Abstract
Usher Syndrome (USH) is a rare disease with hearing loss, retinitis pigmentosa and, sometimes, vestibular dysfunction. A phenotype heterogeneity is reported. Recent evidence indicates that USH is likely to belong to an emerging class of sensory ciliopathies. Olfaction has recently been implicated in ciliopathies, but the scarce literature about olfaction in USH show conflicting results. We aim to evaluate olfactory impairment as a possible clinical manifestation of USH. Prospective clinical study that included 65 patients with USH and 65 normal age-gender-smoking-habits pair matched subjects. A cross culturally validated version of the Sniffin’ Sticks olfaction test was used. Young patients with USH have significantly better olfactory scores than healthy controls. We observe that USH type 1 have a faster ageing olfactory decrease than what happens in healthy subjects, leading to significantly lower olfactory scores in older USH1 patients. Moreover, USH type 1 patients showed significantly higher olfactory scores than USH type 2, what can help distinguishing them. Olfaction represents an attractive tool for USH type classification and pre diagnostic screening due to the low cost and non-invasive nature of the testing. Olfactory dysfunction should be considered among the spectrum of clinical manifestations of Usher syndrome.
Usher syndrome (USH) is a heterogeneous, rare, multisystemic genetic disease, characterized by sensorineural hearing loss, retinitis pigmentosa (RP), and variable vestibular dysfunction. Its prevalence ranges from 3 to 8.0 per 100,000 people1.
Patients with Usher syndrome (USH) often display a complex array of signs and symptoms. The visual, auditory, and vestibular manifestations are well documented, while additional manifestations of the condition have not received much attention. USH is classified into three clinical types2. USH type 1 (USH1) is associated with severe-to-profound sensorineural congenital deafness, vestibular areflexia, and prepuberal onset of RP. USH type 2 (USH2) is characterized by moderate-to-severe hearing impairment, no vestibular impairment, and onset of RP in the first or second decade of life. In type 3 (USH3), the least prevalent type of USH, the post lingual hearing loss onset is progressive while RP and vestibular dysfunction onset is variable3.
While auditory and vestibular testing helps to differentiate among USH types, overlapping and atypical presentations have been described for all three types of Usher syndrome4,5,6,7,8. Specific genetic patterns are associated with each USH phenotype, even while genetic heterogeneity has been reported9. A genotype-phenotype correlation would help to guide genetic evaluation or even make it unnecessary if a perfect correlation is achieved. Next generation sequencing provides a useful tool to reveal clinical misdiagnosis, identify late onset syndromic features, establish novel genotype/phenotype correlations, and to facilitate clinical diagnosis10,11. Yet, our group has observed 10% of cases manifesting atypical presentations (unpublished data).
Improving phenotype may help improving USH diagnosis and genotype-phenotype correlation. Olfaction may be an important link in USH. Nevertheless, very few papers studied olfaction in patients with USH. Seeliger et al.12 studied 8 USH1 and 31 USH2 patients with Sniffin’ Sticks test13. Although no significant olfactory differences were found, it is interesting to observe that patients with USH1 were 20–40 years old and showed a median olfactory score higher than control group (9.7 vs 8.5). They also found a far more rapid decline in olfactory threshold of USH1 that was not explained by normal ageing. Zrada et al.14 observed 22 patients (8 USH1 and 22 USH2), by means of UPSIT age- gender- smoking-habit- matched controls15. Olfactory thresholds were higher than those of controls, although statistical significance was not attained, probably because the study was not adequately powered. In another study, Marietta et al.16 studied the olfactory acuity in a subgroup of USH1C patients. They used UPSIT test in 18 older patients, not pair matched. Although an age decline in olfaction appeared to be greater than normative data, the difference was not significant16.
Patients with ciliopathies affecting the inner ear are frequently deaf and/or persons who exhibit balance difficulties; and patients with retinal ciliopathies often become blind17. Genetics, biochemistry and proteomic approaches have demonstrated that USH proteins are organized in networks in both the eye and the inner ear18. Consequently, a disease that have both systems affected like USH is expectable to be a sensory ciliopathy18. Furthermore, USH has been associated with bronchiectasis, chronic sinusitis, reduced nasal mucociliary clearance19,20 and altered sperm function21, all of which are suggestive of ciliary dyskinesia19.
In recent years a number of papers helped to elucidate the relationship between olfaction and its genetic underpinnings since the pioneering work of Buck and Axel, 199122. Because recent evidence implicated olfaction in sensory ciliopathies18,23,24,25,26,27,28, it is expectable that olfaction is also implicated in USH, however, the few previous studies addressing functional olfactory acuity in patients with USH have showed conflicting results12,14,29.
AIM
Knowing that Usher syndrome is a ciliopathy from a histopathological and molecular standpoint, we have undertaken a prospective recruitment of patients with USH to study their functional olfactory features. This study aimed at identifying and characterizing putative differences in olfactory capacity between patients with USH and controls, as well as among the subtypes of USH.
