Abstract
Objective
To systematically review and meta-analyse the risk–benefit ratio of warfarin users compared with non-warfarin users in patients with atrial fibrillation (AF), who are undergoing dialysis.
Methods
We searched PubMed/MEDLINE, EMBASE, SCOPUS, Web of Science, Cochrane Library, grey literature, conference proceedings, trial registrations and also did handsearch. Cohort studies without language restrictions were included. Two investigators independently conducted a full abstraction of data, risk of bias and graded evidence. Effect estimates were pooled using random-effect models.
Main outcome measure
All-cause mortality, total stroke/thromboembolism and bleeding complications.
Results
14 studies included 37 349 dialysis patients with AF, of whom 12 529 (33.5%) were warfarin users. For all-cause mortality: adjusted HR=0.99 (95% CI 0.89 to 1.10; p=0.825), unadjusted risk ratio (RR)=1.00 (95% CI 0.96 to 1.04; p=0.847). For stroke/thromboembolism: adjusted HR=1.06 (95% CI 0.82 to 1.36; p=0.676), unadjusted incidence rate ratio (IRR)=1.23 (95% CI 0.94 to 1.61; p=0.133). For ischaemic stroke/transient ischaemic attack, adjusted HR=0.91 (95% CI 0.57 to 1.45; p=0.698), unadjusted IRR=1.16 (95% CI 0.84 to 1.62; p=0.370). For haemorrhagic stroke, adjusted HR=1.60 (95% CI 0.91 to 2.81; p=0.100), unadjusted IRR=1.48 (95% CI 0.92 to 2.36; p=0.102). Major bleeding was increased among warfarin users; adjusted HR=1.35 (95% CI 1.11 to 1.64; p=0.003) and unadjusted IRR=1.22 (95% CI 1.07 to 1.40; p=0.003).
Conclusions
Among dialysis patients with AF, warfarin therapy was not associated with mortality and stroke/thromboembolism, but significantly increased the risk of major bleeding. More rigorous studies are essential to demonstrate the effect of warfarin for stroke prophylaxis in dialysis patients with AF.
Keywords: RENAL DISEASE
Key questions.
What is already known about this subject?
Although several studies have described the role of warfarin in dialysis patients with atrial fibrillation, the clinical risk–benefit for stroke prevention has not been fully clarified. Meta-analyses of observational studies have shown that warfarin therapy had no effect for stroke prevention and mortality, but associated with a higher risk of bleeding in these patients. However, they had some major limitations such as quantification of the effect of bias from different types of adjustments across studies, limited interpretation by population heterogeneity, outcomes specification.
What does this study add?
We comprehensively conducted an updated systematic review and meta-analysis on the risk–benefit of warfarin for stroke prevention, with a specific focus on the current controversy using the totality of the applicable evidences, especially when data from randomised controlled trials are not available to address an urgent issue requiring clinical decision-making. We have shown that warfarin therapy was not associated with mortality and stroke/thromboembolism but have significantly increased the risk of major bleeding.
How might this impact on clinical practice?
Clinicians should be aware of the risks associated with warfarin use in these patients, and the clinical decision to prescribe warfarin should comprise an individualised approach that takes into account the risk of stroke and the haemorrhagic complications.
Introduction
End-stage renal disease (ESRD) and atrial fibrillation (AF) are common conditions that often coexist.1 2 Normally, the prevalence of AF increases with age: 1–4% in the general population and >9% in patients 85 years and over.3–5 It is substantially higher in the ESRD undergoing haemodialysis population, with a range of 4.5–27%.6–10 Critically, AF is a potential risk factor for stroke and mortality, particularly in dialysis patients.1 2 11 The risk of mortality and stroke are at 26.9 and 5.2/100 patient-years versus 13.4 and 1.9/100 patient-years compared with those without AF.6
To our knowledge, evidence exists that adjusted-dose warfarin was substantially more efficacious than placebo and antiplatelet agents for stroke prevention in the general AF population.12 13 It is well known that dialysis patients have higher risks of bleeding.14–16 The rate of major bleeding when treated with warfarin raises 10-fold according to the Dialysis Outcomes and Practice Patterns Study.17 Besides the risk of bleeding, some evidence suggests that warfarin might be associated with an increased risk of calciphylaxis18 19 and accelerated vascular calcification in dialysis patients.20–22
Although several observational studies have described the role of warfarin in dialysis patients with AF, the clinical risk–benefit for stroke prevention has not been fully clarified.9 23–35 Three meta-analyses of observational studies have shown that warfarin therapy had no effect for stroke prevention and mortality, but associated with a higher risk of bleeding in these patients.36–38 The major limitations of the studies included: quantification of the effect of bias from different types of adjustments across studies, limited interpretation by population heterogeneity, outcomes specification and lack of recent published literature.
Availability of more robust evidence is crucial to develop guidelines for stroke prevention in these patients. To address this question, we used the totality of the most updated applicable evidences including more participants restricted to dialysis patients and performed comprehensive analyses using all possible and available techniques that lacked in previous meta-analysis studies to specifically focus on current controversy of the risk–benefit of warfarin for stroke prevention, especially when data from randomised controlled trials (RCTs) are not available to address an urgent issue requiring clinical decision-making.
