Abstract
Gene modified or cytokine activated mesenchymal stem cells (MSCs) have been used as a treatment in various types of cancer. Moreover, irradiation is usually applied as either a standard primary or adjuvant therapy. Here, we showed that the expression of TNF related apoptosis-inducing ligand (TRAIL) and Dickouf-3 (Dkk-3), the promising anticancer proteins, increased in murine adipose-derived mesenchymal stromal cells (AD-MSCs) following activation with TNF-α, resulting in the induction of apoptosis in cancer cells. Also, anticancer effects of TNF-α activated AD-MSCs were intensified with irradiation. In vivo results showed that TNF-α preactivated AD-MSCs combined with irradiation decreased tumor size and increased survival rate in tumor bearing mice. On the other hands, both TNF-α preactivated AD-MSCs with or without irradiation prevented metastasis in ling and liver, and increased apoptosis in tumor mass. Finally, flowcytometry assay demonstrated that naïve AD-MSCs combined with irradiation but not TNF-α activated MSCs with irradiation increased Treg population in lymph node and spleen. Altogether, obtained results suggest that TNF-α activated MSCs combined with irradiation therapy can serve as new strategy in breast cancer therapy.
Cancer is currently the second leading cause of death following heart disease and it is expected to be the first leading cause of death in the near future. Furthermore, Breast cancer is the third cause of death following lung and bronchus cancer in women1. Current treatment of breast cancer is based on combination therapy using radiotherapy, chemotherapy and cytotoxic molecules and antibodies targeting cancer cells2.
Radiotherapy as a crucial component in the management of breast cancer, implies high-energy to induce cell death in local tumors. There are a lot of evidences which indicate that the survival of patients is improved by radiotherapy through the reduction of local recurrence3. Radiotherapy is used either for treatment in a primary tumor or after surgery4. Radiotherapy also used as a component in combination therapy alongside with gene therapy5. On the other hands, it has been shown that radiotherapy modulates the tumor microenvironment by enhancing the recruitment of multipotent stromal cells (MSCs)6.
Multipotent stromal cells also known as mesenchymal stem cells are non-hematopoietic stem cells derived from diverse tissues such as bone marrow, umbilical cord and adipose tissue7. MSCs are considered for tumor therapy as a vehicle because they can preferentially migrate towards tumor site and incorporate in tumor stroma and this capability can be improved by radiotherapy8. In addition MSCs do not induce immune reaction in unrelated donor transplantation9.
Previous studies demonstrated that MSCs or gene modified MSCs could inhibit tumor growth10. More recently, some reports showed that antitumor effects of MSCs were improved upon activation with either TNF-α or IFN-γ through the up-regulation of TNF related apoptosis-inducing ligand (TRAIL) and Dickouf-3 (Dkk-3)11. Antitumor effects of TRAIL have been shown in many cancer cell lines without side effects on normal cells. TRAIL induces apoptosis in cancer cells through DR4 and DR5 receptors expressed in turmeric cells12. Because the soluble form of TRAIL exhibits short half-life and reduced efficacy, MSCs can be used as delivery vehicle. It has been shown that TRAIL over-expressed MSCs inhibited tumor growth13. Moreover, several reports have demonstrated that Dkk-3 plays important roles in tumor suppression and declined level of Dkk-3 is reported in diverse types of cancer. Dkk-3 suppresses tumor growth by the inhibition of Wnt signaling as an important signaling in tumorgenesis14.
Radiotherapy increased both expression of DR5 as receptor of TRAIL and MSCs migration toward tumor site15. In this study, we hypothesized that radiotherapy might increase the antitumor effects of cytokine activated AD-MSCs. Here, using in vitro and in vivo study, we investigated whether radiotherapy enhanced the therapeutic effect of TNF-α activated AD-MSCs by monitoring the tumor growth, survival rate, metastasis and regulatory T cell population in spleen and local lymph node.
Results
Proinflammatory cytokines increased expression of TRAIL, Dkk-3 and chemokines in AD-MSCs
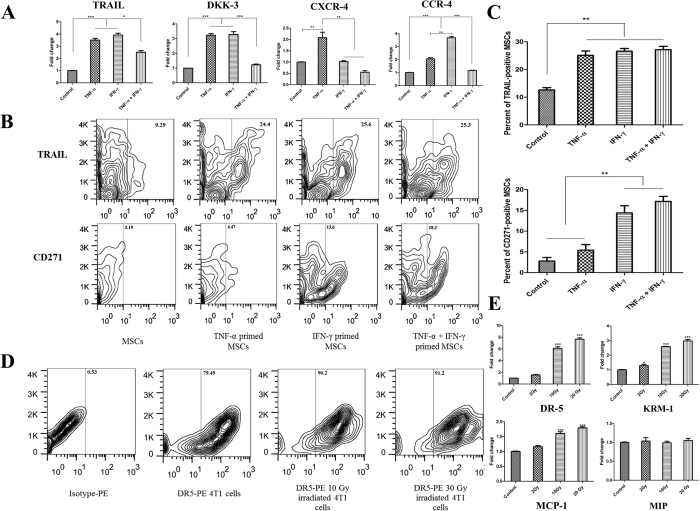
At first step, the expression of TRAIL, DKK-3 and chemokines including CXCR-4 and CCR-4 were analyzed in AD-MSCs after activation with TNF-α, IFN-γ and TNF-α plus IFN-γ in different doses (data not shown). Finally, either TNF-α or IFN-γ was used at 20 ng/ml. In TNF-α plus IFN-γ group, 10 ng/ml of each cytokine was used. Our data showed that preactivation of AD-MSCs with either TNF-α or IFN-γ significantly upregulated the expression of TRAIL and DKK-3 compared to naïve AD-MSC and TNF-α plus IFN-γ activated MSCs (P < 0.001 and P < 0.05 respectively). In addition, CXCR4 and CCR4 expressions were significantly increased by the treatment of TNF-α or IFN-γ, respectively (P < 0.01 and P < 0.001 respectively) (Fig. 1A). At protein level, obtained data showed that expression level of TRAIL was significantly elevated in MSCs following preactivation with either TNF-α or IFN-γ and TNF-α plus IFN-γ (P < 0.01) (Fig. 1B,C). To select best candidate for AD-MSCs preactivation, we checked the immunosuppressive properties of AD-MSCs after MSCs activation either by TNF-α, IFN-γ or TNF-α plus IFN-γ. Our previous data indicated that TNF-α modulates the immunosuppressive effects of AD-MSCs on T cells and Dendritic cells compared to IFN-γ and IFN-γ plus TNF-α16. Moreover, we evaluated the expression of CD271 as a marker which displays the potent immunosuppressive AD-MSCs17. CD271 expression dramatically increased in AD-MSCs upon activation with IFN-γ and TNF-α plus IFN-γ but not TNF-α alone (P < 0.01) (Fig. 1B,C).
