Abstract
Background:
Improved clinical effectiveness and therefore positive modification of multiple sclerosis (MS) with basic therapy can be achieved by long-term regular intake of drugs as prescribed but investigations have shown that a high percentage of patients do not take their medications as prescribed.
Objectives:
We assessed the satisfaction and adherence of patients with MS with their current disease-modifying treatment under clinical practice conditions. We compared different facets of satisfaction as well as their internal relationship and identified predictors in an exploratory manner.
Methods:
Therapy satisfaction in patients with relapsing–remitting multiple sclerosis (THEPA-MS) was a noninterventional, prospective cross-sectional study performed throughout Germany in 2013 and 2014, and included patients with clinically isolated syndrome or relapsing–remitting MS. We applied a standardized approach to document satisfaction and adherence by patient-reported outcomes (Treatment Satisfaction Questionnaire for Medication) as well as by physician ratings.
Results:
Of 3312 patients with a mean age of 43.7 years, 73.3% were women and the mean level of disability according to the Expanded Disability Status Scale was 2.29; 13.3% did not receive any medication at the time of documentation, 21.3% received interferon β1a intramuscularly, 20.7% had interferon β1a subcutaneously, 17.0% had interferon β1b subcutaneously and 23.7% had glatiramer acetate. Adherence rates varied between 60% (lifetime) and 96.5% (current medication). Differences between current medications were found for side effects and convenience scores but not for effectiveness, satisfaction and adherence. Higher global satisfaction and effectiveness were associated with fewer relapses, longer duration of medication, lower disability score and the absence of several side effects.
Conclusion:
In a connected model of patient satisfaction, effectiveness, side effects, convenience and adherence, patients’ individual needs and concerns have to be addressed. Most differences were found with respect to side effects and convenience of treatment. Therefore, an improvement in these two domains seems to be the most promising proximate approach to elevate adherence levels.
Keywords: convenience, multiple sclerosis, patient adherence, patient-centred outcomes research, patient satisfaction, quality of life, side effects, treatment effectiveness
Introduction
Background
Multiple sclerosis (MS) is an immune-mediated chronic inflammatory disease of unclear aetiology which is characterized by demyelination and axonal damage [Compston and Coles, 2008]. Further, MS is the most frequent neurological disease leading to disability and early retirement in young adults. In the initial phase, MS often occurs as clinically isolated syndrome (CIS), for example, blurred vision by one-sided optic neuritis, together with disease signs manifest on magnetic resonance tomography. In about 85% of patients the relapsing–remitting form (RRMS) manifests which is characterized by a pattern of clearly defined relapses that are divided by symptom-free periods [Compston and Coles, 2008]. If not treated, RRMS after about 10 years transitions into the secondary progressive form (SPMS), with increasing disability. To date, MS cannot be cured. A number of disease-modifying treatments (DMTs) have been shown to reduce the number of relapses, to slow progression of disease and thus delay disability. The guidelines released by the German Neurological Society (DGN) issued in 2012 recommend as basic (first-line) treatment for CIS/RRMS interferon (IFN) β1a or β1b preparations, glatiramer acetate (GA), and only with restrictions azathioprine (AZA) or immunoglobulins (IGs) intravenously. IFN and GA require yearlong intramuscular or subcutaneous injections (depending on the drug once daily or once weekly).
In a recent meta-analysis by Giovannoni and colleagues that analysed the data of 50 randomized studies and 19 observational studies in MS, mean discontinuation rates of 17–36% for such therapies were noted [Giovannoni et al. 2012]. The most frequent reasons were side effects and lack of efficacy. The meta-analysis identified flu-like symptoms (IFN) and reactions at the injection site (GA) as the most frequent side effects, in agreement with the prescribing information sheets. The incidence of side effects remained high in studies of more than 2 years’ duration and therapy discontinuations accumulated over time. In another study on therapy adherence in MS, as justification for therapy discontinuation, lack of efficacy was noted in 30–50% and side effects in up to 50% of patients [Kern et al. 2008]. In recent years, much research has been performed on the patient perspective and the importance of patient preference in the choice of treatments and patient adherence to prescribed MS therapy. A recent meta-analysis suggests that greater treatment satisfaction is associated with improved persistence, and with lower regimen complexity or treatment burden [Barbosa et al. 2012].
Satisfaction is a determinant of adherence to treatment [Albrecht and Hoogstraten, 1998; Hirji et al. 2013; Turk-Adawi et al. 2013; Chrystyn et al. 2014; Wong et al. 2015]. Further, it affects health-related decisions, treatment-related behaviours and the impact of treatment outcomes [Lindhiem et al. 2014].
For chronic diseases such as MS, the regular intake or administration of drugs as prescribed by the treating physician, for example, high adherence to therapy, is a prerequisite for treatment success [Osterberg and Blaschke, 2005; Ho et al. 2009].
A number of investigations in various disease areas have shown that a high percentage of patients do not take their medications as prescribed. Claxton and colleagues concluded based on a systematic review that not only patients with chronic diseases such as arterial hypertension or diabetes mellitus (which usually cause little inconvenience or side effects) but also those with cancer have a compliance rate below 80% [Claxton et al. 2001].
With increasing treatment duration, adherence decreases [Vrijens et al. 2008]. Underlying reasons are complex, and as central factors missing understanding of the patients for the need of chronic therapy, but also side effects play an important role [Osterberg and Blaschke, 2005; Horne, 2006; Ho et al. 2009].
