Abstract
Background:
Efficacy of interferon beta in multiple sclerosis (MS) can be dampened in patients who develop neutralizing antidrug antibodies (NAbs). Peginterferon beta1a is an interferon conjugated with a polyethylene glycol (PEG) moiety. Pegylation increases a drug’s half life and exposure, and may also reduce immunogenicity.
Objective:
The objective of this study was to characterize the incidence and impact of immunogenicity to peginterferon beta1a over 2 years in patients with MS.
Methods:
Patients with relapsing–remitting MS (N = 1512) were randomized to subcutaneous peginterferon beta1a 125 μg every 2 or 4 weeks, or placebo, for 1 year; patients in the placebo group were rerandomized to active treatment in year 2. The incidence and titers of binding antibodies (BAbs) and NAbs to interferon and antibodies to PEG (anti-PEG) were assessed in analytically validated assays. The clinical impact of immunogenicity on relapse and magnetic resonance imaging endpoints was evaluated.
Results:
Over 2 years, 6%, less than 1%, and 7% of patients developed anti-interferon BAbs, NAbs, and anti-PEG antibodies, respectively. There was no discernible clinically meaningful effect of antibody status on the pharmacodynamic, efficacy, or safety parameters evaluated, although these analyses were limited by the low incidence of treatment-emergent antibodies.
Conclusion:
The treatment effect of peginterferon beta1a in patients with relapsing–remitting MS is not expected to be attenuated by immunogenicity.
Keywords: immunogenicity, neutralizing antibodies, peginterferon beta1a, pegylation, relapsing–remitting multiple sclerosis
Introduction
Nonpegylated interferon (IFN) beta therapies are well established as safe and effective treatment options for patients with relapsing forms of multiple sclerosis (MS). However, several studies have reported the occurrence of clinically meaningful neutralizing antidrug antibodies (NAbs) against IFN beta in patients treated with commercial IFN beta preparations [Clanet et al. 2002; European Study Group on IFN beta 1b in secondary progressive MS, 1998; Polman et al. 2003; PRISMS Study Group, 1998; Rudick et al. 1998; The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group, 1996]. In addition, efficacy of IFN beta on clinical and MRI measures has been shown to be reduced in patients who develop Nabs [Polman et al. 2002; Paolicelli et al. 2013].
Protein therapeutics generally induce the development of antidrug antibodies. When patients develop NAbs to IFN beta, the NAbs can interfere with binding of IFN to its receptor [Goodin et al. 2007]. The clinical impact of NAbs is difficult to quantify consistently, due to variation between studies in assay methods, timing and frequency of sampling, and criteria for categorizing patients as NAb positive or negative, since antibody status can change over time. Differing clinical assessment methods may also produce variable results, although magnetic resonance imaging (MRI) measures have more consistently shown diminished effect of IFN beta therapy in patients who develop NAbs [Polman et al. 2010; Goodin et al. 2007]. Nonetheless, development of NAbs is recognized as a potential problem with all currently available IFN beta therapies for MS, which can reduce the efficacy of continued treatment, potentially impact the efficacy of other IFN-based treatments, and result in the discontinuation of treatment [Polman et al. 2010; Hartung et al. 2005].
Peginterferon beta1a is a pegylated form of IFN beta1a. Drug modification by conjugation of polyethylene glycol (PEG) molecules (pegylation) may confer several beneficial properties: increased molecular size leads to reduced glomerular filtration rate and increased half life; due to improved stability and solubility of the molecule, plasma concentrations and therefore pharmacologic activity may be further increased; and the PEG moiety may shield the native molecule from proteolytic attack and thereby reduce degradation [Kang et al. 2009; Kieseier and Calabresi, 2012; Harris et al. 2001]. Pegylated products may also have reduced immunogenicity, as the PEG moiety may mask immunoreactive sites on the native molecule [Kieseier and Calabresi, 2012; Harris et al. 2001; Caliceti and Veronese, 2003]. PEG itself is a biologically inert substance [Kieseier and Calabresi, 2012] with a very low risk of generating an adverse immune reaction [Caliceti and Veronese, 2003]. However, several examples of immunogenic responses to pegylated drugs have been reported, including pegylated IFN alphas used in the treatment of hepatitis C [Matsuda et al. 2012; Scagnolari et al. 2012; Halfon et al. 2010] and pegylated thrombopoietin [Rosenberg, 2003], indicating that pegylation does not eliminate immunogenicity altogether. Furthermore, development of antibodies to PEG (anti-PEG Abs) has been observed in animal models and humans [Caliceti and Veronese, 2003; Ishida and Kiwada, 2013; Shimizu et al. 2012]. Therefore, it remains important to evaluate the immunogenic profile of new pegylated drug products; indeed, Food and Drug Administration guidelines recommend that immunogenicity assessments in trials of pegylated therapeutic proteins include assays to detect antibodies to both the protein and the PEG moiety [Food and Drug Administration, 2014].
Data from phase I studies of peginterferon beta1a showed increased half life and exposure (area under the curve and peak concentration) compared with nonpegylated IFN beta1a (30 μg intramuscularly), when administered at various dose levels (63, 125, 188 μg subcutaneously or intramuscularly) [Hu et al. 2012]. In the phase III ADVANCE study, subcutaneous peginterferon beta1a (125 μg) administered every 2 or 4 weeks provided statistically significant improvements in clinical endpoints and MRI measures versus placebo over 1 year, to a greater extent with peginterferon beta1a every 2 weeks, and had a safety profile consistent with that of established IFN therapies for relapsing MS [Calabresi et al. 2014].
