Abstract
Introduction
Status epilepticus (SE) is a life-threatening neurological emergency. SE lasting longer than 120 min and not responding to first-line and second-line antiepileptic drugs is defined as ‘refractory’ (RCSE) and requires intensive care unit treatment. There is currently neither evidence nor consensus to guide either the optimal choice of therapy or treatment goals for RCSE, which is generally treated with coma induction using conventional anaesthetics (high dose midazolam, thiopental and/or propofol). Increasing evidence indicates that ketamine (KE), a strong N-methyl-d-aspartate glutamate receptor antagonist, may be effective in treating RCSE. We hypothesised that intravenous KE is more efficacious and safer than conventional anaesthetics in treating RCSE.
Methods and analysis
A multicentre, randomised, controlled, open-label, non-profit, sequentially designed study will be conducted to assess the efficacy of KE compared with conventional anaesthetics in the treatment of RCSE in children. 10 Italian centres/hospitals are involved in enrolling 57 patients aged 1 month to 18 years with RCSE. Primary outcome is the resolution of SE up to 24 hours after withdrawal of therapy and is updated for each patient treated according to the sequential method.
Ethics and dissemination
The study received ethical approval from the Tuscan Paediatric Ethics Committee (12/2015). The results of this study will be published in peer-reviewed journals and presented at international conferences.
Trial registration number
NCT02431663; Pre-results.
Strengths and limitations of this study.
This is the first randomised controlled study assessing the efficacy of third-line therapy in RCSE in children.
It employs a sequential model approach, which allows efficacy to be demonstrated with a small number of patients.
It assesses the possibility of avoiding endotracheal intubation in the treatment of RCSE.
RCSE is a rare condition, which may result in a longer than originally planned duration of study.
Enrolment of patients already on high dosages of midazolam could require intubation in some.
Introduction
Background and rationale
Status epilepticus (SE) is a life-threatening neurological emergency traditionally defined as ‘an acute epileptic condition characterised by continuous seizures for at least 30 minutes, or by 30 minutes of intermittent seizures without full recovery of consciousness between seizures’.1 Convulsive SE is the most common and harmful form. There is now consensus, based on an improved understanding of the pathophysiology, that any seizure lasting longer than 5 min should be treated as SE.2
SE lasting longer than 120 min and not responding to first-line (benzodiazepines) and second-line (midazolam (MDZ) at high dose, phenytoin and phenobarbital) antiepileptic drugs (AEDs) is defined as ‘refractory’ and requires intensive care unit (ICU) treatment.2 The term ‘super refractory’ defines SE that continues, or recurs, for 24 hours or longer or recurs after withdrawal of the anaesthetic therapy.3
Even with current best practices, neurological sequelae occur in >50% of children with refractory convulsive SE (RCSE).4 5 The mortality rate of RCSE ranges between 2.7% and 5.2%, and increases up to 5–8% when only ICU admissions are taken into account.4 5
There is general consensus regarding the first-line and second-line treatments of SE. Although the types of drugs available are similar in different countries, algorithms/protocols may differ among countries and even among different institutions in the same country; there is currently neither evidence nor consensus to guide either the optimal choice of therapy or treatment goals for RCSE.3 6–8 RCSE is generally treated with coma induction using high-dose MDZ or conventional anaesthetics such as thiopental (TPS) or propofol (PR),6–8 which all require endotracheal intubation, a negative prognostic factor of morbidity and mortality.9–11 All conventional anaesthetics commonly used in RCSE act on inhibitory-γ-aminobutyric acid (GABAA) receptors.6–8 Experimental models suggest that, with continuing seizures, GABAA receptors are internalised in clathrin-coated vesicles, and excitatory N-methyl-d-aspartate (NMDA) receptors are mobilised to the membrane.12 13 This receptor trafficking results in decreased inhibitory control and increased excitation that may foster SE.12 13 Conventional anaesthetics will therefore be less active, making higher doses necessary, which will in turn enhance their adverse events, especially hypotension, requiring vasopressor administration.11 In this scenario, NMDA modulating molecules, such as ketamine (KE), represent an attractive treatment alternative for SE.14
Increasing evidence indicates that KE, a strong NMDA glutamate receptor antagonist, may be effective in treating RCSE.14 Owing to its sympathomimetic action, KE has no cardiac depressant properties and does not cause hypotension.15 KE use does not necessarily require amine administration or mechanical ventilation. Its administration, therefore, does not imply emergent endotracheal intubation, a prognostic factor of increased morbidity and mortality risk in critically ill adults and children.9–11 Between 15% and 39% of emergent endotracheal intubations in adults are associated with one or more complications including severe hypoxaemia, haemodynamic collapse and death.9 11 The complication rate is even higher in the paediatric population, in which acute deterioration can occur rapidly as a result of age-related differences in physiology, oxyhaemoglobin dissociation, oxygen consumption and pulmonary mechanics.10 Large doses of KE and rapid intravenous boluses may cause hallucinations, which are less frequent in children than in adults and can be reduced with benzodiazepine premedication.15 Moreover, KE exerts a neuroprotective action by preventing the transduction of signals to destructive intracellular mechanisms through the blockade of NMDA receptors.15 16
The literature contains good evidence about efficacy and safety of KE in the adult and paediatric RCSE populations.3 14 However, the heterogeneity of prior treatments, timing of KE administration, and KE dosage and duration make available information on seizure responsiveness difficult to interpret.
