Abstract
Whether cytokines can influence the adaptive immune response by antigen-specific γδ T cells during infections or vaccinations remains unknown. We previously demonstrated that, during BCG/Mycobacterium tuberculosis (Mtb) infections, Th17-related cytokines markedly upregulated when phosphoantigen-specific VγVδ2 T cells expanded. In this study, we examined the involvement of Th17-related cytokines in the recall-like responses of Vγ2Vδ2 T cells following Mtb infection or vaccination against TB. Treatment with IL-17A/IL-17F or IL-22 expanded phosphoantigen 4-hydroxy-3-methyl-but-enyl pyrophosphate (HMBPP)-stimulated Vγ2Vδ2 T cells from BCG-vaccinated macaques but not from naïve animals, and IL-23 induced greater expansion than the other Th17-related cytokines. Consistently, Mtb infection of macaques also enhanced the ability of IL-17/IL-22 or IL-23 to expand HMBPP-stimulated Vγ2Vδ2 T cells. When evaluating IL-23 signaling as a prototype, we found that HMBPP/IL-23-expanded Vγ2Vδ2 T cells from macaques infected with Mtb or vaccinated with BCG or Listeria ΔactA prfA*-ESAT6/Ag85B produced IL-17, IL-22, IL-2, and IFN-γ. Interestingly, HMBPP/IL-23-induced production of IFN-γ in turn facilitated IL-23-induced expansion of HMBPP-activated Vγ2Vδ2 T cells. Furthermore, HMBPP/IL-23-induced proliferation of Vγ2Vδ2 T cells appeared to require APC contact and involve the conventional and novel protein kinase C signaling pathways. These findings suggest that Th17-related cytokines can contribute to recall-like expansion and effector function of Ag-specific γδ T cells after infection or vaccination.
Keywords: γδ T cells, IL-17/IL-22, IL-23, Phosphoantigen, Tuberculosis
Introduction
IL-17A/IL-17F, IL-22, and IL-23 cytokines can be classified as Th17/Th22-related cytokines, as Th17 and/or Th22 cells [1, 2] are usually polarized or induced by IL-6, TGF-β, and IL-1β, and expanded by IL-23 [3, 4]. IL-17/IL-22 can be produced by CD4+/CD8+/γδ/NKT cells [5–9], whereas IL-23 is dominantly produced by dendritic cells and macrophages [10]. IL-17A and IL-17F have been shown to exhibit pleiotropic biological activities on neutrophils, fibroblasts, endothelial/epithelia cells [11–13], while IL-22 can regulate keratinocytes involved in skin inflammation [14] or exhibit anti-Mycobacterium tuberculosis (Mtb) effector function in vitro [15]. To date, none of theTh17-related cytokines has been shown to act alone to significantly induce recall-like expansion/effector functions of naïve T cells [16, 17].
Human γδ T cells appear to be nonclassical T cells that contribute to both innate and adaptive immune responses [18–21]. Dominant Vγ2Vδ2 T cells exist only in human/nonhuman primates and remain the sole γδ T-cell subset capable of recognizing phosphoantigen (E)-4-hydroxy-3-methyl-but-enyl pyrophosphate (HMBPP) from Mtb, M. bovis BCG, Listeria monocytogenes, and other pathogens [22, 23]. Although recall-like immune responses of Vγ2Vδ2 T cells have been demonstrated during infections with HMBPP-producing Mtb, BCG, or L. monocytogenes [24, 25], the immune mechanisms by which these memory-like responses develop after infections remain unknown.
Recent studies have elucidated the molecular interaction between the Vγ2Vδ2 TCR and HMBPP presented on APC membranes [26–28], making it possible to focus on HMBPP/TCR-stimulated and cytokine-driven cellular signals required for γδ T-cell responses. Of note, we have recently demonstrated that Mtb and BCG infections can induce high-level expression of Th17-related cytokine genes but not IL-2 [29, 30], and that the upregu-lation of IL-22 and IL-23 coincides with the expansion of Vγ2Vδ2 T cells in lungs and lymphoid organs [25]. We therefore hypothesize that Th17-related cytokines may stimulate recall-like expansion/effector functions of Vγ2Vδ2 T cells in HMBPP-producing microbial infections. To test this hypothesis, we produced and purified these Th17-related cytokines and assessed each of them for the ability to function as growth factors conferring adaptive-like immune responses after infection of macaques with HMBPP-producing Mtb, BCG, or L. monocytogenes.
Results and discussion
Th17 cytokines induce a greater HMBPP-stimulated Vγ2Vδ2 T-cell expansion in BCG-vaccinated monkeys
We previously demonstrated that major expansion of Vγ2Vδ2 T cells coincided with upregulation of Th17-related cytokines during mycobacterial infections [25, 29]. We therefore sought to determine whether IL17-A/IL17-F, IL-22, or IL-23 could facilitate HMBPP activation and expansion of Vγ2Vδ2 T cells in mycobacterial infection. We successfully produced and purified macaque IL-17A, IL-17F, and IL-22 (Fig. 1A, top) by employing our pXmh vector/L. lactis PA1001 expression system [31]. Considerably pure IL-17A, IL-17F, and IL-22 were obtained after Ni-NTA column purification of the concentrated supernatant (Fig. 1A, bottom). Macaque IL-23 heterodimer was nonproducible, so we used recombinant human IL-23 that cross-activates macaque Vγ2Vδ2 T cells.
Figure 1.