On the basis of a systematic literature search, Costello and colleagues listed the following as pivotal factors for low therapy adherence in patients with MS: forgetfulness, fear of the injection, missing efficacy as assessed by the patient, side effects, problems with complex treatment schemes, as well as fatigue [Costello et al. 2008].
With decreasing adherence, the relative risk of relapses increases [Steinberg et al. 2010]. Improved clinical effectiveness and therefore positive modification of MS with basic therapy can be achieved by long-term regular intake of drugs as prescribed. Therapy satisfaction of patients is likely directly linked to adherence.
Objectives
With our study ‘Therapy satisfaction in patients with relapsing–remitting multiple sclerosis’ (THEPA-MS), we aimed to assess the satisfaction of patients with MS with their current DMT under clinical practice conditions. We undertook a standardized, broad approach to document satisfaction and adherence by patient-reported outcomes as well as by physician ratings beyond literature reviews. We intended to compare different facets of satisfaction and their internal relationship, and identify predictors of treatment-related satisfaction in an exploratory, data-driven manner.
Further, we wanted to provide reference values for the Treatment Satisfaction Questionnaire for Medication (TSQM) from a large set of patients with CIS/RRMS being treated with first-line MS basic therapy as specified in the DGN guidelines (IFN of any type, GA, AZA, IGs) [DGN/KKNMS (Kompetenznetzwerk Multiple Sklerose), 2012].
Methods
Participants
THEPA-MS was a multicentre, open, prospective, noninterventional cross-sectional study. Patient data were obtained in 445 neurological practices at a single time point between August 2013 and April 2014 in all parts of Germany. The ethics committee of the Medical Faculty of the Technical University of Dresden approved the study materials. All patients provided written informed consent prior to inclusion. Patients were eligible for documentation if they had a verified MS diagnosis according to the McDonald criteria and met the following criteria: age at least 18 years; CIS/RRMS; received MS first-line therapy (interferon β1a or β1b intramuscularly or subcutaneously; GA subcutaneously; AZA; IGs) as current treatment or were principally eligible for first-line treatment (irrespective of whether it was performed or not) and had received no escalation therapy in the past [Polman et al. 2005].
Measures
In an initial step, physicians were requested to fill out a questionnaire to describe their personal and centre characteristics. For every patient they documented sociodemographic data (age, sex, occupational status, family status).
They also documented MS characteristics and therapy in detail [among others, date of diagnosis/disease duration, current MS diagnosis, current Expanded Disability Status Scale (EDSS) score, number of relapses in the last year, presence of fatigue or depression, MS treatment duration and current MS basic therapy with treatment duration] [Kurtzke, 1983, 2000]. In addition, the following side effects and changes in laboratory parameters related to the current MS first-line therapy (within 3 months prior to the documentation date) were recorded: injection site reaction, pain, flu-like symptoms, headache, acute postinjection reaction, lymphadenopathy, increased fatigue, infection, elevated transaminases, neutropenia, lymphocytopenia, leukopenia and thrombocytopenia. If no current MS therapy was administered, reasons for nontreatment or discontinuation of prior therapy were documented. Further, physicians rated therapy satisfaction and adherence.
The TSQM is an instrument for the assessment of patients’ satisfaction with their current medication [Atkinson et al. 2004, 2005]. The questionnaire (version 1.4) consists of 14 questions. Besides global treatment satisfaction, satisfaction in the dimensions of side effects, efficacy and convenience are rated. Higher levels of satisfaction are expressed as higher TSQM scores. The TSQM has been validated in patients with various chronic diseases (such as migraine, arthritis, depression, asthma and hypertension) and showed high reliability and validity despite the heterogeneity of patient groups [Atkinson et al. 2004].
In addition, four supplemental questions on patient satisfaction (developed for this study) were asked with respect to drug treatment and regularity of drug intake. Patients were asked to fill in answers to all questions themselves, but in the case of physical disability, they could accept help from other people.
Statistical analysis
All statistical comparisons were two tailed and a p value of less than 0.05 indicated statistical significance. Continuous variables were checked for the assumption of data with normal distribution by the Shapiro–Wilk test. Non-Gaussian variables were log transformed before analyses. When no normal distribution could be obtained, only nonparametric tests were applied. For AZA and IGs, only descriptive statistics were presented due to the limited number of patients receiving these medications and the restrictions of the DGN guidelines.
Exhaustive χ2 Automatic Interaction Detection (CHAID) analysis, a nonparametric method for analysing nominal, ordinal and continuous outcomes with multiple Bonferroni adjusted χ2 tests, was used in (classification and) regression tree models (CRTs) with 10-fold cross validation and complete regrouping of predictor variables providing insights on the connection between a set of predictors and the respective outcome [Kass, 1980; Loh, 2014]. Age, sex, disease duration, EDSS score, annual relapse rate, current DMT, number of medication changes, duration of medication intake, presence of adverse events or laboratory changes, current diagnosis of a major depressive episode (MDE) or disease-related fatigue, current family and occupational status were included in the analysis model as potential predicting factors for treatment satisfaction. To validate the results generated by the CRTs, analyses of variance (ANOVA) with medication as an independent variable and analyses of covariance (ANCOVAs) including the same set of predictors were calculated. For effectiveness and global treatment satisfaction (by TSQM), all DMTs were analysed in one approach whereas convenience and side effects were analysed for each DMT separately. Differences in metric variables between groups were assessed with (Bonferroni-adjusted) t tests or Mann–Whitney U tests in the case of non-Gaussian distributions. A linear regression model was estimated with global satisfaction as dependent variable and the effectiveness, side effects and convenience as predictors. For correlations between physician ratings and TSQM scores, Kendall τ-b rank correlation coefficients were calculated.