Methods
Search strategy
We searched the PubMed/MEDLINE, EMBASE, SCOPUS, Web of Science and the Cochrane Library for relevant studies without language restrictions, from inception to 17 January 2016. An extensive search strategy using the terms; warfarin, oral anticoagulation, vitamin K antagonists, coumarins, coumadin, atrial fibrillation, atrial arrhythmias, end-stage renal disease, dialysis, haemodialysis and peritoneal dialysis as keywords or text words or the MeSH terms. The search strategies used for the databases are available in eTable 1. The studies included were based on the PICOTS Framework (see eTable 2).
openhrt-2016-000441supp.pdf (2.1MB, pdf)
The Methods Guide for Effectiveness and Comparative Effectiveness Reviews, 2014 edition was used39 and in accordance with the MOOSE guidelines for conducting and reporting of meta-analyses of observational studies (see eTable 3).40
The reference lists of the studies included, prior systemic reviews and electronic searches from ClinicalTrial.gov, Google Scholar and Jane (Journal/Author Name Estimator) were browsed for identification of additional studies. Relevant abstracts from 2002 to 2015 were searched from major nephrology scientific meetings (European Renal Association–European Dialysis and Transplant Association Congress, American Society of Nephrology; Kidney Week, Renal Week, International Society of Nephrology; World Congress of Nephrology, Annual Dialysis Conference, Annual Conference on Peritoneal Dialysis, International Symposium on Haemodialysis and Annual Symposium on Pediatric Dialysis).
Selection of studies
After deduplication, two investigators (SN and CR) independently reviewed titles and abstracts. Full articles were retrieved if a decision could not be made based on the abstracts. Disagreement regarding the inclusion of a study was resolved by discussion; if a consensus could not be reached, a third party (RA) served as the final arbiter.
Inclusion criteria
For inclusion in the study, the following criteria had to be met (see eTable 2): (1) prospective/retrospective cohort studies regarding AF in dialysis patients; (2) two or more groups of which one group was warfarin users; (3) containing data of mortality, stroke/thromboembolism and bleeding. Exclusion criteria were case–control studies, case series/case report, kidney transplantation patients and <90 days of follow-up. In studies with overlapping samples, data with the longest follow-up period, the most detailed information and/or the most relevant to our outcomes were included.
Data extraction
An extraction form was constructed. Elements abstracted included general trial and patient characteristics, stroke/bleeding risk score, risk of bias assessment and predefined outcomes. SN and CR independently extracted data using a standardised form and RA verified the accuracy. Any disagreement was resolved by RA Missing data or unclear information was sought by contacting the corresponding authors. When this was not possible and they were considered to introduce serious bias, a sensitivity analysis was conducted.
Risk of bias assessment
SN and CR independently assessed the risk of bias using the Newcastle–Ottawa Scale (NOS)41 including selection of the exposed/unexposed cohort, comparability of the study group and the outcome assessment. Studies with a total score ≥8 were defined as the highest quality. Disagreements were resolved by RA if a consensus could not be reached.
Strength of evidence grading
The Grading of Recommended Assessment, Development and Evaluation (GRADE) system, was used to grade the strength of evidence (SOE) based on five key domains; study limitations, consistency, directness, precision and reporting bias.42 The ratings were classified to insufficient-quality, low-quality, moderate-quality or high-quality evidence. SN and CR independently assessed SOE domains for each outcome and resolved the differences by RA.
Outcome measures
The primary outcomes included all-cause mortality, stroke/thromboembolism, ischaemic stroke, haemorrhagic stroke and major bleeding.
Major bleeding was defined according to the International Society on Thrombosis and Haemostasis.43 For reasons of clinical relevance, however, a definition by the investigators of each study and gastrointestinal events that required hospitalisation or related with death were considered as major bleeding (see eTable 4).
The secondary outcomes extracted were death from stroke, cardiovascular death, fatal bleeding and gastrointestinal bleeding. If the quality of warfarin control in terms of the international normalised ratio (INR) or percentage of time in the therapeutic range (TTR) were provided, we explored evidence for dose–response effects.
Statistical analysis
All statistical analyses were performed using STATA statistical software V.14.0 (StataCorp LP). For primary analysis, we restricted to trials published in full-text articles. Generally, incomplete reporting in abstracts limits the ability to describe the quality of trials and is therefore maybe of questionable value.44
To address biases from different types of adjustments across studies, statistical analyses for adjusted and unadjusted risks of outcomes were performed.45 With survival data, log HR and its variance were calculated.46 47 Incidence rate ratios (IRRs) were used when available.48 For primary analysis, the results from multivariable models or propensity score analysis were applied. The overall HRs and IRRs were pooled by random-effect models.49 For studies that reported results separately, the fixed-effects model was used to estimate risk before including the data in the overall analysis.
Homogeneity was assessed using the Cochran Q test, with p<0.10.50 The degree of inconsistency was estimated by I2 and the tau-squared (t2) statistics. The I2 value indicated low (<25%), moderate (25–75%) and high (>75%) heterogeneity,50 while, the t2 value indicated low (≤0.04), moderate (>0.04–<0.36) and high (≥0.36) heterogeneity.51
Publication bias was examined by a contour-enhanced funnel plot of each study's effect size against the precision (1/SE).52 The Funnel plot was assessed by Begg's and Egger's test at p<0.10.53 54 Furthermore, the trim and fill method was used to calibrate for publication bias.55
Preplanned subgroup analyses were conducted to investigate whether associations varied across the studies and the patients' key characteristics. Sensitivity analyses were performed restricted to the highest-quality study, adjustment for key determinants of stroke/bleeding risk scores, post hoc meta-analysis adding unpublished studies, the ‘leave one out’ approach and the analytical methods (multivariate analysis vs propensity score matching analysis). Robustness of findings was determined by consistency of pooled adjusted and unadjusted models using different parameters, that is, HR, RR, IRR. A random-effect univariate meta-regression was then performed to explore heterogeneity.
