Abstract
Idiopathic intracranial hypertension (IIH) is a challenging disorder with a rapid increasing incidence due to a close relation to obesity. The onset of symptoms is often insidious and patients may see many different specialists before the IIH diagnosis is settled. A summary of diagnosis, symptoms, headache characteristics and course, as well as existing evidence of treatment strategies is presented and strategies for investigations and management are proposed.
Keywords: acetazolamide, headache, idiopathic intracranial hypertension, visual loss
Introduction
Idiopathic intracranial hypertension (IIH), or pseudotumor cerebri, formerly called benign intracranial hypertension, is a challenging condition with raised intracranial pressure (ICP) in the absence of identifiable cause [Friedman et al. 2013; Mollan et al. 2014; Friedman, 2014].
In the literature, IIH primarily affects young obese women and the estimated incidence in this population is 20 per 100,000 which is 20-fold higher than in normal-weight individuals [Durcan et al. 1988; Radhakrishnan et al. 1993]. Due to the close relationship with obesity, which has been reported to have increased three-fold over just 15 years in Western countries [WHO, 2013], the incidence of IIH is expected to increase rapidly. As obesity affects children and males to a similar degree as in females, and as the reports of IIH in these groups are accumulating [Bruce et al. 2009; Standridge, 2010] it can be hypothesized that obesity is the main underlying causative factor, and not gender or age. Still, case-control studies with male and female patients matched by weight are needed to clarify the role of gender.
At time of diagnosis various degrees of visual impairment are present in up to 90% of patients with IIH [Craig et al. 2001] and in prior studies an estimated rate of 10–24% progressed to severe and permanent visual impairment [Carta et al. 2004; Durcan et al. 1988]. Visual loss can occur anytime along the course of the disease but is often insidious and as central vision is spared until late in the course of the illness the visual loss is often asymptomatic until profound. In addition, diagnosis is often delayed as the general knowledge of IIH is limited and multiple doctors from various specialties have been consulted before patients are identified. In a new series from our group the visual prognosis after one year was good, probably due to earlier diagnosis, more awareness, a dedicated team effort or a close follow-up [Yri et al. 2012].
Clinical presentation and diagnosis
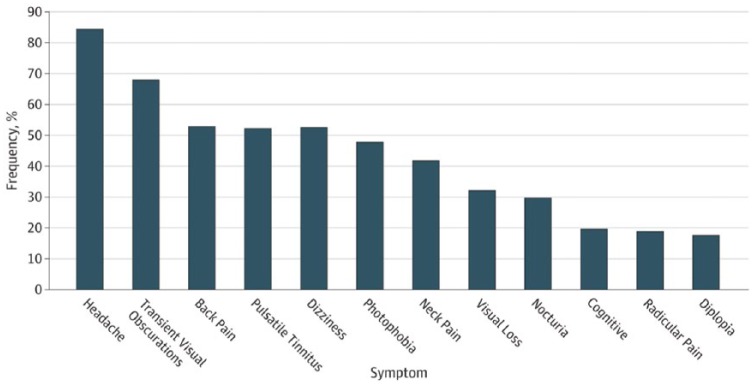
In most patients IIH manifests with severe headache, visual disturbances and bilateral papilledema [Carta et al. 2004; Mollan et al. 2014; Yri and Jensen, 2015; Yri et al. 2012]. The symptoms are clearly summarized [Wall et al. 2014a] (Figure 1) and were confirmed in the recent field testing of diagnostic criteria [Yri and Jensen, 2015].
Figure 1.
Percentage of patients and their presenting symptoms in Idiopathic Intracranial Hypertension, modified from Wall and colleagues [Wall et al. 2014a].
Headache is present in around 93% of patients at the time of diagnosis, usually being constant or occurring daily or nearly daily [Craig et al. 2001; Yri et al. 2012]. It lacks specific features and may mimic chronic migraine, chronic tension-type headaches or both. Headache related to IIH is more likely to be focal than holocranial, and often with pulsating elements. Aggravation by coughing, straining and physical activity is reported by the majority of patients [Yri and Jensen, 2015; Yri et al. 2014b]. The current diagnostic criteria for headache attributed to IIH given by the International Classification of Headache Disorders (ICHD-III beta) [Headache Classification Subcommittee of the International Headache Society, 2013] are presented in Table 1. Recently, these criteria have been field tested and new validated criteria have been proposed [Yri and Jensen, 2015]. Still, the headache attributed to IIH is scarcely studied and none of the existing trials elucidated the effects of current headache treatments. The headaches may also determine the impaired quality of life reported in a study of IIH patients from the Birmingham group [Mulla et al. 2015].