Here we report the immunogenicity profile of peginterferon beta1a over 2 years in the ADVANCE study, characterized using separate assays to determine levels of anti-IFN binding antibodies (BAbs), which bind the IFN molecule without altering biological activity, NAbs to peginterferon beta1a, and anti-PEG Abs. This analysis also sought to evaluate the possible impact of peginterferon beta1a immunogenicity on measures of efficacy and safety.
Materials and methods
Patients and study design
ADVANCE study design and methods have been previously described [Calabresi et al. 2014]. Briefly, ADVANCE is a 2-year, multicenter, randomized, double-blind, parallel-group, phase III study with a 1-year placebo-controlled period. Key inclusion criteria were relapsing–remitting MS [Polman et al. 2005], age 18–65 years, a score of 0–5 on the Expanded Disability Status Scale [Kurtzke, 1983], and at least two clinically documented relapses in the previous 3 years, with at least one occurring within the past 12 months. Patients were excluded if they received previous IFN treatment for MS for over 4 weeks or had discontinued less than 6 months before baseline.
Patients were randomized (1:1:1) to receive subcutaneous injections with prefilled syringes of placebo, peginterferon beta1a 125 μg every 2 weeks, or peginterferon beta1a 125 μg every 4 weeks in the first year. During year 2, patients receiving peginterferon beta1a remained on the same dose frequency and patients receiving placebo were re-randomized to peginterferon beta1a 125 μg every 2 weeks or every 4 weeks.
Protocols and informed consent forms were approved by the appropriate institutional review board for each site, the study was conducted according to International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki, and all patients provided written informed consent before entering the study [International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, 1996; World Medical Association, 2013].
Study procedures and endpoints
The primary endpoint of ADVANCE was annualized relapse rate (ARR) at 48 weeks, based on the number of relapses. The secondary endpoints were new or newly enlarging T2 lesions (relative to baseline MRI), the proportion of patients who had relapsing disease, and the proportion of patients with disability progression.
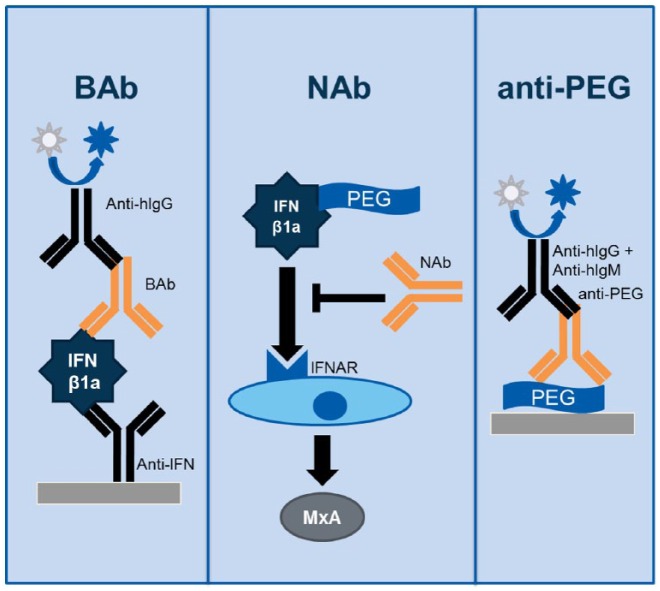
To evaluate immunogenicity, clinical serum samples were collected predose on day 1 and at weeks 8, 20, 36, 48, 60, 72, and 96. Immunogenicity was assessed via three analytically validated assays: an enzyme-linked immunosorbent assay (ELISA) for IFN beta1a BAbs, a cell-based assay for peginterferon beta1a NAbs, and an ELISA for anti-PEG Abs (Figure 1), which are described briefly in Appendix 1 (publication of full methodological details in progress).
Figure 1.
Immunogenicity was assessed via three analytically validated assays: an ELISA for IFN beta1a BAbs, a cell-based assay for peginterferon beta1a NAbs, and an ELISA for anti-PEG Abs.
Ab, antibody; BAb, binding antibody; ELISA, enzyme-linked immunosorbent assay; hIgG, human immunoglobulin G; hIgM, human immunoglobulin M; IFN, interferon; IFNAR, type I IFN receptor; MxA, myxovirus protein A; NAb, neutralizing antibody; PEG, polyethylene glycol.
A tiered testing strategy was used: samples were first tested for presence of BAbs to IFN beta1a; samples positive for BAbs to IFN beta1a were then tested for presence and titer of NAbs to peginterferon beta1a. Since binding is a necessary prerequisite for neutralization, samples negative for BAbs to IFN beta1a were presumed negative for NAbs to peginterferon beta1a. All samples were also tested for presence and titer of anti-PEG Abs.
Statistical analysis
The incidence of each type of antibody was summarized by treatment group and visit based on the number of patients who were at risk. Number at risk was defined as the number of patients whose baseline antibody status was not positive and had at least one positive post-baseline antibody value.
Patients positive for antibodies were further categorized as transient positive (a single positive evaluation, or more than one positive evaluation occurring less than 74 days apart) or persistent positive (consecutive positive tests ⩾74 days apart or a positive evaluation at the final assessment with no further samples available). The 74-day interval was chosen to accommodate the 84 nominal days between time points and the ±5-day visit window. The incidence of transient and persistent positivity was summarized by treatment group.