Figure 1. Preactivetion of MSCs with TNF-α increased the expression of TRAIL, DKK-3 and CXCR-4.
(A) Real-time assay of TRAIL, DKK-3, CXCR-4 and CCR-4 in MSCs after activation with TNF-α, IFN-γ and TNF-α plus IFN-γ for 6 hrs. (B,C) flowcytometry assay of TRAIL and CD271 in AD-MSCs after activation with TNF-α, IFN-γ and TNF-α plus IFN-γ for 24 hrs. (D) Flowcytometry analysis of DR-5 expression in 4T1 cells after irradiation in different doses. (E) Enhanced expression of DR-5, Krm-1 and MCP-1 in 4T1 cells after irradiation confirmed by real-time PCR. All results are expressed by mean ± SD from at least three independent experiments analyzed by ANOVA with Tukey’s posthoc. *P < 0.05, **P < 0.01, ***P < 0.001.
Irradiation enhanced the expression of DR5 and Kremen1 in 4T1 cells
The expression of death receptor 5 (DR-5) and Kremen-1 (Krm-1) as main receptors of TRAIL and DKK-3 respectively, was analyzed after irradiation in 4T1 cells. The expression of DR-5 and Krm-1 at both mRNA and protein level was significantly up-regulated by irradiation compared to non-irradiated 4T1 cells (P < 0.001) (Fig. 1D,E). On the other hands, the expression of important chemokine receptors were evaluated and the expression of MCP-1 was noticeably increased after irradiation in 4T1 cells (P < 0.001) (Fig. 1E).
Irradiation increased antitumor effects TNF-α activated AD-MSCs
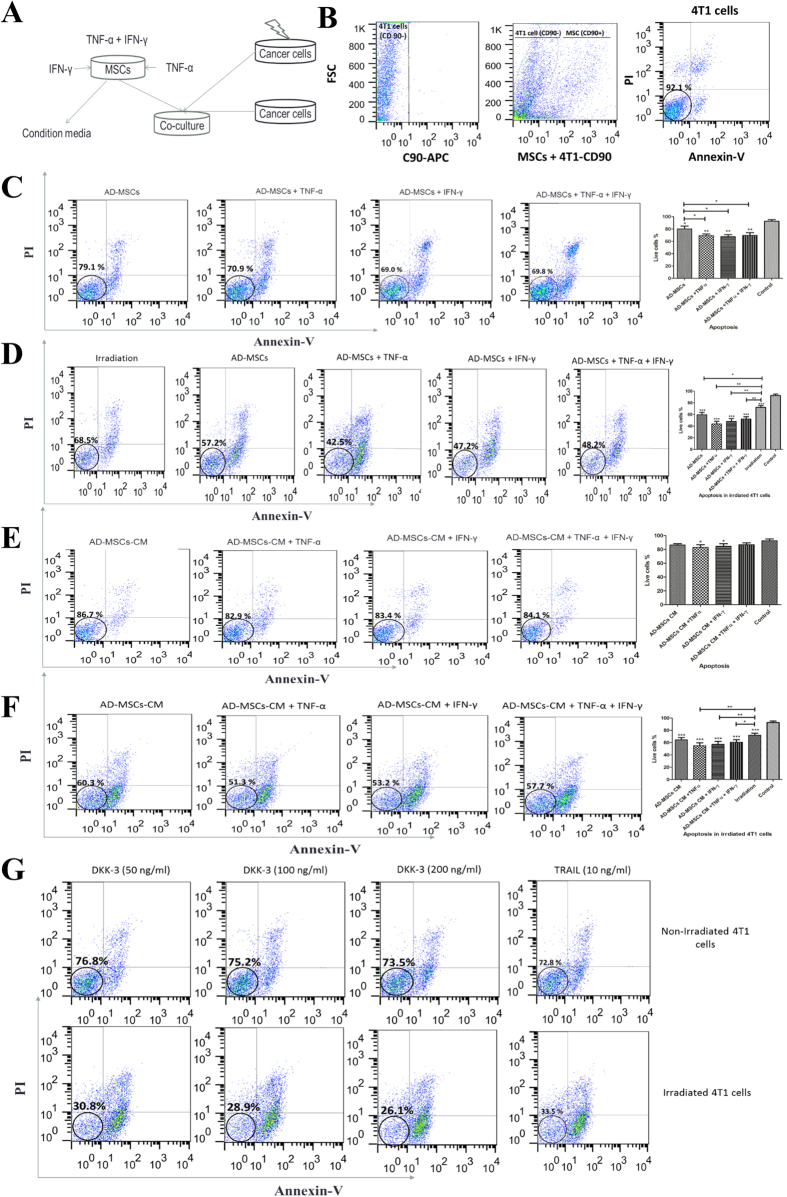
The apoptosis induction in naïve and irradiated 4T1 cells was analyzed following co-culture with naïve and activated AD-MSCs (Fig. 2A). We first isolated MSCs from 4T1 cells using CD90 as a specific marker for AD-MSCs (Fig. 2B). Our results showed that antitumor effects of naïve AD-MSCs were significantly enhanced following pre-activation with proinflammatory cytokines (P < 0.01; Fig. 2C) and this antitumor effects were dramatically increased after 4T1 cells irradiation (P < 0.001; Fig. 2D). As previously reported, MSCs derived condition media (MSCs-CM) can induce apoptosis and post apoptotic necrosis in cancer cells. Therefore, we next analyzed the percentage of live 4T1 cells after 24 hrs culture in the presence of 50% AD-MSCs-CM collected from preactivated AD-MSCs either by TNF-α, IFN-γ or TNF-α plus IFN-γ. Obtained results indicated that antitumor effects of AD-MSCs-CM were remarkably increased in either TNF-α or IFN-γ activated AD-MSCs but not in TNF-α plus IFN-γ group (P < 0.05; Fig. 2E) and the rate of apoptosis and post apoptotic necrosis on 4T1 cells significantly increased after irradiation (P < 0.01; Fig. 2F). On the other hands, irradiation also remarkably increased the apoptosis and post apoptosis necrosis induced by rhTRAIL and rhDkk-3 (P < 0.001; Fig. 2G).
