, USA), 1% penicillin and streptomycin (HyClone), and 50 nM 2-mercaptoethanol (Sigma, USA) in the presence or absence of mouse IL-4 (10 ng/ml; PeproTech, USA) and/or TGF-β1 (15 ng/ml; Cell Signaling, USA). Where appropriate, 1 μM SB431542 (selective inhibitor of TGF-β type 1 receptor kinases) or 10 μg/ml neutralizing antibody to TGf-β (1D11; R&D Systems, USA) was added once in 2 days. After 7 days, the thymuses were harvested and single-cell suspensions were prepared and analyzed for their CD4, CD8, Foxp3, and CD103 expression levels by flow cytometry.
Induction of allergic airway inflammation
C57BL/6 mice were sensitized i.p. with 100 μg of ovalbumin emulsified in 50 μl of PBS containing 2 mg of aluminum hydroxide (Thermo Fisher, USA) on days 0 and 7. Cells sorted as GFP+ Treg cells (2.0 × 105) were transferred to the sensitized mice i.v. 1 day before the initial intranasal challenge (on day 13). The challenge consisted of the intranasal administration of 50 μg of ovalbumin in 50 μl of PBS, and the challenge was performed for three consecutive days (from day 14 to day 16). Animals were anesthetized with isoflurane for pain relief. Twenty-four hours after the last challenge, mice were sacrificed for further analysis (on day 17).
Isolation of bronchoalveolar lavage fluid and lung cells
To obtain bronchoalveolar lavage (BAL) cells, the trachea was cannulated and the lungs were lavaged with 3 ml of PBS. The total and differential cell counts of BAL were determined using a hemocytometer and cytospin preparations stained with Hema-color® for microscopic analysis (Merck, Germany). After collecting BAL cells, the lungs were immediately removed, minced, and placed in 4 ml of complete RPMI 1640 containing 1 U/ml collagenase D and 1 mg/ml DNase I (Roche, USA); they were incubated at 37°C for 1 h with shaking at 200 rpm. After incubation, the cells were pelleted and treated with 2 ml of ACK lysis buffer to remove red blood cells. The remaining tissue was forced through a 70-μm cell strainer, washed thoroughly with complete RPMI 1640, and counted via trypan blue exclusion. Further analysis was sequentially performed.
Histological analysis
After collecting BAL cells, the lungs were infused with neutral buffered 10% formalin to approximate the normal state of inflation, and then harvested. The tissues were preserved in formalin for more than 24 h and then subjected to routine paraffin processing and hematoxylin and eosin staining
Statistical analysis
All data were analyzed using GraphPad Prism software (GraphPad Software, USA). Bar graphs show the percentage of each cell represented as the mean ± standard error of the mean (SEM), and the data were compared using an unpaired t-test.
RESULTS
The activated/memory phenotype Foxp3+CD4+ nTreg fraction was increased in mice with abundant PLZF+ innate T cells in their thymus
To investigate whether PLZF+ T-T CD4 T cells affect the development of Treg cells, we compared the Foxp3+CD4+ nTreg population in the thymuses of WT and CIITATg mice. As previously reported (Lee et al., 2010), the thymus of CIITATg mice contained abundant PLZF+ CD4 T cells that were selected by the MHC class II-dependent T-T interaction (Fig. 1A). We also used CIITATgPIV−/− mice in which most of the CD4 T cells are selected only by MHC class II-self peptide complexes that are presented by the other thymocytes; therefore, a much higher number of PLZF+ innate T cells were detected compared with those of CIITATg mice (Fig. 1A). When we counted the absolute number of thymic and splenic Foxp3+ Treg cells, we could not find any significant difference among WT, CIITATg and CIITATgPIV−/− mice (Fig. 1B). However, CIITATg and CIITATgPIV−/− mice were found to have larger fractions of CD103+ Treg cells than the WT mice (Fig. 1C). These findings indicate that there is a correlation between the number of PLZF+ innate T cells and the number of CD103+ Treg cells in both central and peripheral lymphoid organs.
Fig. 1.

The frequency of CD103+Foxp3+CD4+ naturally occurring (nTreg) cells increased in the presence of thymic PLZF+ innate T cells. Flow cytometric analysis of total thymocytes or splenocytes from 8-week-old WT, CIITATg, and CIITATgPIV−/− C57BL/6 mice assessing the PLZF (A) and Foxp3 (B) expression in CD4+ cells. Representative dot plots (left panel) and summarized results (right panel) are displayed. The numbers in quadrants indicate the percentages of cells. (C) The number of CD103 positive cells among thymic and splenic Foxp3+CD4+ T cells increased in CIITATg and CIITATgPIV−/− mice compared with that in WT mice. The numbers in the histogram (left panel) indicate the percentages of Treg cells that are CD103+ cells. Summarized data of the frequency of the CD103+ fraction of Foxp3+CD4+ cells are shown in the right panel. The bars indicate the group means, ns, not significant; *P < 0.05; **P < 0.001; ***P < 0.0001.