Positive anti-peginterferon beta1a NAbs and anti-PEG Abs were broken down by titer level in case only a subset of positive values were clinically relevant. Cutoffs were set empirically based on titer distributions of all samples. Titer levels of peginterferon beta1a NAbs were categorized as low (⩽50), medium (>50 and ⩽700), or high (>700). Titer levels of anti-PEG Abs were categorized as low (⩽100), medium (>100 and <800), or high (⩾800). Each patient was categorized according to their highest individual sample titer level.
Since antibodies have the potential to impact safety and efficacy regardless of whether pre-existing or induced by treatment, analyses to evaluate the potential impact of immunogenicity on efficacy and safety used categories of ‘never positive’ or ‘ever positive’, including baseline antibody status.
Results
Patients
A total of 1512 patients were randomized and treated with placebo (n = 500), peginterferon beta1a 125 μg every 2 weeks (n = 512), or peginterferon beta1a 125 μg every 4 weeks (n = 500) during year 1 of the study. Demographics and baseline clinical characteristics were similar between treatment groups [Calabresi et al. 2014].
Baseline antibody incidence
Overall, few patients were positive for anti-IFN beta1a BAbs (⩽3% across all groups), antipeginterferon beta1a NAbs (⩽2% across all groups), or anti-PEG Abs (⩽8% across all groups) at baseline. Incidence of antibodies at baseline was balanced between groups (Table 1). A second, pretreatment, baseline assessment was made for the placebo (delayed treatment) group at the beginning of year 2, immediately prior to receiving peginterferon beta1a in the second year, and incidences of baseline positive antibodies remained similar to those observed at the beginning of the study (Table 1).
Table 1.
Baseline antibody status prior to treatment with peginterferon beta1a.
|
Delayed treatment*
|
Peginterferon beta1a 125 μg
|
|||
|---|---|---|---|---|
| Every 2 weeks (n = 228) | Every 4 weeks (n = 227) | Every 2 weeks (n = 512) | Every 4 weeks (n = 501) | |
| Anti-IFN beta1a BAb positive, n (%) | 1 (<1) | 7 (3) | 16 (3) | 8 (2) |
| Antipeginterferon beta1a NAb positive, n (%)** | 0 | 3 (1) | 8 (2) | 2 (<1) |
| Anti-PEG Ab positive, n (%) | 18 (8) | 12 (5) | 25 (5) | 27 (5) |
Delayed treatment groups: patients who received placebo in year 1 and switched to peginterferon beta1a in year 2. Pretreatment baseline measure taken at the beginning of year 2 in these groups.
NAb assay was performed only on anti-IFN BAb-positive samples, per tiered assay design; percentages are given as a proportion of the total safety population.
Ab, antibody; BAb, binding antibody; IFN, interferon; NAb, neutralizing antibody; PEG, polyethylene glycol.
Incidence of treatment-emergent antibodies
The overall incidence of treatment-emergent IFN beta1a BAbs was low. In year 1, the proportion of patients with emerging anti-IFN beta1a BAbs was 3% in the placebo group (n = 13/481), 8% (n = 38/483) in the peginterferon beta1a every 2 weeks, and 4% (n = 20/486) in the peginterferon beta1a every 4 weeks group. Similar results were observed over 2 years in patients treated at any point with peginterferon beta1a, and the overall incidence was 6% (n = 90/1412) (Table 2). Approximately half of the treatment-emergent BAbs to IFN beta1a appeared to be transient (Table 2).
Table 2.
Incidence of treatment-emergent anti-IFN beta1a BAbs at year 1 and over 2 yearsa.
| Year 1 |
Over 2 years |
||||
|---|---|---|---|---|---|
| Placebo (n = 481) | Peginterferon beta1a | Peginterferon beta1a | Peginterferon beta1a | Peginterferon beta1a | |
| every 2 weeks (n = 483) | every 4 weeks (n = 486) | every 2 weeks (n = 706) | every 4 weeks (n = 706) | ||
| Patients with ⩾1 positive anti-IFN BAb result, n (%) | 13 (3) | 38 (8) | 20 (4) | 54 (8) | 36 (5) |
| Transient positive, n (%)b | 12 (2) | 20 (4) | 15 (3) | 25 (4) | 17 (2) |
| Persistent positive, n (%)b | 1 (<1) | 18 (4) | 5 (1) | 29 (4) | 19 (3) |
All patients who received peginterferon beta1a at any point during the study.
Transient positive defined as a single positive evaluation, or more than one positive evaluation occurring less than 74 days apart; persistent positive defined as at least two consecutive positive evaluations that occurred at least 74 days apart or a positive evaluation at the final assessment.
BAb, binding antibody; IFN, interferon.
The overall incidence of treatment-emergent antipeginterferon beta1a NAbs was also low. Per the tiered assay design, only patients who were positive for anti-IFN BAbs were evaluated for the presence of antipeginterferon beta1a NAbs. In year 1, less than 1% of patients in all groups became positive for antipeginterferon beta1a NAbs; 2/13 patients in the BAb-positive placebo group, 4/38 patients in the BAb-positive peginterferon beta1a every 2 weeks group, and 2/20 patients in the BAb-positive peginterferon beta1a every 4 weeks group developed NAbs. The overall incidence of antipeginterferon beta1a NAbs remained less than 1% (n = 13/1431) over 2 years in patients treated at any point with peginterferon beta1a and most had low (range 6–44) or medium (80–581) titer levels (Table 3). Only one patient had high titer levels of NAbs (range 1210–3530). The incidence of transient and persistent antibodies was similar (Table 3). Among patients who received continuous peginterferon beta1a in both years, the incidence of NAbs in year 2 was 5/429 (1%) in the peginterferon beta1a every 2 weeks group, and 2/435 (<1%) in the peginterferon beta1a every 4 weeks group (Supplementary Table 1).