In November 2009, the Paediatric Neurology Unit at the Meyer Children's Hospital (Florence, Italy) adopted a treatment protocol for RCSE including intravenous KE infusion.17 As of January 2013, in order to avoid mechanical ventilation, we have used KE (Ketamina, Molteni SpA, Italy) before considering conventional anaesthetics.18
Our paediatric series17 18 shows that treatment with KE in RCSE is effective and safe, and that its use could be considered before TPS and PR, unless specific contraindications exist. Based on these encouraging results, we designed a nationwide multicentre randomised sequential trial that has been approved by the Italian Medicines Agency and includes 10 paediatric hospitals (EudraCT number 2013-004396-12; ClinicalTrial.gov identification number: NCT02431663).
Objectives
We hypothesised that intravenous KE is more effective and safer than conventional anaesthetics (high-dose MDZ, TPS and/or PR) in treating RCSE.
Primary objective
To assess the efficacy of KE compared with conventional anaesthetics in the treatment of RCSE in children.
Secondary objectives
To assess the short-time safety profile of KE compared with conventional anaesthetics and, in particular, to evaluate the possibility of administering KE, thus avoiding mechanical ventilation.
Methods and analysis
Study design
KETASER01 is an Italian, multicentre, randomised, controlled, open-label, sequentially designed non-profit study (ClinicalTrials.gov identifier: NCT02431663) involving 10 centres/hospitals.
Study setting
Patients will be enrolled and treated in the ICUs at 10 Italian hospitals: (1) Meyer Children's Hospital, Florence; (2) Bambino Gesù Children's Hospital, IRCCS, Rome; (3) Gemelli Hospital, Catholic University, Rome; (4) University Hospital, Padua; (5) Ospedali Riuniti, Ancona; (6) University Hospital, Verona; (7) Burlo Garofolo Institute for Maternal and Child Health, IRCCS, Trieste; (8) Regina Margherita Children's Hospital, Turin; (9) Buzzi Children's Hospital, ICP, Milan; (10) Sant’Orsola-Malpighi University Hospital, Bologna.
Patients will be randomised to the intervention arm or control arm with a computer-assisted system. Block randomisation with fixed size blocks and age stratification (<4, 5 to 10 and 11 to 18 years) will be used.
Eligibility criteria
Inclusion criteria
Patients are eligible if (1) they are aged 1 month to 18 years of age; (2) they present with SE refractory to first-line (benzodiazepines by mouth or by rectum) and second-line (phenytoin 20 mg/kg and/or phenobarbital 20 mg/kg and MDZ up to 6 µg/kg/min) AEDs; (3) their parents provide written consent.
Exclusion criteria
Patients will be excluded if (1) they have a contraindication to the use of any of the drugs in the study protocol; (2) they have a presumed or ascertained pregnancy status; (3) they had already been enrolled in the KETASER01 study for an antecedent RCSE episode.
Patients with RCSE unresponsive to first-line and second-line drugs will be transferred from the neurological department to the ICU. They will be enrolled in the study by the neurologist and anaesthesiologist in the ICU, after assessing the eligibility criteria and obtaining informed consent from their parents.