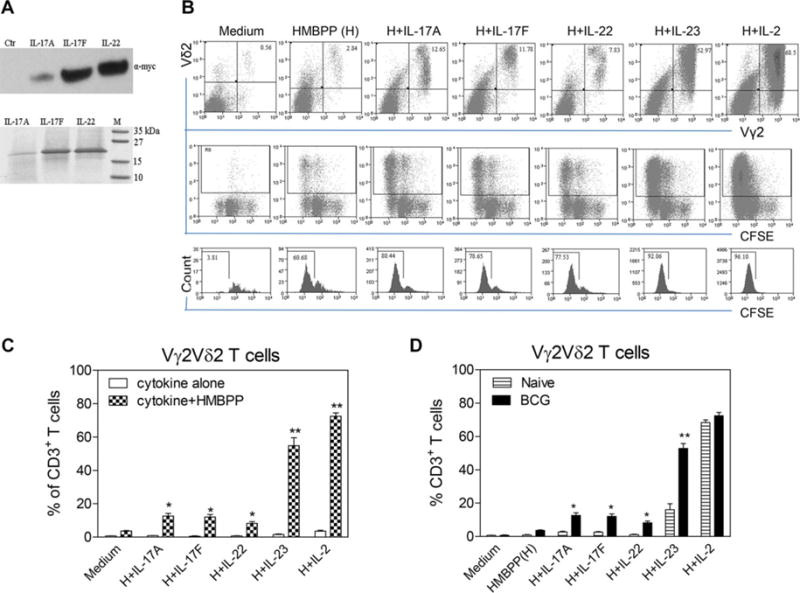
Th17-related cytokines expand Vγ2Vδ2 T cells in an HMBPP-dependent fashion; IL-23 and other Th17-type cytokines induce greater expansion of HMBPP-stimulated Vγ2Vδ2 T cells from BCG-vaccinated monkeys than those from naïve animals. (A) Western blot (upper panel) was performed on culture supernatant samples to assess the presence of recombinant IL-17A, IL-17F, or IL-22 in the culture supernatant of L. lactis, using an anti-c-myc antibody. Loading control (ctr) is concentrated supernatant from induced L. lact1is without recombinant cytokine genes. Blot is representative of two independent experiments. SDS-PAGE (lower panel) was performed to assess the extent to which these concentrated cytokines were purified by Ni-NTA system. Gel is representative of three independent experiments. (B) Representative flow cytometric histograms examining cellular expansion (upper panels) and proliferation (mid and lower panels) of Vγ2Vδ2T cells after 7-day coculture with HMBPP plus IL-17A, IL-17F, IL-22, IL-23, or IL-2. Cells were gated on CD3+ T cells from the BCG-vaccinated macaque (7245). Proliferation was determined based on the percentage dilution of CFSE fluorescence intensity. Flow plots are representative of three independent experiments. (C) Percentages of Vγ2Vδ2T cells in CD3+ T cells expanded after the 7-day culture with media, IL-17A, IL-17F, IL-22, IL-23, or IL-2 in the absence or presence of HMBPP were determined by flow cytometry. Data are shown as means +SEM from three BCG-vaccinated macaques and are pooled from two independent experiments. Recombinant human IL-22 from R&D system was also tested, with similar effects on Vγ2Vδ2 T cells (data not shown). ** p < 0.001, *p < 0.05 (Student t test). (D) PBMCs culture with cytokine was used to assess the effect of BCG vaccination on the ability of Th17-related cytokines and IL-2 to expand HMBPP-stimulated Vγ2Vδ2 T cells. Data are shown as means + SEM of three naïve and three BCG-vaccinated monkeys and are pooled from two independent experiments after the 7-day culture with medium alone, HMBPP, HMBPP + IL-17A, HMBPP + IL-17F, HMBPP + IL-22, HMBPP + IL-23, or HMBPP + IL-2.
PBMC from BCG-infected monkeys were cultured with each of these cytokines in the absence or presence of HMBPP. In the absence of HMBPP, almost no proliferation or expansion of Vγ2Vδ2 T cells was induced by IL-17A, IL-17F, IL-22, or IL-23 in cultures (Fig. 1B and C). In the presence of HMBPP, however, IL-17A/IL-17F, IL-22, or IL-23 induced detectable proliferation/expansion of Vγ2Vδ2 T cells in PBMC from BCG-vaccinated macaques, with IL-23 promoting greater expansion than other Th17-related cytokines (Fig. 1B and C). In contrast, controls including supernatant from L. lactis carrying empty plasmid did not expand HMBPP-stimulated Vγ2Vδ2 T cells (data not shown). Interestingly, while HMBPP and IL-23 coculture of PBMCs from BCG-vaccinated monkeys could expand Vγ2Vδ2 T cells up to the level of 50–60% in CD3+ T cells (Fig. 1B and C), PBMC from naïve macaques exhibited much lower levels of Vγ2Vδ2 T cell expansion by HMBPP and IL-23 or other Th17-type cytokines (Fig. 1D). Of note, IL-17/IL-22 and IL-23 seemed to act differently from IL-2, as IL-2 induced similar expansions of Vγ2Vδ2 T cells from both naïve and BCG-vaccinated animals (Fig. 1D). Thus, while IL-2 acted similarly on γδ T cells regardless of infections, Th17 cytokines induced greater expansion of HMBPP-stimulated Vγ2Vδ2 T cells from BCG-vaccinated monkeys than those from naïve animals.
It is interesting to note that after BCG vaccination or infection, Th17-related cytokines, particularly IL-23, can function as a 2nd signal to induce or enhance the ability of Vγ2Vδ2 T cells to mount in vitro recall-like expansion in response to the 1st stimulation signal from HMBPP engagement of the TCR. This finding is in agreement with our previous in vivo observation demonstrating that Vγ2Vδ2 T cells are able to develop adaptive immune responses during BCG, Mtb, and listerial infections of macaques [25, 32]. The HMBPP/IL-23-induced recall-like expansion of Vγ2Vδ2 T cells induced by Th17-related cytokines is somehow consistent with the upregulation of IL-22 and IL-23 during BCG and Mtb infections of macaques [25, 29]. In fact, up to 1000-fold overexpression of IL-22, IL-23, or IFN-γ can be detected in blood, lymphoid, and lung tissues during BCG/Mtb infection ([29], and data not shown). Such remarkable expression of IL-22, IL-23, and IFN-y is indeed found coincident with the major increases in numbers of circulating and pulmonary Vγ2Vδ2 T cells after BCG and Mtb infections [25, 29]. In contrast, IL-2 is almost undetectable in the blood and lymphoid tissues, along with very low levels of TNF-α, at the time the expansion of Vγ2Vδ2 T cells occurs during active BCG and Mtb infections [25, 29]. Thus, IL-23 and IL-22 are likely among the major cytokines driving the in vivo expansion or recall-like expansion of HMBPP-specific Vγ2Vδ2 T cells during mycobacterial infections.
