Abstract
To evaluate the characteristics of thyroid carcinoma over time, we carried out a retrospective study to illustrate the evolutionary features of thyroid carcinoma. All records of thyroidectomies from the First Affiliated Hospital of Nanjing Medical University from 2008 to 2013 were obtained focusing on pathological diagnosis, size, local lymph node metastasis (LNM) of the tumors. The thyroid cancer detection rate increased from 24.6% to 41.5% significantly (P < 0.05). Papillary thyroid carcinoma (PTC) remained to be the most common type counting 86.4% of all thyroid carcinomas. In all 1,704 PTCs, microPTC (mPTC) with maximum diameter less than or equal to 10 mm has become the dominant form taking up 56.5% of all PTCs in 2013 while only 43.1% in 2008. The mean maximum tumor size has decreased from 17.8 mm to 12.2 mm significantly (P < 0.05). However, the average age, female dominance, and local LNM remained similarly in the past six years. Logistic regression test showed that the determinants for local LNM were age, gender and tumor size. mPTC has become the most common form of thyroid carcinoma detected during thyroidectomies in China while other features of thyroid carcinoma remained similarly in the recent years.
Thyroid cancer is the most common endocrine cancer1. The incidence of thyroid cancer has increased dramatically worldwide, especially in Asian countries including China2,3,4,5,6,7. Thyroid cancer has become the most common form of cancer among Chinese women aged below 30 years old6. Our previous study has showed that by using ultrasound the prevalence of thyroid nodule was 46.6% in population aged above 40 years old8. However, only 7–15% of them are supposed to be cancerous according to the recent guideline from American Thyroid Association (ATA)5. Due to the high prevalence of this clinic scenario, selection of nodules with the highest risks of malignancy for thyroidectomy would be more reasonable9 and cost-effective10. These modalities include clinical evaluation, thyroid function test, ultrasound, FNA and molecular test if possible1,9,11. Although there are currently several guidelines to walk us through the pathway of diagnosing and treating patients with thyroid nodules, some major discrepancies exist among guidelines from different regions and organizations1,5,12,13,14. The cut-off size for performing FNA is one of them. The ATA guideline does not recommend performing FNA routinely for nodules less than 1 cm, however the guidelines from AACE/ETA and Korean Society of Radiology do not hold the cut-off strictly13,14. One the other hand, in real practice a recent study showed that the practitioners generally held a lower threshold for FNA than the cut-off recommended by ATA15. With more surveillance modality being applied for screening for thyroid cancer2,4,16,17,18, it is reasonable to speculate the size of thyroid cancer might decrease annually. However, it is more intriguing to know whether the nature of these tumors remains the same or not as marked by for example the local lymph node metastasis (LNM) one of common parameter for cancer progression specially for those less than 1 cm. Due to that uncertainty, the primary objective of current study was to evaluate the evolutionary features of thyroid carcinoma, especially for the size and the lymph node metastasis.
Materials and Methods
Data were collected from Electronic Record Registration System in the Record Room and Pathological Diagnosis Registration and Reporting system in the Department of Pathology both from the First Affiliated Hospital of Nanjing Medical University, one of the largest academic hospitals of eastern China from 2008 to 2013 when both electronic systems were available since 2008. We reviewed all thyroid pathological diagnosis reports. Clinical data were also reviewed by inquiring the admission number in the Electronic Medical Record system when first available since 2009. All the data were obtained by chart review at a Record Room by X.L., L.Z., D.C., Z.W., H.C. and Y.D. We excluded data without precise documentation for the size of the tumor and those with invalid year. For patients with multiple entries in the same year and/or different years, we only recoded the year when the patient was first admitted in our hospital. Finally we obtained data with valid pathological diagnosis in 6,406 cases in the six year period. The methods of this work were described previously10,19. Among these cases, we obtained 1,973 malignant cases and clinical data were available for 1,710 cases. Clinical data included how the nodule was found, symptoms related to the nodule and the signs during physical examination. We divided the reasons for thyroid nodules consultant into four types: nodule(s) found by the patient, nodule(s) found during routine physical examination, nodule(s) found during consultant for other medical conditions, and for the completion of thyroidectomy after a cancerous nodule resected in other hospitals. Symptoms included discomfort or pain of the front neck, dysphagia, dysphonia or hoarseness and dyspnea. If any of these symptoms occurred, we recorded as positive, otherwise negative. If the nodule(s) or any enlargement of the thyroid gland or local lymph node was palpitated during physical examination, we recorded as positive, otherwise negative.
Generally, thyroidectomies were performed for the following reasons as previously described19: a) worrisome findings from ultrasonography, and/or abnormal lymph node enlargement, b) malignancy suspected from previous thyroid FNA, or inclusive FNA results, c) patients with multiple or bilateral nodules or symptoms of neck or throat compression, or enlargement during follow-up, d) concerning clinical or physical examination findings warranting consideration for removal. A small minority of patients chose thyroidectomy at their own wish, primarily for cosmetic reasons.
Malignancy, if present, was reported as one of the following subtypes: papillary thyroid carcinoma (PTC) including crPTC (clinical relevant papillary thyroid carcinoma, with the maximum diameter larger than 10 mm) and microPTC (mPTC) with the maximum diameter less than or equal to 10 mm; follicular thyroid carcinoma (FTC); medullary thyroid carcinoma (MTC); and anaplastic thyroid carcinoma (ATC). For each case, we collected data confirming the maximum diameter of the tumor and other pathologic features as well. All pathological findings were confirmed by experienced endocrine pathologist (Z.Z.). We divided results according to the chronological sequence from 2008 to 2013.
Lymph node dissection was performed at the discretion of the surgeon based upon clinical and other factors. Most often, a neck ultrasound or computed tomography (CT) scan was performed prior to surgery allowing assessment of neck adenopathy. Frozen sections were routinely used to guide the extent of the surgical procedures. If the nodule was found to be malignant by frozen section and no abnormal lymph nodes were identified on preoperative imaging (or during the surgery), an ipsilateral central lymph node dissection (CLND) from level VI was generally performed. Only when the tumor size was within 10 mm and no abnormal lymph node was found during the operation, CLND was not routinely performed. If abnormal lymph nodes were identified during pre-operative ultrasound or intraoperatively, and the snap-frozen sample showed malignant lesion, a modified lateral lymph node dissection (LLND) was performed. All the samples were formalin fixed paraffin embedded (FFPE) for final histopathology confirmation.