Results
Search strategy and study characteristics
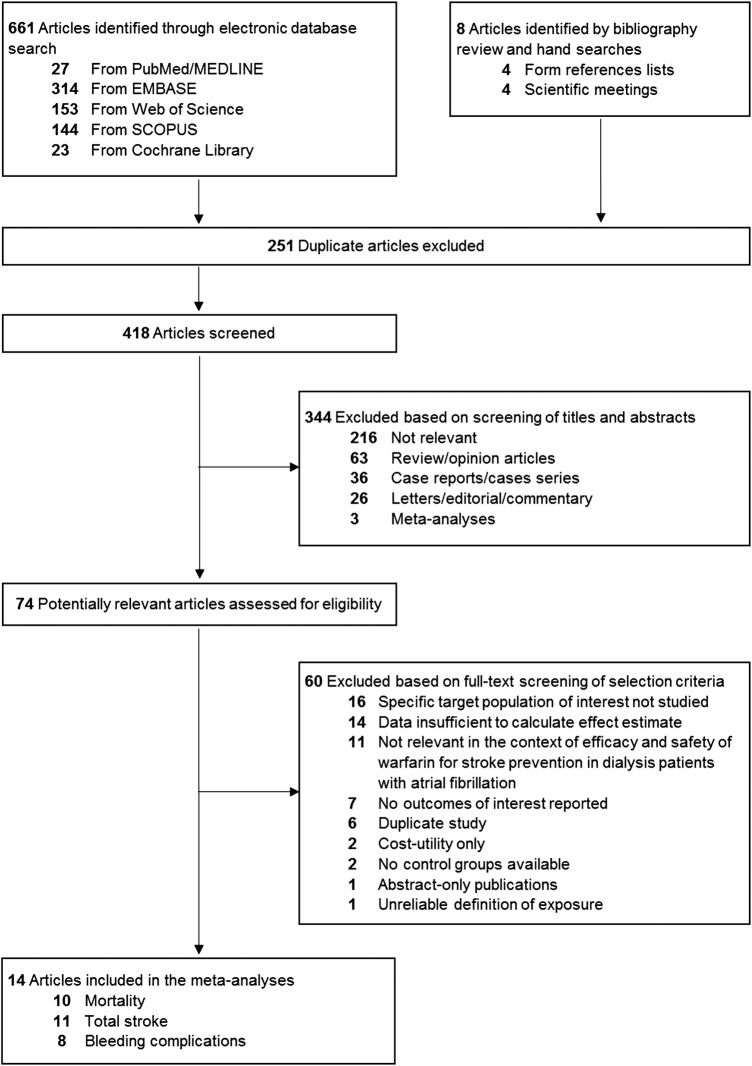
The literature search details are described in figure 1, Bonde et al,26 2014 was included instead of the Denmark cohort study by Olesen et al,56 2012 because it provided more detailed and update information. Thus, 14 full-text studies were identified through database search.9 23–35 Studies of each analysis, measurement and definition of clinical endpoint are provided (see eTables 5 and 6). A total of 37 349 patients were involved, 12 529 (33.5%) were warfarin users and have mainly undergone haemodialysis (8 studies). The databases are described (see eTable 7), and the characteristics of the studies are summarised in table 1.
Figure 1.
Flowchart of the literature review process.
Table 1.
Characteristics of studies included in the meta-analysis
| First author, Year | Study design | Country enrolment | Sample size | Age in years, Mean (SD) | Female, No (%) | Race; White/Caucasian, No (%) | Dialysis modality | Data collection | Follow-up time |
|---|---|---|---|---|---|---|---|---|---|
| Abbott,23 2003 | Retrospective | USA | 123 | NR | NR | NR | HD, PD | 1996–2000 | Mean 2.92±1.1 years |
| Chan,24 2009* | Retrospective | USA | 1671 (1492 for PS-matched 1:1) | 72.4 (10.3) | 890 (59.7) | 1206 (80.8) | HD | 2003–2007 | Mean 1.6 years, 2740 patient-years |
| Wizemann,9 2010 | Prospective | International Collaboration | 3245 | ≤65 (30.9%) 66–75 (35.0%) >75 (34.1%) |
NR | NR | HD | DOPPS I; 1996–2001, DOPPS II; 2002–2004 | 4348 patient-years |
| Winkelmayer,25 2011* | Retrospective | USA | 1185 for PS matched 1:4 | 69.4 (11.9) | 1329 (57.5) | 1498 (64.8) | HD, PD | 1994–2006 | 2287patient-years |
| Bonde,26 2014 | Retrospective | Denmark | 1680 | NR | NR | NR | HD, PD | 1997–2010 | NR |
| Shah,27 2014 | Retrospective | Canada | 1626 | 75.2 (8.3) | 634 (39.0) | NR | HD, PD | 1998–2007 | NR |
| Wakasugi,28 2014 | Prospective | Japan | 60 | 68.1 (8.9) | 21 (35.0) | 0 | HD | 2008–2011 | 110 patient-years |
| Chan,29 2015 | Prospective | USA. Columbia, and the Territory of Puerto Rico | 14 607 | 70.2 (10.8) | 5910 (40.5) | 10 902 (74.6) | HD | 2010–2014 | 7260 patient-years |
| Chan,30 2015 | Retrospective | Hong Kong, China | 271 | 70.4 (11.1) | 109 (40.2) | 0 (0.0) | PD | 1997–2011 | 1.5 years |
| Genovesi,31 2015 | Prospective | Italy | 290 | <65 (20.7%) 65–74 (25.9%) ≥75 (53.4%) |
116 (40.0) | NR | HD | 2010–2012 | 2 years |
| Mitsuma,32 2015 | Retrospective | Japan | 82 | 70.7 (9.6) | 23 (28.0) | 0 (0.0) | HD | 2011–2015 | Mean 3.0 years (423 patient; AF and non-AF) |
| Shen,33 2015 | Retrospective | USA | 12 284 (3658 for PS-matched 1:1) | 61.7 (13.4) | 6284 (51.2) | 6082 (49.5) | HD | 2007–2011 | 16 617 patient-years |
| Wang,34 2015 | Retrospective | New Zealand | 141 | 61.2 (11.3) | 54 (38.3) | 53 (37.6) | HD, PD | 2000–2014 | Mean 3.4±2.5 years |
| Yodogawa,35 2015 | Retrospective | Japan | 84 | 70 (10.4) | 25 (29.8) | 0 (0.0) | HD | 2003–2012 | Mean 3.9 years |
| First author, Year | Warfarin use defined as | Warfarin use, No. (%) | Comparison group | Stroke risk stratification | Bleeding risk stratification | Outcomes reported | Total NOS Score |
|---|---|---|---|---|---|---|---|
| Abbott,23 2003 | Baseline use; day 60 of dialysis | NR | Non-warfarin users (non-specify) | NR | NR | All-cause mortality | 7 |
| Chan,24 2009* | Baseline use; any use in the first 90 days | 746 (44.6) | Non-warfarin users (placebo/clopidogrel/aspirin/clopidogrel+aspirin) | CHADS2 score | NR | All-cause mortality, cardiovascular death, stroke/TE, bleeding events | 8 |