Table 1.
International Classification of Headache Disorders, third edition beta (ICHD-3-beta) criteria for headache attributed to IIH [Headache Classification Subcommittee of the International Headache Society, 2013].
|
IIH, idiopathic intracranial hypertension; CSF, cerebrospinal fluid.
Characteristic for the condition is the presence of a pulsatile tinnitus that is believed to arise from intensified vascular pulsation occurring with high ICP. Although very common, it is often not reported by the patients unless specifically queried about it.
Patients with papilledema often present with transitory visual obscurations which can be a manifestation of increased bulb pressure, retinal ischemia or transient ischemia at the optic nerve caused by papilledema. Increased ICP without papilledema has been reported in unresponsive chronic migraine patients suggesting a diagnosis of IIH without papilledema [De Simone et al. 2014; Mathew et al. 1996; Wang et al. 1998]. However, such a diagnosis is challenging and requires caution with additional clinical or neuroradiological confirmation as suggested in the revised diagnostic criteria for pseudotumor cerebri syndrome (Table 2) [Friedman et al. 2013]. Other symptoms and clinical signs include dizziness, nausea, reduced memory and concentration and horizontal diplopia due to sixth nerve palsy [Carta et al. 2004; Mollan et al. 2014; Yri and Jensen, 2015; Yri et al. 2012]. Recent studies demonstrated a marked cognitive dysfunction [Kharkar et al. 2011; Yri et al. 2014a; Zur et al. 2015] and indicated that impaired executive function, working memory, processing speed and reaction time remained after normalization of ICP and alleviation of the headache and the visual symptoms [Yri et al. 2014a]. It is likely that such cognitive dysfunction could contribute to the substantial loss of work capacity and life quality in patients with IIH and increased focus of the underlying pathophysiology and of their rehabilitation are highly relevant.
Table 2.
Diagnostic criteria for pseudotumor cerebri syndrome [Friedman et al.2013].
|
A diagnosis of pseudotumor cerebri syndrome is definite if the patient fulfills criteria A–E. The diagnosis is considered probable if criteria A–D are met but the measured CSF pressure is lower than specified for a definite diagnosis.
CSF, cerebrospinal fluid; CT, computerized tomography; MRI, magnetic resonance imaging.
Examination
The first step in the neurological examination of a suspected IIH patient is fundoscopy. Although assessment of papilledema may be limited by considerable intra- and inter-observer variability and lack of experience in junior doctors, is it still a quick, simple and accessible bedside test in the emergency room. Test of vision and especially visual field by perimetry are also essential [Carta et al. 2004; Mollan et al. 2014; Skau et al. 2011a] but the test method should be standardized and in most cases needs ophthalmological expertise. Use of optical coherence tomography (OCT) is recommended for quantification of papilledema and the method has been demonstrated to be a valuable additional tool for identification and monitoring of papilledema over time [Skau et al. 2011a; El-Dairi et al. 2007; Rebolleda and Munoz-Negrete, 2009].
The next step is neuroimaging, preferably a magnetic resonance imaging (MRI) scan of the brain. Supplemental MR/CT venography to rule out sinus venous occlusions is essential, since sinus venous occlusions may manifest clinically as isolated intracranial hypertension syndrome in more than one-third of patients [Biousse et al.1999]. Bilateral transverse sinus stenosis, but not occlusions, are frequently reported in IIH patients [Fera et al. 2005; Farb et al. 2003; Higgins et al. 2004]; still, it remains unclear whether this plays a role in the pathogenesis of IIH or just serves as a radiological marker of raised ICP. The other neuroimaging abnormalities of IIH include empty sella, flattening of the posterior aspect of the globe and perioptic subarachnoid space enlargement [Friedman et al. 2013].
In the clinical setting IIH is documented by a lumbar puncture with manometry. To minimize false positive values a standardized procedure is essential with patients placed in a lateral decubitus position on a flat bed, as relaxed as possible, with legs stretched before pressure measurements [King et al. 2002; Whiteley et al. 2006]. Opening pressure values above 25 cm H2O are considered abnormal [Headache Classification Subcommittee of the International Headache Society, 2013; Friedman et al. 2013]. However, it is only a point measurement and with high diurnal ICP variability repeated pressure measurements may be required in patients presenting with an atypical phenotype or with only marginally increased opening pressure.