Fasting serum-free thyroxine (FT4, reference interval: 12.0–22.0 pmol/l), serum-free triiodothyronine (FT3, reference interval: 3.10–6.80 pmol/l), serum thyroid-stimulating hormone (TSH, reference interval: 0.27–4.2 mIU/L), anti-thyroglobulin (anti-Tg or TgAb, reference interval: 0–115 IU/ml), and anti-thyroid peroxidase (anti-TPO or TPoAb, reference interval: 0–34 IU/ml) were measured by chemiluminescent immunoassay (AutoBio Co., Ltd., Zhengzhou, China) before surgery. Coefficients of variation for the assays were all <15%. Serum TPoAb and TgAb were defined as positive and negative if the value of the antibody was higher than the upper reference or lower than the upper reference, respectively.
Quantitative data were shown as mean ± SD or mean ± SE as indicated, compared using One Way ANOVA whereas numbers and percentage were provided for qualitative data. Percentages were compared using the χ2test or χ2test for trend. Logistic regression test was used to determine the risk factors for local LNM. All analyses were additionally corrected for confounders including age, gender, nodule size, year group and presence of Hashimoto thyroiditis. All tests were 2-sided, and a P value < 0.05 was considered statistically significant. Statistical analyses were performed with SPSS software, version 13.0 for Windows (SPSS Inc, Chicago, IL, USA).
Ethics
This study was reviewed and deemed exempt from written informed consent by the Institutional Review Board (IRB) of the First Affiliated Hospital of Nanjing Medical University. The patient records were anonymized and de-identified prior to analysis. It was approved by the IRB for analysis.
Results
Malignancy detection rate from 2008 to 2013
It was found that the rate of malignancy in all thyroid surgeries increased significantly from 2009 to 2013. The malignancy rate of all thyroidectomies increased from 20.6% in 2009 to 41.5% in 2013 significantly (P < 0.05, Table 1). In the meantime the benign rate dropped from 79.4% to 58.5% correspondently (P < 0.05, Table 1).
Table 1. Malignancy and benignity percentage detected during thyroidectomies from 2008 to 2013.
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | Total | P | |
|---|---|---|---|---|---|---|---|---|
| Benign, n(%) | 612(75.4%) | 742(79.4%a) | 746(73.5%a) | 765(70.5%) | 737(64.6%a) | 831(58.5%a,b) | 4433(69.2%) | <0.001 |
| Cancer, n(%) | 200(24.6%) | 192(20.6%a) | 269(26.5%a) | 320(29.5%) | 403(35.4%a) | 589(41.5%a,b) | 1973(30.8%) | |
| Total | 812 | 934 | 1015 | 1085 | 1140 | 1420 | 6406 |
ameans percentages were significantly different compared with previous year; bmeans percentages were significantly different compared with the year 2008.
Pathological diagnosis distribution in all thyroid malignancies from 2008 to 2013
In all 1,973 patients of thyroid malignancies, the percentage of papillary thyroid carcinoma (PTC) has significantly increased from 68.5% in 2008 to 93.4% in 2013 (P < 0.05, Table 2), while other cancer types decreased or remained similar in detection percentage (Table 2). Majority of the patients had unilateral cancer (74.3%) and only 20.7% of the patients had cancer on both lobes of the thyroid gland. The mean numbers of loci were 1.4 ± 0.8 (Table 2)
Table 2. Pathological diagnosis classification of all thyroid malignancies from 2008 to 2013.
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | Total | P for difference | P for trend | |
|---|---|---|---|---|---|---|---|---|---|
| PTC, n(%) | 137(68.5%) | 146(76.0%a) | 230(85.5%a) | 277(86.6%) | 364(90.3%) | 550(93.4%b) | 1704(86.4%) | <0.001 | <0.001 |
| FTC, n(%) | 42(21.0%) | 13(6.8%a) | 12(4.5%) | 15(4.7%) | 13(3.2%) | 12(2.0%b) | 107(5.4%) | <0.001 | <0.001 |
| MTC, n(%) | 6(3.0%) | 2(1.0%) | 5(1.9%) | 6(1.9%) | 9(2.2%) | 7(1.2%) | 35(1.8%) | 0.550 | 0.318 |
| ATC, n(%) | 0(0.0%) | 1(0.5%) | 2(0.7%) | 0(0.0%) | 1(0.2%) | 0(0.0%) | 4(0.2%) | 0.210 | 0.274 |
| Other, n(%) | 15(7.3%) | 30(15.6%a) | 20(7.4%a) | 22(6.9%) | 16(4.0%) | 20(3.4%) | 123(6.2%) | <0.001 | <0.001 |
| Unilateral or Bilateral | |||||||||
| Unilateral | 141(70.5%) | 131(68.2%) | 204(75.8%) | 234(73.1%) | 299(74.2%) | 456(77.4%) | 1465(74.3%) | 0.118 | 0.016 |
| Bilateral | 45(22.5%) | 35(18.2%) | 49(18.2%) | 67(20.9%) | 94(23.3%) | 119(20.2%) | 409(20.7%) | 0.563 | 0.740 |
| Unknown* | 14(7%) | 26(13.5%) | 16(5.9%) | 19(5.9%) | 10(2.5%) | 14(2.4%) | 99(5.0%) | <0.001 | <0.001 |
| Mean numbers of loci (n)** | 1.45 ± 0.76 | 1.46 ± 0.85 | 1.46 ± 0.91 | 1.41 ± 0.73 | 1.49 ± 0.83 | 1.42 ± 0.79 | 1.44 ± 0.81 | 0.826 | |
| All cancer(n) | 200 | 192 | 269 | 320 | 403 | 589 | 1973 | ||
ameans percentages were significantly different compared with previous year; bmeans percentages were significantly different compared with the year 2008; PTC, papillary thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; ATC, anaplastic thyroid carcinoma; other means all the carcinoma could not be classified into previous types including poorly differentiated thyroid cancer, squamous cell carcinomas, B cell lymphomas of thyroid, spindle cell carcinoma, adenoid cystic carcinoma, renal clear cell metastasis and Langerhans cell histiocytosis of the thyroid gland.