| Wizemann,9 2010 | NR | 509 (15.7) | Non-warfarin users (non-specify) | NR | NR | Stroke/TE | 7 |
| Winkelmayer,25 2011* | Baseline use; prescription within 30 days from index date | 249 (10.8) | Non-warfarin users (non-specify) | NR | NR | All-cause mortality, stroke/TE, bleeding events | 7 |
| Bonde,26 2014 | Baseline use | NR | Non-warfarin users (no antithrombotic) | CHA2DS2-VASc score | Modified HAS-BLED score† | All-cause mortality, cardiovascular death | 8 |
| Shah,27 2014 | Baseline use; prescription within 30 days from index date | 756 (46.5) | Non-warfarin users (non-specify) | CHADS2 score | Modified HAS-BLED score‡ | stroke/TE, bleeding events | 8 |
| Wakasugi,28 2014 | Baseline use | 28 (46.7) | Non-warfarin users (non-specify) | CHADS2 score | NR | All-cause mortality, stroke/TE, bleeding events | 7 |
| Chan,29 2015 | Baseline use | 8064 (55.2) | Non-warfarin users (aspirin/dabigatran/rivaroxaban) | CHADS2 score | Outpatient Bleeding Risk Index | stroke/TE, bleeding events | 9 |
| Chan,30 2015 | Baseline use | 67 (24.7) | Non-warfarin users (placebo/aspirin) | CHA2DS2-VASc score | HAS-BLED score | Stroke/thromboembolism | 7 |
| Genovesi,31 2015 | Baseline use; prescription at recruitment or starting within 2 weeks following recruitment | 156 (53.8) | Non-warfarin users (non-specify) | CHA2DS2-VASc score | Modified HAS-BLED score† | All-cause mortality, cardiovascular death, stroke/TE, bleeding events | 8 |
| Mitsuma,32 2015 | Baseline use | 27 (32.9) | Non-warfarin users (non-specify) | NR | NR | All-cause mortality, cardiovascular death | 7 |
| Shen,33 2015 | Baseline use; prescription within 30 days from index date | 1838 (15.0) | Non-warfarin users (non-specify) | CHADS2 score | Modified HAS-BLED score† | All-cause mortality, cardiovascular death, stroke/TE, bleeding events | 9 |
| Wang,34 2015 | Baseline use | 59 (41.8) | Non-warfarin users (placebo/clopidogrel/aspirin) | CHADS2/CHA2DS2-VASc score | HAS-BLED score | All-cause mortality, stroke/TE, bleeding events | 7 |
| Yodogawa,35 2015 | Baseline use | 30 (35.7) | Non-warfarin users (non-specify) | CHADS2 score | NR | All-cause mortality, stroke/TE | 7 |
*Data based on propensity score-matched.
†Modified HAS-BLED score for estimating the risk for bleeding (not included the score related to labile INR).
‡Modified HAS-BLED score for estimating the risk for bleeding (not included the score related to labile INR and alcohol intake). AF, atrial fibrillation; HD, haemodialysis; INR, international normalised ratio; NOS, the Newcastle-Ottawa Scale; NR; not reported; PD, peritoneal dialysis; PS, propensity score; TE, thromboembolism.
The participants' characteristics and medications used at baseline are shown. Thromboembolic and bleeding risk among comparators were similar (see eTables 8 and 9). Comparators were varied across studies including placebo, aspirin, clopidogrel, aspirin–clopidogrel, dabigatran or rivaroxaban. Notably, most of the studies did not provide INR/TTR (see eTable 10). The NOS results have shown high quality in most studies, ranging from 7 to 9 point (see eTable 11).
Outcomes
A meta-analysis can be pooled for seven outcomes: all-cause mortality, cardiovascular death, stroke/thromboembolism, ischaemic stroke, haemorrhagic stroke, major bleeding and gastrointestinal bleeding. A dose–response between INR/TTR and outcomes cannot be estimated due to limited data on warfarin monitoring. The summary of the outcomes and SOE are shown in table 2 and eTable 12.