The α integrin, CD103, is a well-known marker for discriminating activated/memory-like subsets of Treg cells (Huehn et al., 2004). Unlike their CD103− counterparts, CD103+Foxp3+ Treg cells display an activated/memory-like phenotype, expressing multiple adhesion molecules and receptors for inflammatory chemokines. Consistent with these previous reports (Huehn et al., 2004; Stephens et al., 2007), we again confirmed that CD103+ Treg cells in WT mice had an activated/memory-like phenotype from the thymus based on the up-regulation of CD44, CD54, CD127 and inducible T-cell co-stimulator (ICOS) and down-regulation of CD62L (Fig. 2A). This expression pattern of surface molecules was also observed in CD103+ Treg cells of CIITATgPIV−/− mice. Next, we investigated the expression pattern of inhibitory surface molecules and tissue-homing chemokine receptors. Compared with CD103− Treg cells from WT mice, CD103+ Treg cells from either WT or CIITATgPIV−/− mice showed higher expression levels of inhibitory surface molecules (GITR, CD39, CD73 and CTLA-4) (Fig. 2B) and tissue-homing chemokine receptors (CXCR3, CCR4, and CCR5) (Fig. 2C) from the thymus. The reduced level of surface CCR7 also supported the activated/memory-like phenotype. All of these findings demonstrated that CD103+ Treg cells have similar expression patterns of surface molecules, regardless of their origin (WT or CIITATgPIV−/−). Therefore, the altered thymic environment due to the abundance of PLZF+ innate T cells only increased the number of CD103+ Treg cells from the thymus, preserving their well-documented activated/memory-like properties.
Fig. 2.
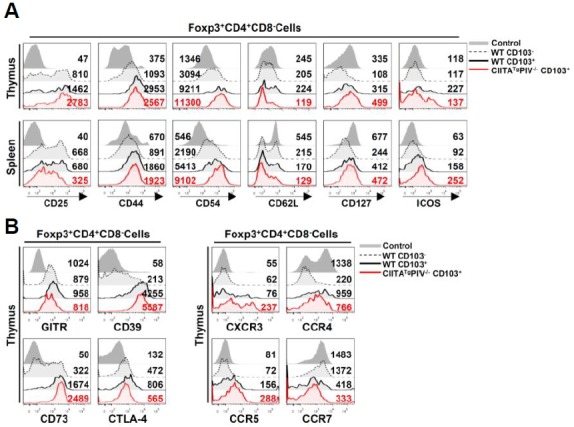
CD103+Foxp3+ regulatory T cells show the activated/memory-like phenotype. The expression patterns of markers associated with the activated/memory-like phenotype (A) were compared among four groups of cells; Foxp3−CD4+ non-Treg cells from WT mice (Control), CD103−Foxp3+CD4+ Treg cells from WT mice (WT CD103−), CD103+Foxp3+CD4+ Treg cells from WT mice (WT CD103+), and CD103+Foxp3+CD4+ Treg cells from CIITATgPIV−/− mice (CIITATgPIV−/− CD103+). These four groups of cells were identified in CD4 SP (CD4+CD8−) thymocytes and splenocytes via flow cytometric analysis after staining with antibodies against CD4, CD8, CD103 and Foxp3. In addition, molecules associated with inhibitory function (B) and chemotaxis (C) were also analyzed in Treg (WT CD103−, WT CD103+, CIITATgPIV−/− CD103+) and non-Treg (Control) cells from CD4 SP thymocytes of WT and CIITATgPIV−/− mice. Numbers in the histogram represent the median fluorescence intensity of surface molecules of the indicated cell population. Data are representative of three independent experiments.
IL-4 produced by thymic PLZF+ inate T cells enhances the generation of activated/memory-like Treg cells
Previous studies have demonstrated that in the thymus of CIITATg and CIITATgPIV−/− mice and itk−/− mice, PLZF+ innate T cells induced the generation of activated/memory-like CD8 (Min et al., 2011; Weinreich et al., 2010) and CD4 (Kang et al., 2015b; Prince et al., 2014a; 2014b) T cells in an IL-4-dependent manner. Based on this, we further investigated whether PLZF+ innate T cells also contributed to the development of activated/memory-like Treg cells in thymus of CIITATg mice. For this, Treg cells in the thymus of CIITATg mice were compared with those of CIITATgIL-4−/− mice as well as CIITATgPlzflu/lu, in which PLZF expression is genetically deficient. In this comparison, we found that the CIITATgPlzflu/lu or CIITATgIL-4−/− mice clearly showed a reduction in activated/memory-like Treg cell frequency compared with the CIITATg mice: it was basically reduced to the level of WT and IL-4−/− control mice (Fig. 3A). These findings strongly suggest a role of IL-4 produced by PLZF+ cells in the generation of activated/memory-like Treg cells in the thymus of CIITATg mice.
Fig. 3.