*Probably due to primary surgeries were done at other hosptials.
**data were presented as mean ± SD.
Clinical information for thyroid cancer from 2009 to 2013
Clinical data were obtained for 1,710 cases mostly since 2009. According to the history of each patient in our EMR, we also documented the clinical related information. We found that more patients found their thyroid nodule during routine physical examinations with only 32% in 2009 increased to 54.3% significantly in 2013 (P < 0.05, Fig. 1 and Supplementary Table 1). In the meantime, the percentage of patients who found the nodules by themselves decreased from 47.1% in 2009 to 35.7% in 2013. The patients with the purpose of receiving completion of thyroidectomy also decrease in this period. About 7% of patients found the nodule issue during check-up for other medical conditions which would be termed as incidental thyroid carcinoma (Fig. 1 and Supplementary Table 1).
Figure 1. Clinical data related to the patients who received thyroidectomy with malignant histology from 2009 to 2013.
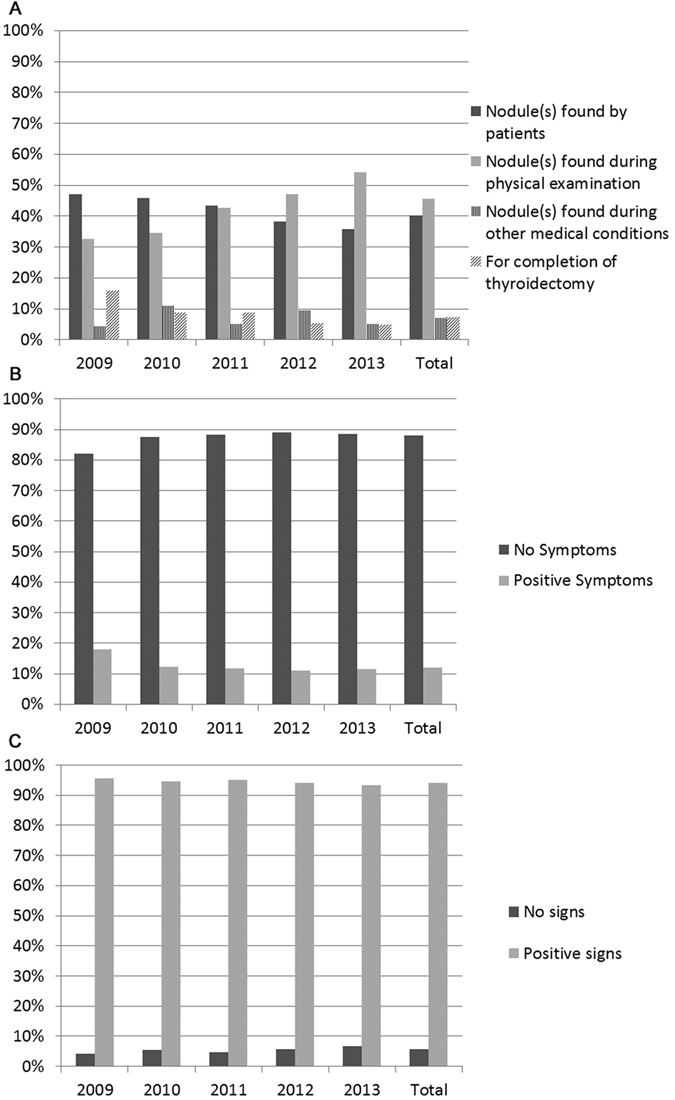
(A) Reasons for thyroid check-up, (B) Symptoms of the patients, (C) Signs during physical examination.
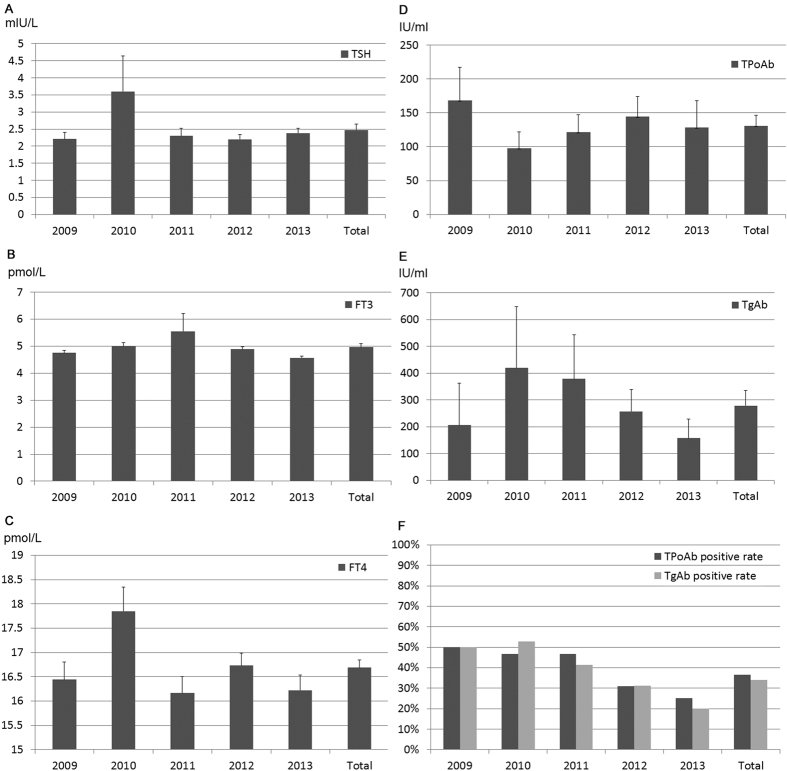
Majority of the patients did not have any clinical related symptoms, only 12% of the patients have different degrees of discomfort including pain and dysphagia etc. (Fig. 1 and Supplementary Table 1). Most of the patients had positive findings including any nodule(s) or type of enlargement of thyroid gland during palpitation of the neck, however 5.8% of the patients didn’t have any positive signs during physical examinations probably due to intra-thyroidal micro-carcinoma in the deep thyroid (Fig. 1 and Supplementary Table 1). During ultrasonography, 38% of the patients had single thyroid nodule and 62% had two or more thyroid nodules. However, the mean maximum size of the thyroid nodule(s) from each patient decreased from 27.6 ± 10.9 in 2009 to 19.7 ± 11.2 in 2013 significantly (Fig. 2 and Supplementary Table 1). The mean levels of thyroid function of these patients were within normal range (Fig. 3 and Supplementary Table 1). The mean levels of thyroid autoantibodies were above normal range, however, the positivity rates for TPoAb and TgAb were all decreased from 2009 to 2013 significantly (Fig. 3 and Supplementary Table 1). The rate of preoperative FNA was all below 10% from 2009 to 2011, however increased gradually from 14.7% in 2012 to 17.2% in 2013 (Fig. 4 and Supplementary Table 1).