Table 2.
Summary of findings and strength of evidence from trials assessing warfarin therapy for atrial fibrillation patients undergoing dialysis
| Heterogeneity |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Studies, (n) | Participants, (n) | Effect estimate (95% CI) | p Value | Q Statistic | p Value | I2 Index (%) | τ2 | Strength of evidence |
| Efficacy outcomes | |||||||||
| All-cause mortality | 723–26 31 33 34 | 8477 | Adjusted HR 0.99 (0.89 to 1.10) | 0.825 | 9.22 | 0.162 | 34.9 | 0.007 | Low (no benefit) |
| 824 25 28 31–35 | 15 797 | Unadjusted RR 1.00 (0.96 to 1.04) | 0.847 | 5.67 | 0.579 | 0.0 | <0.001 | ||
| Cardiovascular death | 424 26 30 31 | 7028 | Adjusted HR 0.94 (0.84 to 1.06) | 0.347 | 1.46 | 0.691 | 0.0 | <0.001 | Low (no benefit) |
| 524 28 31–33 | 14 116 | Unadjusted RR 0.92 (0.74 to 1.14) | 0.467 | 10.20 | 0.037 | 60.8 | 0.024 | ||
| Stroke/thromboembolism | 119 24 25 27–31 33–35 | 26 539 | Adjusted HR 1.06 (0.82 to 1.36) | 0.676 | 25.50 | 0.004 | 60.8 | 0.085 | Low (no benefit) |
| 724 25 27–29 31 33 | 31 723 | Unadjusted IRR 1.23 (0.94 to 1.61) | 0.133 | 26.67 | <0.001 | 77.5 | 0.085 | ||
| Ischemic stroke/TIA (fatal or nonfatal) | 724 25 27 28 30 33 34 | 8584 | Adjusted HR 0.91 (0.57 to 1.45) | 0.698 | 23.55 | 0.001 | 74.5 | 0.260 | Low (no benefit) |
| 724 25 27–29 31 33 | 31 723 | Unadjusted IRR 1.16 (0.84 to 1.62) | 0.370 | 29.33 | <0.001 | 79.5 | 0.136 | ||
| Safety outcomes | |||||||||
| Haemorrhagic stroke (fatal or nonfatal) | 524 25 29 33 34 | 21 262 | Adjusted HR 1.60 (0.91 to 2.81) | 0.100 | 11.26 | 0.024 | 64.5 | 0.231 | Insufficient |
| 524 25 29 31 33 | 30 037 | Unadjusted IRR 1.48 (0.92 to 2.36) | 0.102 | 12.85 | 0.012 | 68.9 | 0.165 | ||
| Major bleeding | 724 25 27 29 31 33 34 | 23 178 | Adjusted HR 1.35 (1.11 to 1.64) | 0.003 | 14.75 | 0.022 | 59.3 | 0.031 | Low (harm) |
| 724 25 27–29 31 33 | 31 723 | Unadjusted IRR 1.22 (1.07 to 1.40) | 0.003 | 12.39 | 0.054 | 51.6 | 0.013 | ||
| Gastrointestinal bleeding | 225 33 | 4843 | Adjusted HR 1.10 (0.82 to 1.46) | 0.527 | 1.47 | 0.225 | 32.0 | 0.014 | Insufficient |
| 325 29 33 | 28 076 | Unadjusted IRR 1.10 (0.93 to 1.31) | 0.273 | 5.78 | 0.056 | 65.4 | 0.014 | ||
HR, hazard ratio; IRR, incidence rate ratio; RR, risk ratio; and TIA, transient ischemic attack.
Mortality outcomes
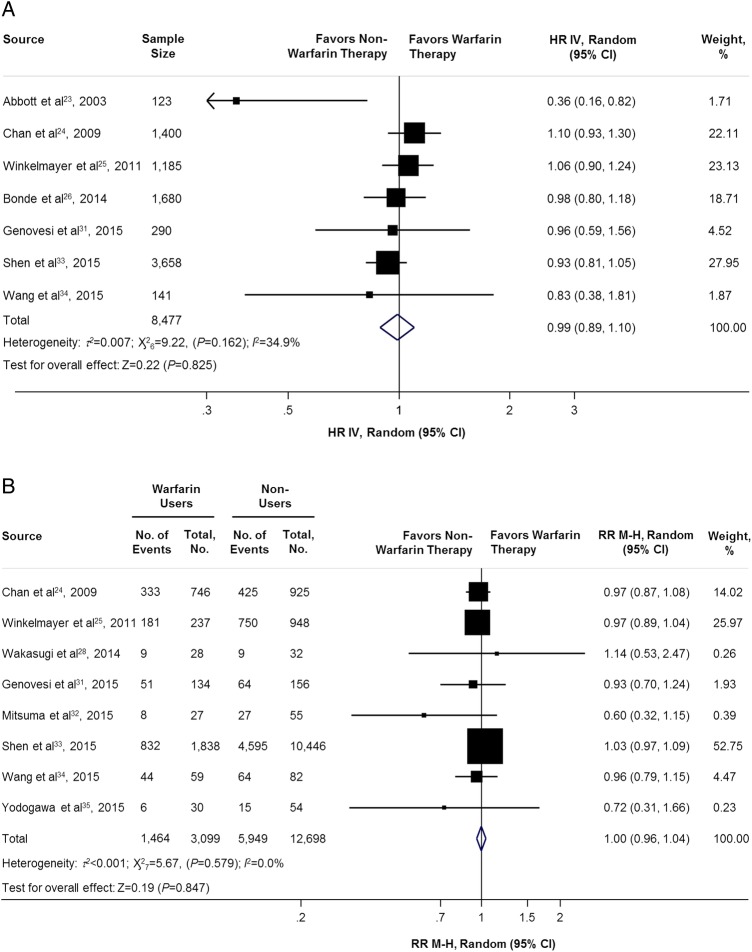
In the adjusted HR analysis (7 studies,23–26 31 33 34 n=8477) and unadjusted analysis (8 studies,24 25 28 31–35 n=15 797), there were no difference in the all-cause mortality between warfarin users or non-warfarin users. The adjusted HR and unadjusted RR were 0.99 (95% CI 0.89 to 1.10; p=0.825; figure 2A) and 1.00 (95% CI 0.96 to 1.04; p=0.847; figure 2B), respectively.