Table 3.
Incidence of treatment-emergent antipeginterferon beta1a NAbs at year 1 and over 2 yearsa.
| Year 1 |
Over 2 years |
||||
|---|---|---|---|---|---|
| Placebo (n = 489) | Peginterferon beta1a | Peginterferon beta1a | Peginterferon beta1a | Peginterferon beta1a | |
| every 2 weeks (n = 491) | every 4 weeks (n = 492) | every 2 weeks (n = 715) | every 4 weeks (n = 716) | ||
| Patients with ⩾1 positive antipeginterferon beta1a NAbs result, n (%)b | 2 (<1) | 4 (<1) | 2 (<1) | 7 (<1) | 6 (<1) |
| Transient positive, n (%)c | 1 (<1) | 2 (<1) | 2 (<1) | 2 (<1) | 5 (<1) |
| Persistent positive, n (%)c | 1 (<1) | 2 (<1) | 0 | 5 (<1) | 1 (<1) |
| Low titer, n (%) | 1 (<1) | 3 (<1) | 1 (<1) | 2 (<1) | 3 (<1) |
| Medium titer, n (%) | 1 (<1) | 1 (<1) | 1 (<1) | 3 (<1) | 3 (<1) |
| High titer, n (%) | 0 | 0 | 0 | 1 (<1) | 0 |
All patients who received peginterferon beta1a at any point during the study.
NAb assay was performed only in anti-IFN BAb-positive samples, per tiered assay design; percentages are given as a proportion of the total number of patients at risk (the number of patients whose baseline antibody was not positive and who had at least one post-baseline immunogenicity assessment).
Transient positive defined as a single positive evaluation, or more than one positive evaluation occurring less than 74 days apart; persistent positive defined as at least two consecutive positive evaluations that occurred at least 74 days apart or a positive evaluation at the final assessment. Results reported as ‘positive titer not determinable’ were considered titer missing. Antipeginterferon NAbs titer levels: low (⩽50), medium (>50 and ⩽700), or high (>700).
BAb, binding antibody; IFN, interferon; NAb, neutralizing antibody.
The incidence of treatment-emergent anti-PEG Abs in year 1 was 5% (24/456) in the placebo group and 6% (30/474) and 9% (43/467) in the peginterferon beta1a every 2 weeks and every 4 weeks groups, respectively. The incidence over 2 years in patients treated at any point with peginterferon beta1a was similar to the incidence in the peginterferon beta1a groups in year 1 (Table 4). Of the 95 treatment-emergent anti-PEG Ab-positive patients, 18 patients in the peginterferon beta1a every 2 weeks group and 35 patients in the peginterferon beta1a every 4 weeks group had persistent anti-PEG reactivity. Most patients with a positive test for anti-PEG Abs had low or medium titer levels (Table 4). For patients positive for anti-PEG Abs at baseline, titers increased over threefold across the study in 4/43 and 2/39 patients receiving peginterferon beta1a every 2 weeks or every 4 weeks, respectively.
Table 4.
Incidence of treatment-emergent anti-PEG Abs at year 1 and over 2 yearsa.
| Year 1 |
Over 2 years |
||||
|---|---|---|---|---|---|
| Placebo (n = 456) | Peginterferon beta1a | Peginterferon beta1a | Peginterferon beta1a | Peginterferon beta1a | |
| every 2 weeks (n = 474) | every 4 weeks (n = 467) | every 2 weeks (n = 681) | every 4 weeks (n = 682) | ||
| Patients with ⩾1 positive anti-PEG Abs result, n (%) | 24 (5) | 30 (6) | 43 (9) | 40 (6) | 55 (8) |
| Transient positive, n (%)b | 17 (4) | 19 (4) | 18 (4) | 22 (3) | 20 (3) |
| Persistent positive, n (%)b | 7 (2) | 11 (2) | 25 (5) | 18 (3) | 35 (5) |
| Low titer, n (%) | 15 (3) | 18 (4) | 24 (5) | 26 (4) | 32 (5) |
| Medium titer, n (%) | 9 (2) | 12 (3) | 17 (4) | 13 (2) | 21 (3) |
| High titer, n (%) | 0 | 0 | 2 (<1) | 1 (<1) | 2 (<1) |
All patients who received peginterferon beta1a at any point during the study.
Transient positive defined as a single positive evaluation, or more than one positive evaluation occurring less than 74 days apart; persistent positive defined as at least two consecutive positive evaluations that occurred at least 74 days apart or a positive evaluation at the final assessment. Results reported as ‘positive titer not determinable’ were considered low titer. Anti-PEG titer levels: low (⩽100), medium (>100 and <800), or high (⩾800).
Ab, antibody; PEG, polyethylene glycol.
Impact of antibody status on efficacy and safety of peginterferon beta1a
Assessment of the impact of antibody status on efficacy and safety was confounded by low numbers of patients with positive antibody results. However, based on the available data for patients with baseline or treatment-emergent antibodies, we observed that, at the end of year 1, unadjusted ARR (the primary endpoint of ADVANCE) was lower for patients treated with peginterferon beta1a every 2 weeks or every 4 weeks versus placebo regardless of antibody status (Table 5). Better outcomes were also observed in patients treated with peginterferon beta1a in comparison to placebo-treated patients, regardless of antibody status, for both MRI and clinical secondary endpoints (the number of new or newly enlarging T2 lesions, the proportion of patients who had relapsing disease, and the proportion of patients with disability progression) at year 1 (Supplementary Table 2).