Figure 2. Ultrasound features of the nodules of the patients who had thyroidectomy with malignant histology from 2009 to 2013.
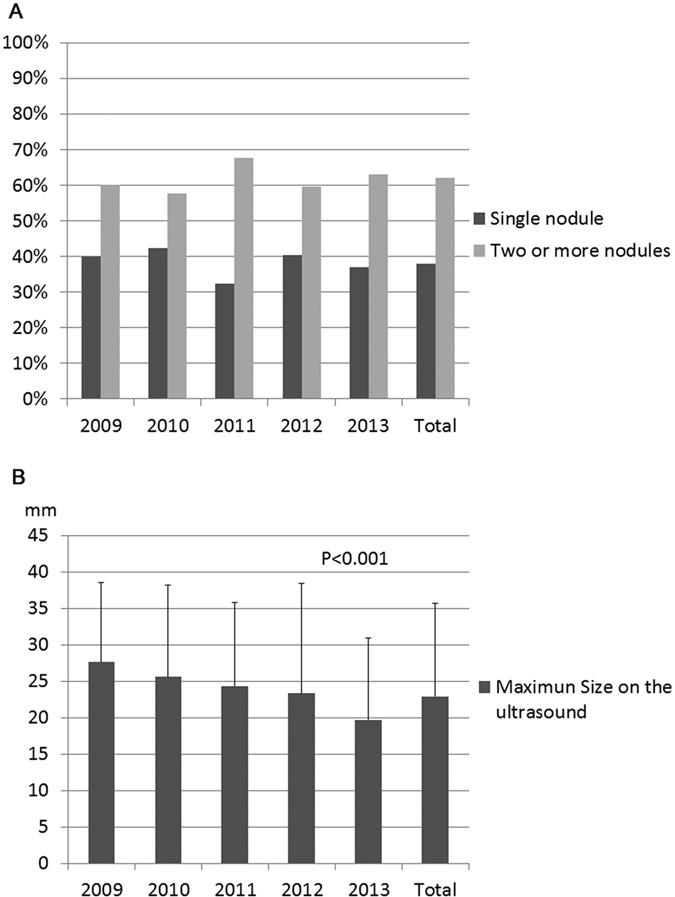
(A) Multi-nodularity of the patients. (B) Mean maximum size of the largest nodule during ultrasonography, data were express as mean ± SD.
Figure 3. Thyroid function and autoimmune status of the patients who received thyroidectomy with malignant histology from 2009 to 2013.
(A) Mean levels of TSH (normal reference range 0.27–4.20 mIU/L). (B) Mean levels of FT3 (normal reference range 3.10–6.80 pmol/L). (C) Mean levels of FT4 (normal reference range 12.00–22.00 pmol/L). (D) Mean levels of TPoAb (normal reference range <34.0 IU/ml). (E) Mean levels of TgAb (normal reference range <115.0 IU/ml). (F) Positivity rates of TPoAb and TgAb. Data were express as mean ± SE.
Figure 4. Preoperative FNA rates among the patients who received thyroidectomy with malignant histology from 2009 to 2013.
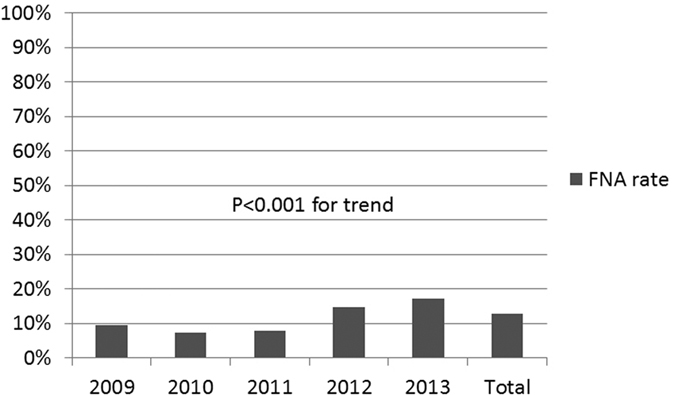
P < 0.001 for trend from 2009 to 2013.
Characteristics of papillary thyroid carcinoma changes from 2008 to 2013
Due to the dominance of PTC, we analysed the detailed features of PTC. The mean age of all the PTC patients was 44.0 ± 13.6 years old, 79.8% being female (Table 3). It was documented that the mean size of PTC dropped significantly from 17.8 ± 14.3 mm in 2008 to 12.2 ± 9.5 mm in 2013 (P < 0.05, Table 3). We divided all the PTC into two groups mPTC and crPTC. It was found that the percentage of mPTC was increased from 43.1% in 2008 to 56.5% in 2013 significantly (P < 0.05, Table 3). With mPTC being the dominant form of PTC in 2013, the other features including mean age, gender distribution and percentage of LNM remained without significant changes in the past six years annually or in total (P > 0.05, Table 3).
Table 3. Characteristics of PTCs from 2008 to 2013.