We used IBM SPSS version 23.0 (IBM Corporation, Armonk, NY, USA) for all statistical computations.
Results
Patient characteristics
A total of 3312 evaluable patients with MS were documented by their attending physician. Mean age was 43.7 years (±11.3 standard deviation, SD) and 73.2% of patients were women. Nearly half of patients (45.5%) worked full time, 15.6% worked part time (7.9% as a result of MS) and 17.0% of patients were permanently unable to work (16.3% as a result of MS). A total of 50.1% of patients were married and 20.7% lived with a partner. The vast majority of patients were diagnosed with RRMS (96.5%), followed by 3.5% with CIS. Looking at the number of relapses within the past 12 months prior to study entry, 62.1% of the patients were relapse free, 27.7% had one relapse, 7.9% had two relapses and 1.9% were affected by three or more relapses. The mean duration of disease was 9.04 years (±7.08 SD) and the level of disability by EDSS was estimated between 2.29 (mean ± 1.55 SD) and 2.0 (median). On average, patients were 34.1 ± 10.4 years old at first occurrence of MS symptoms, and 35.6 ± 10.5 years at diagnosis of MS. Of all 3312 patients, 441 (13.3%) did not receive any medication at the time of documentation, 704 (21.3%) patients received IFN β1a intramuscularly, 687 (20.7%) had IFN β1a subcutaneously, 564 (17.0%) had IFN β1b subcutaneously, 785 (23.7%) had GA and only a small number were prescribed AZA (n = 37, 1.1%) and IG (n = 6, 0.2%). For further analyses, 3269 patients were eligible.
A total of 270 patients (8.2%) had only been treated in the past but were not currently receiving treatment. Average duration of the MS basic therapy last prescribed was 2.7 ± 3.1 years. The decision to terminate the therapy had primarily been made by the patients (65.6%). In total, 47.8% of patients terminated therapy on account of side effects; 191 patients (5.8%) had never received MS treatment, and of those, the decision against therapy was essentially taken by the patient in 76.4% of cases. The most frequent reasons to decide against therapy were fear of side effects (38.2%) and lack of trust in efficacy (28.8%).
For 96.5% of patients on current MS therapy, doctors reported having the overall impression that the patients were adherent. Among working patients, the adherence rate was slightly higher (97.2%) compared with patients who were not employed (95.6%, p = 0.037). A total of 1326 of 3312 patients (40.0%) reported that they discontinued therapy or had missed an injection. As the general reasons and reason for the discontinuation of therapy, ‘side effects in general’ (41.9% and 25.3%, respectively) and ‘problems at the injection site’ (33.1% and 15.2%, respectively) were most frequently reported.
Interruptions of MS therapy were noted to occur more than once a week in 5.7% of patients, once a week in 7.2%, about once a month in 18.0%, and about once in 3 months in 23.9% of patients (missing data in 11.7%).
Global treatment satisfaction and effectiveness
An overview for all TSQM scores by different DMTs can be found in Table 1.
Table 1.
Treatment satisfaction of different DMTs in patients with MS by TSQM.
| TSQM scores | No DMT |
IFN β1a intramuscularly |
IFN β1a subcutaneously |
IFN β1b subcutaneously |
GA |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
n = 441 |
n = 704 |
n = 687 |
n = 564 |
n = 785 |
||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Global satisfaction* | 47.7 | 31.32 | 72.3 | 20.36 | 72.3 | 19.74 | 72.6 | 21.03 | 71.4 | 20.48 |
| Effectiveness* | 50.9 | 26.51 | 70.0 | 22.65 | 68.1 | 22.39 | 67.6 | 23.48 | 66.5 | 22.90 |
| Side effects* | 60.9 | 37.19 | 67.7 | 27.09 | 73.4 | 26.85 | 77.6 | 25.53 | 83.9 | 23.65 |
| Convenience* | 63.8 | 27.40 | 70.2 | 21.72 | 74.2 | 20.11 | 70.1 | 20.70 | 68.8 | 19.51 |
ANOVA result: p < 0.001.
DMT, disease-modifying treatment; GA, glatiramer acetate; IFN, interferon; SD, standard deviation; TSQM, Treatment Satisfaction Questionnaire for Medication.
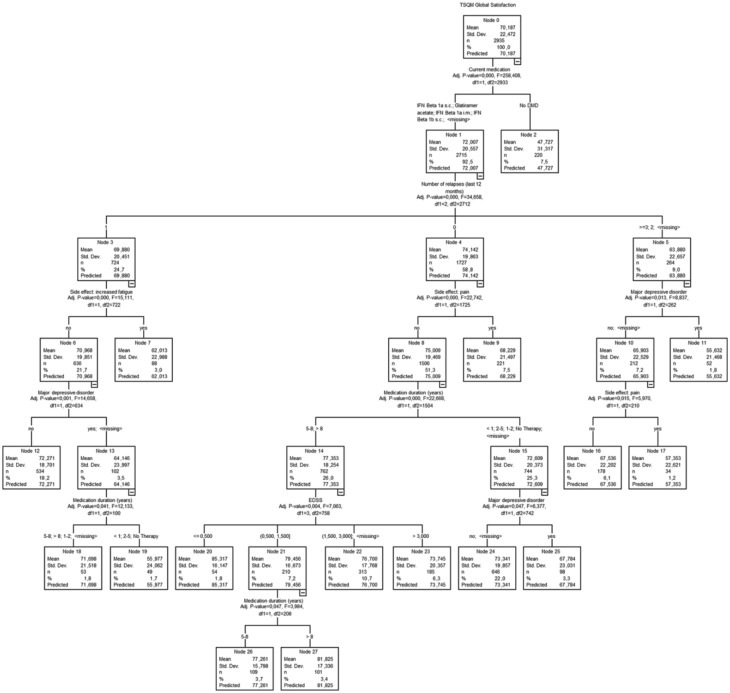
The main difference in global treatment related satisfaction was lower values for patients having dropped their last DMT in comparison to currently treated patients independently of the specific current DMT (ANOVA: p < 0.001, Table 1). The same result was present in the regression tree analysis of global satisfaction (n = 2935, Figure 1). Further, higher global satisfaction was associated with fewer relapses, longer duration of medication, the absence of a MDE, a lower EDSS score and the absence of side effects like pain and fatigue. These results could be confirmed by a comprehensive ANCOVA for all predictors of the tree model (with all p values < 0.01) except fatigue.