Table 5.
| Year 1 |
Over 2 years |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n=500) | Peginterferon beta-1a |
Peginterferon beta-1a |
Peginterferon beta-1a |
Peginterferon beta-1a |
||||||
| every 2 weeks (n=512) | every 4 weeks (n=500) | every 2 weeks (n=438) | every 4 weeks (n=438) | |||||||
| Never positive | Ever positive | Never positive | Ever positive | Never positive | Ever positive | Never positive | Ever positive | |||
| Anti-IFN beta-1a BAbs | n | 500 | 458 | 54 | 472 | 28 | 384 | 54 | 403 | 35 |
| ARR | 0.41 | 0.28 | 0.19 | 0.30 | 0.12 | 0.21 | 0.17 | 0.29 | 0.18 | |
| Anti-peginterferon beta-1a NAbs | n | 500 | 500 | 12 | 496 | 4 | 427 | 11 | 433 | 5 |
| ARR | 0.41 | 0.27 | 0.00 | 0.29 | 0.00 | 0.21 | 0.10 | 0.29 | 0.11 | |
| Anti-PEG Abs | n | 500 | 456 | 56 | 430 | 70 | 388 | 50 | 378 | 60 |
| ARR | 0.41 | 0.27 | 0.24 | 0.29 | 0.25 | 0.21 | 0.19 | 0.29 | 0.26 | |
ARR calculated as the total number of relapses that occurred during the period for all patients in each group, divided by the total number of patient-years followed in the period.
’Never positive’ indicates patients without antibodies to peginterferon beta-1a, ‘ever positive’ indicates patients positive at any timepoint, including baseline.
Patients who were treated with peginterferon beta-1a in both Year 1 and Year 2.
Ab, antibody; ARR, annualized relapse rate, BAb, binding antibody; IFN, interferon; NAb, neutralizing antibody.
Among patients who received peginterferon beta1a in both years 1 and 2, efficacy results were generally similar at year 1 and over 2 years (Table 5); in fact further reductions in ARR were observed in the peginterferon beta1a every 2 weeks group [Kieseier et al. 2015]. There was no evidence of worsening with longer-term treatment in patients who were ever positive for antibodies to IFN or PEG.
There was no discernible effect of antibody status on the incidence of adverse events (AEs), including serious AEs and injection-site reactions (Table 6).
Table 6.
| Peginterferon beta1a |
Peginterferon beta1a |
||||
|---|---|---|---|---|---|
| every 2 weeks$ (n = 438) | every 4 weeks$ (n = 439) | ||||
| Never positive | Ever positive | Never positive | Ever positive | ||
| Anti-IFN BAbs | n | 384 | 54 | 404 | 35 |
| Patients with ⩾1 AE, n (%) | 375 (98) | 50 (93) | 392 (97) | 34 (97) | |
| Patients with ⩾1 serious AE, n (%) | 65 (17) | 7 (13) | 98 (24) | 8 (23) | |
| Patients with ⩾1 AE related to study treatment, n (%) | 359 (93) | 49 (91) | 379 (94) | 33 (94) | |
| Patients discontinuing due to AE, n (%) | 6 (2) | 3 (6) | 9 (2) | 0 | |
| Patients with ISR, n (%) | 282 (73) | 40 (74) | 272 (67) | 22 (63) | |
| Antipeginterferon NAbs | n | 427 | 11 | 434 | 5 |
| Patients with ⩾1 AE, n (%) | 417 (98) | 8 (73) | 421 (97) | 5 (100) | |
| Patients with ⩾1 serious AE, n (%) | 71 (17) | 1 (9) | 106 (24) | 0 | |
| Patients with ⩾1 AE related to study treatment, n (%) | 400 (94) | 8 (73) | 408 (94) | 4 (80) | |
| Patients discontinuing due to AE, n (%) | 8 (2) | 1 (9) | 9 (2) | 0 | |
| Patients with ISR, n (%) | 315 (74) | 7 (64) | 291 (67) | 3 (60) | |
| Anti-PEG Abs | n | 388 | 50 | 379 | 60 |
| Patients with ⩾1 AE, n (%) | 377 (97) | 48 (96) | 366 (97) | 60 (100) | |
| Patients with ⩾1 serious AE, n (%) | 63 (16) | 9 (18) | 88 (23) | 18 (30) | |
| Patients with ⩾1 AE related to study treatment, n (%) | 362 (93) | 46 (92) | 358 (94) | 54 (90) | |
| Patients discontinuing due to AE, n (%) | 9 (2) | 0 | 8 (2) | 1 (2) | |
| Patients with ISR, n (%) | 286 (74) | 36 (72) | 256 (68) | 38 (63) | |
‘Never positive’ indicates patients without antibodies to peginterferon beta1a, ‘ever positive’ indicates patients positive at any timepoint, including baseline.
Patients who were treated with peginterferon beta1a in both year 1 and year 2 (safety population).
Ab, antibody; AE, adverse event; BAb, binding antibody; IFN, interferon; ISR, injection site reaction; NAb, neutralizing antibody; PEG, polyethylene glycol.
Discussion
We evaluated the risk of generating an immunogenic response and explored the impact of antibodies to peginterferon beta1a on efficacy and safety measures. The first assessment evaluated the risk of generating de novo antibodies in antibody-naïve patients. The second assessment evaluated the risk of antibodies affecting safety and efficacy, whether those antibodies were present prior to treatment (such as from other IFN therapies or environmental exposure to PEG) or generated during the course of treatment with peginterferon beta1a.