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | Total | P for difference | P for trend | |
|---|---|---|---|---|---|---|---|---|---|
| Age(year) | 43.5 ± 14.8 | 43.5 ± 13.9 | 45.9 ± 14.7 | 44.5 ± 13.7 | 43.5 ± 13.1 | 43.5 ± 13.1 | 44.0 ± 13.6 | ||
| Male, n(%) | 29(21.2%) | 31(21.2%) | 42(18.3%) | 49(17.7%) | 87(23.9%) | 106(19.3%) | 344(20.2%) | 0.394 | 0.976 |
| Female, n(%) | 108(78.8%) | 115(78.8%) | 188(81.7%) | 228(82.3%) | 277(76.1%) | 444(80.7%) | 1360(79.8%) | ||
| Mean Diameter(mm)* | 17.8 ± 14.3 | 16.8 ± 11.9 | 16.6 ± 12.2 | 15.4 ± 11.2 | 14.4 ± 11.8 | 12.2 ± 9.5a,b | 14.6 ± 11.5 | <0.001 | |
| crPTC, n(%) | 78(56.9%) | 94(64.4%) | 141(61.3%) | 154(55.6%) | 183(50.3%) | 239(43.5%a,b) | 889(52.2%) | <0.001 | <0.001 |
| mPTC, n(%) | 59(43.1%) | 52(35.6%) | 89(38.7%) | 123(44.4%) | 181(49.7%) | 311(56.5%a,b) | 815(47.8%) | <0.001 | <0.001 |
| Postive LNM, n(%) | 37(27.0%) | 51(34.9%) | 73(31.7%) | 84(30.3%) | 122(33.5%) | 167(30.4%) | 534(31.3%) | 0.659 | 0.907 |
| Negative LNM, n(%) | 32(23.4%) | 37(25.3%) | 49(21.3%) | 48(17.3%) | 80(22.0%) | 134(24.4%) | 380(22.3%) | 0.276 | 0.703 |
| No LN Resected, n(%) | 68(49.6%) | 58(39.7%) | 108(47.0%) | 145(52.3%) | 162(44.5%) | 249(45.3%) | 790(46.4%) | 0.158 | 0.670 |
| Total | 137 | 146 | 230 | 277 | 364 | 550 | 1704 |
ameans percentages were significantly different compared with previous year; bmeans percentages were significantly different compared with the year 2008; crPTC, clinical relevant papillary thyroid carcinoma; mPTC, micro papillary thyroid carcinoma; LNM, lymph node metastasis; LN, lymph node.
*Data were presented as mean ± SD.
In further analysis, we compared the positive lymph node metastasis (LNM) rate between mPTC and crPTC in each year which showed positive LNM was all higher significantly in crPTC group than in mPTC group (Table 4) except that only similar trend was revealed in 2011 although without significance (P = 0.055, Table 4). No significance was revealed when comparison of positive LNM percentage was made in consecutive two years or in total six years both in mPTC and in crPTC groups. In general, evidenced positive LNM in all PTC, mPTC and crPTC was 31.3% (Table 3), 20.6% and 41.2% respectively (Table 4) without clear upward or downward trend in the past six years.
Table 4. Rate of local lymph node metastasis comparison between mPTC and crPTC from 2008 to 2013.
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | Total | P for difference | P for the trend | |
|---|---|---|---|---|---|---|---|---|---|
| Positive LNM in mPTC, n(%) | 9(15.3%) | 8(15.4%) | 14(15.7%) | 30(24.4%) | 40(22.1%) | 67(21.5%) | 168(20.6%) | 0.456 | 0.128 |
| Positive LNM in crPTC, n(%) | 28(35.9%) | 43(45.7%) | 59(41.8%) | 54(35.1%) | 82(44.8%) | 100(41.8%) | 366(41.2%) | 0.398 | 0.596 |
| P value* | 0.007 | <0.001 | <0.001 | 0.055 | <0.001 | <0.001 | <0.001 |
*Rate of Positive LNM was compared between mPTC and crPTC in each year. LNM, lymph node metastasis; mPTC, micro papillary thyroid carcinoma; crPTC, clinical relevant papillary thyroid carcinoma.
Determinants of local lymph node metastasis in PTC, mPTC and crPTC
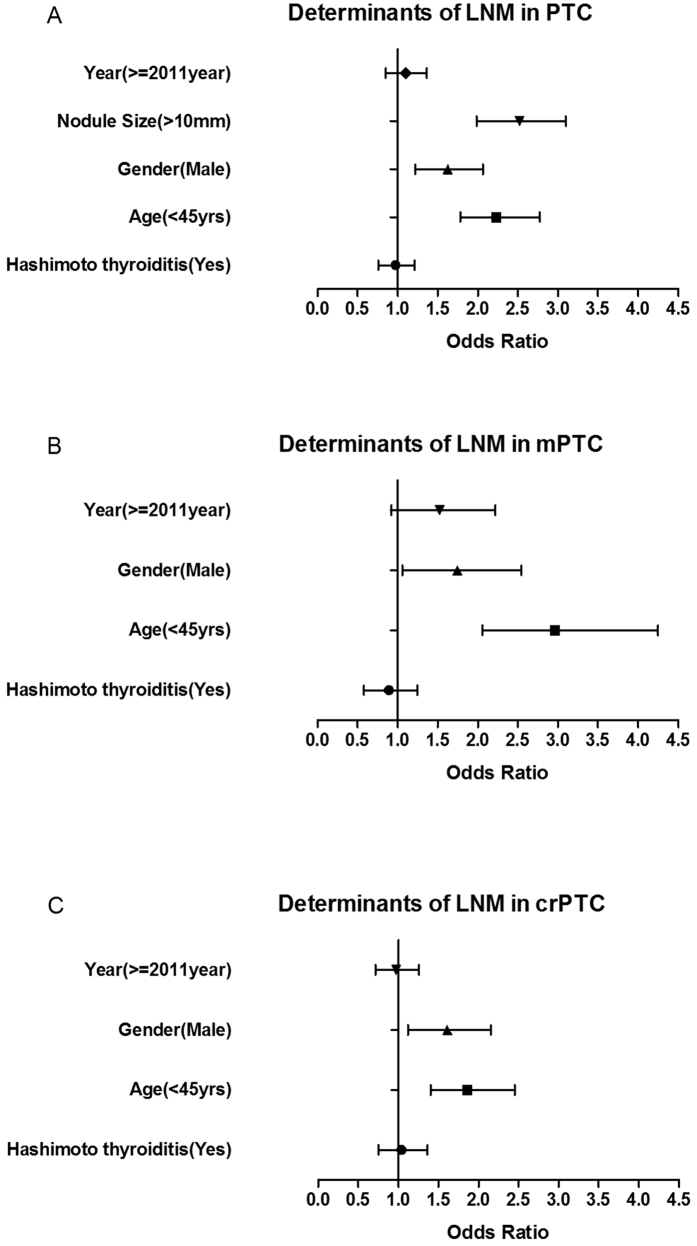
We also determined the risk factors for local LNM in PTC, mPTC and crPTC. As showed in Fig. 5 and Supplementary Table 2 the LNM was positively related to nodule size >10 mm (OR = 2.480, CI 1.985–3.098, P < 0.001), male (OR = 1.587, CI 1.220–2.064, P = 0.001) and age below 45 years old (OR = 2.226, CI 1.786–2.774, P < 0.001) after adjustment for other covariates in all PTCs. Consistent findings were revealed in mPTC and crPTC groups (Fig. 5 and Supplementary Tables 3 and 4). However, the year and presence of Hashimoto’s thyroiditis were not related to LNM (Fig. 5 and Supplementary 2–4).