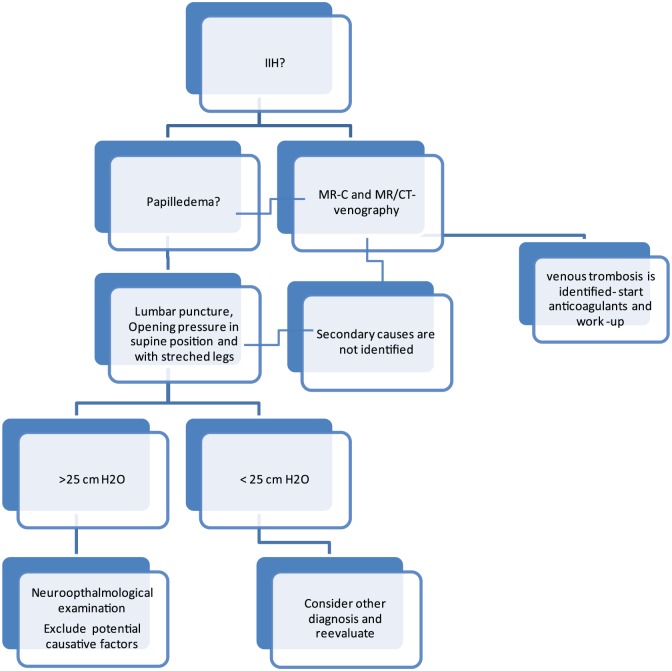
Based on the existing evidence and clinical experience the following diagnostic plan has now been developed in our academic center and can be recommended for systematic testing in other settings (Figure 2).
Figure 2.
Proposal of a diagnostic work-up and strategy in patients with possible idiopathic intracranial hypertension.
Management
The target of IIH management is to reduce ICP with the main goals of preservation of vision and relief of headache. Over the years the management strategy for IIH has been based on clinical experience, but in recent times, well designed clinical trials assessing acetazolamide and weight loss have been published [Ball et al. 2011; Sinclair et al. 2010; Wall et al. 2014b].
Weight reduction
Weight loss plays a significant role in IIH management. Originally, this was based on case reports and small open series but was recently confirmed in a prospective cohort trial [Sinclair et al. 2010]. Twenty-five patients with IIH were treated with a very low calorie diet (425 kcal/day) for three months following a three-month observational control period. Upon diet, patients reduced their weight dramatically (15% of body weight) resulting in a significant reduction in ICP, headaches and papilledema. In another open-labeled design study of newly diagnosed IIH, the CSF pressure decreased markedly in patients with ⩽3.5% reduction of body mass index (BMI), in contrast to patients with no weight loss [Skau et al. 2011b]. Large scale randomized controlled studies with long-term follow up are still warranted to verify these findings.
Bariatric surgery can be offered to these overweight patients for the faster weight reduction and a review of case reports or small case series has been published [Fridley et al. 2011]. Altogether, the 62 included patients reported 92% resolution of IIH symptoms postsurgery. Specifically, papilloedema regressed in 34 of 35 subjects and a mean ICP reduction of 25 cm H2O was reported. The average weight loss was impressive with a 45.4 kg decrease and the BMI was reduced by 16 kg/m2 [Fridley et al. 2011]. On the indication of severe IIH with rapid loss of vision and morbid obesity, bariatric surgery appears promising; but the complication rate has been very high and randomized controlled trials with at least some years of follow-up protocols are required to establish the long-term outcome and the side effects.
Drug treatments
The first drug of choice in IIH management is acetazolamide, an old diuretic with a significant carbonic anhydrase inhibitor effect. The efficacy of acetazolamide in IIH has been investigated in two randomized studies. The first by Ball and colleagues was an open-label randomized pilot study of acetazolamide versus no acetazolamide, including 25 patients in each arm [Ball et al. 2011]. However, 48% of patients in the active group could not to tolerate the medication and stopped or reduced the planned dose up to a maximum of 1500 mg. The authors concluded that a very large controlled study was needed to demonstrate a moderate treatment effect. The reported side effects were acroparesthesia, dysgeusia, fatigue, gastrointestinal symptoms, and nephrolithiasis. The other study was a multicenter, randomized, double-masked, placebo-controlled study of acetazolamide in 165 participants with IIH and showed a small but significant beneficial effect of acetazolamide on visual function [Wall et al. 2014b]. For obvious ethical reasons only patients with mild visual loss were included which may explain that only a limited effect of treatment could be demonstrated. The clinical significance of the shown visual improvement and of other symptoms thus remains to be determined.