This study showed very low immunogenicity with peginterferon beta1a over 2 years. The overall incidence of treatment-emergent anti-IFN BAbs at any point over 2 years in patients receiving peginterferon beta1a dosed every 2 or 4 weeks was 6%, and the overall incidence of NAbs was less than 1%. For both BAbs and NAbs, appearance of antibodies was transient in approximately half of the patients who developed antidrug antibodies. A small minority of patients were positive for anti-IFN antibodies at baseline; this may relate to prior IFN treatment, or could reflect false positive reactivity in the assays due to the statistically derived cut point. As patients on long-term IFN therapy prior to this trial were excluded from enrollment, there are insufficient data to determine whether antibodies generated against nonpegylated IFNs will be cross reactive to peginterferon beta1a.
Anti-PEG Abs were detected in the serum of approximately 6% of patients at baseline. This background level of anti-PEG Abs is consistent with general exposure to PEG from processed foods and over-the-counter medicines such as antacids [Sheftel, 2000]. A similar proportion of patients in the placebo group (5%) developed ‘treatment-emergent’ anti-PEG Abs during year 1. Although cross-study comparisons are complicated by different assay formats and the lack of a true positive control [Verhoef et al. 2014], this is in a comparable range to the 13% anti-PEG Ab incidence determined by serology in a subgroup of patients receiving nonpegylated drug in a study of pegasparaginase [Armstrong et al. 2007]. Treatment-emergent anti-PEG Abs also developed in a similar proportion of patients receiving peginterferon beta1a (~7%) over 2 years. Approximately half of patients developing anti-PEG Abs were only transiently positive, and the majority had low or medium antibody titers. Among patients who were anti-PEG Ab positive at baseline, only 6 of 82 patients had more than a threefold increase in anti-PEG Ab titer levels during treatment.
The low rate of immunogenicity, with NAbs detected in less than 1% of patients over 2 years, is at the lowest end of the range of immunogenicity observed with interferon treatments for MS. The incidence of NAbs emerging during treatment with nonpegylated IFN betas has been reported as ranging from 2% to 6% in trials of intramuscular IFN beta1a (Avonex, Biogen, Cambridge, MA, USA), from 12% to 28% with subcutaneous IFN beta1a (Rebif, Merck Serono, Geneva, Switzerland), and from 28% to 47% with subcutaneous IFN beta1b (Betaferon/Betaseron, Bayer-Schering, Leverkusen, Germany) [Bertolotto et al. 2004]. Rates reported in clinical trials may vary due to differences in assay methods, timing of sampling and so on [Sorenson et al. 2005; Ross et al. 2000]. Differences between IFN beta products have been attributed to differences in the method of production (e.g. IFN beta1a is produced in mammalian cells, whereas IFN beta1b is produced in Escherichia coli cells) and related differences in glycosylation, as well as to dosage and frequency of administration [Goodin et al. 2007; Bertolotto et al. 2014; Ross et al. 2000]. While some of these factors may contribute to the lower immunogenicity observed with peginterferon beta1a, for example reduced frequency of administration, it is probable that pegylation also contributes to a large extent to the favorable immunogenicity profile, either directly or through the extended pharmacokinetic profile.
For each type of antibody (BAbs, NAbs, and anti-PEG Abs), results for active treatment groups over the entire 2-year treatment period remained consistent with year 1 results; therefore, we believe that 2 years was an adequate duration to characterize immunogenicity. Ongoing monitoring in the ATTAIN study [ClinicalTrials.gov identifier: NCT01332019], an extension of ADVANCE, will reveal whether there may be a longer-term trend for increasing NAbs incidence; however, previous studies have shown that patients who have remained NAb negative during the first 18 or 24 months of IFN beta therapy rarely develop NAbs with continuing treatment [Sorensen et al. 2005]. A longer duration of treatment in the commercial setting may be required to reveal any effect of antibody status on clinical efficacy, particularly given the small number of patients developing antibodies.
The NAb assay measures ability to neutralize IFN activity in vitro, which is anticipated to predict potential in-vivo neutralization. An immunogenic response is most likely to have a clinically relevant impact if a patient develops persistent NAbs at a sufficiently high in vitro titer [Polman et al. 2003, 2010; Goodin et al. 2007]. However, so few patients had persistent NAb positivity at any titer level (<1% overall, and only one patient with high titer; range 1210–3530) that reliable assessment of any potential impact on efficacy or safety on this basis was not feasible. Instead, we categorized patients according to their status as ever positive or never positive, for each type of antibody. This permitted assessment of potential impact on efficacy and safety regardless of whether antibodies were treatment induced or pre-existing, and of potential impact of anti-PEG Abs as well as antipeginterferon beta1a NAbs. Anti-IFN BAbs were included for completeness, although only NAbs have been reported to impact safety or efficacy of nonpegylated IFN beta [Polman et al. 2010; Paolicelli et al. 2013]. The data from this trial showed no discernible effect of IFN BAbs or NAbs or anti-PEG Abs on clinical efficacy, improvement in MRI measures, or AEs.
Favorable pharmacokinetic characteristics of peginterferon beta1a could also help explain the lack of apparent impact of antibodies, including NAbs, on efficacy in this study, by allowing for dosing over the inhibitory concentration in a similar manner as has been used with hemophilia factors [Abshire, 2004]. Future experience in patients with NAbs from prior IFN beta treatment could help distinguish between this hypothesis, not reaching a clinically relevant titer for NAbs, or simply the low number of NAb-positive patients.