Figure 2. Irradiation increased antitumor effects of either cytokine preactivated AD-MSCs or cytokine preactivated AD-MSCs derived condition media.
(A) Schematic diagram. (B) 4T1 cells were separated from MSCs by negative selection using CD90. Annexin-PI based flowcytometry analysis were used to check apoptosis and post apoptotic necrosis after co-culture for 24 hrs. Apoptosis assay was executed in (C) naïve or preactivated AD-MSCs and 4T1 cells co-culture, (D) naïve or preactivated AD-MSCs and irradiated 4T1 cells co-culture, (E) naïve or preactivated AD-MSCs derived condition media and 4T1 cells co-culture, (F) naïve or preactivated AD-MSCs derived condition media and irradiated 4T1 cells co-culture. rhDkk-3 and rhTRAIL were used as positive controls. Flowcytometry assay of induced apoptosis by (G) rhDKK-3 and rhTRAIL in 4T1 and irradiated 4T1 cells are presented. All results are expressed by mean ± SD from at least three independent experiments analyzed by ANOVA with Tukey’s posthoc. *P < 0.05, **P < 0.01, ***P < 0.001.
Irradiation combined with TNF-α activated MSCs decreased tumor size and improved survival of mice
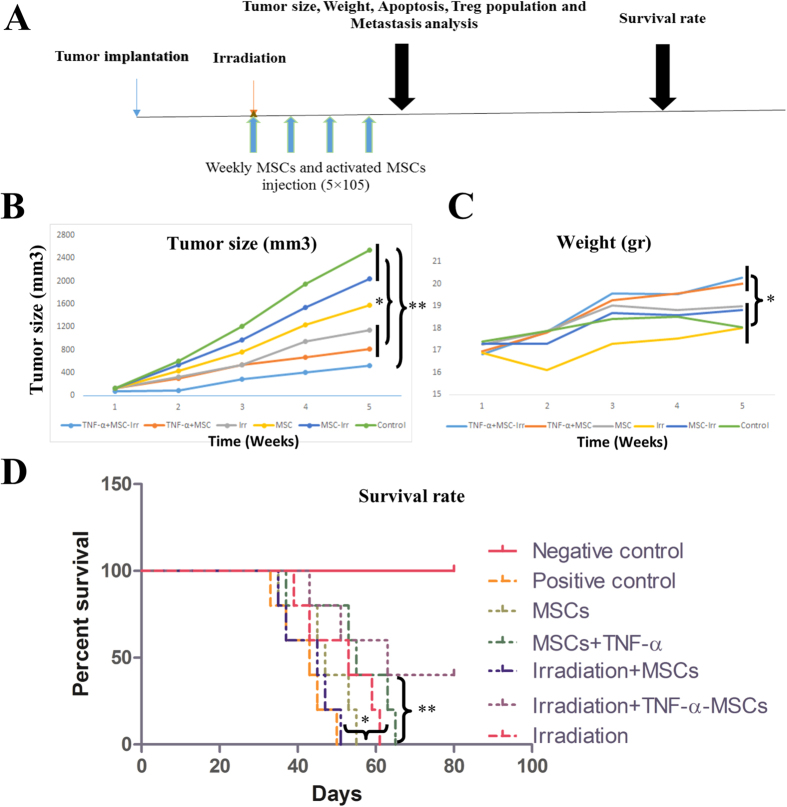
Based on our current and previous studies, TNF-α was the best candidate for AD-MSCs preactivation. The schematic timeline of in vivo experiment is presented in Fig 3A. Tumor size in mice which received TNF-α preactivated AD-MSCs combined with irradiation was measured in comparison to the rest of mice (P < 0.01; Fig. 3B). TNF-α activated AD-MSCs or irradiation significantly decreased the tumor size compared to naïve MSCs or naïve MSCs with irradiation groups (P < 0.05; Fig. 3B). On the other hands, the body weight of mice as a possible indicator of quality of life, was measured. The body weight loss in TNF-α preactivated AD-MSCs plus irradiation and TNF-α preactivated AD-MSCs groups was significantly improved compared to the other groups (P < 0.05; Fig. 3C). Mice which received TNF-α preactivated AD-MSCs with irradiation treatment survived more than other groups (P < 0.01; Fig. 3D). Moreover, survival rate significantly increased in TNF-α activated AD-MSCs and irradiation groups compared to the groups of naïve MSCs with or without irradiation (P < 0.05; Fig. 3D).