Furosemide and other diuretics are sometimes used in IIH, either alone or in combination with acetazolamide but there are no randomized controlled trials supporting its effect. Further evidence is thus required before furosemide or other diuretics can be recommended. Topiramate, an antiepileptic and a migraine prophylactic, has become increasingly popular as a management option in IIH [Celebisoy et al. 2007; Linde et al. 2013]. The appeal for this drug stems from the combined effect as carbonic anhydrase inhibitor, although weaker than acetazolamide, a migraine preventive, and its positive side effect of appetite suppression and weight loss. Reported adverse effects are very similar to acetazolamide with paresthesia, fatigue, and gastrointestinal symptoms in addition to dizziness, coordination, and gait problems.
The effect of topiramate and acetazolamide was compared in an open-label study of 40 IIH patients, with a main focus on visual fields [Celebisoy et al. 2007]. Both drugs showed an improvement over time with no significant difference between them with respect to visual function. However, the effect of topiramate could be explained by a greater weight loss in this group. As headache is such a predominant and disabling feature in patients with IIH, topiramate is appealing to prescribe as a monotherapy or in combination with acetazolamide. However, treatments are often hampered by the marked side effects. In migraine treatment the adherence to topiramate is under 50% [Linde et al. 2013]. In addition cognitive symptoms may occur even with low doses of topiramate, and it is a highly undesirable adverse effect in a patient population already hampered by severe headaches and various degrees of cognitive dysfunction.
So far, there are no guidelines or relevant studies that would provide recommendations for drug treatment duration. Our clinical practice is to taper the medication slowly down when the visual symptoms and papilledema are improved, the CSF-pressure is normalized, and 5–10% weight loss is achieved. We recommend regular follow-up visits of the patients, so an eventual relapse can be identified and medication reinstituted.
Other interventions
Several surgical procedures for IIH have been reported but none of them have been subjected to randomized controlled trials and the evidence is still lacking. Neurosurgical intervention with insertion of a ventriculoperitoneal shunt (VPS) is not indicated in the vast majority of IIH-cases but should be reserved for cases with rapid or malignant loss of vision (i.e. fulminant or malignant IIH) [Thambisetty et al. 2007]. A recent systematic review summarizing the effect and the complications of different surgical options and stenting for IIH concluded that no evidence was available to support the use of any technique in particular [Lai et al. 2014]. Considering a risk of various complications associated with surgical interventions, in many settings repeated spinal taps are employed with daily or weekly intervals but the evidence is entirely clinical. The choice of procedure is therefore largely dependent on local expertise, resources, and preference.
Optic nerve sheath fenestration
Studies assessing the safety and efficacy of optic nerve sheath fenestration (ONSF) are small and uncontrolled and there have been no prospective trials. Chandrasekaran and colleagues reviewed 32 patients in an observational case series including 13 diagnosed with IIH. Visual function improved significantly post-operatively, but in 11 of 32 patients additional CSF diversion was required after the ONSF procedure [Chandrasekaran et al. 2006]. Another series of 78 patients with ONSF, 62 of which had a unilateral diversion [Alsuhaibani et al. 2011] suggested that a unilateral procedure may be sufficient in relief of pressure in both eyes. Complications to ONSF include traumatic optic neuropathy, retinal vascular occlusion, pupil dilatation, and diplopia [Spitze et al. 2014]. Overall outcome on headache and quality of life are not reported.
CSF diversion
Types of CSF diversion include: lumboperitoneal shunts (LPS) or VPS. In earlier days also ventriculojugular and ventriculoatrial shunting have been performed. There are no prospective controlled trials to guide procedure choice but shunts with variable flow valves are reported to be superior [Hickman et al. 2014].
In one of the largest retrospective case note reviews of LPS and VPS shunts, including 53 IIH patients, improvements from baseline were noted in VAs after 6 months and 12 months. However, at 6 month follow up, 68% of patients still reported persistent or recurrent headache and after 12 months this number was increased to 77% [Sinclair et al. 2011]. Other reported IIH symptoms, such as tinnitus and diplopia remained unchanged in 28% of patients and low pressure headaches were also reported post-procedurally. Although appearing effective in reducing the early ICP-related signs and symptoms, complications included shunt blockage, infection, abdominal and back pain, intracranial hypotension and tonsillar herniation. Shunt revisions were required in 51% with multiple revisions needed in 30% [Sinclair et al. 2011].