Patients and Methods
All participants provided informed written consent. The study followed the Declaration of Helsinki 2013 on Biomedical Research Involving Human Participants and was approved by the Ethics Committee of the Faculty of Medicine, University of Coimbra, Portugal.
Sixty-five patients with USH (49.2 ± 15.1 years old; 18 females and 47 males) were prospectively recruited from the Otorhinolaryngology consultation of the Centro Hospitalar e Universitário de Coimbra, Portugal. They were pair-matched with 65 healthy controls (controls 49.2 ± 14.9 years old) for age, sex, and smoking habits, so that a total of 130 participants were studied.
All participants received a full ENT clinical examination, as well as a structured interview, a rhinologic examination including nasal endoscopy and a standardized Sniffin’ Sticks test (Burghart GmbH, Wedel, Germany) culturally validated to the Portuguese population (SnSt-pt)30. Briefly, this test comprised three subtests, namely odor threshold (T, tested by means of a single staircase procedure), odor discrimination (D, 3-alternative forced choice) and odor identification (I, 4-alternative forced choice). Results of the 3 subtests are typically summed up and presented as a composite TDI score, as review by Hummel et al.31.
Eligible patients had a documented neurosensory hearing loss and RP, fulfilling the clinical criteria for USH1 or USH2, as defined by the USH consortium2. Exclusion criteria included participants with known factors affecting olfaction, such as post-traumatic olfactory dysfunction, sinonasal disease, malignant tumor, recent radiotherapy or chemotherapy, post-upper respiratory tract infection, use medication known to interfere in olfaction32, and Parkinson´s and Alzheimer´s disease.
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS), version 22.0 (SPSS, Inc., Chicago, IL). The normality of continuous variables was tested with the Kolmogorov-Smirnov test. Age is displayed as mean and standard deviation. Olfactory data is presented as median and interquartile range. The Mann-Whitney U test or Student’s t-test test was used whichever appropriate. In spite of the main measure being the Sniffin’ Sticks TDI scores, and though it would be justifiable not to perform multiple comparisons adjustment in planned comparisons using each one of the partial measures (T, D, and I sub-scales of the Sniffin’ Sticks TDI scale), the fact is that those sub-scales are not perfectly independent. As a result, the multiple comparisons problem arises, and is sorted out through the Holm’s correction, which controls the Family-Wise Error Rate, and the Benjamini-Hosper correction, which controls the False Discovery Rates. Spearman´s rank correlation coefficient was calculated to verify the relationship between subgroups tested for olfaction. Multiple linear regression with previous bootstrapping was used to correlate olfaction in relation to age and Usher subtype. All tests were two-tailed and statistical significance was accepted at the p < 0.05 level.
Results
Sixty-five USH patients (49.2 ± 15.1 years old; 18 females/47 males) were pair-matched with 65 healthy controls (49.2 ± 14.9 years old; 18 f/47 m).
Patients with USH included 22 USH1 (43.4 ± 16.7 [18–77] years old; 5 f/17 m) that were compared to 22 healthy pair matched controls (43.3 ± 16.1 years old; 5 f/17 m), and another 43 USH2 (52.2 ± 13.5 years old; 13 f/30 m) that were pair matched to 43 healthy controls (52.2 ± 13.5 [32–78] years old; 13 f/30 m).
Total USH patients’ vs controls
USH patients′ olfactory scores were compared to pair matched controls. Patients with USH had significantly better SnSt- composite TDI olfaction scores than healthy controls (32.8 [28.5–37.4]) vs (30.0 [28.6–31.3]), (p = 0.002). Both USH1 and USH2 showed better discrimination scores than controls, with large standardized effect sizes (Table 1).
Table 1. Sniffin’ Sticks Portuguese version olfactory test results comparing Usher and Usher type patient’s vs controls.
| Patients | Controls | STS | p | Holm’s Correction (α) | Benjamini-Hosper (α) | ||
|---|---|---|---|---|---|---|---|
| USH | T | 8.3 (5.1–11.0) | 7.8 (5.8–8.3) | −1.585 | 0.113 | 0.025 | 0.033 |
| D | 11 (9–12) | 10 (9–11) | −3.416 | 0.001 | 0.017 | 0.017 | |
| I | 13 (11–14) | 13 (12–13) | −0.476 | 0.634 | 0.050 | 0.050 | |
| TDI | 32.8 (28.5–37.4) | 30.0 (28.6–31.3) | −3.095 | 0.002 | – | – | |
| USH1 | T | 8.1 (4.6–12.5) | 7.9 (5.8–9.4) | −0.646 | 0.518 | 0.050 | 0.050 |
| D | 12.5 (9.3–13.8) | 9.5 (9–11) | −2.212 | 0.027 | 0.017 | 0.017 | |
| I | 14 (11.3–15) | 13 (12–13) | −1.104 | 0.270 | 0.025 | 0.033 | |
| TDI | 38.5 (28.2–41.1) | 30.9 (29.1–32.3) | −1.623 | 0.105 | – | – | |
| USH2 | T | 8.3 (5.5–10.8) | 7.5 (5.5–8.3) | −1.794 | 0.073 | 0.025 | 0.033 |
| D | 11 (9–12) | 10 (9–11) | −2.613 | 0.009 | 0.017 | 0.017 | |
| I | 12 (11–14) | 13 (12–13) | 0.072 | 0.943 | 0.050 | 0.050 | |
| TDI | 32.3 (28.8–35) | 29.8 (27.5–30.5) | −2.856 | 0.004 | – | – |
STS: Standardized Test Statistic. TDI: composite score of T, D and I. T: threshold; D: discrimination; I: Identification. Highlighted differences should be retained as statistically significant differences, according to the alpha level presented either by the Holm’s or the Benjamini-Hochberg correction for multiple comparisons.