Figure 5. Determinants of Lymph Node Metastasis in PTC, mPTC and crPTC.
Logistic regression was used to determine the factors for local lymph node metastasis (LNM) in PTC, mPTC and crPTC after adjustments for other covariates.
Discussion
Our study reported significantly increased malignancy detection rate in the past six years reaching 41.5% in 2013 which was favorably higher than 28.1% in Malaysia4 and 6.7% in Germany20. However, this figure was still lower than 56% in a study from Brigham and Women’s Hospital (BWH), one of the major affiliates of Harvard Medical School in the United States21. The discrepancy behind this might be related to different algorithms physicians or surgeons were using for patients with thyroid nodules reflecting different background of medical insurance and cost. Using ultrasound as one single tool might not be accurate enough for preoperative diagnosis22. Combination of FNA, standardized cytology reporting system, repeated biopsy for undetermined thyroid nodules and molecular diagnosis may provide more precise preoperative diagnosis and result in a higher malignancy detection rate9,11,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37. One important factor might be low FNA rate in our cohort. We only had 12.8% of the patients who received FNA preoperatively from 2009 to 2013, however, at BWH 100% of patients received FNA before surgery21. Similar situation was also found in Germany which also resulted a much lower malignancy rate20. We also found that preoperative FNA was significantly increased during these year which correlated to the increased cancer detection rate implying the FNA remains the main step when diagnosing thyroid nodule(s) as suggested by ATA guidelines5,23. It is reasonable to believe a multi-disciplinary approach including a routine preoperative FNA might be needed to achieve higher malignancy detection rate in the future21,28.
With higher malignancy detection rate, the major form of malignancy remained to be PTC reaching 93.4% in 2013 and 86.4% averagely in the six-year period which were both higher than previous reports38. This significantly increased distribution of PTC in all thyroid cancer might be due to patient selection bias and the more recognition of high risk appearances for PTC under the ultrasound. Several ultrasonographic features have been shown to be related to thyroid malignancy, for example, marked hypoechogenicity, irregular or microlobulated margins, a taller than wide configuration (anteroposterior dimension greater than transverse dimension), microcalcification, or chaotic arrangement of intranodular vascular images and presence of metastatic lymph nodes or extracapsular growth5. Among them, microcalcification is believed to have a high specificity for PTC12,23. The question rises whether FNA or surgery is needed for when highly suspicious ultrasound features are reported in a sub-centimeter lesion. According the recent guideline from ATA, these sub-centimeter nodules might not need FNA in the absence of highly suspicious LN based on the fact that vast majority of these microPTC (if confirmed) either progress very slow or not at all39. However, according to the data from the current study, this was not entirely true. We did found that around 20% of all these micro-PTC already showed the positive local lymph node metastasis. If we set the FNA cut-off as nodules with maximum diameter larger than 1 cm as recommended by ATA, all these microPTCs with LNM could not have been confirmed histologically. On the other hand, for the rest 80% of the micro-PTCs, the ATA guideline seemed to guide the right way at least from the perspective of LNM. The question would lie in more about when we need to be more aggressive and when to me more conservative for those highly suspicious nodule within 1 cm. Our analysis suggests that for male patients with younger age (<45 years old), more attention may be needed to be paid as the risk of LNM in this group of patients is high. There has been a major area of controversy that whether these sub-centimeter PTCs needed to be treated as crPTC40,41. Some investigators advocated in favor of not performing further treatment in addition to initial thyroid surgery and some even suggest to remove the cancer label from indolent lesions2,42, whereas others suggested an aggressive surgical approach followed by radioiodine ablation therapy40. Dr Miyauchi compared the outcome of immediate surgery and active surveillance for patients with microPTC43. The oncological outcomes were similarly excellent, but the incidences of unfavorable events were definitely higher in the immediate surgery group. He has suggested that active surveillance is now recommended as the best choice for patients with low-risk mPTC. A recent publication44 suggested specific recommendations with regard to the optimal selection of patients with microPTCs for an active surveillance. They described a risk-stratified clinical decision-making framework44. This framework was composed with three major factors, tumor/neck US characteristics, patient characteristics and medical team characteristics44. They have described as inappropriate to observe or follow-up the patients if there is evidence of aggressive cytology on FNA (rare), or subcapsular locations adjacent to recurrent laryngeal nerve (RLN), or evidence of extrathyroidal extension or clinical evidence of invasion of RLN or trachea (rare) or N1 disease at initial evaluation, etc. Although in our data we have not been able to show all the detailed information described above, they do mention the LNM as a factor to include the patient as the candidate for surgery and FNA should be done for these sub-centimeter lesions to exclude aggressive form of thyroid cancer. We found this framework would be more reasonable to apply when facing individual patient.
The main limitation of current study was that all the data were from a single academic center with a retrospective nature. Patient selection bias was unavoidable. We only compared the local LNM, other pathological features such as extrathyroidal extension, capsule invasion were not compared which are also recognized as important prognostic information45. There were a certain percentage of lymph nodes not resected during the thyroidectomies. We did not have robust follow-up evidence, as suggested by many studies that PTC as the major form of thyroid cancer is generally indolent in nature and longer time of follow-up is often suggested46,47,48,49. For a future study, we hope to analyze the follow up data as to further confirm our points.