IL-23 or IL-17/IL-22 is able to expand HMBPP-activated Vγ2Vδ2 T cells in Mtb-infected macaques
To authenticate HMBPP/Th17 cytokine-driven expansion of Vγ2Vδ2 T cells in BCG-vaccinated macaques, we examined whether Mtb infection also enhanced the ability of Th17 cytokines to expand HMBPP-activated Vγ2Vδ2 T cells. In prospective experiments before and after infection, we found that Vγ2Vδ2 T cells in PBMC collected after Mtb infection of macaques [33] were able to mount in vitro recall-like expansion in response to costimulation with HMBPP and IL-17/22 or IL-23 (Fig. 2A and B). In a separate study, naïve macaques were first vaccinated with saline, BCG, and attenuated Mtb mutant (ΔlysA ΔsecA2), respectively, as we previously described [34], and 3 months later were infected with Mtb. The recall-like expansion of Vγ2Vδ2 T cells was also seen after Mtb infection of naïve, BCG-, or MtbΔlysA ΔsecA2-vaccinated macaques (Fig. 2C). Here, Mtb infection appears to be the primary driving force for in vitro recall like expansion of Vγ2Vδ2 T cells, as the magnitude of γδ T-cell expansion is similar after Mtb infection of naïve and vaccinated macaques.
Figure 2.
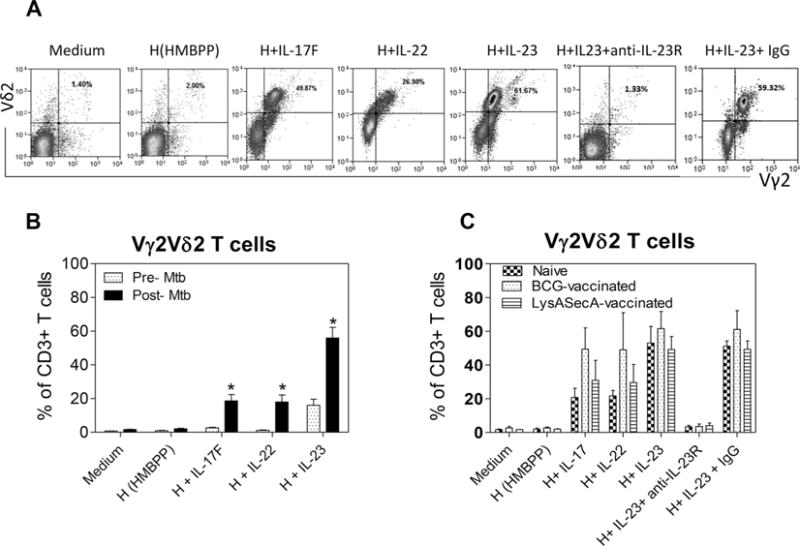
Primary Mtb infection of macaques promotes the ability of IL-23 or IL-17/IL-22 to expand HMBPP-stimulated Vγ2Vδ2 T cells, and IL-23-driven expansion is reduced by blockade of IL-23 signaling. (A) Flow cytometry was performed to assess Th17-related cytokines for the ability to expand HMBPP-activated Vγ2Vδ2T cells in PBMCs. Data shown is representative of two Mtb-infected macaques. (B) Data were compiled from the in vitro stimulation experiments, as described above in Figure 1, using PBMCs before and after Mtb infection. Data are shown as mean + SEM (three macaques) and are from one experiment representative of two experiments performed. *p < 0.05 (Student t test). (C) PBMCs of macaques, which were vaccinated first with BCG, attenuated Mtb mutant (ΔlysA ΔsecA2) or saline, and 3 months later infected with Mtb, were obtained and assessed for recall expansion of Vγ2Vδ2 T cells by Th17-type cytokines and HMBPP in the in vitro stimulation experiments as described above. Postinfection PBMCs were obtained 6–7 weeks after Mtb infection. Anti-IL-23R Ab and control mouse IgG were used at a concentration of 5 (μg/mL. Data are shown as mean + SEM (n = four macaques) and are pooled from two independent experiments.
IL-23-driven expansion is reduced by blockade of IL-23 signaling in Mtb-infected macaques
We assessed cytokine/receptor (R) involvement in HMBPP/Th17 cytokine-driven recall-like expansion of Vγ2Vδ2 T cells. We evaluated IL-23R expression and anti-IL23R blockade as prototypes, because IL-23 caused greater expansion of HMBPP-stimulated γδ T cells than did other Th17 cytokines; and anti-human IL-23R Ab had been shown to cross-react with macaque IL-23R. Here, our focus on IL-23 blockade is also justified by the finding that in vivo recall expansion of Vγ2Vδ2 T cells correlates with upregulation of IL-23, but not IL-2 or TNF-α [25, 29]. Since HMBPP specifically activates Vγ2Vδ2 T cells only, we explored whether IL-23R expression was detectable in HMBPP/IL-23-expanded γδ T cells. Although cytokine receptors were often difficult to detect/distinguish by flow cytometry [35], we found that IL-23R was apparently expressed on HMBPP/IL-23-expanded Vγ2Vδ2 T cells (Fig. 3A and B). In parallel, we examined whether IL-23/IL-23R interaction leading to expansion of HMBPP-activated Vγ2Vδ2 T cells could be blocked by anti-IL-23R blocking Ab, as IL-23 signaling involves Janus kinase 2, STAT2, and positive feedback enhancement [36, 37]. We found that anti-IL23R, but not IgG isotype control, could indeed block IL-23-induced expansion of HMBPP-stimulated Vγ2Vδ2 T cells (Fig. 2A and C), indicating that the IL-23/IL-23R signaling mediates expansion of HMBPP-stimulated Vγ2Vδ2 T cells. The remarkable IL-23 blockade suggests that IL-23 provides cosignaling and feedback-enhancing effects [37] on the expansion of Vγ2Vδ2 T cells.
Figure 3.