Interventions
The experimental arm: KE
KE is administered starting with an initial bolus of 2–3 mg/kg followed by continuous infusion of 10 µg/kg/min, increasing the infusion rate by 5–10 µg/kg/min every 10 min up to a maximum of 100 µg/kg/min, with every increment being preceded by a bolus of 1–2 mg/kg. KE is always administered in association with 2–4 µg/kg/min MDZ. For patients treated with continuous infusion of MDZ (second-line therapy) for <5 days, the dosage of the benzodiazepine is reduced from 6 to 2 µg/kg/min, to prevent emergence reactions. For patients treated with MDZ for 5 or more days, the dose of the benzodiazepine is reduced from 6 to 3–4 µg/kg/min to avoid seizure occurrence secondary to abrupt benzodiazepine withdrawal and prevent emergence reactions.19 Dosages above 4 µg/kg/min, although previously not efficacious, could interfere with the evaluation of the effectiveness of KE.
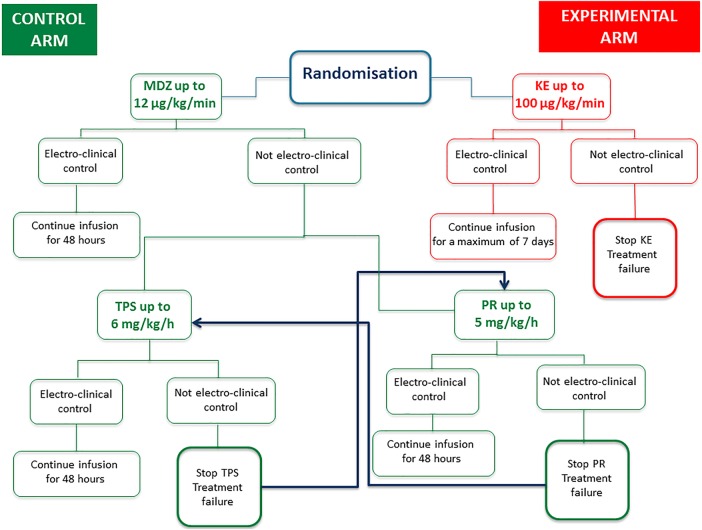
In case of RCSE resolution, the effective dosage of KE is continued for a minimum of 48 hours up to a maximum of 7 days, based on the EEG features of a continuous recording analysed by a neurologist. In the case of no response (persistence of SE at the maximum treatment dose) or adverse events, the drug is discontinued and treatment failure is declared. KE is discontinued gradually by reducing the starting dose by 25% every 12 hours for infusion dosages between 50 and 100 µg/kg/min; withdrawal may be more rapid (25% of the starting dose every 6 hours) for dosages <50 µg/kg/min or a shorter duration of infusion (48 hours; see figure 1).
Figure 1.
Flow chart. MDZ, midazolam; KE, ketamine; PR, propofol; TPS, thiopental.
The control arm: MDZ and (TPS and/or PR)
The administration of conventional anaesthetics for the treatment of RCSE follows the current guidelines that consider MDZ at anaesthetic dosage as the first therapeutic option, followed by PR and/or TPS. The decision to administer PR or TPS first is at the clinician's discretion.
MDZ is administered as follows: using increasing doses of MDZ up to a maximum of 12 µg/kg/min, the dosage is increased by 2 µg/kg/min every 5 min, with every increment being preceded by a bolus of 0.15 mg/kg. In the case of RCSE resolution, the effective dosage of MDZ is continued for 48 hours. In the case of no response (persistence of SE at the maximum treatment dose) or adverse events, MDZ is discontinued and treatment is continued with PR or TPS.
During the weaning process, MDZ is decreased by 1 µg/kg/min every 15 min if the infusion duration was <72 hours; otherwise, weaning is to be performed more slowly. In fact, studies conducted on weaning from BDZ showed a higher incidence of tolerance and, therefore, of abstinence, in patients who received higher doses and for a longer period (≥3 days).19 We recommend a reduction of 10–15% of the initial infusion dose every 6–8 hours in patients receiving infusions for short periods (<3 to 5 days), and a reduction of 10–20% per day in patients receiving infusions for longer periods (>5 to 7 days).