Figure 3. TNF-α activated MSCs combined with irradiation increased survival rate and weight of mice and decreased tumor size.
(A) Schematic diagram of treatment strategy. Mice was inoculated with 1 × 106 4T1 cells and 2 weeks later, tumor mass (tumors were tangible) were irradiated with 6 Gy followed with 4 times weekly intratumoral MSCs injection for four weeks. (B) Tumor size and body weight (C) were assayed every 2 days with digital caliper and animal weight scale respectively. (D) Survival rate were assessed by a log-rank test based on the Kaplan–Meier method. All results are expressed by mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Combination therapy of irradiation with TNF-α activated MSCs prevented lung and liver metastasis and induced apoptosis in 4T1 cells
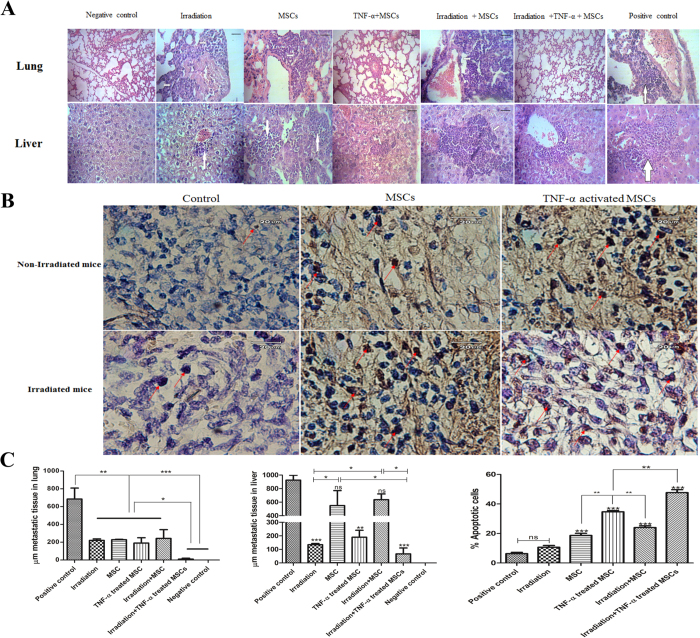
The metastasis in liver and lung was assessed by measuring μm metastatic tissues in whole lung or liver organs. In all treatment groups, metastasis in lung was noticeably decreased compared to the control group (P < 0.01; Fig. 4A,C). Interestingly, combination therapy of TNF-α preactivated MSCs with irradiation dramatically abrogated metastasis in lung to a greater extent compared to other treatment groups (P < 0.05 and P < 0.001 respectively; Fig. 4A,C). Surprisingly, liver metastasis was remarkably reduced in irradiation, TNF-α activated AD-MSCs and TNF-α preactivated MSCs plus irradiation groups but not in naïve AD-MSCs and naïve AD-MSCs plus irradiation groups (Fig. 4A,C). We next analyzed the induced apoptosis in tumor tissues one week after last AD-MSC injection. The tumor in mice that received MSCs or preactivated MSCs contained more apoptotic cells compared to irradiation and control group (Fig. 4B,C). Additionally, both TNF-α preactivated AD-MSCs and TNF-α preactivated MSCs plus irradiation significantly induced apoptosis in the tumor cells compared to the naïve AD-MSCs and naïve MSCs plus irradiation respectively (P < 0.01). Moreover, apoptotic rate in tumor cells of mice which received TNF-α preactivated MSCs plus irradiation was significantly higher than that of mice with single treatment of TNF-α preactivated MSCs (P < 0.01; Fig. 4C).
Figure 4. TNF-α activated MSCs combined with irradiation inhibited lung and liver metastasis and induced apoptosis in tumor mass.
(A) Mice was inoculated with 1 × 106 4T1 cells and 2 weeks later, tumor mass (tumors were tangible) were irradiated with 6 Gy followed with 4 times weekly intratumoral MSCs injection for four weeks. One week after last injection, lung and live tissues were collected and the metastasis were checked by hematoxylin and eosin (H&E) staining. (B) One week after last injection induced apoptosis in tumor mass were analyzed by TUNNEL test. (C) The metastasis were quantified by measuring the presence of μmeter (μm) metastatic tissues in lung and liver. Apoptotic activities were quantified by measuring the percent of apoptotic bodies per 2,000 nuclei. All results are expressed by mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
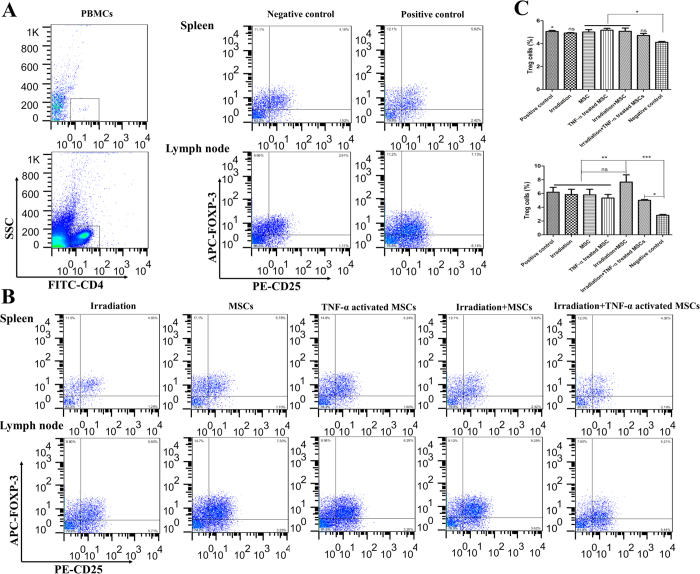
TNF-α activated MSCs with irradiation did not induce Treg cells in lymph node and spleen
One of the important effects of MSCs is Treg induction in the tumor environment. Therefore, we analyzed Treg populations in spleen and local lymph node marked by detecting CD4+, CD25+ and FOXP-3+ cells (Fig. 5A). Tumor generation in mice significantly increased Treg population in both spleen and lymph node compared to healthy mice (Fig. 5A). Interestingly, Treg percentage in spleen was significantly increased in mice which received either MSCs, TNF-α preactivated MSCs or MSCs plus irradiation but not in irradiation and TNF-α preactivated MSCs plus irradiation groups (Fig. 5B,C upper panel). Furthermore, naïve AD-MSCs plus irradiation dramatically induced Treg cells in local lymph nodes (P < 0.001; Fig. 5B,C lower panel). Treg population in TNF-α preactivated MSCs plus irradiation group was increased, but to a lower extent than that in naïve MSCs plus irradiation group (P < 0.05; 5 ± 0.5 vs 7.65 ± 1.5; Fig. 5B,C).