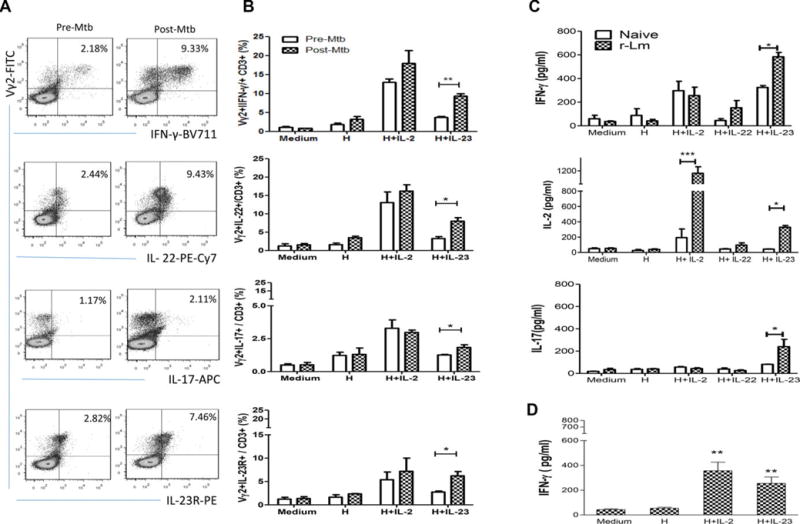
HMBPP/IL-23-expanded Vγ2Vδ2 T cells from macaques infected with Mtb or vaccinated with BCG or rListeria ΔactA prfA*-ESAT6/Ag85B can produce IL-17, IL-22, IL-2, and IFN-γ. (A) Flow cytometry was used to assess the ability of HMBPP/IL-23-expanded Vγ2Vδ2 T cells (top panel) to produce IFN-γ, IL-22, and IL-17 or express IL-23R in PBMCs collected before (pre-Mtb) and week two after (post-Mtb) infection of macaques with Mtb. Cells were gated on CD3+ cells (see gating strategy in Supporting Information Fig. 1). Plots are from one macaque representative of three each analyzed in two independent experiments with similar results. (B) Numbers of Vγ2Vδ2 T effector cells producing IFN-γ, IL-22, IL-17, or expressing IL-23R, were assessed among total CD3+ T cells by in vitro stimulation with HMBPP/cytokines followed by intracellular cytokine staining as described in the Materials and Methods using PBMCs collected before and week 2 after Mtb infection of three macaques. (C) Concentration of IFN-γ, IL-2, and IL-17 in the supernatants collected at day 5 from cultured PBMCs of four naïve and four rListeria ΔactA prfA*-vaccinated macaques was analyzed by ELISA. (D) Concentration of IFN-γ in the supernatants collected at day 5 from cultured PBMCs of BCG-vaccinated macaques was analyzed by ELISA. (B–D) Data are shown as mean + SEM of (B and D) three or (C) four macaques. Data are from one experiment representative of two experiments performed. *p < 0.05; **p < 0.01; ***p < 0.001 (Student t test).
HMBPP/IL-23-expanded Vγ2Vδ2 T cells produce IL-17, IL-22, IL-2, and IFN-γ
We then sought to determine whether HMBPP/IL-23-expanded Vγ2Vδ2 T cells from infected macaques exhibited effector function for producing IL-17/IL-22 and Th1-type cytokines. To this end, PBMC from macaques infected with Mtb, or vaccinated with BCG or Listeria ΔactAprfA* mutant [32] were cocultured with HMBPP and IL-23, and assessed for the ability to produce IL-17, IL-22, IL-4, IFN-γ, and TGF-β using intracellular cytokine staining and ELISA assays. Interestingly, intracellular cytokine staining and flow cytometry analysis revealed that HMBPP/IL-23-expanded Vγ2Vδ2 T cells from Mtb-infected macaques produced IFN-y, IL-22, and IL-17 (Fig. 3A and B, Supporting Information Fig. 1). Consistently, ELISA of culture supernatants showed that copious amounts of IFN-γ, IL-2, and IL-17 were secreted by HMBPP/IL-2-expanded Vγ2Vδ2 T cells from macaques immunized with attenuated recombinant (r) Listeria ΔactA prfA*-ESAT6/Ag85B vaccine (Fig. 3C), with very little production of IL-4 or TGF-β (data not shown). The IFN-γ production was also seen in HMBPP/IL-23 costimulation of Vγ2Vδ2 T cells from BCG-vaccinated macaques (Fig. 3D). Thus, HMBPP/IL-23 costimulation of PBMC from mycobacterium or listeria-infected macaques appears to differentiate Vγ2Vδ2 T cells into T effector cells producing IL-17, IL-22, IL-2, and IFN-γ. The results suggest that mycobacterial or listerial infection may prime or sensitize Vγ2Vδ2 T cells for HMBPP/IL-23-mediated differentiation. It has recently been shown that a two-week coculture protocol using HMBPP plus IL-1β, IL-6, IL-23, and TGF-β cocktails followed by IL-2 and/or PMA/ionomycin stimulation could polarize naïve human Vγ2Vδ2 T cells into effector cells capable of producing IFN-γ, IL-17, and IL-22 [17] or dominantly producing IL-17 [16]. Our results suggest that mycobacterial/listerial infections or immunization of macaques may indeed allow Vγ2Vδ2 T cells to bypass many other cytokines signals required for producing IFN-γ, IL-17, and IL-22. Such simplicity implicates the adaptive-like differentiation feature of Vγ2Vδ2 T cells.
HMBPP/IL-23-activated Vγ2Vδ2 T effector cells can produce IFN-γ in addition to IL-17, IL-22, and IL-2, and somehow may differ from CD4+ Th17 cells which exhibit IL-23-driven domi- nant production of IL-17 and IL-22 [38]. The production of IFN-γ by Vγ2Vδ2 T cells may be the intrinsic immune feature of HMBPP activation of Vγ2Vδ2 TCR [39], as large amounts of IFN-γ can also be produced after HMBPP-activated Vγ2Vδ2 T cells are expanded by IL-2 ([40, 41], Fig. 3) and by variant IL-4 [35]. This intrinsic immune feature may help to explain why cHMBPP/IL-2 expansion/differentiation of these Vγ2Vδ2 T effector cells producing IFN-γ and other anti-TB cytokines during Mtb infection can increase resistance to TB in macaques [42].