TPS is administered as follows: an initial bolus of 1–2 mg/kg, increasing the speed of continuous infusion by 1 mg/kg/hour every 30 min, always preceding with a bolus of 2 mg/kg, up to a maximum dosage of 6 mg/kg/hour. In the case of RCSE resolution, the effective dosage of TPS is continued for 48 hours. In the case of no response (persistence of SE at the maximum treatment dose) or adverse events, the drug is discontinued and treatment is continued with PR, if provided in the hospital, or treatment failure is declared.
During the weaning process TPS is discontinued gradually by reducing the starting dose by 25% every 3 hours. Phenobarbital therapy (5 mg/kg given twice) is initiated during TPS reduction.
PR is administered as follows: an initial bolus of 1–2 mg/kg, increasing the speed of continuous infusion by 1 mg/kg/hour every 5 min, always preceding with a bolus of 1–2 mg/kg, up to a maximum dosage of 5 mg/kg/hour. In the case of RCSE resolution, the effective dosage of PR is continued for 48 hours. In the case of no response (persistence of SE at the maximum treatment dose) or adverse events, the drug is discontinued and treatment failure is declared.
During the weaning process PR is gradually discontinued by reducing the initial dose by 10% every 12 hours if the maximal dose of 5 mg/kg/h has been reached and administered for 48 hours; withdrawal may be more rapid for smaller doses or infusion durations (see figure 1).
Relevant concomitant care and interventions
The antiepileptic therapy is administered simultaneously with the study drugs and the choice of AED is at the discretion of the clinician. Supportive therapy (eg, amine), when necessary, is allowed (see table 1).20 21
Table 1.
Table timeline
| Pre-enrolment | Enrolment | Allocation | T1 | T2 |
||
|---|---|---|---|---|---|---|
| 0–48 hours | 48 hours to 7 days | |||||
| Enrolment | ||||||
| Treatment failure to first-line and second-line antiepileptic drugs | X | |||||
| Eligibility assessment | X | |||||
| Administration PIM III20 and STESS21 | X | |||||
| Informed consent | X | |||||
| Allocation | X | |||||
| Interventions | ||||||
| Administration of MDZ/TPS/PR or KE up to a maximum or up to SE resolution | X | |||||
| Administration of maximum dosage of MDZ/TPS/PR up to reduction-withdrawal | X | |||||
| Administration of maximum dosage of KE up to reduction-withdrawal | X | X | ||||
| Evaluation | ||||||
| Type and blood dosage of antiepileptic drugs in progress | X | X | ||||
| Aetiological classification | X | X | ||||
| Control of RSE | X | X | X | |||
| Need for mechanical ventilation | X | X | X | |||
| Frequency of seizures from outcome assessment to hospital discharge | X | X | ||||
| Adverse events | X | X | X | |||
| Tolerability | X | X | X | |||
| Other variables (laboratory investigations, monitoring video-EEG, monitoring cardiorespiratory parameters, etc) | X | X | X | |||
KE, ketamine; MDZ, midazolam; PIM, Paediatric Index of Mortality; PR, propofol; RSE, Refractory Status Epilepticus; STRESS, Status Epilepticus Severity Score; TPS, thiopental.
Sample size
A sample size of 57 patients was estimated assuming 80% power, an α error of 5%, a percentage of success in the experimental arm of 85% and a percentage of success in the control arm of 60%. The study adopts a sequential design with a non-truncated triangular test.22
Outcomes
Primary outcome
The control of SE up to 24 hours after the withdrawal of therapy is defined by the following EEG features: (1) appearance of suppression-burst pattern and/or; (2) appearance of widespread β activity and/or (3) appearance of slow activity in the absence of widespread or lateralised, continuous or subcontinuous, and periodic abnormalities (periodic lateralised epileptiform discharges, eg).
Definition of treatment success
No recurrence of SE from the highest dose of the study drugs until the 24th hour after withdrawal of the therapy.
Definition of treatment failure
The study treatments (control arm and treatment arm) end when a therapeutic failure is declared, namely:
Therapy completely failed to control SE;
Recurrence of SE while therapy was being tapered or within 24 hours of its withdrawal;
Withdrawal of the study drug due to adverse events defined according to the Common Toxicity Criteria for Adverse Events (CTCAE);23
Death during treatment with the study drugs or within 24 hours after their withdrawal.