Figure 5. MSCs combined with irradiation but not TNF-α activated MSCs combined with irradiation increased Treg population in lymph node and spleen.
(A) Regulatory T cells (Treg) population were analyzed by CD4+, CD25+ and FOXP-3+ in lymph node and spleen one week after last injection using flowcytometry. (B) Representative documentation of Treg cells by flowcytometry and (C) percentage of Treg cells in lymph node and spleen.
Discussion
MSC based cancer therapy have been recently demonstrated in several studies. Previous researches indicated that MSC therapy may lead to either promotion or prevention of tumor growth depending on the route or time point of injection, and type of tumor models18,19. Regarding breast cancer, Leng et al. demonstrated that human umbilical cord-MSCs (hUC-MSCs) inhibited tumor angiogenesis and induced apoptosis in MDA-MB-231 based breast cancer model in Nude mice20. More recently, it has been shown that MSCs derived from iPSCs do not promote tumor growth21. Moreover, MSCs inserted with IL-222, IL-1223, IL-2124, TRAIL25 and DKK-326 genes have been used in wide range of cancer models with promising results. Conversely, it has been indicated that cancer stroma resident MSCs have increased tumor growth through the promotion of cancer metastasis, providing a carcinoma stem cell niche and cytokine networks27,28.
In the present study, we demonstrated that TNF-α activated MSCs combined with irradiation is more advantageous comparing with either irradiation or TNF-α activated MSCs alone. We used TNF-α activated MSCs instead TRAIL-expressing MSCs because, MSCs activation by TNF-α increased expression of TRAIL, DKK-3 and migratory protein in MSCs altogether, modulates immunosuppressive effects of MSCs and is easier to apply compared to gene insertion by delivery systems such as an adenoviral or lentiviral vectors. Obtained data showed that expression of TRAIL and Dkk-3 as main antitumor factors was increased in AD-MSCs following activation with TNF-α. Here, we focused on TRAIL and DKK-3 proteins, because previous studies showed that anticancer effects of MSCs are mainly related to these factors13,29. Also, Lee et al., showed that siRNA inhibition of either TRAIL or DKK-3 strongly inhibit anticancer effects of MSCs in MDA cell line11. We further showed that irradiation enhanced susceptibility of 4T1 cells to activated AD-MSCs as well as MSC derived condition medium through the up-regulation of DR5 and Krm-1. We demonstrated that TRAIL and Dkk-3 efficiently induced apoptosis and post apoptosis necrosis in 4T1 cells, implying that 4T1 cells are sensitive to both TRAIL and Dkk-3. Moreover, irradiation increased 4T1 sensitivity to anticancer effects of TRAIL and Dkk-3 through upregulation of DR-5 and Krm-1 receptors. These data are in parallel with reported results by Kim et al., demonstrating increased susceptibility of glioma cell line to anticancer effects of TRAIL-expressing MSCs. Also, they showed that migration capability of MSCs toward tumor site increased by irradiation through enhanced secretion of IL-8 by irradiated tumor cell15. Previous studies indicated that TRAIL is a potent anticancer protein and that its effects can be synergistically increased by chemotherapy and irradiation15,30. TRAIL induced apoptosis in cancer cells through DR4/DR5 in human and DR5 in mice as well as activating caspase pathway demonstrated in lung, breast, and glioblastoma cancer cells31. Moreover, some researches showed that apoptotic cell-derived RNA and DNA can activate MSCs and other immune cells through TLRs such as TLR-3 and provides a feed forward reaction, leading to the increase in TRAIL expression and subsequent tumor suppression32. On the other hands, there are several reports which showed that Dkk-3 implies antitumor effects through cell cycle inhibition via Wnt-β-catenin inhibition33, EGFR blocking34 and more recently, apoptosis induction35. Previous investigations reported that Wnt-β-catenin inhibition and EGFR blocking are potential treatment for cancer36,37. On the other hands, it has been shown that MSCs derived condition media inhibited cancer cells growth38. Condition media derived Dkk-3 induced apoptosis through mitochondrial and Fas death receptor pathways35. In addition, condition medium derived from MSCs down-regulated the expression of vascular endothelial growth factor partially by miR-16 and suppressed angiogenesis39.