HMBPP/IL-23-induced production of IFN-γ facilitates IL-23-induced expansion of Vγ2Vδ2 T cells
It is currently unknown whether Ag/IL-23-induced production of selected cytokines can in turn facilitate IL-23 expansion of Ag-specific T cells, although IL-23 is one of the essential cytokines driving IL-17 production by αβ CD4+ T cells [1, 2] and by Vγ2Vδ2 T cells [16, 17]. Now, the production of IFN-γ or IL-17 by HMBPP/IL-23-expanded Vγ2Vδ2 T cells raised a mechanistic question as to whether HMBPP/IL-23-driven production of IFN-γ or IL-17 could exert positive-feedback regulation of Vγ2Vδ2 T-cell proliferation and expansion. To address this question, we set up cytokine-neutralizing experiments to examine if anti-IFN-γ or anti-IL-17 neutralizing antibody could reduce or inhibit the HMBPP/IL-23-induced expansion of Vγ2Vδ2 T cells from infected macaques. Interestingly, anti-IFN-γ neutralizing antibody, but not anti-IL-17, anti-IL-4, or anti-TGF-β, significantly inhibited the HMBPP/IL-23-induced recall-like expansion of Vγ2Vδ2 T cells at a dose-dependent fashion (Supporting Information Fig. 2A and B). The IFN-γ involvement appears to consist with the in vivo upregulation of IFN-γ and coincident expansion of Vγ2Vδ2 T cells in Mtb/BCG infection [25, 29]. Although there was no upregulation of IL-2 during in vivo expansion of Vγ2Vδ2 T cells in mycobacterial infections [25, 29], neutralizing anti-IL-2 Ab moderately reduced HMBPP/IL-23-mediated expansion of Vγ2Vδ2 T cells in Mtb-infected subjects (data not shown). Therefore, these results demonstrate that while HMBPP/IL-23-activated Vγ2Vδ2 T cells produce IFN-γ, IL-4, IL-2, TGF-β, and IL-17, HMBPP/IL-23-induced IFN-y production facilitates IL-23-mediated recall-like expansion of Vγ2Vδ2 T cells from infected macaques, implicating mutual regulation of γδ T cells by IL-23 and IFN-γ.
HMBPP/IL-23-induced proliferation of Vγ2Vδ2 T cells requires APC contact
Since infection or vaccination could enhance the ability of Th17-related cytokines to expand HMBPP-stimulated Vγ2Vδ2 T cells, we asked whether such in vitro recall-like responses of HMBPP-stimulated γδ T cells might be attributed partially to an augmented cross-talk [41] between quickly activated γδ T cells and APC from infection-primed macaques. We again focused on IL-23, as IL-23 expanded Vγ2Vδ2 T cells more efficiently than did IL-17 and IL-22. To this end, we cultured purified Vγ2+ T cells alone without monocytes/macrophages (tentatively termed APC). Vγ2Vδ2 T cells were purified from PBMC of BCG-vaccinated macaques using immunomagnetic beads as previously described [32], labeled with CFSE, and assessed for proliferation without APC in the presence of IL-23 plus HMBPP, IL-2 plus HMBPP, or HMBPP alone. Interestingly, in the absence of APC, IL-23 plus HMBPP induced little proliferation of Vγ2+ T cells, whereas IL-2 plus HMBPP stimulated appreciable proliferation of Vγ2+ T cells (Supporting Information Fig. 3A/upper panel). This initial result suggested that physical APC contact and/or additional signal from APC were required for the ability of IL-23 to expand HMBPP-stimulated Vγ2Vδ2 T cells. To address this, we made use of a trans-well coculture system in which Vγ2+ T cells labeled with CFSE were placed in the upper chamber and Vγ2-depleted PBMC containing APC were seeded in the lower compartment. This non-contact system allowed free transport and permeability of media and cytokines/soluble factors, but prevented Vγ2+ T cells from direct contact with APC. IL-2 plus HMBPP reproducibly induced APC-independent proliferation of activated Vγ2+ T cells (Supporting Information Fig. 3A/lower panel). In contrast, IL-23 failed to induce proliferation and expansion of HMBPP-activated Vγ2+ T cells in the absence of APC in the trans-well coculture system (Supporting Information Fig. 3A/lower panel). To confirm this, monocytes were isolated through adherence and cocultured with purified Vγ2+ T cells for proliferation assay. When autologous monocytes were added back to the culture of Vγ2+ T cells, IL-23 induced proliferation of HMBPP-stimulated Vγ2Vδ2 T cells (Supporting Information Fig. 3B). These results suggest that cell-cell contact between Vγ2Vδ2 T cells and APC was required for IL-23-induced proliferation of HMBPP-stimulated Vγ2Vδ2 T cells.
It is currently unknown why APC contact is needed for recall like expansion of Vγ2Vδ2 T cells by HMBPP/IL-23. One would speculate that high-level expression of butyrophilin 3A1 on APC is needed to trigger the HMBPP-driven TCR activation signaling and IL-23 signaling. However, this speculation is not supported by the fact that T-cell presentation of HMBPP can occur [26] and that IL-2/HMBPP costimulation can still induce proliferation of Vγ2Vδ2 T cells without APC. It is likely that HMBPP/IL-23-induced recall like expansion of Vγ2Vδ2 T cells may involve additional signal(s) and/or cytokine(s) from APC. Further studies employing better model systems are needed to elucidate detailed sequential and complex cytokines/signals for HMBPP/IL-23-driven responses in the context of APC contacts. Nevertheless, the current APC experiment demonstrates that APC contact is needed for HMBPP/IL-23-driven responses.