Dural venous sinus stenting
The causal role of venous sinus compression in IIH is debated and stenting of the dural venous system is controversial. Several smaller case series and retrospective studies have been published and no prospective, randomized controlled trials have yet been performed, probably due to the ethical considerations. Resolution of tinnitus, improvements in headaches, visual function, and papilledema is commonly reported after dural venous sinus stenting, but none of these endpoints are statistically confirmed [Higgins et al. 2002; Radvany et al. 2013; Riggeal et al. 2013]. However, in a larger study of 52 patients immediate, and in most cases longstanding, amelioration of signs and symptoms was obtained [Ahmed et al. 2011]. Complications reported in the literature include stent migration, stent thrombosis, restenosis, and vessel perforation [Ahmed et al. 2011; Higgins et al. 2002; Radvany et al. 2013; Riggeal et al. 2013].
Headache management and prognosis
Although headache is the most frequent symptom in patients with IIH, headache management is a field yet to be explored, since the existing treatment trials have been focused on ICP changes or visual outcome rather than a headache relief.
In many patients IIH headache responds well to management of ICP by cerebrospinal fluid withdrawal or medical treatment, with most improvement occurring within the first month [Yri et al. 2014b]. Immediate headache relief is typically seen after the diagnostic lumbar puncture with CSF removal [Yri and Jensen, 2015]. In a small prospective acetazolamide study, headache prevalence was reduced from 68% to 43% in acetazolamide-treated patients, compared to a reduction from 72% to 65% in a control group [Ball et al. 2011]. However, no significant treatment effects were noted with respect to headache disability in a larger, randomized placebo-controlled study of acetazolamide [Wall et al. 2014b].
Data on the long-term outcome of headache in IIH are limited. In a prospective follow-up study by Yri and colleagues, 43% of patients either recovered totally or reported only infrequent headache (⩽1 day/month) after 1 year while another 43% were left with a persistent chronic headache even after intracranial hypertension and visual symptoms had resolved [Yri et al. 2014b], supporting theories that headache in IIH is attributed to more complex mechanisms than ICP elevation alone [Ekizoglu et al. 2012]. Persistent headache has a major impact on quality of life in IIH but the causes of headache chronification are yet unknown. Coexisting migraine and tension-type headache, medication overuse, depression and anxiety which are common in IIH, may have a role in chronic post-IIH headaches, and should be recognized and treated simultaneously [Friedman and Rausch, 2002; Kleinschmidt et al. 2000]. In the recent study predictors for a favorable outcome of headache 12 months after diagnosis were younger age at onset and a high diagnostic opening pressure. This may relate to an early diagnosis or fast initiation of relevant treatment in these patients [Yri et al. 2014b].
The visual prognosis appears to have improved in recent years, but IIH is still a complex disorder to handle, multiple medical specialties need to be involved and the relapse rate is high at up to 40% [Best et al. 2013; Kesler et al. 2004; Ko et al. 2011; Shah et al. 2008]. In our IIH populations of 44 and 40 patients, respectively, the visual function were almost normalized in all subjects after 12 months whereas the major long-term complications were headache and impaired cognition [Yri et al. 2014b; Yri et al. 2012].
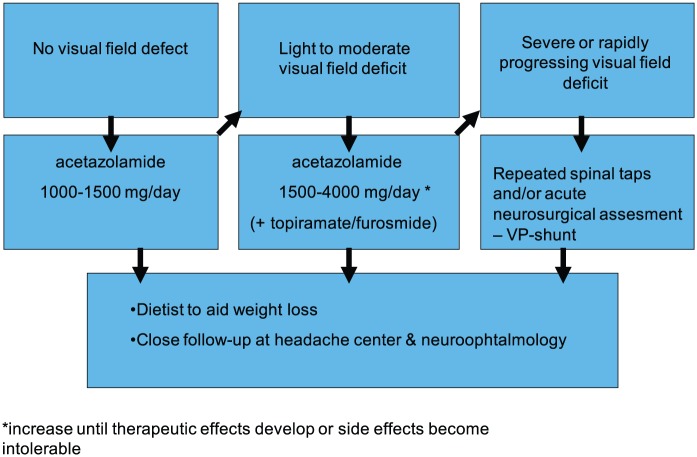
Despite the severe headache and a potential treat to visual function, compliance to treatment and follow-up is surprisingly poor. Therefore, we recommend patient education and close long term follow-up with weight control. From a clinical perspective, we also recommend that the treatment strategy should be stratified according to disease severity (Figure 3). Other issues such as patient tolerability, compliance and the available health care service should also be considered.