USH1 VS USH2
USH1 and USH2 are distinguishable in terms of hearing loss and vestibular function. Patients with USH1 and USH2 were compared regarding olfaction capabilities and USH1 showed significantly higher TDI and identification scores than USH2 (Table 2). Effect size was 1.27 with a posteriori power of 99.87%.
Table 2. Sniffin’ Sticks Portuguese version olfactory test results comparing olfaction between USH1 with USH2.
| USH1 | USH2 | STS | p | Holm’s Correction (α) | Benjamini-Hochberg (α) | |
|---|---|---|---|---|---|---|
| T | 8.1 (4.6–12.5) | 8.3 (5.5–10.8) | −4.020 | <0.001 | 0.017 | 0.017 |
| D | 12.5 (9.3–13.8) | 11 (9–12) | 0.093 | 0.926 | 0.050 | 0.050 |
| I | 14 (11.3–15) | 12 (11–14) | −3.360 | 0.001 | 0.025 | 0.033 |
| TDI | 38.5 (28.2–41.1) | 32.3 (28.8–35) | −4.261 | <0.001 | – | – |
STS: standardized test statistic. TDI: composite score of T, D and I. T: threshold test; D: discrimination test; I: Identification test. Highlighted differences should be retained as statistically significant differences, according to the alpha level presented either by the Holm’s or the Benjamini-Hochberg correction for multiple comparisons.
Usher syndrome olfactory age decline
A deeper look showed a significant olfactory ageing decline in patients with USH. Because olfaction is known to decline with ageing30 and to adequately evaluate how olfactory ability is affected in USH patients, it is important to rule out ageing´s effect on olfaction. Consequently, after considering the normal age effect on olfaction based on our normative data30 (n = 203, that compares well with original data from Hummel et al.31), a multiple regression model was obtained. Patients with USH show a significantly faster age decline in olfaction. This effect is particularly relevant regarding USH1 patients: TDI = 50,010 − 0,401 × age; T = 16,549 − 0,188 × age; D = 16,248 − 0,124 × age; and I = 17,096 − 0,127 × age (Table 3).
Table 3. Linear regression values show the ageing olfactory loss in USH1 patients, compared with normative data.
| Correlation |
Regression |
||||
|---|---|---|---|---|---|
| Coefficient | P | Coefficient | 95% confidence interval | p | |
| T | −0.792 | <0.001 | −0.188 | −0.268 a −0.134 | 0.001 |
| D | −0.824 | <0.001 | −0.124 | −0.192 a −0.093 | 0.002 |
| I | −0.842 | <0.001 | −0.127 | −0.175 a −0.082 | 0.001 |
| TDI | −0.872 | <0.001 | −0.401 | −0.575 a −0.295 | 0.002 |
TDI = composite score of T, D and I olfactory tests. T = threshold test; D = discrimination test; I = Identification test. All the p-values remained statistically significant either by the Holm’s or the Benjamini-Hosper correction for multiple comparisons.
After correcting for normal olfactory ageing decline, patients with USH1 show a significantly faster olfactory decline with age than healthy subjects with a R2 = 0.703 and a power of 99.99% (Table 4).
Table 4. Decreased olfactory function related to USH1 after adjusting for age related olfactory loss.
| Healthy subjects age dependent olfactory decrease |
Usher Syndrome age dependent olfactory decrease |
|||||
|---|---|---|---|---|---|---|
| Mean | 95% CI | p | Mean | 95% CI | P | |
| T | −0.032 | −0.058 to −0.006 | 0.017 | −0.156 | −0.225 to −0,087 | <0.001 |
| D | −0.018 | −0.037 to +0.001 | 0.067 | −0.106 | −0.157 to −0.056 | 0.001 |
| I | −0.025 | −0.040 to −0.010 | 0.001 | −0.102 | −0.142 to −0.062 | <0.001 |
| TDI | −0.075 | −0.112 to −0.038 | <0.001 | −0.326 | −0.476 to −0.177 | <0.001 |
TDI: composite score of T, D and I olfactory tests. T: threshold test; D: discrimination test; I: Identification test.; CI – confidence interval. Highlighted differences should be retained as statistically significant differences.