We have provided a thorough characterization of the immunogenic profile of peginterferon beta1a, evaluating immune responses to both the IFN portion and the PEG moiety. Our findings do not suggest a need to routinely monitor patients receiving peginterferon beta1a for development of antipeginterferon NAbs or anti-PEG Abs. Despite limitations to the interpretation of results regarding the impact of antidrug antibodies on treatment effect, the low incidence of immunogenicity, including extremely low frequency of antipeginterferon NAbs developing at high titer levels, means that few patients are at risk of experiencing impaired efficacy during treatment with peginterferon beta1a due to immunogenicity. Overall, these results show that the treatment effect of peginterferon beta1a in patients with relapsing–remitting MS is not expected to be attenuated by immunogenicity.
Supplementary Material
Acknowledgments
The authors were assisted in the preparation of this manuscript by Alexandra Silveira of Biogen (Cambridge, MA, USA) and Samantha Stanbury, a professional medical writer contracted to CircleScience (Tytherington, UK). Writing support was funded by the study sponsor. All authors were involved in reviewing the manuscript critically for important intellectual content. The authors had full editorial control of the manuscript and provided their final approval of all content. ClinicalTrials.gov identifier: NCT00906399.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study was sponsored by Biogen (Cambridge, MA, USA). S.D. Newsome has participated in scientific advisory boards for Biogen, Genzyme, and Novartis, and has received research support (paid directly to the institution) from Biogen and Novartis. B.C. Kieseier has received personal compensation for activities with Bayer Schering, Biogen, Merck Serono, Novartis, Roche, Sanofi-Aventis, and TEVA Neurosciences as a lecturer, research support from Bayer Schering, Biogen, Merck Serono, and Teva Neurosciences, and is currently an employee of Biogen (although he was not affiliated to Biogen at the time of study conduct and data analysis). R. Bermel has received consulting fees for activities from Biogen, Novartis, Genentech, Genzyme and Mallinkrodt and research support (paid directly to the institution) from Biogen and Novartis. J.T. White, Y. Cui, A. Seddighzadeh, S. Hung, M. Crossman, and M. Subramanyam are all current or former employees of Biogen.
Contributor Information
Joleen T. White, Biogen, Cambridge, MA, USA
Scott D. Newsome, Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD, USA
Bernd C. Kieseier, Biogen, Cambridge, MA, USA Department of Neurology, Heinrich-Heine University, Düsseldorf, Germany.
Robert A. Bermel, Department of Neurology, Mellen Center for Multiple Sclerosis, Cleveland Clinic, Cleveland, OH, USA
Yue Cui, Biogen, Cambridge, MA, USA.
Ali Seddighzadeh, Biogen, Cambridge, MA, USA.
Serena Hung, Biogen, Cambridge, MA, USA.
Mary Crossman, Biogen, Cambridge, MA, USA.
Meena Subramanyam, 14 Cambridge Center, Building 6A, Floor 6, Office K01, Cambridge, MA 02142, USA.
References
- Abshire T. (2004) Dose optimization of recombinant factor VIIa for control of mild to moderate bleeds in inhibitor patients: improved efficacy with higher dosing. Semin Hematol 41: 3–7. [DOI] [PubMed] [Google Scholar]
- Armstrong J., Hempel G., Koling S., Chan L., Fisher T., Meiselman H., et al. (2007) Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer 110: 103–111. [DOI] [PubMed] [Google Scholar]
- Bertolotto A., Deisenhammer F., Gallo P., Sölberg Sørensen P., et al. (2004) Immunogenicity of interferon beta: differences among products. J Neurol 251(Suppl. 2): II15–II24. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Kieseier B., Arnold D., Balcer L., Boyko A., Pelletier J., et al. (2014) Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol 13: 657–665. [DOI] [PubMed] [Google Scholar]
- Caliceti P., Veronese F. (2003) Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev 55: 1261–1277. [DOI] [PubMed] [Google Scholar]
- Clanet M., Radue E., Kappos L., Hartung H., Hohlfeld R., Sandberg-Wollheim M., et al. (2002) A randomized, double-blind, dose-comparison study of weekly interferon beta-1a in relapsing MS. Neurology 59: 1507–1517. [DOI] [PubMed] [Google Scholar]
- European Study Group on interferon beta-1b in secondary progressive MS (1998) Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. Lancet 352: 1491–1497. [PubMed] [Google Scholar]
- Food and Drug Administration (2014) Guidance for industry: immunogenicity assessment for therapeutic protein products. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm338856.pdf (accessed August 2014).