HMBPP/IL-23 expansion of Vγ2Vδ2 T cells involves conventional and novel protein kinase C pathways
It has recently been shown that activation of Vγ2Vδ2 T cells by IPP plus IL-2 initiates the activation of both conventional PKC (α/β) and novel PKC (PKC θ) isoforms [43]. Data suggest that PKC θ appears to be ligand-dependent, whereas activation of PKC α/β tends to be driven by growth factor IL-2. Since IL-23 differed from IL-2 in preferential expansion of Vγ2Vδ2 T cells from infected or vaccinated macaques, we examined whether HMBPP/IL-23-induced activation involved different PKC isoforms. To address this, we assessed PKC α/β inhibitor GO6976 and PKC θ inhibitor rottlerin, respectively, for the ability to inhibit HMBPP/IL-23-induced proliferation of Vγ2Vδ2 T cells. The results showed that either GO6976 or rottlerin indeed inhibited the HMBPP/IL-23-induced proliferation of Vγ2Vδ2 T cells (Supporting Information Fig. 3C), suggesting that both PKC α/β and PKC θ are the downstream signaling kinases involved in HMBPP/IL-23-induced proliferation of Vγ2Vδ2 T cells. It is noteworthy that IL-23 signaling alone involves Janus kinase 2, STAT2 and positive feedback expansion of Th17 cells [36, 37]. It is likely that IL-23 may additionally involve the PKC α/β signaling, whereas HMBPP predominantly engages in the PKC θ pathway [43].
Thus, the current study provides evidence supporting the hypothesis that Th17-related cytokines, particularly IL-23, are involved in the in vitro recall-like responses of Vγ2Vδ2 T cells after infection with HMBPP-producing Mtb and other microbes. These findings provide rationale for the manipulation of HMBPP/IL-23-driven Vγ2Vδ2 T-cell responses for the enhancement of antimicrobial immunity.
Materials and methods
Animals, vaccination, or infection
Four- to eight-year-old cynomolgus macaques with 3–5 kg body weights were used in this study. Monkeys were vaccinated with saline or BCG (106 CFU) as previously described [25], infected with Mtb (500 CFU) via bronchoscope-guided spread into bron-choalveolar interface [33], or immunized with recombinant (r) Listeria ΔactΔprfA* vaccine expressing ESAT6/Ag85B (108 CFU) via oral/intratracheal vaccination [32]. All animals were maintained and used in accordance with guidelines of the institutional animal care and use committee.
Expression, purification, and characterization of recombinant macaque IL-17A, IL-17F, and IL-22
The coding sequence of macaque IL-17A, IL-17F, or IL-22 cDNA was cloned into the pXmh vector to express a protein tagged with C-terminal 6-his, and electroporated into Lactococcus lactis stain PA1001 for secretion of each of these cytokines as we previously described [31]. Vector pXmh is a derivative of L. lactis expression vector pPA3, by which expressed protein contains a c-myc tag for Western blotting analysis with anti c-myc antibody (clone 9E10, Sigma-Aldrich) and a 6-his tag for purification [35]. Details in production, purification, and characterization are described in the Supporting Information section. Approximately 150 μg of IL-17A and 300 μg of IL-17F or IL-22 were purified from 1 L culture supernatant based on the measurement with BCA protein assay [35].
In vitro stimulation and expansion of Vγ2Vδ2 T cells by HMBPP and Th17-related cytokines
PBMCs were isolated from EDTA-treated blood from macaques using Ficoll-Paque Plusdensity gradient centrifugation and then cultured with RPMI1640 media supplemented with 2 mM glu-tamine, 50 U/mL of penicillin and 50 μg/mL of streptomycin, and containing 10% FBS (Invitrogen). Cells (1 × 106/mL) were cultured in U-bottomed 96-well plates in the absence or presence of 50 ng/mL of HMBPP (provided by Dr. Hassan Jomaa from Justus-liebig-University Giessen, Giessen, Germany), and then supplemented at day 0, 3, and 5 with 20 U/mL hIL-2 (Sigma-Aldrich), 100 ng/mL hIL-23 (R&D Systems, Minneapolis, MN), 100 ng/mL of recombinant macaque IL-17A, IL-17F, or IL-22 (recombinant human IL-22 from R&D was also used, with similar effects on Vγ2Vδ2 T cells). At day 7, cells were harvested for surface or intracellular cytokine staining and flow cytometry analyses.
Isolation of Vγ2+ T cells, CFSE labeling, and proliferation assays
This is essentially the same as previously described. [44]. Vγ2+ T cells were purified from PBMCs through incubation with purified mouse-anti-human Vγ2 Ab (Clone: 7A5, Pierce), followed by goat-anti-mouse IgG microbeads (Miltenyi Biotec, Germany) at 4°C for 20 min for each incubation. Cells were subjected to positive magnetic separation, and Vγ2+ T cells and unbound cells fractions were collected, respectively. The purity of positively selected Vγ2+ T cells was over 95%, as assessed by flow cytometry.
Purified Vγ2+ T cells were labeled with CFSE by using Cell-trace CFSE cell proliferation kit (Invitrogen) following the manufacturer’s protocol. Then Vγ2+ T cells and Vγ2-depleted PBMCs were cultured in the upper and lower chambers, respectively, in the transwell system (Corning, Acton, MA), which could separate Vγ2+ from other cells by a permeable membrane (pore size 0.4 (μm). Cells were cultured in chambers for 7 days with media only, HMBPP, HMBPP plus IL-2, or HMBPP plus IL-23 and collected for proliferation assay. In parallel, PBMCs from same monkeys were labeled with CFSE and cultured in U-bottomed 96-well plates at indicated conditions.
For coculture of purified Vγ2+ T cells with monocytes, Vγ2+ T cells were isolated as described above and monocytes were enriched by adherence to tissue culture flask as we previously described [44]. Purified Vγ2+ T cells were labeled with CFSE and then either left alone or mixed with monocytes in 96-well plates. In these two systems, cells were cultured for 7 days with media only, HMBPP, HMBPP plus IL-2, or HMBPP plus IL-23 and collected for proliferation assay.