Figure 3.
Proposal of a stratified treatment strategy for idiopathic intracranial hypertension according to symptom severity.
Conclusion and recommendations
IIH is a challenging and serious disease with a significant burden on the individual and the society. We suggest organization of specific national IIH teams for awareness, management and research. In a setting with a dedicated IIH team consisting of neuro-ophthalmologist, neurologist and dietician and an easy access to frequent follow-up visits, it should be possible to adjust the medical treatment individually depending on disease course and symptomatology. The number of surgical interventions should be minimized and only employed for the malignant cases or rapid progression. The visual prognosis seems to have improved in recent years while persistent headache still poses a significant long-term problem for the patients. The incidence of IIH is in rapid progression in the wake of the global endemic obesity problem and expertise is absolutely required for scientific progress and a better outcome.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Rigmor Højland Jensen, Danish Headache Center, Department of Neurology, Rigshospitalet-Glostrup, University of Copenhagen, Denmark.
Aleksandra Radojicic, Neurology Clinic, Clinical Center of Serbia, Belgrade, Serbia.
Hanne Yri, Danish Headache Center, Department of Neurology, University of Copenhagen, Rigshospitalet-Glostrup, 2600 Glostrup, Denmark.
References
- Ahmed R., Wilkinson M., Parker G., Thurtell M., Macdonald J., McCluskey P., et al. (2011) Transverse sinus stenting for idiopathic intracranial hypertension: a review of 52 patients and of model predictions. AJNR Am J Neuroradiol 32: 1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsuhaibani A., Carter K., Nerad J., Lee A. (2011) Effect of optic nerve sheath fenestration on papilledema of the operated and the contralateral nonoperated eyes in idiopathic intracranial hypertension. Ophthalmology 118: 412–414. [DOI] [PubMed] [Google Scholar]
- Ball A., Howman A., Wheatley K., Burdon M., Matthews T., Jacks A., et al. (2011) A randomised controlled trial of treatment for idiopathic intracranial hypertension. J Neurol 258: 874–881. [DOI] [PubMed] [Google Scholar]
- Best J., Silvestri G., Burton B., Foot B., Acheson J. (2013) The Incidence of Blindness Due to Idiopathic Intracranial Hypertension in the UK. Open Ophthalmol J 7: 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biousse V., Ameri A., Bousser M. (1999) Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology 53: 1537–1542. [DOI] [PubMed] [Google Scholar]
- Bruce B., Kedar S., Van Stavern G., Monaghan D., Acierno M., Braswell R., et al. (2009) Idiopathic intracranial hypertension in men. Neurology 72: 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta A., Bertuzzi F., Cologno D., Giorgi C., Montanari E., Tedesco S. (2004) Idiopathic intracranial hypertension (pseudotumor cerebri): descriptive epidemiology, clinical features, and visual outcome in Parma, Italy, 1990 to 1999. Eur J Ophthalmol 14: 48–54. [DOI] [PubMed] [Google Scholar]
- Celebisoy N., Gokcay F., Sirin H., Akyurekli O. (2007) Treatment of idiopathic intracranial hypertension: topiramate vs acetazolamide, an open-label study. Acta Neurol Scand 116: 322–327. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran S., McCluskey P., Minassian D., Assaad N. (2006) Visual outcomes for optic nerve sheath fenestration in pseudotumour cerebri and related conditions. Clin Experiment Ophthalmol 34: 661–665. [DOI] [PubMed] [Google Scholar]
- Craig J., Mulholland D., Gibson J. (2001) Idiopathic intracranial hypertension; incidence, presenting features and outcome in Northern Ireland (1991-1995). Ulster Med J 70: 31–35. [PMC free article] [PubMed] [Google Scholar]
- De Simone R., Ranieri A., Montella S., Cappabianca P., Quarantelli M., Esposito F., et al. (2014) Intracranial pressure in unresponsive chronic migraine. J Neurol 261: 1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan F., Corbett J., Wall M. (1988) The incidence of pseudotumor cerebri. Population studies in Iowa and Louisiana. Arch Neurol 45: 875–877. [DOI] [PubMed] [Google Scholar]