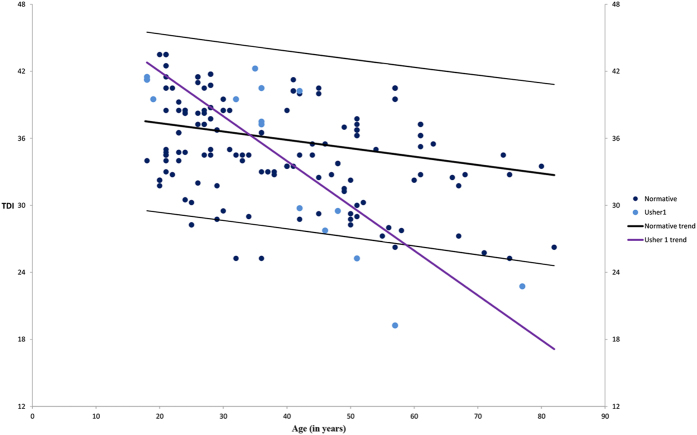
The significant olfactory decline in patients with USH1, not attributable to the olfactory ageing effect, is displayed in Fig. 1. Normative data is presented for comparison purposes (Fig. 1).
Figure 1. USH1 faster olfactory ageing decline, not expected by normal age olfaction decline (p < 0.001).
USH1: Usher type 1. TDI: composite score of Sniffin’ Sticks olfaction test.
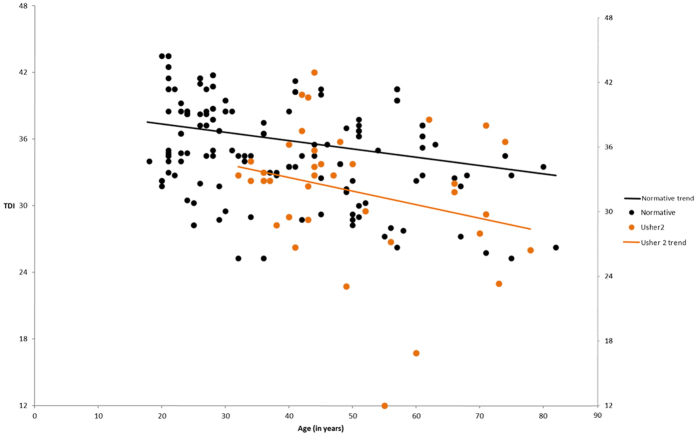
Considering patients with USH2, none of the models were statistically significant. Olfactory ageing effect is presented in Fig. 2.
Figure 2. USH2 olfactory ageing decline comparing to the healthy population (p > 0.05).
USH2: Usher type 2. TDI: composite score of Sniffin’ Sticks olfaction test.
When comparing patients with USH1 younger or older than the mean age of the group (43 years old), the older ones show significantly lower TDI scores (11.9 ± 1.7 vs 4.9 ± 2.0, p < 0.001) (Table 5). Such effect is not seen in patients with USH2.
Table 5. Olfaction scores comparing USH, USH1 and USH2 patients below and above 43 years old (mean age of USH1), after correcting for normal ageing decline.
| Age |
STS | P | Holm’s Correction (α) | Benjamini-Hochberg (α) | |||
|---|---|---|---|---|---|---|---|
| <43yr | ≥43yr | ||||||
| USH | T | 10.4 ± 2.3 | 6.8 ± 3.2 | −4.020 | <0.001 | 0.017 | 0.017 |
| D | 11.8 ± 1.9 | 10.1 ± 2.0 | 0.093 | 0.926 | 0.050 | 0.050 | |
| I | 13.6 ± 1.5 | 11.6 ± 2.6 | −3.360 | 0.001 | 0.025 | 0.033 | |
| TDI | 35.8 ± 4.8 | 29.7 ± 6.8 | −4.261 | <0.001 | |||
| USH1 | T | 11.9 ± 1.7 | 4.9 ± 2.0 | −3.915 | <0.001 | 0.017 | 0.017 |
| D | 12.9 ± 1.2 | 8.4 ± 1.1 | −3.112 | <0.001 | 0.050 | 0.050 | |
| I | 14.4 ± 1.0 | 10.2 ± 1.6 | −3.201 | <0.001 | 0.025 | 0.033 | |
| TDI | 39.1 ± 3.5 | 24.9 ± 4.1 | −3.120 | <0.001 | |||
| USH2 | T | 9 ± 1.9 | 7.5 ± 3.3 | 0.223 | 0.232 | 0.017 | 0.017 |
| D | 10.8 ± 1.9 | 10.4 ± 1.9 | 0.466 | 0.491 | 0.050 | 0.050 | |
| I | 12.8 ± 1.6 | 11.9 ± 2.7 | 0.409 | 0.432 | 0.025 | 0.033 | |
| TDI | 32.7 ± 3.8 | 30.7 ± 6.9 | 0.516 | 0.532 | |||
USH: Usher syndrome patients. USH1: Usher type 1 patients. USH2: Usher type 2 patients. TDI: composite score of T, D and I olfaction tests. T: threshold test; D: discrimination test; I: Identification test. Highlighted differences should be retained as statistically significant differences, according to the alpha level presented either by the Holm’s or the Benjamini-Hochberg correction for multiple comparisons.