Figure 1.
Regression tree analysis of global satisfaction (TSQM, N = 2935). DMD, disease-modifying drug; EDSS, Expanded Disability Status Scale; IFN, interferon; i.m., intramuscular; s.c., subcutaneous; TSQM, Treatment Satisfaction Questionnaire for Medication.
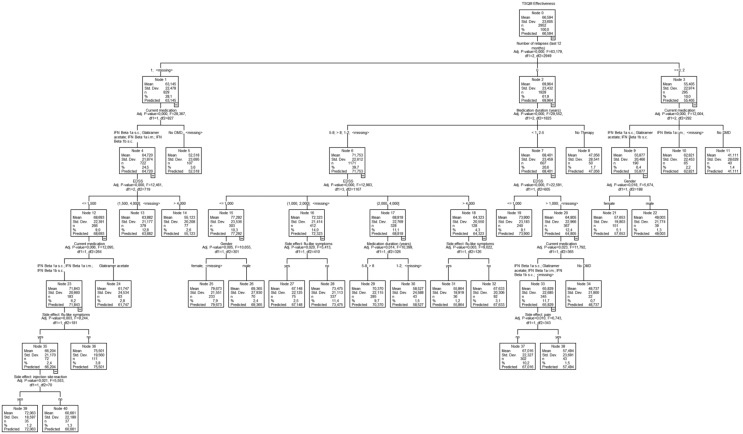
In a similar set of analyses, the effectiveness score presented lowest ratings for the previously treated patients without current medication compared with all other patients (ANOVA: p < 0.001). Also, GA showed a small significant difference (p = 0.045) compared with IFN β1a intramuscularly. In CRT analysis, fewer relapses were connected with higher effectiveness (Figure 2). The type of medication (as suggested by the ANOVA results), lower EDSS, sex (in favour of female patients), longer duration of medication, the absence of flu-like symptoms and pain also predicted better effectiveness ratings. On a global level, the ANCOVA results confirm the extracted predictors except the two side effects on a 0.01% level.
Figure 2.
Regression tree analysis of effectiveness (TSQM, N = 2952). DMD, disease-modifying drug; EDSS, Expanded Disability Status Scale; IFN, interferon; i.m., intramuscular; s.c., subcutaneous; TSQM, Treatment Satisfaction Questionnaire for Medication.
Side effects and treatment convenience
Nearly half of the patients (42.8%) reported side effects, most commonly skin reactions at the injection site (24.9%) and influenza-like symptoms (23.6%), followed by pain at the injection site (13.9%). Concerning the degree of severity of the side effects, increased fatigue was most frequently classified as moderate (49.0%), while the other side effects were predominantly assessed as mild. Being asked which application form they would prefer, 79.3% chose oral application, 11.8% subcutaneous injection, 6.8% intramuscular injection and 2.1% infusion.
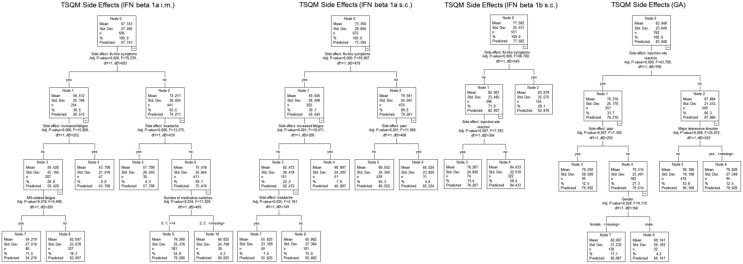
Most differences in TSQM scores between DMTs were found by analysing the side effects score. Only IFN β1a subcutaneously and IFN β1b subcutaneously did not differ from each other in a statistically significant way (p = 0.067). All other paired comparisons were significant at least on a 1% level (Table 1). Patients with GA reported fewer side effects than patients with other DMTs. For all IFN β DMTs, the presence of flu-like symptoms was the main predictor of a bad side effects score in CRTs (Figure 3). Subsequently, presence of pain, injection site reactions, headache or fatigue was also associated with a decreased side effects score across the DMTs.
Figure 3.
Regression tree analyses of side effects (TSQM, N = 2680). GA, glatiramer acetate; IFN, interferon; i.m., intramuscular; MS, multiple sclerosis; s.c., subcutaneous; TSQM, Treatment Satisfaction Questionnaire for Medication.