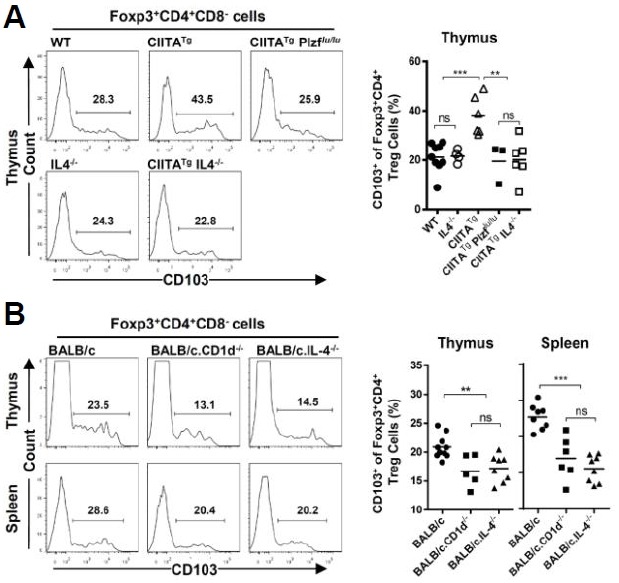
The generation of activated/memory-like regulatory T cells depends on IL-4 produced by thymic PLZF+ innate T cells. (A) CD103 expression profile from Treg cells (Foxp3+CD4+CD8−) in the thymus of WT, CIITATg, CIITATgPlzfflu/lu, IL-4−/− and CIITATgIL-4−/− C57BL/6 mice. Representative flow cytometry data (left panel) and a cumulative summary (right panel) of the percentage of CD103+ cells among Foxp3+CD4+ Treg cells are presented. Numbers in the histogram indicate the percentages of the CD103+ population among Treg cells. (B) Expression pattern of CD103 from Treg cells (Foxp3+CD4+CD8−) in the thymus and spleen of WT, CD1d−/− and IL-4−/− BALB/c mice. Representative flow cytometry data (left panel) and a cumulative summary (right panel) of the percentage of CD103+ cells among Foxp3+CD4+ Treg cells are presented. Numbers in the histogram indicate the percentages of the CD103+ population among Treg cells. ns, not significant; **P < 0.001; ***P < 0.0001.
Next, we evaluated whether IL-4 plays this role in WT mice. Among various strains of wild type mice, BALB/c strain is known to have a much higher number of PLZF+ innate T cells in the thymus than C57BL/6 mice (Lai et al., 2011; Lee et al., 2013). Most of the PLZF+ innate T cells in the BALB/c thymus are CD1d-dependent iNKT cells. Therefore, we compared the CD103+ Treg population in the thymus of WT BALB/c mice with that of BALB/c.CD1d−/− mice, in which iNKT cells could not develop. We also used BALB/c.IL-4−/− mice to assess the role of IL-4 in the development of CD103+ Treg cells in the BALB/c strain. Notably, we more frequently found activated/memory-like Treg cells from WT BALB/c mice than from BALB/c.CD1d−/− and BALB/c.IL-4−/− mice in both the thymus and the spleen (Fig. 3B). This also supports the previous finding from CIITATg mice that IL-4 produced by the increased number of PLZF+ innate T cells is set in motion to generate activated/memory-like Treg cells from the thymus.
TGF-β signaling is responsible for the generation of activated/memory-like Treg cells in the thymus
To determine whether IL-4 alone can induce the activated/memory-like phenotype of Treg cells, we performed fetal thymic organ culture (FTOC) in the presence of IL-4. It was shown that IL-4 alone was unable to induce this phenotype of Treg cells (Fig. 4A); instead, it reinforced the effect of TGF-β, which was reported to be a potent factor driving CD103 expression on conventional T cells (El-Asady et al., 2005; Hadley et al., 1999; Kilshaw and Murant, 1991; Robertson et al., 2001; Wang et al., 2004) as well as iTreg cells (Siewert et al., 2008). Moreover, this TGF-β-induced effect during FTOC was almost completely inhibited by a specific inhibitor of activin-like kinase 5 (ALK5) (SB-431542) and TGF-β neutralizing antibody (1D11) (Fig. 4B), confirming the capacity of TGF-β to induce the expression of CD103 on Treg cells during their ontogeny. Then, to validate whether TGF-β signaling is actually responsible for the increased CD103+ Treg cells in vivo, bone marrow chimeras were established. Based on the fact that Smad proteins are major mediators of intracellular TGF-β signaling (Li and Flavell, 2008), mixed bone marrow cells from CD45.1+ CIITATg mice and CD45.2+ WT or Smad4−/− mice were transferred to irradiated CD45.1/CD45.2+ CIITATgPIV−/− mice, in which both WT and Smad4−/− thymocytes can be positively selected by MHC class II-expressing double positive thymocytes. Flow cytometry analysis was performed 8 weeks after bone marrow transfer, and the CD103+ fraction of Smad4−/− Treg cells was compared with that of WT bone marrow-derived Treg cells. As shown in Fig. 4C, the frequency of CD103+ Treg cells was markedly lower in the Smad4−/− compartment than that from CIITATg cells, while there was no significant difference in the frequency of CD103+ cells between Treg cells derived from WT and CIITATg bone marrow cells. These findings indicate that TGF-β signaling is important for the generation of activated/memory-like Treg cells in an environment where there are abundant PLZF+ innate T cells. Taking these findings together, we suggest that TGF-β is indispensable for the activated/memory-like phenotype of nTreg cells in cooperation with IL-4.
Fig. 4.