Secondary outcomes
Number of patients requiring mechanical ventilation during treatment with KE;
Frequency of seizures during treatment, from the time at which the maximum dose of study drugs was reached until outcome assessment;
Frequency of seizures from outcome assessment to hospital discharge;
Number of patients requiring drugs for cardiovascular support (amines);
Number of patients who respond to alternative therapy administered after a treatment failure in their study arm;
Number of patients requiring treatment withdrawal due to adverse events;
Mortality rate;
Duration of mechanical ventilation;
Duration of stay in the ICU;
Total length of hospital stay.
Data collection methods
An electronic case report form (eCRF) with security input rules has been developed in order to ensure accurate data collection. Personal data are made anonymous and codified by the system. Demographic, clinical and anamnestic data are recorded at the time of enrolment and throughout follow-up. Laboratory tests and continuous EEG recordings are included in the patient file. The final efficacy outcome is recorded in each eCRF for the sequential analysis. All adverse events are collected in the ‘adverse event’ section of the eCRF. Serious adverse events are also immediately reported to the European Medicines Agency (EudraVigilance).
Statistical methods
As the trial follows a randomised controlled sequential model, the assessment of efficacy is updated after each patient concludes treatment.22 Sequential methods comprise a commonly used frequentist approach to control inflation of the false-positive error rate generated by multiple tests. This method of analysis regards only the primary outcome and has the advantage of allowing early discontinuation of the study in case of clear superiority of the intervention arm or clear futility of the treatment. The sequential analysis model allows early termination of the trial in case of large differences between the two groups in terms of efficacy. Secondary outcomes are reported in a descriptive analysis as proportions, averages and medians.
Data monitoring
The coordinating centre (Meyer Children's Hospital, Florence) oversees the activity of the participating sites through regular visits. The coordinating centre itself is supported by the local internal Clinical Trial Office for internal audits.
Ethics and dissemination
The study was approved by the Tuscan Paediatric Ethics Committee, on 21 October 2014, and was registered on the site ClinicalTrial.gov (number: NCT02431663). Any amendment will be submitted to the local Ethics Committee. Signed informed consent is required from both parents (see online supplementary file). We will disseminate the results of our study via presentations at international conferences and publications in peer-reviewed journals.
bmjopen-2016-011565supp.pdf (22.3KB, pdf)
Footnotes
Contributors: AR, LI, ML, SDM, AB and RG conceptualised the research design, wrote the research protocol, secured funding and are coordinating the project team. AB and GF were responsible for the sample size calculation and statistical methods, and for the acquisition of data. KSM contributed to the preparation of the manuscript. LDS and AP coordinated the management of study drugs. FV, RB, SP, SS, AP and PB contributed to the design of the study protocol. RB, FS, LF, SP, DB, AP, SS, PB, EF, EC, DM, PC, RM, RV, AC, AW, MM, MCM, EF, LM and FV contributed to the implementation of the study at the 10 Italian sites. All the authors have read and approved the final version of the protocol.
Funding: Molteni Pharmaceuticals contributed €15 000 to the study. Meyer Children's Hospital, Viale Pieraccini 24, Florence, Italy, is the independent sponsor. Only the independent sponsor will have access to the final trial data set.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The study received ethical approval from the Tuscany Paediatric Ethics Committee, Florence, Italy (12/2015).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The results of this study will be published in peer-reviewed journals and presented at international conferences.