The faster olfactory loss after adjusting for normal age related olfactory loss comparisons achieved a power of at least 91.12% in the all comparisons.
Discussion
The present study shows that patients with USH have significantly better TDI scores than healthy controls. Moreover, patients with USH1 show significantly higher TDI scores than patients with USH2, what can help to phenotypically distinguish them, having important clinical implications. A cut-off age of 43 years could be used. We observe that patients with USH1 have a faster decrease in olfactory function than what happens in healthy subjects, leading to much lower olfactory scores in older patients with USH1.
Data presented in this study indicates that olfactory tests may provide a valuable tool in characterizing and categorizing USH types. Although auditory and vestibular testing helps to distinguish typical USH patients, additional phenotypic abnormalities are necessary to aid in differentiating among atypical cases. Because of that, the current phenotype–genotype correlation is insufficient to predict the likely causative mutation, which makes sequencing of all known USH genes an often necessary but difficult way to identify the underlying genetic defect in affected patients.
The few papers addressing olfaction in Usher have shown conflicting results12,14,16,29. Our study tried to solve some methodology problems that highly limited the strength of conclusions from those papers. Being a rare disease, with such heterogeneous phenotypes, it is difficult to investigate a sample size with a sufficient power to confirm possible differences in olfaction among patients with Usher12,14,16. While some papers trust on the subjects’ self-assessment of olfaction, it is unreliable, and testing olfactory function is necessary33. Moreover, some previous studies used non-standardized and not validated olfactory testing14. Additionally, because patients were not matched for well-known factors to affect olfaction13,34,35,36 including age, gender and smoking habits12,14,37, those studies must be interpreted with caution38.
The better olfaction observed in young USH1 may be easily explained by the adaptive intramodal neuroplasticity and cross-modal sensory reorganization associated with blindness and by the behavioral compensations taking place in the remaining senses, including those in the tactile, auditory and olfactory domains39,40,41,42. Recent studies on olfaction in blind persons have shown that early blindness may affect olfactory processing39,41,43,44,45. Not all studies have found superior olfactory capabilities in the different olfaction areas46,47,48, but discrepancies are not surprising because most studies enrolled few patients, lacked matched-pair controls and did not use standardized and cultural validated olfactory testing procedures46,47,49,50. Patients with USH2 may have shown less significant differences because when a person is afflicted by combined visual and hearing impairment, it becomes more difficult for other sensory systems to compensate39.
The higher scores observed in younger patients with USH, in particular in USH1, may be a compensatory olfactory effect of deaf-blindness. There is growing evidence that congenitally blind individuals outperform age- and gender matched, normally sighted persons in tactile, auditory and possibly also olfactory tasks39,41,44, but such effect is not seen in late-blindness patients41,45,51. Thus, our results are not surprising in view of the fact that both hearing and visual loss occur earlier and in a more severe manner in patients with USH144.
The number of cases used to perform each regression was different due to the sampling of such a rare disease, where patients with USH1 were 22 and USH2 43. In spite of the different number of cases used in both regressions, statistical power was similar thus both regressions are valid and comparable in terms of conclusions achieved. The regression models showed a power superior to 91% in all comparisons performed. When the observed effect sizes are large (between 1.27 and 4.24), a nearly null type II error exists and the p-value achieved (p < 0.001) represent a true positive value between groups. The difference between patients with USH1 and USH2 concerning TDI scores represent an effect size of 1.27 which means that the achieved a posteriori power with a sample of 22 and 43 patients is 99.87%. Therefore, we must conclude there is no bias related to the different number of patients in each group and consequently, the observed dispersion is related to the olfactory features of patients with USH.
Data linking USH to a ciliopathy is grounded on histopathological and molecular studies24,27. Most of the USH proteins have been localized at or around ciliary photoreceptor structures in the retina (as well as other ciliated tissues, such as olfactory epithelium)14,21,52,53. On the other hand, a considerable body of evidence indicates that olfactory dysfunction might well be associated with ciliary disorders18,25,27,53,54. Proteins related to the USH have a periciliary localization and play a role in ciliogenesis and ciliary maintenance of olfactory cilia55. Furthermore, given the evidence linking ciliary abnormalities to USH- related pathology in the retina18,56, inner ear18,57, nasal27,52,58 and tracheal mucosa19,59,60, and sperm21, the consequences of a genetic olfactory cilia impairment21,24,52,56 may well explain the faster decrease in olfaction seen in this study´s patients with USH1.
Although it is the biggest study on olfaction in patients with USH to our knowledge, the results must be regarded with caution until larger series confirm it. Consequently, and because of the rarity of USH, multicenter studies are needed to investigate olfactory function in these rare cases. Moreover, a possible supra-threshold effect may have not been studied due to the possible ceiling effect of Sniffin’ Sticks, something that was not explored41.