In this study, we showed that tumor growth, survival rate and metastasis were not significantly altered by naïve (without cytokine activation) AD-MSCs injection. However, TNF-α preactivated AD-MSCs significantly inhibited tumor growth, metastasis and improved survival rate in tumor bearing mice and this antitumor effects were remarkably intensified by single dose irradiation one day prior to initial administration of cells. In the present study, AD-MSCs were injected by intratumoral route, because previous results showed that intratumoral injection of MSCs exhibited potent anticancer effects with more maintenance and stability in comparison to other injection routes40. Furthermore, intratumoral injection provided enough numbers of MSCs in tumor site. Also, it has been shown that MSCs are mostly trapped in lung when injected intravenously41. We used irradiation as an adjuvant therapy that have increased antitumor effects of modified MSCs reported by several reports. Balyasnika et al., showed TRAIL expressing MSCs in combination with irradiation promoted survival rate in brain tumor bearing mice42. More recently, it has been shown that irradiation enhanced curative effects of IL-12 expressing MSCs in murine metastatic hepatoma by inducing MSCs localization and increasing apoptotic activity43. Radiotherapy enhanced tumor cell sensitivity to TRAIL by inducing expression of DR-4/DR-5 in human or DR-5 in mice confirmed in wide range of tumor such as lung, colon, head and neck, prostate and breast cancer. Furthermore, it has been found that radiotherapy also induced stress pathways and death signaling in independent manner in cancer cells44.
Finally, it has been demonstrated that the number and activity of Treg increased by the soluble factors secreted from tumor associated MSCs, such as IL-10 and TGF-β45. Therefore, we here examined Treg population in spleen and lymph node following tumor inoculation. Interestingly, naïve MSCs but not TNF-α preactivated MSCs with irradiation induced Treg development in lymph node. This effect is possibly related to MSC recruitment from other tissues towards tumor mass. Because it has been previously shown that irradiation increased the migratory properties of MSCs by inducing the secretion of VEGF, tumor growth factor-β (TGF-β) and platelet-derived growth factor (PGEF) by tumor cells. Furthermore, enhanced level of TGF-β and PGEF in tumor cells can directly contribute to the development of Treg cells in tumor microenvironment6.In preactivated MSCs, it seems that high level of apoptosis induction decreased the tumor cell mass as a main source of soluble factor release which in turn recruit MSCs and induce Treg cells. Moreover, some studies showed that TNF-α modulated the immunosuppressive effects of MSCs16,46.
In conclusion, these data suggest that intratumoral delivery of TNF-α preactivated MSCs immediately after single dose irradiation could be a potential strategy for cancer treatment through the strongest antitumor effects as well as significant suppressive action on metastasis of breast cancer.
Material and Methods
Mice and tumor lines
Six to eight weeks old female Balb/c mice from Pasteur institute, Tehran, Iran were used according to institutional guidelines approved by Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran. All mice were kept in approved animal facility with ad libitum access to food and water under 12 hrs dark and light. Breast adenocarcinoma (4T1) mouse cancer cells and mouse embryonic fibroblast (MEF) cell lines were provided from Pasteur institute, Tehran, Iran. Mouse adipose derived MSCs (AD-MSCs) were isolated and confirmed based on our previous study16,47. Mice were divided into 7 groups including healthy group (tumor negative group), tumor control, Single irradiation, MSCs, TNF-α activated MSCs, MSCs plus irradiation, TNF-activated MSCs plus irradiation.
Reagents and antibodies
All used antibodies including PE conjugated TRAIL, PE conjugated CD271, FITC conjugated CD4, PE conjugated CD25, APC conjugated FOXP-3, PE conjugated DR-5 and APC conjugated CD90 were purchased from eBioscience company (San Diego, USA). The rhTRAIL and rhDKK-3 proteins were provided form PeproTech and Sino Biological companies, respectively (Seoul, South Korea and North Wales, USA, respectively).
Real-time PCR
Real-time PCR procedure was executed based on the 1 μg/μl cDNA in all samples. Primer pairs for all considered genes are listed in Supplementary Table-1. The exact mRNA expression was normalized to the expression level of GAPDH.
Irradiation
4T1 and MEF cells were irradiated with different doses including 5, 10 and 30 Gy provided by Gammacell 3000 Elan source. Irradiation of the animals was performed two weeks after tumor induction using a Co-60 unit (Imatron, Canada) as the gamma radiation source at a dose 6 Gy single. Tumor bearing mice were fixed using an acrylic chamber to avoid mice movement and precisely expose the tumors of the mice. The body of mice except tumor site was shielded using a specially designed lead apparatus.
Cocultures of AD-MSCs and 4T1 Cells
AD-MSCs were cultured in low glucose Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin at 37 °C with 5% CO2. 4T1 cancer cells were grown in Roswell Park Memorial Institute (RPMI 1640) with 10% FBS and 1% penicillin/streptomycin. AD-MSCs were activated by 20 ng/ml TNF-α 24 hrs before co-culture. After 24 hrs, AD-MSCs were twice washed by PBS and 1 × 105 AD-MSCs were cocultured with naïve and irradiated 4T1 cells in ratio of 1:1 for 24 hrs. For condition medium (CM) preparation, after AD-MSCs activation by TNF-α, the medium was replaced and maintained for another 24 hrs. After 24 hrs, condition medium was collected and stored in −70 °C for further analysis. Naïve and irradiated 4T1 cells were cultured with 50% activated AD-MSCs derived CM and 50% normal culture medium for 24 hrs.
Flow Cytometry
4T1 cancer cells were separated from AD-MSCs cells by negative selection using APC conjugated CD90 antibody. Apoptosis was evaluated using Annexin & PI apoptosis kit (eBioscience, San Diego, USA) in naïve and irradiated 4T1 cells according to the manufacture’s instruction. The regulatory T cells (Treg) population was analyzed by Treg kit based on the manufacture’s protocol in spleen and local lymph node.