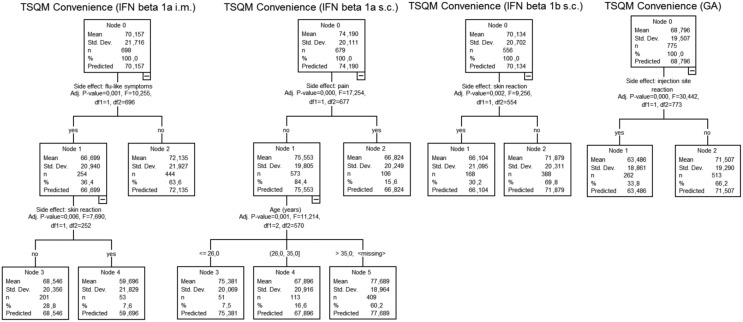
Looking at the results for convenience, IFN β1a intramuscularly, IFN β1b subcutaneously and GA received comparable ratings. Patients receiving IFN β1a subcutaneously reported higher convenience for their treatment (p < 0.01 versus IFN β1a intramuscularly and IFN β1b subcutaneously; p < 0.001 versus GA) whereas patients having dropped their last DMT presented lower convenience score values (p < 0.001 versus IFNs, p = 0.016 versus GA). Predicting factors for treatment convenience were dominated by potential side effects (Figure 4).
Figure 4.
Regression tree analyses of convenience (TSQM, N = 2708). IFN, interferon; GA, glatiramer acetate; i.m., intramuscular; s.c., subcutaneous; TSQM, Treatment Satisfaction Questionnaire for Medication.
Facets of satisfaction
A linear regression analysis provided insights on the plain (nonhierarchical) connection between the components of the satisfaction with the global satisfaction score. The model fit (adjusted R² = 0.493, p < 0.001) and the β coefficients (effectiveness: β = 0.421, p < 0.001; side effects: β = 0.218, p < 0.001; convenience: β = 0.294, p < 0.001) of the analysis provided further general information about the relationship between facets of satisfaction in patients with MS.
In addition to the patient-generated TSQM scores, physicians rated adherence, overall side effects, overall convenience and overall satisfaction with the current medication. An overview of rank correlation coefficients between those parameters is given in Table 2. Physicians’ rating of patients’ adherence showed small but still highly significant correlations with other physician ratings and TSQM scores. Focusing on physicians’ ratings, convenience turned out to be the parameter with the highest correlations.
Table 2.
Correlational analyses of satisfaction, side effects, convenience and adherence between patients’ and physicians’ ratings.
| Physician: ADHER | Physician: SE | Physician: CONV | Physician: SAT | Patient: TSQM EFF | Patient: TSQM SE | Patient: TSQM CONV | |
|---|---|---|---|---|---|---|---|
| Physician: ADHER | – | ||||||
| Physician: SE | −0.116* | – | |||||
| Physician: CON | −0.164* | 0.372* | – | ||||
| Physician: SAT | −0.145* | 0.370* | 0.579* | – | |||
| Patient: TSQM EFF | −0.067* | 0.146* | 0.216* | 0.282* | – | ||
| Patient: TSQM SE | −0.058* | 0.321* | 0.201* | 0.219* | 0.227* | – | |
| Patient: TSQM CON | −0.089* | 0.130* | 0.293* | 0.235* | 0.264* | 0.254* | – |
| Patient: TSQM GS | −0.092* | 0.157* | 0.247* | 0.335* | 0.508* | 0.288* | 0.392* |
Kendall τ-b: p < 0.001.
ADHER, adherence (no/yes); CONV, convenience; EFF, effectiveness; GS, global satisfaction; Patient, rating by patient (via TSQM); Physician, rating by physician; SAT, satisfaction; SE, side effects; TSQM, Treatment Satisfaction Questionnaire for Medication.
With respect to adherence, higher correlations were found for convenience and satisfaction than for side effects (Table 2). Strongest associations between physicians and patients were found for the same domains (e.g. side effects rated by physicians with side effects of TSQM) supporting the validity of the given results. Again, the relationship between TSQM scores was described by correlational coefficients matching with the results of the linear regression model.
Discussion
In the present observational study, we surveyed patients with MS treated by first-line injectable treatment (as specified in the DGN guideline) [DGN/KKNMS, 2012] about their general and specific treatment satisfaction.
We were able to collect a comprehensive dataset providing standardized information about several aspects of treatment satisfaction and adherence for the injectable MS treatments at the time of the start of the survey. We showed that global treatment satisfaction and perceived effectiveness did not differ in general between DMTs. Scores of side effects and convenience varied between DMTs depending on their type of application and pharmaceutical ingredient. For all facets of satisfaction, we identified predictors and were able to validate patient-reported values against physicians’ ratings.
Treatment satisfaction
The importance of the patient’s perspective on treatments for various indications including MS has been highlighted in recent years [US Department of Health and Human Services FDA Center for Drug Evaluation and Research, 2006; Coons et al. 2009; Rieckmann et al. 2015]. However, the majority of studies focused on quality of life and adherence or persistence of therapy, while patient satisfaction with treatment has only been assessed in a few specific studies. We chose the TSQM as an established generic instrument for the assessment of patient satisfaction, which has been validated in several chronic indications including MS and which is available in several languages including German [Atkinson et al. 2004].
Besides general reference values for patients in a mostly stable treatment environment, we extracted patterns of satisfaction with data-driven regression tree analyses. According to our results, patients’ opinion about the effectiveness and the closely related overall satisfaction is built upon their experience with the current DMT. A relapse-free treatment was the best predictor for high ratings, followed by a progression-free treatment and the absence of further side effects like pain, fatigue and depression symptoms. The decision about a successful treatment course or the need for a change of DMT may mostly be made between 1 and 5 years of treatment, which was indicated by the positive association of short-term and long-term treatment duration with higher satisfaction compared with mid-term durations. Such patterns may become important when healthcare professionals have to address treatment expectations, improve communication with the patient, and manage side effects of treatment [Brandes et al. 2009].