Figure 2.
Adjusted and unadjusted of all-cause mortality comparing warfarin users versus non-warfarin users. HR IV, hazard ratio inverse variance method; RR M-H, risk ratio Mantel-Haenszel method.
For cardiovascular death, the adjusted HR (4 studies,24 26 31 33 n=7028) and unadjusted RR (5 studies,24 28 31–33 n=14 116) were 0.94 (95% CI 0.84 to 1.06; p=0.347; see eFigure 1A) and 0.92 (95% CI 0.74 to 1.14; p=0.467; see eFigure 1B), respectively.
Stroke/thromboembolism outcomes
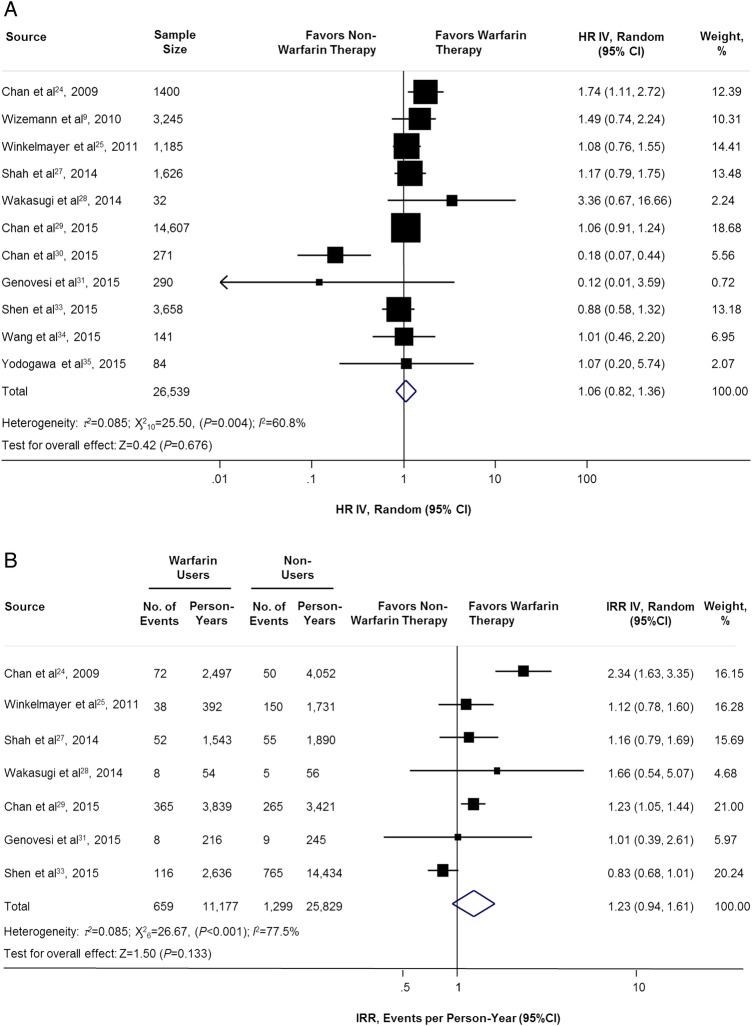
The adjusted HR (11 studies,9 24 25 27–31 33–35 n=26 539) and unadjusted IRR (7 studies,24 25 27–29 31 33 n=31 723) have shown no significant association of stroke/thromboembolism between warfarin users and non-warfarin users: HR=1.06 (95% CI 0.82 to 1.36; p=0.676; figure 3A), IRR=1.23 (95% CI 0.94 to 1.61; p=0.133; figure 3B).
Figure 3.
Adjusted and unadjusted of stroke/thromboembolism comparing warfarin users versus non-warfarin users. HR IV, hazard ratio inverse variance method; IRR IV, incidence rate ratio inverse variance method.
For ischaemic stroke/TIA, adjusted HR (7 studies,24 25 27 28 30 33 34 n=8584) and unadjusted IRR (7 studies,24 25 27–29 31 33 n=31 723) between warfarin users and non-warfarin users were 0.91 (95% CI 0.57 to 1.45; p=0.698; see eFigure 2A) and 1.16 (95% CI 0.84 to 1.62; p=0.370; see eFigure 2B), respectively.
Similarly, no association of haemorrhagic stroke among warfarin users and non-warfarin users was found. The adjusted HR (5 studies,24 25 29 33 34 n=21 262) and unadjusted IRR (5 studies,24 25 29 31 33 n=30 037) were 1.60 (95% CI 0.91 to 2.81; p=0.100; see eFigure 3A) and 1.48 (95% CI 0.92 to 2.36; p=0.102; see eFigure 3B), respectively.