References
- 1.Proposal for revised clinical and electrographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1981;22:489–501. 10.1111/j.1528-1157.1981.tb06159.x [DOI] [PubMed] [Google Scholar]
- 2.Brophy GM, Bell R, Claassen J et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. 10.1007/s12028-012-9695-z [DOI] [PubMed] [Google Scholar]
- 3.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain 2011;134:2802–18. 10.1093/brain/awr215 [DOI] [PubMed] [Google Scholar]
- 4.Raspall-Chaure M, Chin RF, Neville BG et al. Outcome of pediatric convulsive status epilepticus: a systematic review. Lancet Neurol 2006;5:769–79. 10.1016/S1474-4422(06)70546-4 [DOI] [PubMed] [Google Scholar]
- 5.Chin RF, Neville BG, Peckham C et al. Incidence, cause and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet 2006;368:222–31. 10.1016/S0140-6736(06)69043-0 [DOI] [PubMed] [Google Scholar]
- 6.Abend NS, Duglas DT. Treatment of refractory status epilepticus. Literature review and a proposed protocol. Pediatr Neurol 2008;38:377–80. 10.1016/j.pediatrneurol.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Sofou K, Kristjansdòttir R, Papachatzakis NE et al. Management of prolonged seizures and status epilepticus in childhood: a systematic review. J Child Neurol 2009;24:918–26. 10.1177/0883073809332768 [DOI] [PubMed] [Google Scholar]
- 8.Fernandez A, Claassen J. Refractory status epilepticus. Curr Opin Crit Care 2012;18:127–31. 10.1097/MCC.0b013e32835132cc [DOI] [PubMed] [Google Scholar]
- 9.Griesdale DE, Bosma TL, Kurth T et al. Complications of endotracheal intubation in the critically ill. Int Care Med 2008;34:1835–42. 10.1007/s00134-008-1205-6 [DOI] [PubMed] [Google Scholar]
- 10.Carroll CL, Spinella PC, Corsi JM et al. Emergent endotracheal intubations in children: be careful if it's late when you intubate. Pediatr Crit Care Med 2010;11:343–8. 10.1097/PCC.0b013e3181ce6d19 [DOI] [PubMed] [Google Scholar]
- 11.Schmutzhard E, Pfausler B. Complications of the management of status epilepticus in the intensive care unit . Epilepsia 2011;52:39–41. 10.1111/j.1528-1167.2011.03233.x [DOI] [PubMed] [Google Scholar]
- 12.Wasterlain CG, Chen JW. Mechanistic and pharmacologic aspects of status epilepticus and its treatment with new antiepileptic drugs. Epilepsia 2008;49:63–73. 10.1111/j.1528-1167.2008.01928.x [DOI] [PubMed] [Google Scholar]
- 13.Naylor DE. Glutamate and GABA in the balance: convergent pathways sustain seizures during status epilepticus. Epilepsia 2010;5l:106–9. [DOI] [PubMed] [Google Scholar]
- 14.Zeiler FA, Teitelbaum J, Gillman LM et al. NMDA Antagonists for refractory seizures. Neurocrit Care 2014;20:502–13. 10.1007/s12028-013-9939-6 [DOI] [PubMed] [Google Scholar]
- 15.Craven R. Ketamine. Anaesthesia 2007;62:48–53. 10.1111/j.1365-2044.2007.05298.x [DOI] [PubMed] [Google Scholar]
- 16.Shibuta S, Varathan S, Mashimo T. Ketamine and thiopental sodium: individual and combined neuroprotective effects on cortical cultures exposed to NMDA or nitric oxide. Br J Anaesth 2006;97:517–24. 10.1093/bja/ael192 [DOI] [PubMed] [Google Scholar]
- 17.Rosati A, L'Erario M, Ilvento L et al. Efficacy and safety of ketamine in refractory status epilepticus children. Neurology 2012;79:2355–8. 10.1212/WNL.0b013e318278b685 [DOI] [PubMed] [Google Scholar]
- 18.Ilvento L, Rosati A, Marini C et al. Ketamine in refractory convulsive status epilepticus in children avoids endotracheal intubation. Epilepsy Behav 2015;49:343–6. 10.1016/j.yebeh.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 19.Franck LS, Naughton I, Winter I. Opioid and benzodiazepine withdrawal symptoms in paediatric intensive care patients. Intensive Crit Care Nurs 2004;20:344–51. 10.1016/j.iccn.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 20.Straney L, Clements A, Parslow RC et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med 2013;14:673–81. 10.1097/PCC.0b013e31829760cf [DOI] [PubMed] [Google Scholar]
- 21.Rossetti AO, Logroscino G, Milligan TA et al. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol 2008;255:1561–6. 10.1007/s00415-008-0989-1 [DOI] [PubMed] [Google Scholar]
- 22.Whiteheand J, Stratton I. Group sequential clinical trials whit triangular continuation regions. Biometrics 1983;39:227–36. 10.2307/2530822 [DOI] [PubMed] [Google Scholar]
- 23.Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS 31 March 2003. http://ctep.cancer.gov, Publish Date: 9 August 2006.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-011565supp.pdf (22.3KB, pdf)