- El-Dairi M., Holgado S., O’Donnell T., Buckley E., Asrani S., Freedman S. (2007) Optical coherence tomography as a tool for monitoring pediatric pseudotumor cerebri. J AAPOS 11: 564–570. [DOI] [PubMed] [Google Scholar]
- Ekizoglu E., Baykan B., Orhan E., Ertas M. (2012) The analysis of allodynia in patients with idiopathic intracranial hypertension. Cephalalgia 32: 1049–1058. [DOI] [PubMed] [Google Scholar]
- Farb R., Vanek I., Scott J., Mikulis D., Willinsky R., Tomlinson G., et al. (2003) Idiopathic intracranial hypertension: the prevalence and morphology of sinovenous stenosis. Neurology 60: 1418–1424. [DOI] [PubMed] [Google Scholar]
- Fera F., Bono F., Messina D., Gallo O., Lanza P., Auteri W., et al. (2005) Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J Neurol 252: 1021–1025. [DOI] [PubMed] [Google Scholar]
- Fridley J., Foroozan R., Sherman V., Brandt M., Yoshor D. (2011) Bariatric surgery for the treatment of idiopathic intracranial hypertension. J Neurosurg 114: 34–39. [DOI] [PubMed] [Google Scholar]
- Friedman D. (2014) The pseudotumor cerebri syndrome. Neurol Clin 32: 363–396. [DOI] [PubMed] [Google Scholar]
- Friedman D., Liu G., Digre K. (2013) Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 81: 1159–1165. [DOI] [PubMed] [Google Scholar]
- Friedman D., Rausch E. (2002) Headache diagnoses in patients with treated idiopathic intracranial hypertension. Neurology 58: 1551–1553. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache Society (2013) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33: 629–808. [DOI] [PubMed] [Google Scholar]
- Hickman S., Raoof N., Panesar H., McMullan J., Pepper I., Sharrack B. (2014) Visual outcomes from shunting for idiopathic intracranial hypertension. Neuro-opthalmology 38(6): 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J., Gillard J., Owler B., Harkness K., Pickard J. (2004) MR venography in idiopathic intracranial hypertension: unappreciated and misunderstood. J Neurol Neurosurg Psychiatry 75: 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J., Owler B., Cousins C., Pickard J. (2002) Venous sinus stenting for refractory benign intracranial hypertension. Lancet 359: 228–230. [DOI] [PubMed] [Google Scholar]
- Kesler A., Hadayer A., Goldhammer Y., Almog Y., Korczyn A. (2004) Idiopathic intracranial hypertension: risk of recurrences. Neurology 63: 1737–1739. [DOI] [PubMed] [Google Scholar]
- Kharkar S., Hernandez R., Batra S., Metellus P., Hillis A., Williams M., Rigamonti D. (2011) Cognitive impairment in patients with Pseudotumor Cerebri Syndrome. Behav Neurol 24: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Mitchell P., Thomson K., Tress B. (2002) Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 58: 26–30. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J., Digre K., Hanover R. (2000) Idiopathic intracranial hypertension: relationship to depression, anxiety, and quality of life. Neurology 54: 319–324. [DOI] [PubMed] [Google Scholar]
- Ko M., Chang S., Ridha M., Ney J., Ali T., Friedman D., et al. (2011) Weight gain and recurrence in idiopathic intracranial hypertension: a case-control study. Neurology 76: 1564–1567. [DOI] [PubMed] [Google Scholar]
- Lai L., Danesh-Meyer H., Kaye A. (2014) Visual outcomes and headache following interventions for idiopathic intracranial hypertension. J Clin Neurosci 21: 1670–1678. [DOI] [PubMed] [Google Scholar]
- Linde M., Mulleners W., Chronicle E., McCrory D. (2013) Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev 6: CD010610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew N., Ravishankar K., Sanin L. (1996) Coexistence of migraine and idiopathic intracranial hypertension without papilledema. Neurology 46: 1226–1230. [DOI] [PubMed] [Google Scholar]
- Mollan S., Markey K., Benzimra J., Jacks A., Matthews T., Burdon M., et al. (2014) A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol 14: 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulla Y., Markey K., Woolley R., Patel S., Mollan S., Sinclair A. (2015) Headache determines quality of life in idiopathic intracranial hypertension. J Headache Pain 16: 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan K., Ahlskog J., Cross S., Kurland L., O’Fallon W. (1993) Idiopathic intracranial hypertension (pseudotumor cerebri). Descriptive epidemiology in Rochester, Minn, 1976 to 1990. Arch Neurol 50: 78–80. [DOI] [PubMed] [Google Scholar]