IL-4 augments the TGF-β-induced acquisition of the activated/memory-like phenotype of Treg cells. (A) Fetal thymuses from E15.5 C57BL/6 mice were cultured in the presence or absence of murine IL-4, murine TGF-β1 or both for 7 days. Two to three fetal thymic lobes were used for each condition, and pooled cells were analyzed by flow cytometry. The representative FACS data (left panel) and calculated frequency of CD103+ cells among Foxp3+ CD4 SP (CD4+CD8−) thymocytes are displayed as a cumulative summary (right panel). Numbers in quadrants indicate the percentage of cells and bars in the summary indicate the mean value. (B) TGF-β receptor signaling is necessary to induce the CD103 expression of Treg cells. FTOC was performed with murine TGF-β1 for 7 days in the presence or absence of an ALK5 chemical inhibitor (SB-431542) or TGF-β1 blocking antibodies. Representative FACS data of CD4 SP thymocytes (left panel) and their cumulative summary (right panel) are shown. Numbers in quadrants indicate the percentages of cells and bars in the summary indicate the mean value. (C) T cell-depleted bone marrow cells from CD45.1+ CIITATg were mixed with those of WT or Smad4−/− C57BL/6 mice, respectively, at a 1:1 ratio. The mixed bone marrow cells were transferred to lethally irradiated CD45.1/CD45.2+ CIITATgPIV−/− recipients via intravenous injection. At 6–7 weeks after the transfer, the thymocytes and splenocytes of bone marrow chimera were analyzed by flow cytometry: CD45.1+ CD45.2− cells were considered to be generated from CIITATg bone marrow and CD45.22-cells thymocytes from WT or Smad4−/− bone marrow. CD103+ fractions among Foxp3+CD4+ Treg cells were compared between CIITATg and WT or Smad4−/− populations in individual mice. •, CIITATg+ WT ➔ CIITATgPIV−/−, n = 5; ○, CIITATg + Smad4−/− 4 ➔CIITATg PIV−/−, n = 6. ns, not significant; *P < 0.05; ***P < 0.0001.
Treg cells of CIITATgPIV−/− mice exhibited enhanced suppressive activity during airway inflammation
To determine whether the activated/memory-like phenotype of Treg cells from CIITATgPIV−/− mice is directly related to their suppressive function, an in vitro suppressive assay was performed following a previously described method (Collison and Vignali, 2011). As expected, Treg cells of CIITATgPIV−/− mice suppressed the proliferation of effector T cells in a dose-dependent manner, indicating their competence for performing suppression. Treg cells from CIITATgPIV−/− mice suppressed effector T cell proliferation more efficiently under 1:1 (Teff:Treg) culture conditions than WT Treg cells. However, they did not show higher suppressive activity under conditions of fewer Treg cells than effector T cells (Fig. 5A). It has been reported that CD103+ Treg cells are not superior regarding the suppression of effector T cell proliferation under some in vitro culture conditions compared with the CD103− subset (Chang et al., 2012; Stephens et al., 2007). Therefore, we decided to assess the in vivo suppressive function of Treg cells from CIITATgPIV−/− mice using the ovalbumin-induced airway hypersensitivity model based on a previous report describing that Treg cells accumulate and attenuate the inflammatory response in the lungs of ovalbumin-induced allergic mice in a CCR4-dependent manner (Faustino et al., 2013; Saito et al., 2008). C57BL/6 mice were first sensitized by an intraperitoneal injection of ovalbumin emulsified in an aluminum-based adjuvant on days 0 and 7. Then, 2 × 105 Treg cells isolated from Foxp3-GFP knock-in C57BL/6 or CIITATgPIV−/− mice were transferred to the sensitized mice 1 day before the first intranasal ovalbumin challenge. After 3 days of consecutive intranasal ovalbumin challenge, the severity of airway inflammation was assessed 24 h after the final ovalbumin challenge (on day 17). Differential BAL cell counts revealed an intense influx of immune cells, including eosinophils, neutrophils, macrophages and lymphocytes, into the airways of mice exposed to ovalbumin compared with that in intact mice (Fig. 5B). Flow cytometry analysis of the ovalbumin-challenged lung showed the severe inflammatory status, with the percentage of infiltrated eosinophils being six times higher (CD11c− MHCII− Siglec-Fhi-Gr-1int cells; Fig. 5C) than in PBS-challenged control lung. Intriguingly, Treg cells from Foxp3-GFP knock-in CIITATgPIV−/− mice significantly protected the infiltration of eosinophils and neutrophils into both the BAL and the lung, whereas Treg cells of Foxp3-GFP knock-in C57BL/6 mice did not show any significant protection in this disease model (Figs. 5B and 5C). Additionally, histological analysis again clearly supported the attenuated cellular infiltration in the lungs of the mice that received Treg cells from Foxp3-GFP knock-in CIITATgPIV−/− mice (Fig. 5D). All of these findings strongly suggest that Treg cells from CIITATgPIV−/− mice have more potent in vivo suppressive activity on Th2-mediated allergic airway inflammation than Treg cells from WT mice.
Fig. 5.