Satisfaction of patients treated with conventional DMTs has been reported in several current studies, in which, however, ratings on TSQM varied substantially: in a study of 568 patients who applied a new device for subcutaneous self injection of IFN β1b, on TSQM effectiveness at 12 weeks was rated at 49.7 (±18.6) points, convenience at 55.0 (±16.1) and global satisfaction at 47.3 (±19.6), while the side-effect subscale was not reported [Boeru et al. 2013].
Various first-line therapies were jointly assessed in the Spanish COMPLIANCE study (subcutaneous IFN β1b in 23%, intramuscular IFN β1a in 21%, subcutaneous IFN β1a in 37% and GA in 19%) [Saiz et al. 2015].
The mean score for effectiveness was 64.0 (±20.8), for side effects 63.4 (±22.0), for convenience 60.2 (±19.2) and for global satisfaction 69.8 (±10.2). On all scales, compliant patients had higher scores compared with noncompliant patients. Further, in the controlled TENERE study [ClinicalTrials.gov identifier: NCT00883337], in 101 patients on subcutaneous IFN β1a, effectiveness was rated at 59.3, for side effects 71.4, for convenience 61.9 and for global satisfaction 61.0 [Vermersch et al. 2014]. Compared with the conventional injectable therapies, the novel oral MS therapies achieved substantially higher satisfaction on TSQM: in the TENERE study, for teriflunomide, all scores were substantially higher (depending on the dose) with effectiveness rated at 63–67 points, side effects at 93–95 points, convenience at 88–90 points and global satisfaction at 68–69 points. Our values of satisfaction and effectiveness were slightly higher than those reported in the cited studies, which may be a result of the broad and noninterventional character of the study where a stable treatment situation may be likely assumed.
In a cross-sectional survey with 310 patients on fingolimod, TSQM scores were highest for side effects (79.4), followed by convenience (71.7), effectiveness (70.1) and global satisfaction (68.9); relatively higher scores were observed among treatment-experienced patients [Hanson et al. 2013].
In the publication on the EPOC study which compared the switch from GA or beta interferons to fingolimod versus staying on the injectable DMT, no absolute TSQM values were reported [Calkwood et al. 2014]. However, patients switched to oral therapy improved on the effectiveness scale depending on the original injectable DMT by 12–18 points, on the side effects scale by 9–31 points and on the convenience scale by 38–44 points (values for global satisfaction were not given).
Treatment adherence
It is well known that physicians’ and patients’ views on various aspects of a disease may vary. For example, Rothwell and colleagues showed that patients with MS and possibly those with other chronic diseases are less concerned than their clinicians about physical disability in their illness [Rothwell et al. 1997].
Assessment and perception of therapeutic risks vary among patients, doctors and regulators [Clanet et al. 2014].
It has been reported in several studies that adherent patients report greater satisfaction with DMT with regard to convenience and effectiveness [Twork et al. 2007; Treadaway et al. 2009; Saiz et al. 2015].
It was interesting to note that physicians in our study had the impression that 96.5% of patients receiving therapy were adherent to treatment, which was clearly above the expected level. However, at least a quarter of patients missed doses during the last 3 months. This is in line with the large Global Adherence Project, in which patients with RRMS reported nonadherence or missing DMT injections in the previous month in 25%, most frequently due to forgetfulness or injection-related reasons [Devonshire et al. 2011].
Several other studies confirm lower adherence rates in MS. In a systematic review of 24 studies published between 2001 and 2011, Menzin and colleagues reported adherence rates in patients with MS ranging from 41% to 88% [Menzin et al. 2013]. Like Halpern and colleagues, they found higher weighted mean adherence rates for intramuscular IFN β1a administered once a week (69.4%), and subcutaneous IFN β1a administered every other day (63.8%) than for subcutaneous IFN β1b administered three times a week (58.4%) and GA administered daily (56.8%) [Halpern et al. 2011]. Further, there was a numerically greater risk of MS relapse or disease progression among nonadherent patients compared with those categorized as adherent patients, with findings statistically significant in two out of four analysed studies. Hansen and colleagues also executed a data-driven approach to research patient adherence in MS by evaluating German claims data between 2001 and 2009 and finding only 30–40% of patients to be adherent to treatment over a period of 2 years [Hansen et al. 2015]. Agashivala and colleagues showed in another retrospective study on adherence to MS DMTs in 2013 that higher adherence rates were seen in an oral DMT compared with self-injection DMTs [Agashivala et al. 2013].
Limitations
When interpreting the results of the present study, some further methodological considerations need to be taken into account. The study used an observational design, which may lead to unknown bias in the selection of patients with MS (i.e. underrepresentation of critically ill individuals) [Delgado-Rodriguez and Llorca, 2004]. No explicit exclusion criteria for documentation were stated to avoid selection bias.
Further, neurologists willing to participate in the survey may be a positive selection of physicians with a particular interest and knowledge in the field of MS management. Adherent patients are more likely to provide their informed consent for study participation [Van Onzenoort et al. 2011].
The unusual high adherence rate may result from the fact that assessment of adherence was reduced to a single item for physicians and, therefore, may have limited the precision of the adherence estimation. The majority of the included patients (92.3%) presented an EDSS below or equal to 5.5, limiting the generalizability of the results for patients with severe disabilities. Additional non-MS-specific medications were not included in the survey. Accordingly, there was no further information available about the symptomatic treatment of side effects like nonsteroidal anti-inflammatory medication for flu-like symptoms and their potential influence on adherence and satisfaction.