Olfactory dysfunction should be considered in the spectrum of clinical manifestations associated with Usher syndrome. Patients with USH present better olfactory scores than controls, especially younger USH1. The olfaction difference between USH1 and USH2, as well as the major decline in olfactory ability seen in USH1, but not in USH2, may contribute to distinguish USH types and represent an attractive tool for pre diagnostic screening due to the low cost and non-invasive nature of the procedure.
Additional Information
How to cite this article: Ribeiro, J. C. et al. Accelerated age-related olfactory decline among type 1 Usher patients. Sci. Rep. 6, 28309; doi: 10.1038/srep28309 (2016).
Acknowledgments
The authors are grateful to the families who made this research possible. We also thank Filipe Silva for his role on data preparation and paper revision, Ana Margarida Amorim for ENT examination and counselling and Luis Filipe Silva for referring some patients, Ana Veloso, Elisabete Grade, Luis Santos, Carla Antunes and Mafalda Fernandes for their help in data collection. This work was supported in part by EUR-USH E-rare4/001/2012E, UID/NEU/04539/2013; FcB-FAUN Investigator Travel Grant Program (Forschung contra Blindheit - Initiative Usher-Syndrome and the FAUN foundation), and the Else Kröner-Fresenius Stiftung (http://www.ekfs.de/; grant number 2015_A71). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. All participants provided informed written consent. The study followed the Declaration of Helsinki 2013 on Biomedical Research Involving Human Participants and was approved by the Ethics Committee of the Faculty of Medicine, University of Coimbra, Portugal.
Footnotes
Author Contributions J.C.R., A.P. and E.D.S. conducted the experiments. J.C.R. and B.O. analyzed the data and performed statistical analysis. J.C.R., B.O., N.A. and T.H. wrote the main manuscript text. J.C.R., P.P., N.A., T.H., A.P. and E.D.S. designed the experiments protocol. All authors reviewed the manuscript.
References
- Sadeghi M., Kimberling W., Tranebjœrg L. & Möller C. The prevalence of Usher syndrome in Sweden: A nationwide epidemiological and clinical survey. Audiol. Med . 2, 220–228 (2004). [Google Scholar]
- Smith R. et al. Clinical diagnosis of the Usher syndromes. Usher Syndrome Consortium. Am. J. Med. Genet. 50, 32–38 (1994). [DOI] [PubMed] [Google Scholar]
- Yan D. & Liu X. Z. Genetics and pathological mechanisms of Usher syndrome. J. Hum. Genet. 55, 327–335 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Kelly F. et al. Clinical Aspects of Usher Syndrome and the USH2A Gene in a Cohort of 433 Patients. JAMA ophthalmology 133, 157–164, 10.1001/jamaophthalmol.2014.4498 (2015). [DOI] [PubMed] [Google Scholar]
- Astuto L. M. et al. CDH23 Mutation and Phenotype Heterogeneity: A Profile of 107 Diverse Families with Usher Syndrome and Nonsyndromic Deafness. The American Journal of Human Genetics 71, 262–275, 10.1086/341558 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal S. et al. Clinical and genetic studies in Spanish patients with Usher syndrome type II: description of new mutations and evidence for a lack of genotype–phenotype correlation. Clin. Genet. 68, 204–214, 10.1111/j.1399-0004.2005.00481.x (2005). [DOI] [PubMed] [Google Scholar]
- Malm E., Ponjavic V., Möller C., Kimberling W. J. & Andréasson S. Phenotypes in Defined Genotypes Including Siblings with Usher Syndrome. Ophthalmic Genet . 32, 65–74, 10.3109/13816810.2010.536064 (2011). [DOI] [PubMed] [Google Scholar]
- Ness S. et al. Genetic homogeneity and phenotypic variability among Ashkenazi Jews with Usher syndrome type III. J. Med. Genet. 40, 767–772 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C. Usher syndrome: from genetics to pathogenesis. Annual review of genomics and human genetics 2, 271–297 (2001). [DOI] [PubMed] [Google Scholar]
- Millan J. M. et al. An Update on the Genetics of Usher Syndrome. Journal of Ophthalmology 2011, 10.1155/2011/417217 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saihan Z., Webster A., Luxon L. & Glindzicz M. B. Update on Usher syndrome. Curr. Opin. Neurol. 22, 19–27 (2009). [DOI] [PubMed] [Google Scholar]
- Seeliger M. et al. Comparative study of visual, auditory, and olfactory function in Usher syndrome. Graefe’s Arch Clin Exp Ophthalmol 237, 301–307 (1999). [DOI] [PubMed] [Google Scholar]
- Hummel T., Sekinger B., Wolf S., Pauli E. & Kobal G. ‘Sniffin’sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 22, 39–52 (1997). [DOI] [PubMed] [Google Scholar]