Our study has filled in the blanks of thyroid research by showing the evolutionary trend of thyroid carcinoma from a single academic center in China. The major findings of the current study lie in that mPTC has become the dominant form of thyroid cancer without significant change in local LNM in recent years. Although the detection rate of mPTC was indeed increased from 43.1% to 56.5%, the local lymph node metastasis was generally around 20% suggesting than around 80% of these microPTCs did not have evidence of cancer progression as manifested by LNM. Our findings support this hypothesis that an increasing number of thyroidectomies are being performed for small and often indolent disease questioning the current algorithms surgeons are using for patients with thyroid nodules in China. Our study provided some evidence for physicians or surgeons that clinical judgments are very important independent of the nodule size especially when high risk ultrasound appearances or suspicious LN were present at first clinical consult. A risk-stratified clinical decision-making framework might be useful when making decisions for these sub-centimeter lesions especially when microPTC is confirmed by FNA, however further study is needed to illustrate this issue.
Additional Information
How to cite this article: Liu, X. et al. Evolutionary features of thyroid cancer in patients with thyroidectomies from 2008 to 2013 in China. Sci. Rep. 6, 28414; doi: 10.1038/srep28414 (2016).
Supplementary Material
Acknowledgments
The study was supported by National Science Foundation of China (number 81270897) and grants from Jiangsu Health International Exchange Program (Xiaoyun Liu), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Footnotes
Author Contributions X.L. carried out analysis, co-designed the study and wrote the manuscript; X.L., L.Z., D.C., Z.W. and H.C. collected the data. M.S. and H.L. performed the thyroidectomies. Z.Z. interpreted the pathology results. X.L., Y.D., J.C. and E.K.A contributed to study design; X.L. wrote the initial manuscript. X.L. and L.Z. contributed to the statistics of this study and interpreted the data. T.Y. and X.W. co-directed the project, co-designed the study and revised the manuscript. T.Y. and X.W. are the guarantors of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- Burman K. D. & Wartofsky L. CLINICAL PRACTICE. Thyroid Nodules. N Engl J Med 373, 2347–2356, 10.1056/NEJMcp1415786 (2015). [DOI] [PubMed] [Google Scholar]
- Brito J. P., Hay I. D. & Morris J. C. Low risk papillary thyroid cancer. BMJ (Clinical research ed.) 348, g3045, 10.1136/bmj.g3045 (2014). [DOI] [PubMed] [Google Scholar]
- Han M. A. et al. Current status of thyroid cancer screening in Korea: results from a nationwide interview survey. Asian Pacific journal of cancer prevention : APJCP 12, 1657–1663 (2011). [PubMed] [Google Scholar]
- Kilfoy B. A. et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer causes & control: CCC 20, 525–531, 10.1007/s10552-008-9260-4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen B. R. et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association 26, 1–133, 10.1089/thy.2015.0020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians 66, 115–132, 10.3322/caac.21338 (2016). [DOI] [PubMed] [Google Scholar]
- Brito J. P., Kim H. J., Han S. J., Lee Y. S. & Ahn H. S. Geographic Distribution and Evolution of Thyroid Cancer Epidemic in South Korea. Thyroid: official journal of the American Thyroid Association , 10.1089/thy.2016.0057 (2016). [DOI] [PubMed] [Google Scholar]
- Guo H. et al. The prevalence of thyroid nodules and its relationship with metabolic parameters in a Chinese community-based population aged over 40 years. Endocrine 45, 230–235, 10.1007/s12020-013-9968-0 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang Y. Z. et al. Value of TIRADS, BSRTC and FNA-BRAF V600E mutation analysis in differentiating high-risk thyroid nodules. Scientific reports 5, 16927, 10.1038/srep16927 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Y. et al. Annual financial impact of thyroidectomies for nodular thyroid disease in china. Asian Pacific journal of cancer prevention : APJCP 15, 5921–5926 (2014). [DOI] [PubMed] [Google Scholar]
- Singh Ospina N. et al. Diagnostic accuracy of ultrasound-guided fine needle aspiration biopsy for thyroid malignancy: systematic review and meta-analysis. Endocrine , 10.1007/s12020-016-0921-x (2016). [DOI] [PubMed] [Google Scholar]
- Levine R. A. Current guidelines for the management of thyroid nodules. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 18, 596–599, 10.4158/ep12071.co (2012). [DOI] [PubMed] [Google Scholar]
- Gharib H. et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Journal of endocrinological investigation 33, 1–50 (2010). [PubMed] [Google Scholar]
- Moon W. J. et al. Ultrasonography and the ultrasound-based management of thyroid nodules: consensus statement and recommendations. Korean journal of radiology 12, 1–14, 10.3348/kjr.2011.12.1.1 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch H. B., Burman K. D., Cooper D. S., Hennessey J. V. & Vietor N. O. A 2015 Survey of Clinical Practice Patterns in the Management of Thyroid Nodules. The Journal of clinical endocrinology and metabolism, jc20161155 , 10.1210/jc.2016-1155 (2016). [DOI] [PubMed] [Google Scholar]
- Brito J. P., Morris J. C. & Montori V. M. Thyroid cancer: zealous imaging has increased detection and treatment of low risk tumours. BMJ (Clinical research ed.) 347, f4706, 10.1136/bmj.f4706 (2013). [DOI] [PubMed] [Google Scholar]
- Brito J. P., Gionfriddo M., Morris J. C. & Montori V. M. Overdiagnosis of thyroid cancer and graves’ disease. Thyroid : official journal of the American Thyroid Association 24, 402–403, 10.1089/thy.2013.0425 (2014). [DOI] [PubMed] [Google Scholar]
- Trimboli P. et al. Ultrasound sensitivity for thyroid malignancy is increased by real-time elastography: a prospective multicenter study. The Journal of clinical endocrinology and metabolism 97, 4524–4530, 10.1210/jc.2012-2951 (2012). [DOI] [PubMed] [Google Scholar]
- Liu X. et al. Coexistence of Histologically Confirmed Hashimoto’s Thyroiditis with Different Stages of Papillary Thyroid Carcinoma in a Consecutive Chinese Cohort. International journal of endocrinology 2014, 769294, 10.1155/2014/769294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienhold R., Scholz M., Adler J. R., C G. N. & Paschke R. The management of thyroid nodules: a retrospective analysis of health insurance data. Deutsches Arzteblatt international 110, 827–834, 10.3238/arztebl.2013.0827 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa L. et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 111, 508–516, 10.1002/cncr.23116 (2007). [DOI] [PubMed] [Google Scholar]