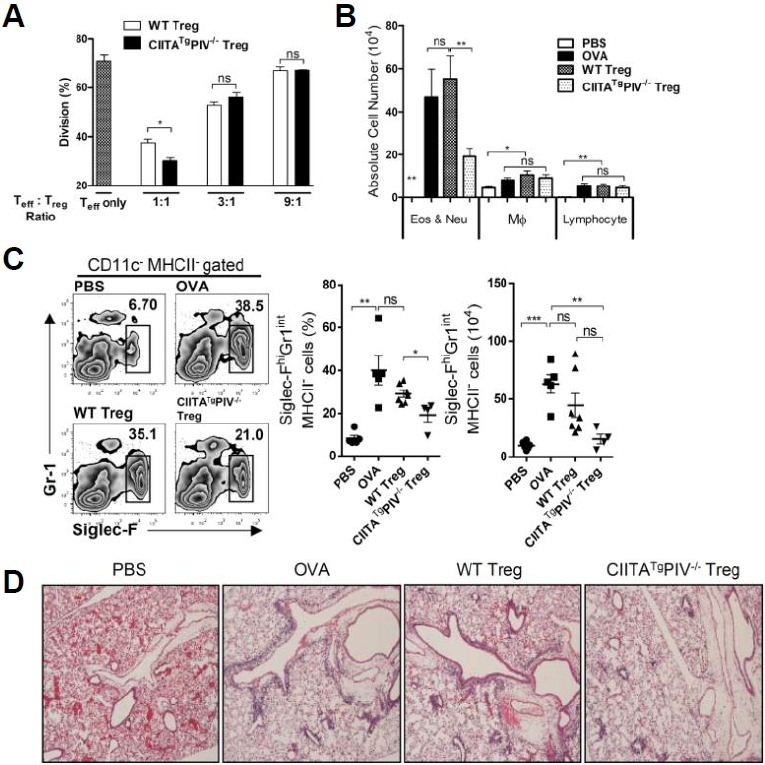
Airway inflammation is efficiently suppressed by Treg cells from the CIITATgPIV−/− mice. (A) CFSE-labeled CD4+GFP− effector T cells (Teff) isolated from Foxp3-GFP knock-in mice were mixed with CD4+GFP+ Treg cells from CD45.1 Foxp3-GFP-knock-in WT or CIITATgPIV−/− C57BL/6 mice at various ratios (Teff only, or Teff:Treg 1:1, 3:1, and 9:1), and stimulated with anti-CD3/CD28 microbeads (bead-to-cell ratio, 1:1) for 3 days. Proliferation was assessed by measuring the dilution of CFSE in CD45.1− effector T cells by flow cytometry. A representative result from three independent experiments is shown. (B–D) The ovalbumin-sensitized C57BL/6 mice at days 0 and 7, were treated with 2.0 × 105 of CD4+GFP+ Treg cells isolated from CD45.1+Foxp3-GFP knock-in WT or CIITATgPIV−/− C57BL/6 mice 1 day before three consecutive intranasal doses of ovalbumin (on days 1416). These mice were sacrificed on day 17, and we obtained differential counts of cytospin preparations from BAL cells (B), flow cytometry data of lung eosinophils (C), and lung histology sections stained with hematoxylin/eosin for the analysis of cellular inflammation (D). For the eosinophil count, cells harvested from lungs were stained for I-Ab, CD11c, Siglec-F, and Gr-1, and the Siglec-FhiGr-1int fraction in I-Ab−CD11c− cells was analyzed (C). Numbers in boxes indicate the percentages of eosinophils. The cumulative data of the percentages (middle panel) and absolute numbers (right panel) of eosinophils from the lung are shown. Bars indicate the mean value ± SEM. ns, not significant; *P < 0.05; **P < 0.001; ***P < 0.0001. Original magnification of lung photographs, ×40.
DISCUSSION
This study revealed a synergistic role of IL-4 produced by PLZF+ innate T cells in the process of TGF-β-dependent intrathymic generation of activated/memory-like Foxp3+ Treg cells. It is generally accepted that naturally occurring Foxp3+ Treg cells acquire an activated/memory-like phenotype when they encounter a specific antigen in the periphery (Horwitz et al., 2008; Zhao et al., 2008). However, there are several lines of evidences showing that a small number of CD103+ Treg cells with the activated/memory-like phenotype still develop from the WT thymus (Annacker et al., 2005; Stephens et al., 2007), which was reconfirmed in the present study (Fig. 1). What we additionally showed in this paper was that more Treg cells in CIITATgPIV−/−, CIITATg and Wt BALB/c mice than in their counterparts, developed with the activated/memory-like phenotype (high expression of CD44, CD54, CD103, CD127 and ICOS, and lower expression of CD62L; Fig. 2). As previously reported, PLZF+ innate T cells were strongly responsible for creating the IL-4 rich conditions (Alonzo and Sant’Angelo, 2011; Lai et al., 2011; Lee et al., 2009; Min et al., 2011; Weinreich et al., 2010). The significant reduction in the number of CD103+ activated/memory-like Treg cells shown in CIITATgPlzflu/lu and CIITATgIL-4−/− mice, compared with that in CIITATg mice, demonstrates the importance of both PLZF+ innate T cells and IL-4 for the acquisition of this phenotype (Fig. 3A). This was also the case in BALB/c.CD1d−/−, BALB/c.IL-4−/− and BALB/c mice, indicating that the IL-4-dependent effect has physiological relevance (Fig. 3B).
Several groups previously reported the effects of IL-4 on the development and function of Treg cells (Dardalhon et al., 2008; Maerten et al., 2005; Skapenko et al., 2005; Wei et al., 2007). IL-4 partially prevents the apoptosis of nTreg cells and preserves their suppressive ability by maintaining the mRNA levels of Foxp3 and CD25 (Maerten et al., 2005). In the case of iTreg cell generation, the role of IL-4 is somewhat controversial. One group suggested that IL-4 and IL-13 play important roles in the extrathymic generation of Foxp3+CD25+ Treg cells via an antigen-dependent process (Skapenko et al., 2005), while other groups reported that IL-4 inhibits the TGF-β mediated generation of Treg cells (Dardalhon et al., 2008; Wei et al., 2007). In the present study, we reported on the peculiar role of IL-4 in the intrathymic acquisition of the activated/memory-like phenotype of Treg cells. Interestingly, similar to the intrinsic property of TGF-β to induce CD103 molecules in activated T cells (El-Asady et al., 2005; Mackay et al., 2013), the enhanced CD103 expression during the intrathymic development of nTreg cells in CIITATg and CIITATgPIV−/− mice was also dependent on TGF-β (Fig. 4C). IL-4 alone could not induce this effect, but it had a synergistic effect on TGF-β during this process, as shown in FTOC (Figs. 4A and 4B).