Bleeding outcomes
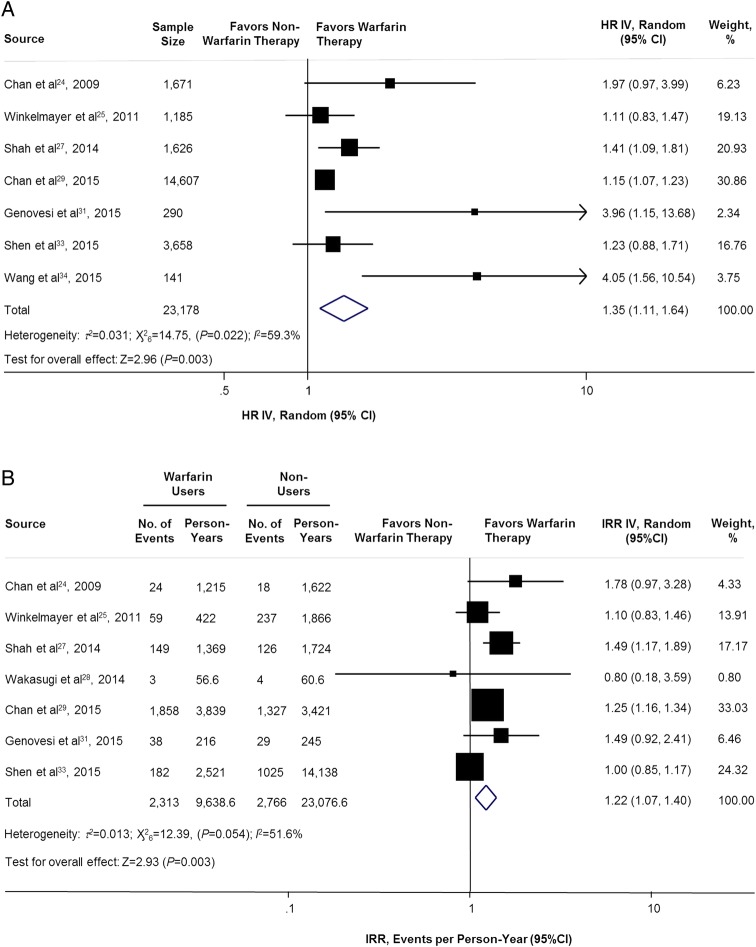
Not surprisingly, warfarin therapy was associated with an increased risk of major bleeding for adjusted (7 studies,24 25 27 29 31 33 34 n=23 178) and unadjusted (7 studies,24 25 27–29 31 33 n=31 723) analyses. The adjusted HR and unadjusted IRR were 1.35 (95% CI 1.11 to 1.64; p=0.003; figure 4A) and 1.22 (95% CI 1.07 to 1.40; p=0.003; figure 4B), respectively.
Figure 4.
Adjusted and unadjusted of major bleeding comparing warfarin users versus non-warfarin users. HR IV, hazard ratio inverse variance method; IRR IV, incidence rate ratio inverse variance method.
However, there was no association in gastrointestinal bleeding among warfarin users and non-warfarin users. The adjusted HR (2 studies,25 33 n=4843) and unadjusted IRR (3 studies,25 29 33 n=28 076) were 1.10 (95% CI 0.82 to 1.46; p=0.527; see eFigure 4A) and 1.10 (95% CI 0.93 to 1.31; p=0.273; see eFigure 4B), respectively.
Subgroup analysis
When the data was pooled as unadjusted IRRs, study design and location were the significant sources of heterogeneity of stroke/thromboembolism, and haemorrhagic stroke. Additionally, sample size was also a source of heterogeneity of haemorrhagic stroke. However, subgroup analyses of all-cause mortality, cardiovascular death, and ischaemic stroke/TIA were similar to the primary results. Several preplanned subgroup analyses could not be performed because of limited statistical power, that is, CHADS2/CHA2DS2VASc score, and bleeding risk score such as the HAS-BLED score. Furthermore, clinically and statistically meaningful conditions that increase the risks of major bleeding were defined; warfarin used in haemodialysis patients; sample size; and the location of the study. Details are presented in eTable 13.
Sensitivity analysis
After restricting the analyses to the highest-quality study, stroke/bleeding risk score and adding an unpublished study, it illustrated that there was no effect on the main findings. Results are summarised (eTables 14–16). The ‘leave one out’ analyses were performed (eTable 17). All-cause mortality, ischaemic stroke/TIA, major bleeding and gastrointestinal bleeding appeared to be robust. However, after the removal of Shen et al,33 warfarin reduced cardiovascular death (RR=0.79 (95% CI 0.68 to 0.91; p=0.001)) and increased stroke/thromboembolism (IRR 1.36 (95% CI 1.05 to 1.75; p=0.019)). Interestingly, after the removal of Chan et al29 2015 and Shen et al,33 warfarin increased the risk of haemorrhagic stroke: HR=1.99 (95% CI 1.03 to 3.84; p=0.040), and IRR=1.63 (95% CI 1.25 to 2.11; p<0.001), respectively.
Finally, a multivariate analysis or propensity score matching approach did not affect the main findings, except for adjusted HR for major bleeding. The pooled estimated of major bleeding was no longer associated with warfarin therapy when using propensity score matching results (HR=1.16 (95% CI 0.93 to 1.44; p=0.181; eTable 18).