Breast cancer Model, AD-MSCs Infusion and Tissue Collection
Breast cancer model in Balb/c mice were induced by subcutaneous injection of 0.2 ml Phosphate buffered saline (PBS) containing 1 × 106 4T1 cells to the right hind limb of the mice. After two weeks, when the tumor size was tangible, mice were irradiated with 6 Gy. After one day, naïve and TNF-α activated AD-MSCs were weekly injected into tumor area for 4 weeks. One week after last injection, liver, lung and tumor tissues were collected for further analysis.
Tumor size measurement
The tumor dimensions were measured every two days by using a digital caliper and the tumor volume was calculated with the following formula: tumor volume [mm3] = (length [mm]) × (width [mm]) 2 × 0.52.
Lung and liver metastasis
Lung and liver tissues were fixed by 70% formalin and were stained with Hematoxylin and Eosin (H&E) dyes according to the previous studies48.
TUNEL assay
Tissue were fixed with paraformaldehyde and embedded in paraffin. In vivo apoptosis were analyzed using In Situ Cell Death Detection Kit, POD (Roche, Mannheim, Germany) based on manufacture’s protocol. In each group, at least ten area were selected to detect the apoptosis. Apoptotic activities were quantified by measuring the percent of apoptotic bodies per 2,000 nuclei.
Statistical analysis
Obtained results were analyzed by GraphPad Prism version 4.0 and SPSS 16.0. Two-tailed Student’s t-test and ANOVA with post-hoc Tukey’s Multiple Comparison tests were used for differences between two groups and more than two groups respectively. Kaplan- Meier test was used for survival and comparison of groups was determined by Log-rank (Mantel-Cox). P value <0.05 was considered as statistically significant.
Additional Information
How to cite this article: Mohammadpour, H. et al. Irradiation enhances susceptibility of tumor cells to the antitumor effects of TNF-α activated adipose derived mesenchymal stem cells in breast cancer model. Sci. Rep. 6, 28433; doi: 10.1038/srep28433 (2016).
Supplementary Material
Acknowledgments
This work was partially supported by grants from Iran National Science Foundation and Tarbiat Modares University, Tehran, Iran. We thank Dr. Hyung‐Sik Kim for final proof of manuscript and Ms. Somayeh Yousefi for kind helps in mice handling and sampling.
Footnotes
Author Contributions H.M. wrote the main manuscript text and prepared all figures. H.M., A.A.P. and M.N.Z. made substantial contributions to conception and design. H.M., M.N.Z. and A.A.S. made substantial contributions to acquisition of data and analysis and interpretation of data. All authors participated in revising the article critically for important intellectual content. A.A.P. gave final approval of the submitted manuscript. All authors reviewed the manuscript.
References
- Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2015. CA Cancer. J. Clin. 65, 5–29 (2015). [DOI] [PubMed] [Google Scholar]
- Jagsi R. Progress and controversies: radiation therapy for invasive breast cancer. CA Cancer. J. Clin. 64, 135–152 (2014). [DOI] [PubMed] [Google Scholar]
- Whelan T. J. et al. Long-term results of hypofractionated radiation therapy for breast cancer. N. Engl. J. Med. 362, 513–520 (2010). [DOI] [PubMed] [Google Scholar]
- Group E. B. C. T. C. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 378, 1707–1716 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibault J.-E. et al. Personalized radiation therapy and biomarker-driven treatment strategies: a systematic review. Cancer. Metastasis. Rev. 32, 479–492 (2013). [DOI] [PubMed] [Google Scholar]
- Klopp A. H. et al. Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer. Res. 67, 11687–11695 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P. et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nature. Med. 19, 35–42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug. Deliv. Rev. 64, 739–748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell. Res. 20, 510–518 (2010). [DOI] [PubMed] [Google Scholar]
- Sasportas L. S. et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc. Natl. Acad. Sci. 106, 106, 4822–4827 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. H., Yoon N., Reneau J. C. & Prockop D. J. Preactivation of human MSCs with TNF-α enhances tumor-suppressive activity. Cell. stem. cell. 11, 825–835 (2012). [DOI] [PubMed] [Google Scholar]
- Refaat A., Abd Rabou A. & Reda A. TRAIL combinations: The new ‘trail’for cancer therapy (Review). Oncol. Lett. 7, 1327–1332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop M. et al. Antitumor effects of TRAIL-expressing mesenchymal stromal cells in a mouse xenograft model of human mesothelioma. Cancer. Gene. Ther. 22, 44–54 (2015). [DOI] [PubMed] [Google Scholar]
- Veeck J. & Dahl E. Targeting the Wnt pathway in cancer: the emerging role of Dickkopf-3. Biochim. Biophys. Acta. 1825, 18–28 (2012). [DOI] [PubMed] [Google Scholar]
- Kim S. M. et al. Irradiation Enhances the Tumor Tropism and Therapeutic Potential of Tumor Necrosis Factor‐Related Apoptosis‐Inducing Ligand‐Secreting Human Umbilical Cord Blood‐Derived Mesenchymal Stem Cells in Glioma Therapy. Stem. Cell 28, 2217–2228 (2010). [DOI] [PubMed] [Google Scholar]
- Mohammadpour H., Pourfathollah A. A., Zarif M. N. & Tahoori M. T. TNF-α modulates the immunosuppressive effects of MSCs on dendritic cells and T cells. Int. Immunopharmacol. 28, 1009–1017 (2015). [DOI] [PubMed] [Google Scholar]