- Goodin D., Frohman E., Hurwitz B., O’Connor P., Oger J., Reder A., et al. (2007) Neutralizing antibodies to interferon beta: assessment of their clinical and radiographic impact: an evidence report: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 68: 977–984. [DOI] [PubMed] [Google Scholar]
- Halfon P., Perusat S., Bourliere M., Bronowicki J., Trimoulet P., Benhamou Y., et al. (2010) Neutralizing antibodies to interferon-alpha and circulating interferon in patients with chronic hepatitis C non-responding to pegylated interferon plus ribavirin re-treated by pegylated interferon-alpha-2a and ribavirin (ANRS HC16 GAMMATRI substudy). J Med Virol 82: 2027–2031. [DOI] [PubMed] [Google Scholar]
- Harris J., Martin N., Modi M. (2001) Pegylation: a novel process for modifying pharmacokinetics. Clin Pharmacokinet 40: 539–551. [DOI] [PubMed] [Google Scholar]
- Hartung H., Munschauer F., III, Schellekens H. (2005) Significance of neutralizing antibodies to interferon beta during treatment of multiple sclerosis: expert opinions based on the Proceedings of an International Consensus Conference. Eur J Neurol 12: 588–601. [DOI] [PubMed] [Google Scholar]
- Hu X., Miller L., Richman S., Hitchman S., Glick G., Liu S., et al. (2012) A novel PEGylated interferon beta-1a for multiple sclerosis: safety, pharmacology, and biology. J Clin Pharmacol 52: 798–808. [DOI] [PubMed] [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (1996) ICH harmonized tripartite guideline – guideline for good clinical practice: E6(R1) (1996). Available at: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf (accessed August 2015). [PubMed]
- Ishida T., Kiwada H. (2013) Anti-polyethyleneglycol antibody response to PEGylated substances. Biol Pharm Bull 36: 889–891. [DOI] [PubMed] [Google Scholar]
- Kang J., Deluca P., Lee K. (2009) Emerging PEGylated drugs. Expert Opin Emerg Drugs 14: 363–380. [DOI] [PubMed] [Google Scholar]
- Kieseier B., Arnold D., Balcer L., Boyko A., Pelletier J., Liu S., et al. (2015) Peginterferon beta-1a in multiple sclerosis: 2-year results from ADVANCE. Mult Scler 21: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieseier B., Calabresi P. (2012) PEGylation of interferon-beta-1a: a promising strategy in multiple sclerosis. CNS Drugs 26: 205–214. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- Matsuda F., Torii Y., Enomoto H., Kuga C., Aizawa N., Iwata Y., et al. (2012) Anti-interferon-alpha neutralizing antibody is associated with nonresponse to pegylated interferon-alpha plus ribavirin in chronic hepatitis C. J Viral Hepat 19: 694–703. [DOI] [PubMed] [Google Scholar]
- Paolicelli D., D’Onghia M., Pellegrini F., Direnzo V., Iaffaldano P., Lavolpe V., et al. (2013) The impact of neutralizing antibodies on the risk of disease worsening in interferon beta-treated relapsing multiple sclerosis: a 5 year post-marketing study. J Neurol 260: 1562–1568. [DOI] [PubMed] [Google Scholar]
- Polman C., Bertolotto A., Deisenhammer F., Giovannoni G., Hartung H., Hemmer B., et al. (2010) Recommendations for clinical use of data on neutralising antibodies to interferon-beta therapy in multiple sclerosis. Lancet Neurol 9: 740–750. [DOI] [PubMed] [Google Scholar]
- Polman C., Kappos L., White R., Dahlke F., Beckmann K., Pozzilli C., et al. (2003) Neutralizing antibodies during treatment of secondary progressive MS with interferon beta-1b. Neurology 60: 37–43. [DOI] [PubMed] [Google Scholar]
- Polman C., Reingold S., Edan G., Filippi M., Hartung H., Kappos L., et al. (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria’. Ann Neurol 58: 840–846. [DOI] [PubMed] [Google Scholar]
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group (1998) Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352: 1498–1504. [PubMed] [Google Scholar]
- Rosenberg A. (2003) Immunogenicity of biological therapeutics: a hierarchy of concerns. Dev Biol (Basel) 112: 15–21. [PubMed] [Google Scholar]
- Ross C., Clemmesen K., Svenson M., Sørensen P., Koch-Henriksen N., Skovgaard G., et al. (2000) Immunogenicity of interferon-beta in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Danish Multiple Sclerosis Study Group. Ann Neurol 48: 706–712. [PubMed] [Google Scholar]
- Rudick R., Simonian N., Alam J., Campion M., Scaramucci J., Jones W., et al. (1998) Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology 50: 1266–1272. [DOI] [PubMed] [Google Scholar]
- Scagnolari C., Trombetti S., Solda A., Milella M., Gaeta G., Angarano G., et al. (2012) Development and specificities of anti-interferon neutralizing antibodies in patients with chronic hepatitis C treated with pegylated interferon-alpha. Clin Microbiol Infect 18: 1033–1039. [DOI] [PubMed] [Google Scholar]
- Sheftel V. (2000) Indirect Food Additives and Polymers: Migration and Toxicology (1st ed.). Boca Raton, FL: Lewis Publishers CRC Press LLC. [Google Scholar]
- Shimizu T., Ichihara M., Yoshioka T., Ishida Y., Nakagawa S., Kiwada H. (2012) Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biol Pharm Bull 35: 1336–1342. [DOI] [PubMed] [Google Scholar]
- Sorensen P., Koch-Henriksen N., Ross C., Clemmesen K., Bendtzen K.; on behalf of the Danish Multiple Sclerosis Study Group. (2005) Appearance and disappearance of neutralizing antibodies during interferon-beta therapy. Neurology 65: 33–39. [DOI] [PubMed] [Google Scholar]
- The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group (1996) Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: experience during the first three years. Neurology 47: 889–894. [DOI] [PubMed] [Google Scholar]
- Verhoef J., Carpenter J., Anchordoquy T., Schellekens H., et al. (2014) Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov Today 19: 1945–1952. [DOI] [PubMed] [Google Scholar]
- World Medical Association (2013) Declaration of Helsinki – ethical principles for medical research involving human subjects. Available at: http://www.wma.net/en/30publications/10policies/b3/ (accessed October 2015). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.