- Brito J. P. et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism 99, 1253–1263, 10.1210/jc.2013-2928 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thyroid Association Guidelines Taskforce on Thyroid, N. et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid : official journal of the American Thyroid Association 19, 1167–1214, 10.1089/thy.2009.0110 (2009). [DOI] [PubMed] [Google Scholar]
- Alexander E. K. et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. The New England journal of medicine 367, 705–715, 10.1056/NEJMoa1203208 (2012). [DOI] [PubMed] [Google Scholar]
- Alexander E. K. et al. Multicenter clinical experience with the afirma gene expression classifier. The Journal of clinical endocrinology and metabolism 99, 119–125, 10.1210/jc.2013-2482 (2014). [DOI] [PubMed] [Google Scholar]
- Cibas E. S. et al. Indications for thyroid FNA and pre-FNA requirements: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagnostic cytopathology 36, 390–399, 10.1002/dc.20827 (2008). [DOI] [PubMed] [Google Scholar]
- Cibas E. S. & Ali S. Z. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid : official journal of the American Thyroid Association 19, 1159–1165, 10.1089/thy.2009.0274 (2009). [DOI] [PubMed] [Google Scholar]
- Gupta A. et al. A standardized assessment of thyroid nodules in children confirms higher cancer prevalence than in adults. The Journal of clinical endocrinology and metabolism 98, 3238–3245, 10.1210/jc.2013-1796 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson J. L. Minimizing unnecessary surgery for thyroid nodules. N Engl J Med 367, 765–767, 10.1056/NEJMe1205893 (2012). [DOI] [PubMed] [Google Scholar]
- Kim M. I. & Alexander E. K. Diagnostic use of molecular markers in the evaluation of thyroid nodules. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 18, 796–802, 10.4158/EP12183.RA (2012). [DOI] [PubMed] [Google Scholar]
- Nikiforov Y. E. et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. The Journal of clinical endocrinology and metabolism 96, 3390–3397, 10.1210/jc.2011-1469 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov Y. E. et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. The Journal of clinical endocrinology and metabolism 94, 2092–2098, 10.1210/jc.2009-0247 (2009). [DOI] [PubMed] [Google Scholar]
- Nikiforov Y. E., Yip L. & Nikiforova M. N. New strategies in diagnosing cancer in thyroid nodules: impact of molecular markers. Clinical cancer research : an official journal of the American Association for Cancer Research 19, 2283–2288, 10.1158/1078-0432.CCR-12-1253 (2013). [DOI] [PubMed] [Google Scholar]
- Hsiao S. J. & Nikiforov Y. Molecular approaches to thyroid cancer diagnosis. Endocrine-related cancer , 10.1530/erc-14-0166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip L. et al. A clinical algorithm for fine-needle aspiration molecular testing effectively guides the appropriate extent of initial thyroidectomy. Annals of surgery 260, 163–168, 10.1097/sla.0000000000000215 (2014). [DOI] [PubMed] [Google Scholar]
- Krane J. F., Cibas E. S., Alexander E. K., Paschke R. & Eszlinger M. Molecular analysis of residual ThinPrep material from thyroid FNAs increases diagnostic sensitivity. Cancer cytopathology 123, 356–361, 10.1002/cncy.21546 (2015). [DOI] [PubMed] [Google Scholar]
- Eszlinger M., Hegedus L. & Paschke R. Ruling in or ruling out thyroid malignancy by molecular diagnostics of thyroid nodules. Best practice & research. Clinical endocrinology & metabolism 28, 545–557, 10.1016/j.beem.2014.01.011 (2014). [DOI] [PubMed] [Google Scholar]
- Othman N. H., Omar E. & Naing N. N. Spectrum of thyroid lesions in hospital Universiti Sains Malaysia over 11years and a review of thyroid cancers in Malaysia. Asian Pacific journal of cancer prevention : APJCP 10, 87–90 (2009). [PubMed] [Google Scholar]
- Ito Y. et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World journal of surgery 34, 28–35, 10.1007/s00268-009-0303-0 (2010). [DOI] [PubMed] [Google Scholar]
- Ertorer M. E., Tutuncu N. B. & Ozyilkan O. Incidental papillary microcarcinoma of the thyroid. Asian Pacific journal of cancer prevention : APJCP 8, 631–634 (2007). [PubMed] [Google Scholar]
- Wang S. F. et al. Clinical features and prognosis of patients with benign thyroid disease accompanied by an incidental papillary carcinoma. Asian Pacific journal of cancer prevention : APJCP 14, 707–711 (2013). [DOI] [PubMed] [Google Scholar]
- Nikiforov Y. E. et al. Nomenclature Revision for Encapsulated Follicular Variant of Papillary Thyroid Carcinoma: A Paradigm Shift to Reduce Overtreatment of Indolent Tumors. JAMA oncology , 10.1001/jamaoncol.2016.0386 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H. et al. Incidences of Unfavorable Events in the Management of Low-Risk Papillary Microcarcinoma of the Thyroid by Active Surveillance Versus Immediate Surgery. Thyroid : official journal of the American Thyroid Association 26, 150–155, 10.1089/thy.2015.0313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito J. P., Ito Y., Miyauchi A. & Tuttle R. M. A Clinical Framework to Facilitate Risk Stratification When Considering an Active Surveillance Alternative to Immediate Biopsy and Surgery in Papillary Microcarcinoma. Thyroid : official journal of the American Thyroid Association 26, 144–149, 10.1089/thy.2015.0178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Bethesda Categorization of Thyroid Nodule Cytology and Prediction of Thyroid Cancer Type and Prognosis. Thyroid : official journal of the American Thyroid Association 26, 256–261, 10.1089/thy.2015.0376 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici M. et al. Long- versus short-interval follow-up of cytologically benign thyroid nodules: a prospective cohort study. BMC medicine 14, 11, 10.1186/s12916-016-0554-1 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong N. et al. Long-term, treatment-free survival in select patients with distant metastatic papillary thyroid cancer. Endocrine connections 3, 207–214, 10.1530/ec-14-0097 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante C. et al. The natural history of benign thyroid nodules. Jama 313, 926–935, 10.1001/jama.2015.0956 (2015). [DOI] [PubMed] [Google Scholar]
- Nou E. et al. Determination of the optimal time interval for repeat evaluation after a benign thyroid nodule aspiration. The Journal of clinical endocrinology and metabolism 99, 510–516, 10.1210/jc.2013-3160 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.