In our ex vivo FTOC, CD103 expression was increased in both Foxp3+ and Foxp3− CD4 T cell populations. During thymocyte development, the cooperation of TGF-β and Runx3 induces CD103 expression in CD8 single positive (SP) thymocytes and CD4+ Treg cells (Grueter et al., 2005). The main regulator of Runx3 expression in CD8 SP thymocytes is IL-7, but IL-4 has also been shown to induce Runx3 expression in these cells (Park et al., 2010). Moreover, we recently found that IL-4 produced by PLZF+ innate T cells could induce the expression of Runx3 in CD4-lineage thymocytes with low-affinity TCR (Kang et al., 2015b). Taking these findings, IL-4 seemed to enhance the TGF-β dependent CD103 expression in Foxp3− thymocytes with low-affinity TCR as well as Treg cells via the induction of Runx3 expression.
We used CD103 as a major marker of activated/memory-like Treg cells. Murine Treg cells can be divided into two subsets based on their CD103 expression (Banz et al., 2003; Huehn et al., 2004; Lehmann et al., 2002) CD103−/− Treg cells resemble naïve T cells, recirculating through the lymph nodes. In contrast, the CD103+ Treg cell subset has the typical features of activated/memory-like cells. Regarding suppressive activity, CD103+ activated/memory-like Treg cells were initially reported to be more potent suppressors, both in vitro and in vivo, than CD103− Treg cells (Huehn et al., 2004). However, in subsequent studies, CD103+ Treg cells showed no difference in the suppression of effector T cell proliferation and cytokine production in vitro compared with the CD103− subset, when anti-CD3 antibodies were used as a polyclonal stimulator (Chang et al., 2012; Stephens et al., 2007). Consistent with these reports, Treg cells from CIITATgPIV−/− mice attenuated the severity of ovalbumin-induced airway inflammation more efficiently than their WT counterparts, while an in vitro suppressive assay showed significantly different suppression between the two types of Treg cell only under culture conditions with high Treg cell numbers. Unlike in the in vitro system, the in vivo activity of Treg cells depends on their capacity to migrate into inflamed sites (Chang et al., 2012; Faustino et al., 2013). In skin inflammation model, only CD103+ Treg cells efficiently migrated into inflamed skin (Huehn et al., 2004), and the in vivo suppressor function of tumor-derived activated/memory-like Treg cells depended on CCR5 expression (Chang et al., 2012). Moreover, a high level of CCR4 expression in activated Treg cells was indispensable for attenuating allergic airway inflammation (Faustino et al., 2013). Consistent with this, Treg cells from CIITATgPIV−/− mice also had a higher expression level of tissue-homing chemokine receptors, such as CXCR3, CCR5 and CCR4, than those from WT mice (Fig. 2C).
PLZF+ innate T cells have received considerable attention because of their unique intrathymic selection through the T-T interaction and pleiotropic production of cytokines as well as their role in the intrathymic generation of effector/memory type CD8 T cells (Lai et al., 2011; Min et al., 2011; Weinreich et al., 2010) and CD4 T cells (Kang et al., 2015b; Prince et al., 2014a; 2014b). In this study, we demonstrated that PLZF+ innate T cells also contribute to the acquisition of the activated/memory phenotype in nTreg cells in WT BALB/c and CIITATg mice. Taking these findings, PLZF+ innate T cells modulate the intrathymic development of both effector and Treg cells by producing IL-4.
Acknowledgments
We thank H. Acha-Orbea (University of Lausanne, Switzerland) for providing the PIV−/− mice and Dr. A. Rudensky (University of Washington, USA) for the Foxp3-IRES-GFP knock-in mice. This work was sponsored by Support for Creative-Pioneering Researchers through Seoul National University in 2014, and also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI13C0954).