Flow cytometry
This was done as we previously described [24, 33, 44]. Cells were harvested at day 7 after culture and stained with Pacific blue-conjugated anti-CD3 (Clone SP34-2, BD, Franklin Lakes, NJ), PE conjugated anti-CD4 (clone OKT4, eBioscience, San Diego, CA), FITC-conjugated Vγ2 (Clone 7A5, Pierce, Rockford, IL) and purified Vδ2 (clone 15D, Pierce) in combination of allophycocyanin-conjugated goat anti mouse IgG (Dako, Carpinteria, CA). After staining, cells were fixed with 2% formaldehyde-PBS (Protocol Formalin, Kalamazoo, MI) and subjected to run on a CyAn ADP flow cytometer (DakoCytomation, Carpinteria, CA). Lymphocytes were gated based on forward- and side-scatters, and pulse width and at least 40 000 gated events were analyzed by using Summit Data Acquisition and Analysis Software (Dako Cytomation). Further special gates and quadrants for data analysis were determined on nonstaining, specific antibody staining, and isotype control antibody background staining.
Intracellular cytokine staining
This was done as we previously described [24, 33, 44]. Briefly, PBMCs were used in each reaction (round bottom 96-well plate) to measure T cells that could produce IFN-γ, TNF-α, IL-17, IL-22, and IL-23 in the absence or presence of HMBPP stimulation in vitro. Lymphocytes were incubated for 1 h with medium in presence of CD28 (1 μg/mL) and CD49d (1 μg/mL) mAbs in a 200 μL final volume in round bottom 96-well plates at 37°C, 5% CO2, followed by 5 h incubation in the presence of brefeldin A (GolgiPlug, BD). After a total of 6-h incubation, cells in 96-well plate were transferred into 5 mL polystyrene round bottom tubes (BD) for surface and intracellular staining. Cells were washed once with 2% FBS-PBS and stained at RT for at least 15–30 min with surface marker Abs (CD3 and Vγ2/Vδ2) and washed twice with 2% FBS-PBS. Cells were permeabilized for 45 min (cytofix/cytoperm, BD) and washed twice by Perm buffer (BD), and then stained another 45 min for anti-IFN-γ Brilliant Violet 711 (clone 4S.B3, Biolegend) or anti-IL-23R-PE (clone 218213, R&D Systems), anti-IL-17A-allophycocyanin (clone eBio64DEC17, eBioscience), and anti-IL-22 PE-Cy7 (clone 142928, R&D Systems), and repeated Perm wash twice. Last, cells were resuspended in 2% formaldehyde-PBS (Kalamazoo, MI) and subjected to flow cytometry analysis.
ELISA
PBMCs from naïve, vaccinated or infected monkeys were cultured for 7 days with media, HMBPP, HMBPP+IL-2, or HMBPP+IL-23. The supernatants at day 5 or 7 were collected to measure the level of IFN-γ, IL-2, or IL-17 (kit from R&D system) by ELISA as we previously described [45].
Cytokine neutralization, IL-23R blocking, and inhibition of PKC activation
For neutralization experiments, PBMCs from BCG-vaccinated monkeys were cultured with media, HMBPP, HMBPP+IL-23, HMBPP+IL-23+anti-IFN-γ (1 or 5 μg/mL), HMBPP+IL-23+anti-IL-4 (5 μg/mL), HMBPP+IL-23+ anti-TGF-β (5 μg/mL) or HMBPP+IL-23+ anti-IL-17 (5 μg/mL), anti-IL-23R (5 μg/mL, R&D) or mouse IgG (5 μg/mL), with cytokine and Ab supplemented at day 0, 3, 5. At day 7, the cells were collected and stained. The following neutralizing Abs were used: anti-IFN-γ (clone MD-1; eBioscience), and anti-TGF-β (clone 9016; R&D systems), anti-IL-17 (Clone 133617, R&D Systems,), anti-IL-23R (clone 3D7, R&D Systems). Other primary and secondary mAbs or mouse isotype control IgG were listed previously [40].
For PKC inhibition experiment, PBMCs from BCG-vaccinated monkeys were cultured with media alone, HMBPP, HMBPP+IL-2, or HMBPP+IL-23; PKC inhibitors Gö6976 (500 nM, Calbiochem, San Diego, CA) and rottlerin (5 μM, Calbiochem) were added at days 3 and 5, respectively, as previously described [45]. At day 7, the cells were collected and stained.
Statistical analysis
Statistical analysis was done by using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA). Data were analyzed by Student t test (parametric method) or by Mann-Whitney test (non-parametric method). p < 0.05 was considered significant. Only p values < 0.05 were shown in the text.
Supplementary Material
Acknowledgments
We thank other Chen ZW lab staff for technical assistance. This work was supported by the following research grants: the National Program Project grant (ZX10003); the National Institutes of Health R01 grants (OD015092 or RR13601, HL64560, and AI106590, all to Z.W.C.).