- Zrada S., Braat K., Doty R. & Laties A. Olfactory loss in Usher Syndrome: another sensory deficit? Am J of Med Genet 64, 602–603 (1996). [DOI] [PubMed] [Google Scholar]
- Doty R. L., Shaman P., Kimmelman C. P. & Dann M. S. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. The Laryngoscope 94, 176–178 (1984). [DOI] [PubMed] [Google Scholar]
- Marietta J. et al. Usher’s syndrome type IC: Clinical studies and fine-mapping the disease locus. Ann. Otol. Rhinol. Laryngol. 106, 123–128 (1997). [DOI] [PubMed] [Google Scholar]
- Silva E. & Pinazo-Durán M. The ciliopathies and their relationship with ophthalmology. Archivos de la Sociedad Espanola de Oftalmologia 88, 165 (2013). [DOI] [PubMed] [Google Scholar]
- Sorusch N., Wunderlich K., Bauss K., Nagel-Wolfrum K. & Wolfrum U. Usher syndrome protein network functions in the retina and their relation to other ciliopathies. Retinal Degenerative Diseases 527–533 (Springer, 2014). [DOI] [PubMed] [Google Scholar]
- Bonneau D. et al. Usher syndrome type I associated with bronchiectasis and immotile nasal cilia in two brothers. J. Med. Genet. 30, 253–254 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallocchi M., Sisson J. H. & Cosgrove D. Biochemical characterization of native Usher protein complexes from a vesicular subfraction of tracheal epithelial cells. Biochemistry 49, 1236–1247 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D. G., Fishman G. A., Mehta R. S. & Kretzer F. L. Abnormal Sperm and Photoreceptor Axonemes in Usher’s Syndrome. Arch. Ophthalmol. 104, 385–389, 10.1001/archopht.1986.01050150085033 (1986). [DOI] [PubMed] [Google Scholar]
- Buck L. & Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187 (1991). [DOI] [PubMed] [Google Scholar]
- Hilgert N., Smith R. J. H. & Van Camp G. Forty-six genes causing nonsyndromic hearing impairment: Which ones should be analyzed in DNA diagnostics? Mutation Research/Reviews in Mutation Research 681, 189–196 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins P. M., McEwen D. & Martens J. R. Olfactory Cilia: Linking Sensory Cilia Function and Human Disease. Chem. Senses 34, 451–464, 10.1093/chemse/bjp020 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone A. et al. Clinical evidence of decreased olfaction in Bardet–Biedl syndrome caused by a deletion in the BBS4 gene. Am. J. Med. Genet. A 132, 343–346 (2005). [DOI] [PubMed] [Google Scholar]
- Badano J. L., Mitsuma N., Beales P. L. & Katsanis N. The Ciliopathies: An Emerging Class of Human Genetic Disorders. Annual Review of Genomics and Human Genetics 7, 125–148, 10.1146/annurev.genom.7.080505.115610 (2006). [DOI] [PubMed] [Google Scholar]
- Piatti G., De Santi M. M., Brogi M., Castorina P. & Ambrosetti U. Emerging ciliopathies: are respiratory cilia compromised in Usher syndrome? Am. J. Otolaryngol. 35, 340–346, 10.1016/j.amjoto.2014.01.010 (2014). [DOI] [PubMed] [Google Scholar]
- Van Wijk E. et al. Usher syndrome and Leber congenital amaurosis are molecularly linked via a novel isoform of the centrosomal ninein-like protein. Hum. Mol. Genet. 18, 51–64, 10.1093/hmg/ddn312 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K. L. et al. Human olfactory marker protein maps close to tyrosinase and is a candidate gene for Usher syndrome type I. Hum. Mol. Genet. 2, 115–118 (1993). [DOI] [PubMed] [Google Scholar]
- Ribeiro J. C. et al. Cultural adaptation of the Portuguese version of the “Sniffin’ Stick” smell test: reliability, validity, and normative data. PLoS One , 10.1371/journal.pone.0148937 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T., Kobal G., Gudziol H. & Mackay-Sim A. Normative data for the “Sniffin’Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur. Arch. Otorhinolaryngol. 264, 237–243 (2007). [DOI] [PubMed] [Google Scholar]
- Lötsch J. et al. Olfactory drug effects approached from human-derived data. Drug Discov. Today 20, 1398–1406 (2015). [DOI] [PubMed] [Google Scholar]
- Landis B., Hummel T., Hugentobler M., Giger R. & Lacroix J. Ratings of overall olfactory function. Chem. Senses 28, 691–694 (2003). [DOI] [PubMed] [Google Scholar]
- Doty R. L. & Kamath V. The influences of age on olfaction: a review. Front. Psychol . 5, 10.3389/fpsyg.2014.00020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty R. L. & Cameron E. L. Sex differences and reproductive hormone influences on human odor perception. Physiol. Behav. 97, 213–228, 10.1016/j.physbeh.2009.02.032 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lötsch J., Reichmann H. & Hummel T. Different odor tests contribute differently to the evaluation of olfactory loss. Chem. Senses 33, 17–21, 10.1016/j.drudis.2015.06.012 (2008). [DOI] [PubMed] [Google Scholar]