Among the strengths of the study is its large scale, with complete coverage of all regions in Germany, and strong focus on the ambulatory setting rather than on university or specialist centres. Our study was not designed to assess medication effects.
Conclusion
On the road to adherence, we have to overcome several obstacles. In a connected model of patient’s satisfaction, perceived effectiveness, side effects, convenience and adherence, patients’ individual needs and concerns have to be addressed. In this study, several factors were identified to play an important role in patients’ perception of their treatment, and therefore, are likely to mediate or moderate their adherence to the current treatment. Most differences were found with respect to side effects and convenience of treatment. Therefore, an improvement in these two domains seems to be the most promising proximate approach to elevate adherence levels. Quality information by the physician on the strengths and weaknesses of different treatments is also a necessary step to create realistic expectations which will form the basis for treatment satisfaction. Decreased disease activity and consequently higher quality of life as a result of long-term therapy with increased adherence may be bound to orally administered therapies, as they represent a highly promising way to more convenience and fewer application-related side effects.
Acknowledgments
Input to the protocol and report was given by David Pittrow, MD, PhD, Seefeld, Germany.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Genzyme, Germany.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TZ received personal compensation from Biogen Idec, Bayer, Novartis, Sanofi, Teva, Synthon for consulting services. Additionally, he received financial support for research activities from Bayer, Biogen Idec, Novartis, Teva and Sanofi Aventis. JK is an employee of Genzyme, a Sanofi company. RH declared no conflict of interest.
Contributor Information
Rocco Haase, Center of Clinical Neuroscience, Neurological Clinic, University Clinic Carl Gustav Carus Dresden, TU Dresden, Germany.
Jennifer S. Kullmann, Genzyme, a Sanofi company, Neu-Isenburg, Germany
Tjalf Ziemssen, Multiple Sklerose Zentrum, Zentrum für klinische Neurowissenschaften, Universitätsklinik Carl Gustav Carus, Technische Universität Dresden, Fetscherstr. 74, 01307 Dresden, Germany.
References
- Agashivala N., Wu N., Abouzaid S., Wu Y., Kim E., Boulanger L., et al. (2013) Compliance to fingolimod and other disease modifying treatments in multiple sclerosis patients, a retrospective cohort study. BMC Neurol 13: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht G., Hoogstraten J. (1998) Satisfaction as a determinant of compliance. Community Dent Oral Epidemiol 26: 139–146. [DOI] [PubMed] [Google Scholar]
- Atkinson M., Kumar R., Cappelleri J., Hass S. (2005) Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM Version II) among outpatient pharmacy consumers. Value Health 8(Suppl. 1): S9–S24. [DOI] [PubMed] [Google Scholar]
- Atkinson M., Sinha A., Hass S., Colman S., Kumar R., Brod M., et al. (2004) Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa C., Balp M., Kulich K., Germain N., Rofail D. (2012) A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence 6: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeru G., Milanov I., De Robertis F., Kozubski W., Lang M., Rojas-Farreras S., et al. (2013) Extaviject(R) 30g device for subcutaneous self-injection of interferon beta-1b for multiple sclerosis: a prospective European study. Med Devices (Auckl) 6: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes D., Callender T., Lathi E., O’Leary S. (2009) A review of disease-modifying therapies for MS: maximizing adherence and minimizing adverse events. Curr Med Res Opin 25: 77–92. [DOI] [PubMed] [Google Scholar]
- Calkwood J., Cree B., Crayton H., Kantor D., Steingo B., Barbato L., et al. (2014) Impact of a switch to fingolimod versus staying on glatiramer acetate or beta interferons on patient- and physician-reported outcomes in relapsing multiple sclerosis: post hoc analyses of the EPOC trial. BMC Neurol 14: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrystyn H., Small M., Milligan G., Higgins V., Gil E., Estruch J. (2014) Impact of patients’ satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med 108: 358–365. [DOI] [PubMed] [Google Scholar]
- Clanet M., Wolinsky J., Ashton R., Hartung H., Reingold S. (2014) Risk evaluation and monitoring in multiple sclerosis therapeutics. Mult Scler 20: 1306–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton A., Cramer J., Pierce C. (2001) A systematic review of the associations between dose regimens and medication compliance. Clin Ther 23: 1296–1310. [DOI] [PubMed] [Google Scholar]
- Compston A., Coles A. (2008) Multiple sclerosis. Lancet 372: 1502–1517. [DOI] [PubMed] [Google Scholar]
- Coons S., Gwaltney C., Hays R., Lundy J., Sloan J., Revicki D., et al. (2009) Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO good research practices task force report. Value Health 12: 419–429. [DOI] [PubMed] [Google Scholar]
- Costello K., Kennedy P., Scanzillo J. (2008) Recognizing nonadherence in patients with multiple sclerosis and maintaining treatment adherence in the long term. Medscape J Med 10: 225. [PMC free article] [PubMed] [Google Scholar]
- Delgado-Rodriguez M., Llorca J. (2004) Bias.J Epidemiol Community Health 58: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire V., Lapierre Y., Macdonell R., Ramo-Tello C., Patti F., Fontoura P., et al. (2011) The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol 18: 69–77. [DOI] [PubMed] [Google Scholar]
- DGN/KKNMS (2012). German Neurological Society (DGN). Leitlinie Zur Diagnose Und Therapie Der Ms [Guidelines for MS Diagnosis and Treatment]. [Google Scholar]