REFERENCES
- Alonzo E.S., Sant’Angelo D.B. Development of PLZF-expressing innate T cells. Curr. Opin. Immunol. 2011;23:220–227. doi: 10.1016/j.coi.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annacker O., Coombes J.L., Malmstrom V., Uhlig H.H., Bourne T., Johansson-Lindbom B., Agace W.W., Parker C.M., Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J. Exp. Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banz A., Peixoto A., Pontoux C., Cordier C., Rocha B., Papiernik M. A unique subpopulation of CD4+ regulatory T cells controls wasting disease, IL-10 secretion and T cell homeostasis. Eur. J. Immunol. 2003;33:2419–2428. doi: 10.1002/eji.200324205. [DOI] [PubMed] [Google Scholar]
- Bayer A.L., Yu A.X., Malek T.R. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J. Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- Bird L. Regulatory T cells nurtured by TGFβ. Nat. Rev. Immunol. 2010;10:466–466. doi: 10.1038/nri2812. [DOI] [PubMed] [Google Scholar]
- Burchill M.A., Yang J.Y., Vogtenhuber C., Blazar B.R., Farrar M.A. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- Chang L.Y., Lin Y.C., Kang C.W., Hsu C.Y., Chu Y.Y., Huang C.T., Day Y.J., Chen T.C., Yeh C.T., Lin C.Y. The indispensable role of CCR5 for in vivo suppressor function of tumor-derived CD103+ effector/memory regulatory T cells. J. Immunol. 2012;189:567–574. doi: 10.4049/jimmunol.1200266. [DOI] [PubMed] [Google Scholar]
- Choi E.Y., Park W.S., Jung K.C., Chung D.H., Bae Y.M., Kim T.J., Song H.G., Kim S.H., Ham D.I., Hahn J.H., et al. Thymocytes positively select thymocytes in human system. Hum. Immunol. 1997;54:15–20. doi: 10.1016/s0198-8859(97)00012-8. [DOI] [PubMed] [Google Scholar]
- Choi E.Y., Jung K.C., Park H.J., Chung D.H., Song J.S., Yang S.D., Simpson E., Park S.H. Thymocyte-thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity. 2005;23:387–396. doi: 10.1016/j.immuni.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Collison L.W., Vignali D.A. In vitro Treg suppression assays. Methods Mol. Biol. 2011;707:21–37. doi: 10.1007/978-1-61737-979-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V., Awasthi A., Kwon H., Galileos G., Gao W., Sobel R.A., Mitsdoerffer M., Strom T.B., Elyaman W., Ho I.C., et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Asady R., Yuan R., Liu K., Wang D., Gress R.E., Lucas P.J., Drachenberg C.B., Hadley G.A. TGF-β-dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J. Exp. Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino L., da Fonseca D.M., Takenaka M.C., Mirotti L., Florsheim E.B., Guereschi M.G., Silva J.S., Basso A.S., Russo M. Regulatory T cells migrate to airways via CCR4 and attenuate the severity of airway allergic inflammation. J. Immunol. 2013;190:2614–2621. doi: 10.4049/jimmunol.1202354. [DOI] [PubMed] [Google Scholar]
- Gottschalk R.A., Corse E., Allison J.P. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter B., Petter M., Egawa T., Laule-Kilian K., Aldrian C.J., Wuerch A., Ludwig Y., Fukuyama H., Wardemann H., Waldschuetz R., et al. (2005) Runx3 regulates integrin αE/CD103 and CD4 expression during development of CD4−/CD8+ T cells. J. Immunol. 175:1694–1705. doi: 10.4049/jimmunol.175.3.1694. [DOI] [PubMed] [Google Scholar]
- Hadley G.A., Rostapshova E.A., Gomolka D.M., Taylor B.M., Bartlett S.T., Drachenberg C.I., Weir M.R. Regulation of the epithelial cell-specific integrin, CD103, by human CD8+ cytolytic T lymphocytes. Transplantation. 1999;67:1418–1425. doi: 10.1097/00007890-199906150-00005. [DOI] [PubMed] [Google Scholar]
- Horwitz D.A., Zheng S.G., Gray J.D. Natural and TGF-β-induced Foxp3+CD4+ CD25+ regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Huehn J., Siegmund K., Lehmann J.C., Siewert C., Haubold U., Feuerer M., Debes G.F., Lauber J, Frey O, Przybylski G.K., et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J. Exp. Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.H., Min H.S., Lee Y.J., Choi B., Kim E.J., Lee J., Kim J.R., Cho K.H., Kim T.J., Jung K.C., et al. Analyses of the TCR repertoire of MHC class II-restricted innate CD4+ T cells. Exp. Mol. Med. 2015a;47:e154. doi: 10.1038/emm.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.H., Park H.J., Yum H.I., Park S.P., Park J.K., Kang E.H., Lee J.I., Lee E.B., Park C.G., Jung K.C., et al. Thymic low affinity/avidity interaction selects natural Th1 cells. J. Immunol. 2015b;194:5861–5871. doi: 10.4049/jimmunol.1401628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karecla P.I., Bowden S.J., Green S.J., Kilshaw P.J. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin αM290 β7 (αEβ7) Eur. J. Immunol. 1995;25:852–856. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- Kilshaw P.J., Murant S.J. Expression and regulation of β-7 (β-P) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur. J. Immunol. 1991;21:2591–2597. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- Lai D., Zhu J., Wang T., Hu-Li J., Terabe M., Berzofsky J.A., Clayberger C., Krensky A.M. KLF13 sustains thymic memory-like CD8+ T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. J. Exp. Med. 2011;208:1093–1103. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Jung K.C., Park S.H. MHC class II-dependent T-T interactions create a diverse, functional and immunoregulatory reaction circle. Immunol. Cell. Biol. 2009;87:65–71. doi: 10.1038/icb.2008.85. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Jeon Y.K., Kang B.H., Chung D.H., Park C.G., Shin H.Y., Jung K.C., Park S.H. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. J. Exp. Med. 2010;207:237–246. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.J., Holzapfel K.L., Zhu J., Jameson S.C., Hogquist K.A. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann J., Huehn J., de la Rosa M., Maszyna F., Kretschmer U., Krenn V., Brunner M., Scheffold A., Hamann A. Expression of the integrin αEβ7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc. Natl. Acad. Sci. USA. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.O., Flavell R.A. TGF-β: A master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Kim M.G., Gourley T.S., McCarthy B.P., Sant’Angelo D.B., Chang C.H. An alternate pathway for CD4 T cell development: thymocyte-expressed MHC class II selects a distinct T cell population. Immunity. 2005;23:375–386. doi: 10.1016/j.immuni.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Li W., Sofi M.H., Rietdijk S., Wang N., Terhorst C., Chang C.H. The SLAM-Associated protein signaling pathway is required for development of CD4+ T cells selected by homotypic thymocyte interaction. Immunity. 2007;27:763–774. doi: 10.1016/j.immuni.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lio C.W., Hsieh C.S. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang P., Li J., Kulkarni A.B., Perruche S., Chen W. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- Mackay L.K., Rahimpour A., Ma J.Z., Collins N., Stock A.T., Hafon M.L., Vega-Ramos J., Lauzurica P., Mueller S.N., Stefanovic T., et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Maerten P., Shen C., Bullens D.M., Van Assche G., Van Gool S., Geboes K., Rutgeerts P., Ceuppens J.L. Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. J. Auto-immun. 2005;25:112–120. doi: 10.1016/j.jaut.2005.04.001. [DOI] [PubMed] [Google Scholar]
- McHugh R.S., Whitters M.J., Piccirillo C.A., Young D.A., Shevach E.M., Collins M., Byrne M.C. CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Min H.S., Lee Y.J., Jeon Y.K., Kim E.J., Kang B.H., Jung K.C., Chang C.H., Park S.H. MHC Class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T Cells. J. Immunol. 2011;186:5749–5757. doi: 10.4049/jimmunol.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang W., Beckett O., Ma Q., Li M.O. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Bae Y.M., Kim T.J., Ha I.S., Kim S., Chi J.G., Lee S.K. HLA-DR expression in human fetal thymocytes. Hum. Immunol. 1992;33:294–298. doi: 10.1016/0198-8859(92)90338-n. [DOI] [PubMed] [Google Scholar]
- Park J.H., Adoro S., Guinter T., Erman B., Alag A.S., Catalfamo M., Kimura M.Y., Cui Y., Lucas P.J., Gress R.E., et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat. Immunol. 2010;11:257–264. doi: 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A.L., Kraus Z., Carty S.A., Ng C., Yin C.C., Jordan M.S., Schwartzberg P.L., Berg L.J. 2014a doi: 10.4049/jimmunol.1302059. Alonzo, E.S., and Sant’Angelo, D.B. (2011). Development of PLZF-expressing innate T cells. Curr. Opin. Immunol. 23, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A.L., Watkin L.B., Yin C.C., Selin L.K., Kang J., Schwartzberg P.L., Berg L.J. Innate PLZF+CD4+ αβ T cells develop and expand in the absence of Itk. J. Immunol. 2014b;193:673–687. doi: 10.4049/jimmunol.1302058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P.E., Petrone A.L., Ponath P.D. Differentiation and expansion of T cells with regulatory function from human peripheral lymphocytes by stimulation in the presence of TGF-β. J. Immunol. 2005;174:1446–1455. doi: 10.4049/jimmunol.174.3.1446. [DOI] [PubMed] [Google Scholar]
- Robertson H., Wong W.K., Talbot D., Burt A.D., Kirby J.A. Tubulitis after renal transplantation: demonstration of an association between CD103+ T cells, transforming growth factor β1 expression and rejection grade. Transplantation. 2001;71:306–313. doi: 10.1097/00007890-200101270-00024. [DOI] [PubMed] [Google Scholar]
- Saito K., Torii M., Ma N., Tsuchiya T., Wang L., Hori T., Nagakubo D., Nitta N., Kanegasaki S., Hieshima K., et al. Differential regulatory function of resting and preactivated allergen-specific CD4+ CD25+ regulatory T cells in Th2-type airway inflammation. J. Immunol. 2008;181:6889–6897. doi: 10.4049/jimmunol.181.10.6889. [DOI] [PubMed] [Google Scholar]
- Siewert C., Lauer U., Cording S., Bopp T., Schmitt E., Hamann A., Huehn J. Experience-driven development: effector/memory-like αE+Foxp3+ regulatory T cells originate from both naive T cells and naturally occurring naive-like regulatory T cells. J. Immunol. 2008;180:146–155. doi: 10.4049/jimmunol.180.1.146. [DOI] [PubMed] [Google Scholar]
- Skapenko A., Kalden J.R., Lipsky P.E., Schulze-Koops H. The IL-4 receptor α-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25− CD4+ precursors. J. Immunol. 2005;775:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- Stephens G.L., Andersson J., Shevach E.M. Distinct subsets of FoxP3+ regulatory T cells participate in the control of immune responses. J. Immunol. 2007;178:6901–6911. doi: 10.4049/jimmunol.178.11.6901. [DOI] [PubMed] [Google Scholar]
- Treiner E., Lantz O. CD1d- and MR1-restricted invariant T cells: of mice and men. Curr. Opin. Immunol. 2006;18:519–526. doi: 10.1016/j.coi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vignali D.A.A., Collison L.W., Workman C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Yuan R., Feng Y., El-Asady R., Farber D.L., Gress R.E., Lucas P.J., Hadley G.A. Regulation of CD103 expression by CD8+ T cells responding to renal allografts. J. Immunol. 2004;172:214–221. doi: 10.4049/jimmunol.172.1.214. [DOI] [PubMed] [Google Scholar]
- Wei J., Duramad O., Perng O.A., Reiner S.L., Liu Y.J., Qin F.X. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc. Nat’l. Acad. Sci. USA. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich M.A., Odumade O.A., Jameson S.C., Hogquist K.A. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat. Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Zhang C., Yi T., Lin C.L., Todorov I., Kandeel F., Forman S., Zeng D. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112:2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]