- Landis B. N., Konnerth C. G. & Hummel T. A Study on the Frequency of Olfactory Dysfunction. The Laryngoscope 114, 1764–1769, 10.1097/00005537-200410000-00017 (2004). [DOI] [PubMed] [Google Scholar]
- Doty R. L., McKeown D. A., Lee W. W. & Shaman P. A Study of the Test-retest Reliability of Ten Olfactory Tests. Chem. Senses 20, 645–656, 10.1093/chemse/20.6.645 (1995). [DOI] [PubMed] [Google Scholar]
- Kupers R. et al. Neural correlates of olfactory processing in congenital blindness. Neuropsychologia 49, 2037–2044 (2011). [DOI] [PubMed] [Google Scholar]
- Rombaux P., Potier H., Markessis E., Duprez T. & Hummel T. Olfactory bulb volume and depth of olfactory sulcus in patients with idiopathic olfactory loss. Eur. Arch. Otorhinolaryngol. 267, 1551–1556 (2010). [DOI] [PubMed] [Google Scholar]
- Cuevas I., Plaza P., Rombaux P., De Volder A. G. & Renier L. Odour discrimination and identification are improved in early blindness. Neuropsychologia 47, 3079–3083 (2009). [DOI] [PubMed] [Google Scholar]
- Luers J. C. et al. Do the blinds smell better? Eur. Arch. Otorhinolaryngol. 271, 1933–1937 (2014). [DOI] [PubMed] [Google Scholar]
- Rombaux P. et al. Increased olfactory bulb volume and olfactory function in early blind subjects. Neuroreport 21, 1069–1073 (2010). [DOI] [PubMed] [Google Scholar]
- Beaulieu-Lefebvre M., Schneider F. C., Kupers R. & Ptito M. Odor perception and odor awareness in congenital blindness. Brain Res. Bull. 84, 206–209 (2011). [DOI] [PubMed] [Google Scholar]
- Wan C. Y., Wood A. G., Reutens D. C. & Wilson S. J. Early but not late-blindness leads to enhanced auditory perception. Neuropsychologia 48, 344–348 (2010). [DOI] [PubMed] [Google Scholar]
- Smith R. S., Doty R. L., Burlingame G. K. & McKeown D. A. Smell and taste function in the visually impaired. Percept. Psychophys. 54, 649–655 (1993). [DOI] [PubMed] [Google Scholar]
- Rosenbluth R., Grossman E. S. & Kaitz M. Performance of early-blind and sighted children on olfactory tasks. Perception-London 29, 101–110 (2000). [DOI] [PubMed] [Google Scholar]
- Çomoğlu Ş. et al. Olfactory Function Assessment of Blind Subjects Using the Sniffin’Sticks Test. Otolaryngology–Head and Neck Surgery 153, 286–290 (2015). [DOI] [PubMed] [Google Scholar]
- Murphy C. & Cain W. S. Odor identification: The blind are better. Physiol. Behav. 37, 177–180 (1986). [DOI] [PubMed] [Google Scholar]
- Schwenn O., Hundorf I., Moll B., Pitz S. & Mann W. [Do blind persons have a better sense of smell than normal sighted people?]. Klinische Monatsblatter fur Augenheilkunde 219, 649–654 (2002). [DOI] [PubMed] [Google Scholar]
- Wakefield C. E., Homewood J. & Taylor A. J. Cognitive compensations for blindness in children: an investigation using odour naming. Perception -London 33, 429–442 (2004). [DOI] [PubMed] [Google Scholar]
- Arden G. B. & Fox B. Increased incidence of abnormal nasal cilia in patients with retinitis pigmentosa. Nature 279, 534–536 (1979). [DOI] [PubMed] [Google Scholar]
- Kulkarni S. S., Karkhanis V. S. & Joshi J. M. Usher’s syndrome: Can primarily be a primary ciliary disorder? Lung India 31, 301–302, 10.4103/0970-2113.135791 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk N., Lösl M., Schröder N. & Gießl A. Specialized Cilia in Mammalian Sensory Systems. Cells 4, 500–519 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen F. et al. Impact of the Usher syndrome on olfaction. Hum. Mol. Genet. 10.1093/hmg/ddv490 (2015). [DOI] [PubMed] [Google Scholar]
- Barrong S. D. et al. Ultrastructure of connecting cilia in different forms of retinitis pigmentosa. Arch. Ophthalmol. 110, 706–710 (1992). [DOI] [PubMed] [Google Scholar]
- Shinkawa H. & Nadol J. B. Histopathology of the inner ear in Usher’s syndrome as observed by light and electron microscopy. Ann. Otol. Rhinol. Laryngol. 95, 313–318 (1986). [DOI] [PubMed] [Google Scholar]
- Aparisi M. J. et al. Study of USH1 Splicing Variants through Minigenes and Transcript Analysis from Nasal Epithelial Cells. PLoS One 8, e57506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengot M. et al. Nasal ciliary beat frequency and beat pattern in retinal ciliopathies. Invest. Ophthalmol. Vis. Sci. 53, 2076–2079 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutland J. & Cole P. J. Nasal mucociliary clearance and ciliary beat frequency in cystic fibrosis compared with sinusitis and bronchiectasis. Thorax 36, 654–658 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]