Abbreviations
- CFSE
5(6)-Carboxyfluorescein N-hydroxysuccinimidyl ester
- HMBPP
4-hydroxy-3-methyl-but-enyl pyrophosphate
- Mtb
Mycobacterium tuberculosis
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 3.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 4.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 5.Shin HC, Benbernou N, Fekkar H, Esnault S, Guenounou M. Regulation of IL-17, IFN-gamma and IL-10 in human CD8(+) T cells by cyclic AMP-dependent signal transduction pathway. Cytokine. 1998;10:841–850. doi: 10.1006/cyto.1998.0375. [DOI] [PubMed] [Google Scholar]
- 6.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 8.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 9.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–333. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 12.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 13.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 15.Zeng G, Chen CY, Huang D, Yao S, Wang RC, Chen ZW. Membrane-bound IL-22 after de novo production in tuberculosis and anti-mycobacterium tuberculosis effector function of IL-22+ CD4+ T cells. J Immunol. 2011;187:190–199. doi: 10.4049/jimmunol.1004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caccamo N, La Mendola C, Orlando V, Meraviglia S, Todaro M, Stassi G, Sireci G, et al. Differentiation, phenotype, and function of interleukin-17-producing human Vgamma9Vdelta2 T cells. Blood. 2011;118:129–138. doi: 10.1182/blood-2011-01-331298. [DOI] [PubMed] [Google Scholar]
- 17.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A- and IL-22-producing human Vgamma2Vdelta2 T cells. J Immunol. 2010;184:7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Born WK, Reardon CL, O’Brien RL. The function of gammadelta T cells in innate immunity. Curr Opin Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 20.Fournie JJ, Bonneville M. Stimulation of gamma delta T cells by phosphoantigens. Res Immunol. 1996;147:338–347. doi: 10.1016/0923-2494(96)89648-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZW, Letvin NL. Adaptive immune response of Vgamma2Vdelta2 T cells: a new paradigm. Trends Immunol. 2003;24:213–219. doi: 10.1016/s1471-4906(03)00032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morita CT, Mariuzza RA, Brenner MB. Antigen recognition by human gamma delta T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22:191–217. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 23.Eberl M, Hintz M, Reichenberg A, Kollas AK, Wiesner J, Jomaa H. Microbial isoprenoid biosynthesis and human gammadelta T cell activation. FEBS Lett. 2003;544:4–10. doi: 10.1016/s0014-5793(03)00483-6. [DOI] [PubMed] [Google Scholar]
- 24.Ryan-Payseur B, Ali Z, Huang D, Chen CY, Yan L, Wang RC, Collins WE, et al. Virus infection stages and distinct Th1 or Th17/Th22 T-cell responses in malaria/SHIV coinfection correlate with different outcomes of disease. J Infect Dis. 2011;204:1450–1462. doi: 10.1093/infdis/jir549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacte-rial infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei H, Huang D, Lai X, Chen M, Zhong W, Wang R, Chen ZW, et al. Definition of APC presentation of phosphoantigen (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate to Vgamma2Vdelta 2 TCR. J Immunol. 2008;181:4798–4806. doi: 10.4049/jimmunol.181.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Henry O, Distefano MD, Wang YC, Raikkonen J, Tanaka MY, Morita CT. Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vgamma2Vdelta2 T cells. J Immunol. 2013;191:1029–1042. doi: 10.4049/jimmunol.1300658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, Beddoe T, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human gammadelta T cells. Nat Immunol. 2013;14:908–916. doi: 10.1038/ni.2665. [DOI] [PubMed] [Google Scholar]
- 29.Qiu L, Huang D, Chen CY, Wang R, Shen L, Shen Y, Hunt R, et al. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpressionofIL-22, MIP-1alpha, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-beta, TIM1, and TLR2 but low antigen-specific cellular responses. J Infect Dis. 2008;198:1514–1519. doi: 10.1086/592448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang D, Qiu L, Wang R, Lai X, Du G, Seghal P, Shen Y, et al. Immune gene networks of mycobacterial vaccine-elicited cellular responses and immunity. J Infect Dis. 2007;195:55–69. doi: 10.1086/509895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng G, Chen J, Zhong L, Wang R, Jiang L, Cai J, Yan L, et al. NSOM- and AFM-based nanotechnology elucidates nano-structural and atomic-force features of a, Y. pestis V immunogen-containing particle vaccine capable of eliciting robust response. Proteomics. 2009;9:1538–1547. doi: 10.1002/pmic.200800528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan-Payseur B, Frencher J, Shen L, Chen CY, Huang D, Chen ZW. Multieffector-functional immune responses of HMBPP-specific Vgamma2Vdelta2 T cells in nonhuman primates inoculated with Listeria monocytogenes {Delta}actA prfA*. J Immunol. 2012;189:1285–1293. doi: 10.4049/jimmunol.1200641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CY, Huang D, Yao S, Halliday L, Zeng G, Wang RC, Chen ZW, et al. IL-2 simultaneously expands Foxp3+T regulatory and T effector cells and confers resistance to severe tuberculosis (TB): implicative Treg-T effector cooperation in immunity to TB. J Immunol. 2012;188:4278–4288. doi: 10.4049/jimmunol.1101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA. Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27:4709–4717. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan Z, Wang R, Lee Y, Chen CY, Yu X, Wu Z, Huang D, et al. Tuberculosis-induced variant IL-4 mRNA encodes a cytokine functioning as growth factor for (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-specific Vgamma2Vdelta2 T cells. J Immunol. 2009;182:811–819. doi: 10.4049/jimmunol.182.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison PJ, Ballantyne SJ, Kullberg MC. Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology. 2011;133:397–408. doi: 10.1111/j.1365-2567.2011.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 38.Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergisti-cally with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen ZW. Immune biology of Ag-specific gammadelta T cells in infections. Cell Mol Life Sci. 2011;68:2409–2417. doi: 10.1007/s00018-011-0703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali Z, Shao L, Halliday L, Reichenberg A, Hintz M, Jomaa H, Chen ZW. Prolonged (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate-driven antimicrobial and cytotoxic responses of pulmonary and systemic Vgamma2Vdelta2 T cells in macaques. J Immunol. 2007;179:8287–8296. doi: 10.4049/jimmunol.179.12.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eberl M, Roberts GW, Meuter S, Williams JD, Topley N, Moser BA. A rapid crosstalk of human gammadelta T cells and monocytes drives the acute inflammation in bacterial infections. PLoS Pathog. 2009;5:e1000308. doi: 10.1371/journal.ppat.1000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CY, Yao S, Huang D, Wei H, Sicard H, Zeng H, Jomaa MH, et al. Phosphoantigen/IL2 expansion and differentiation of Vgamma2Vdelta2 T cells increase resistance to tuberculosis in nonhu-man primates. PLoS Pathog. 2013;9:e1003501. doi: 10.1371/journal.ppat.1003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cipriani B, Knowles H, Chen L, Battistini L, Brosnan CF. Involvement of classical and novel protein kinase C isoforms in the response of human V gamma 9V delta 2 T cells to phosphate antigens. J Immunol. 2002;169:5761–5770. doi: 10.4049/jimmunol.169.10.5761. [DOI] [PubMed] [Google Scholar]
- 44.Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC, Chen ZW, et al. Differentiation, distribution and gammadelta T cell-driven regulation of IL-22-producing T cells in tuberculosis. PLoS Pathog. 2010:e1000789. doi: 10.1371/journal.ppat.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gong G, Shao L, Wang Y, Chen CY, Huang D, Yao S, Zhan X, et al. Phosphoantigen-activated V gamma 2V delta 2 T cells antagonize IL-2-induced CD4+CD25+Foxp3+T regulatory cells in mycobacterial infection. Blood. 2009;113:837–845. doi: 10.1182/blood-2008-06-162792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