- Kuçi S. et al. CD271 antigen defines a subset of multipotent stromal cells with immunosuppressive and lymphohematopoietic engraftment-promoting properties. haematologica. 95, 651–659 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell. Death. Differ. 19, 1505–1513 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. S. Mesenchymal stem cells: angels or demons? Biomed. Res. Int. 2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng L. et al. Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy. Biomaterials. 35, 5162–5170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q. et al. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc. Natl. Acad. Sci. 112, 530–535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene. Ther. 11, 1155–1164 (2004). [DOI] [PubMed] [Google Scholar]
- Hong X., Miller C., Savant-Bhonsale S. & Kalkanis S. N. Antitumor treatment using interleukin‐12‐secreting marrow stromal cells in an invasive glioma model. Neurosurgery 64, 1139–1147 (2009). [DOI] [PubMed] [Google Scholar]
- Kim N. et al. IL-21-Expressing Mesenchymal Stem Cells Prevent Lethal B-Cell Lymphoma Through Efficient Delivery of IL-21, Which Redirects the Immune System to Target the Tumor. Stem. Cells. Dev. 24, 2808–2821 (2015). [DOI] [PubMed] [Google Scholar]
- Kim S. M. et al. Gene therapy using TRAIL-secreting human umbilical cord blood–derived mesenchymal stem cells against intracranial glioma. Cancer. Res. 68, 9614–9623 (2008). [DOI] [PubMed] [Google Scholar]
- Abarzua F. et al. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer. Res. 65, 9617–9622 (2005). [DOI] [PubMed] [Google Scholar]
- Karnoub A. E. et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 449, 557–563 (2007). [DOI] [PubMed] [Google Scholar]
- Joyce J. A. & Pollard J. W. Microenvironmental regulation of metastasis. Nat. Rev. Cancer. 9, 239–252 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.-J. et al. TRAIL-engineered bone marrow-derived mesenchymal stem cells: TRAIL expression and cytotoxic effects on C6 glioma cells. Anticancer. Res. 34, 729–734 (2014). [PubMed] [Google Scholar]
- Buchsbaum D. J. et al. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin. Cancer. Res. 9, 3731–3741 (2003). [PubMed] [Google Scholar]
- Almasan A. & Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine. Growth. Factor. Rev. 14, 337–348 (2003). [DOI] [PubMed] [Google Scholar]
- Chen G. Y. & Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeck J. et al. Wnt signalling in human breast cancer: expression of the putative Wnt inhibitor Dickkopf-3 (DKK3) is frequently suppressed by promoter hypermethylation in mammary tumours. Breast. Cancer. Res. 10, 1–11 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadpour H., Pourfathollah A. A., Zarif M. N. & Khalili S. Key role of Dkk3 protein in inhibition of cancer cell proliferation: An in silico identification. J. Theor. Biol. 393, 98–104 (2016). [DOI] [PubMed] [Google Scholar]
- Takata A. et al. Dkk-3 induces apoptosis through mitochondrial and Fas death receptor pathways in human mucinous ovarian cancer cells. Int. J. Gynecol. Cancer. 25, 372–379 (2015). [DOI] [PubMed] [Google Scholar]
- Chartier C. et al. Therapeutic targeting of tumor-derived R-spondin attenuates β-catenin signaling and tumorigenesis in multiple cancer types. Cancer. Res, 0561.2015 (2015). [DOI] [PubMed] [Google Scholar]
- Troiani T. et al. Increased TGF-α as a mechanism of acquired resistance to the anti-EGFR inhibitor cetuximab through EGFR–MET interaction and activation of MET signaling in colon cancer cells. Clin. Cancer. Res. 19, 6751–6765 (2013). [DOI] [PubMed] [Google Scholar]
- Mohammadpour H. & Majidzadeh-A. K. Antitumor effect of conditioned media derived from murine MSCs and 5-aminolevulinic acid (5-ALA) mediated photodynamic therapy in breast cancer in vitro. Photodiagnosis. Photodyn. Ther. 12, 238–243 (2015). [DOI] [PubMed] [Google Scholar]
- Lee J.-K. et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLos One 8, e84256 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S. et al. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene. Ther. 18, 488–495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. H. et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell. stem. cell. 5, 54–63 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balyasnikova I. V. et al. Intranasal delivery of mesenchymal stem cells significantly extends survival of irradiated mice with experimental brain tumors. Mol. Ther. 22, 140–148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong K. Y. et al. Irradiation‐induced localization of IL‐12‐expressing mesenchymal stem cells to enhance the curative effect in murine metastatic hepatoma. Int. J. Cancer. 137, 721–730 (2015). [DOI] [PubMed] [Google Scholar]
- Kroemer G., Galluzzi L., Kepp O. & Zitvogel L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013). [DOI] [PubMed] [Google Scholar]
- Huang B. et al. Gr-1+ CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer. Res. 66, 1123–1131 (2006). [DOI] [PubMed] [Google Scholar]
- English K., Barry F. P., Field-Corbett C. P. & Mahon B. P. IFN-γ and TNF-α differentially regulate immunomodulation by murine mesenchymal stem cells. Immunology. lett. 110, 91–100 (2007). [DOI] [PubMed] [Google Scholar]
- Mohammadpour H., Pourfathollah A. A., Nikougoftar Zarif M. & Hashemi S. M. Increasing proliferation of murine adipose tissue-derived mesenchymal stem cells by TNF-α plus IFN-γ. Immunopharmacol. Immunotoxicol. 38, 68–76 (2016). [DOI] [PubMed] [Google Scholar]
- Fischer A. H., Jacobson K. A., Rose J. & Zeller R. Hematoxylin and eosin staining of tissue and cell sections. Cold. Spring. Harbor. Protocols 2008, pdb. prot4986 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.