- Giovannoni G., Southam E., Waubant E. (2012) Systematic review of disease-modifying therapies to assess unmet needs in multiple sclerosis: tolerability and adherence. Mult Scler 18: 932–946. [DOI] [PubMed] [Google Scholar]
- Halpern R., Agarwal S., Dembek C., Borton L., Lopez-Bresnahan M. (2011) Comparison of adherence and persistence among multiple sclerosis patients treated with disease-modifying therapies: a retrospective administrative claims analysis. Patient Prefer Adherence 5: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Schussel K., Kieble M., Werning J., Schulz M., Friis R., et al. (2015) Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: a retrospective cohort study. PLoS One 10: e0133279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson K., Agashivala N., Stringer S., Balantac Z., Brandes D. (2013) A cross-sectional survey of patient satisfaction and subjective experiences of treatment with fingolimod. Patient Prefer Adherence 7: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirji I., Gupta S., Goren A., Chirovsky D., Moadel A., Olavarria E., et al. (2013) Chronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient’s perspective. Health Qual Life Outcomes 11: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho P., Bryson C., Rumsfeld J. (2009) Medication adherence. Circulation 119: 3028–3035. [DOI] [PubMed] [Google Scholar]
- Horne R. (2006) Compliance, adherence, and concordance: implications for asthma treatment. Chest 130: 65S–72S. [DOI] [PubMed] [Google Scholar]
- Kass G. (1980) An exploratory technique for investigating large quantities of categorical data. J R Stat Soc Series C 29: 119–127. [Google Scholar]
- Kern S., Reichmann H., Ziemssen T. (2008) [Adherence to neurologic treatment. Lessons from multiple sclerosis]. Nervenarzt 79: 877–878, 880–872, 884–876 passim. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. (1983) Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. (2000) Natural history and clinical outcome measures for multiple sclerosis studies. why at the present time does EDSS scale remain a preferred outcome measure to evaluate disease evolution? Neurol Sci 21: 339–341. [DOI] [PubMed] [Google Scholar]
- Lindhiem O., Bennett C., Trentacosta C., McLear C. (2014) Client preferences affect treatment satisfaction, completion, and clinical outcome: a meta-analysis. Clin Psychol Rev 34: 506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh W. (2014) Fifty years of classification and regression trees. Int Stat Rev 82: 329–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzin J., Caon C., Nichols C., White L., Friedman M., Pill M. (2013) Narrative review of the literature on adherence to disease-modifying therapies among patients with multiple sclerosis.J Manag Care Pharm 19: S24–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg L., Blaschke T. (2005) Adherence to medication. N Engl J Med 353: 487–497. [DOI] [PubMed] [Google Scholar]
- Polman C., Reingold S., Edan G., Filippi M., Hartung H., Kappos L., et al. (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald criteria’. Ann Neurol 58: 840–846. [DOI] [PubMed] [Google Scholar]
- Rieckmann P., Boyko A., Centonze D., Elovaara I., Giovannoni G., Havrdova E., et al. (2015) Achieving patient engagement in multiple sclerosis: a perspective from the multiple sclerosis in the 21st Century Steering Group. Mult Scler Relat Disord 4: 202–218. [DOI] [PubMed] [Google Scholar]
- Rothwell P., McDowell Z., Wong C., Dorman P. (1997) Doctors and patients don’t agree: cross sectional study of patients’ and doctors’ perceptions and assessments of disability in multiple sclerosis. BMJ 314: 1580–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz A., Mora S., Blanco J. (2015) Therapeutic compliance of first line disease-modifying therapies in patients with multiple sclerosis. Compliance study. Neurologia 30: 214–222. [DOI] [PubMed] [Google Scholar]
- Steinberg S., Faris R., Chang C., Chan A., Tankersley M. (2010) Impact of adherence to interferons in the treatment of multiple sclerosis: a non-experimental, retrospective, cohort study. Clin Drug Investig 30: 89–100. [DOI] [PubMed] [Google Scholar]
- Treadaway K., Cutter G., Salter A., Lynch S., Simsarian J., Corboy J., et al. (2009) Factors that influence adherence with disease-modifying therapy in MS. J Neurol 256: 568–576. [DOI] [PubMed] [Google Scholar]
- Turk-Adawi K., Oldridge N., Tarima S., Stason W., Shepard D. (2013) Cardiac rehabilitation patient and organizational factors: what keeps patients in programs? J Am Heart Assoc 2: e000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twork S., Nippert I., Scherer P., Haas J., Pohlau D., Kugler J. (2007) Immunomodulating drugs in multiple sclerosis: compliance, satisfaction and adverse effects evaluation in a German multiple sclerosis population. Curr Med Res Opin 23:1209–1215. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services FDA Center for Drug Evaluation and Research (2006) Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes 4: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Onzenoort H., Menger F., Neef C., Verberk W., Kroon A., De Leeuw P., et al. (2011) Participation in a clinical trial enhances adherence and persistence to treatment: a retrospective cohort study. Hypertension 58: 573–578. [DOI] [PubMed] [Google Scholar]
- Vermersch P., Czlonkowska A., Grimaldi L., Confavreux C., Comi G., Kappos L., et al. (2014) Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler 20: 705–716. [DOI] [PubMed] [Google Scholar]
- Vrijens B., Vincze G., Kristanto P., Urquhart J., Burnier M. (2008) Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ 336: 1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Chow Y., Chen P., Wong S., Fielding R. (2015) A longitudinal analysis on pain treatment satisfaction among Chinese patients with chronic pain: predictors and association with medical adherence, disability, and quality of life. Qual Life Res 24: 2087–2097. [DOI] [PubMed] [Google Scholar]