- Radvany M., Solomon D., Nijjar S., Subramanian P., Miller N., Rigamonti D., et al. (2013) Visual and neurological outcomes following endovascular stenting for pseudotumor cerebri associated with transverse sinus stenosis. J Neuroophthalmol 33: 117–122. [DOI] [PubMed] [Google Scholar]
- Rebolleda G., Munoz-Negrete F. (2009) Follow-up of mild papilledema in idiopathic intracranial hypertension with optical coherence tomography. Invest Ophthalmol Vis Sci 50: 5197–5200. [DOI] [PubMed] [Google Scholar]
- Riggeal B., Bruce B., Saindane A., Ridha M., Kelly L., Newman N., et al. (2013) Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis. Neurology 80: 289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V., Kardon R., Lee A., Corbett J., Wall M. (2008) Long-term follow-up of idiopathic intracranial hypertension: the Iowa experience. Neurology 70: 634–640. [DOI] [PubMed] [Google Scholar]
- Sinclair A., Burdon M., Nightingale P., Ball A., Good P., Matthews T., et al. (2010) Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ 341: c2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A., Kuruvath S., Sen D., Nightingale P., Burdon M., Flint G. (2011) Is cerebrospinal fluid shunting in idiopathic intracranial hypertension worthwhile? A 10-year review. Cephalalgia 31: 1627–1633. [DOI] [PubMed] [Google Scholar]
- Skau M., Milea D., Sander B., Wegener M., Jensen R. (2011a) OCT for optic disc evaluation in idiopathic intracranial hypertension. Graefes Arch Clin Exp Ophthalmol 249: 723–730. [DOI] [PubMed] [Google Scholar]
- Skau M., Sander B., Milea D., Jensen R. (2011b) Disease activity in idiopathic intracranial hypertension: a 3-month follow-up study. J Neurol 258: 277–283. [DOI] [PubMed] [Google Scholar]
- Spitze A., Malik A., Lee A. (2014) Surgical and endovascular interventions in idiopathic intracranial hypertension. Curr Opin Neurol 27: 69–74. [DOI] [PubMed] [Google Scholar]
- Standridge S. (2010) Idiopathic intracranial hypertension in children: a review and algorithm. Pediatr Neurol 43: 377–390. [DOI] [PubMed] [Google Scholar]
- Thambisetty M., Lavin P., Newman N., Biousse V. (2007) Fulminant idiopathic intracranial hypertension. Neurology 68: 229–232. [DOI] [PubMed] [Google Scholar]
- Wall M., Kupersmith M., Kieburtz K., Corbett J., Feldon S., Friedman D., et al. (2014a) The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol 71: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall M., McDermott M., Kieburtz K., Corbett J., Feldon S., Friedman D., et al. (2014b) Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA 311: 1641–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Silberstein S., Patterson S., Young W. (1998) Idiopathic intracranial hypertension without papilledema: a case-control study in a headache center. Neurology 51: 245–249. [DOI] [PubMed] [Google Scholar]
- Whiteley W., Al-Shahi R., Warlow C., Zeidler M., Lueck C. (2006) CSF opening pressure: reference interval and the effect of body mass index. Neurology 67: 1690–1691. [DOI] [PubMed] [Google Scholar]
- WHO (2013) Global Database on Body Mass Index. 2013. [Google Scholar]
- Yri H., Fagerlund B., Forchhammer H., Jensen R. (2014a) Cognitive function in idiopathic intracranial hypertension: a prospective case-control study. BMJ Open 4: e004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yri H., Jensen R. (2015) Idiopathic intracranial hypertension: Clinical nosography and field-testing of the ICHD diagnostic criteria. A case-control study. Cephalalgia 35: 553–562. [DOI] [PubMed] [Google Scholar]
- Yri H., Ronnback C., Wegener M., Hamann S., Jensen R. (2014b) The course of headache in idiopathic intracranial hypertension: a 12-month prospective follow-up study. Eur J Neurol 21:1458–1464. [DOI] [PubMed] [Google Scholar]
- Yri H., Wegener M., Sander B., Jensen R. (2012) Idiopathic intracranial hypertension is not benign: a long-term outcome study. J Neurol 259: 886–894. [DOI] [PubMed] [Google Scholar]
- Zur D., Naftaliev E., Kesler A. (2015) Evidence of multidomain mild cognitive impairment in idiopathic intracranial hypertension. J Neuroophthalmol 35: 26–30. [DOI] [PubMed] [Google Scholar]