Meta-regression
Warfarin use was not associated with adjusted and unadjusted risk for all-cause mortality, cardiovascular death and ischaemic stroke. However, a history of hypertension was associated with an increased risk of stroke/thromboembolism; adjusted HR=1.09 per percentage of the difference (95% CI 1.00 to 1.18; p=0.048; see eFigure 5A). A history of hypertension and prior stroke/TIA were found to increase the risk of haemorrhagic stroke; adjusted HR=1.35 per percentage of the difference (95% CI 1.00 to 1.82; p=0.050; see eFigure 5B) and unadjusted IRR=1.20 per percentage of the difference (95% CI 1.01 to 1.42; p=0.042; see eFigure 5C), respectively. Moreover, a history of diabetes mellitus and prior stroke/TIA were a risk for unadjusted of major bleeding (IRR=1.05 (95% CI 1.00 to 1.11) per percentage of the difference; p=0.049; see eFigure 5D, IRR=1.06 (95% CI 1.01 to 1.12) per percentage of the difference; p=0.025; see eFigure 5E, respectively). eTable 19 shows the estimated effects.
Publication bias
There was no evidence of publication bias by Begg's or Egger's test, with the exception of unadjusted RR for all-cause mortality (p=0.030 for Egger's) and adjusted HR for major bleeding (p=0.035 and p=0.018 for Begg's and Egger's, respectively). After calibration for publication bias by the trim and fill method, the results did not substantively alter the pooled effect estimate from the primary analysis (eTable 20). The contour-enhanced funnel plots are illustrated in eFigure 6.
Discussion
This systematic review and meta-analysis had challenged the value of warfarin for stroke prevention in dialysis patients with AF. The main findings indicated that warfarin therapy does not mitigate the risk of death and total stroke, but is associated with a significant increased risk of major bleeding by 35%. According to the GRADE system, the SOE for the association was low or insufficient.
Evidence has shown that diminished kidney function is related to stroke associated with AF and may be characterised as an independent risk factor for stroke.57 58 Nevertheless, the rigorous mechanism of the complex interrelationship among dialysis patients, AF and stroke are not well established. Despite the traditional risk factors, weak evidence suggests that warfarin might increase the risk of ischaemic stroke by accelerated vascular calcification in dialysis patients.20–22 In this study, however, no association between warfarin and ischaemic stroke was shown.
On the other hand, there are several mechanisms regarding warfarin and the risk of bleeding in dialysis patients.2 59 Moreover, the routine practice of dialysis requires systemic anticoagulation with heparin to prevent clotting that may increase the risk of further bleeding. More importantly, the risk of bleeding can be aggravated by the combination of antiplatelet therapy and comorbid illness. Notably, no apparent impact on all-cause mortality was found from the increased risk of major bleeding in this study. This may be due to indifference of haemorrhagic stroke and GI bleeding, which are the prognostic factors for mortality between warfarin users and non-warfarin users. Giving that the patients in our study were prescribed anticoagulants due to high thromboembolism risk and low bleeding risk, the lack of difference in mortality may demonstrate the efficacy of warfarin. The sensitivity analysis supported the fact that difference of major bleeding disappeared with the propensity matched results.
Generally, the benefit of warfarin for stroke prevention in AF needs to be outweighed against bleeding risks. As recommended by international guidelines, the CHADS2/CHA2DS2VASc score are frequently used for risk stratification of stroke and help to guide towards oral anticoagulation therapy.60–62 They have been fully clarified and validated in the general AF population, but are still limited in dialysis patients, as well as bleeding risk.
Within the general AF population receiving warfarin, the incidence rate of stroke/thromboembolism was estimated to be 1.66% per year with an acceptable risk of major bleeding ranging from 1.40% to 3.40%.63 A recent Chinese cohort,64 reported an annual rate of major bleeding of 9.7% in non-warfarin users. The authors speculate that anticoagulation therapy may be suitable for patients with ESRD with AF who had CHADS2≥4 or CHA2DS2VASc≥6, based on data stating that the annual risk of ischaemic stroke was 9.9% and 10.0%, respectively. More evidence is needed to confirm these specific cut-off points.
Notably, beside the risks of bleeding, dialysis patients appear to present with atherothrombotic stroke rather than embolic stroke due to their high risk of developing atherosclerotic disease. Therefore, this situation could elucidate the reduced benefit of warfarin for stroke prevention in AF.
The prescribed warfarin in practice among dialysis patients with AF was highly variable in this review, ranging from 10.8% to 55.2%. This is not unexpected as there is a lack of a reliable protocol for anticoagulation decision-making in this special population. Regarding several international guidelines,65–67 there is no further recommendation of warfarin use in dialysis patients with AF because of the indefinite risk–benefit. However, the 2014 guidelines from the American Heart Association, the American College of Cardiology Foundation and the Heart Rhythm Society60 recommended warfarin use with a target INR of 2.0–3.0 for primary stroke prevention in high-risk patients (CHADS2/CHA2DS2VASc ≥ 2 points).
Our findings are consistent with the meta-analysis by Li et al,36 Dahal et al,37 and Liu et al38 that warfarin therapy cannot prevent strokes in dialysis patients with AF, but associated with a higher risk of bleeding. Another meta-analysis conducted by Providência et al,68 2014 to evaluate efficacy and safety of warfarin in chronic kidney disease with nonvalvular AF demonstrated in a subgroup analysis of dialysis patients that warfarin exhibited a protective effect and did not increase risk of bleeding. However, only 1 study with dialysis patients by Olsen et al56 was included, leading to inconclusive results. Indeed, it should be noted that there are key differences among the previous and the current study. First, this study explicitly identified the population that was limited to patients with AF, who are undergoing dialysis, while Li et al36 and Liu et al38 included studies in patients who underwent dialysis and kidney transplant, who had wide variation in their kidney function. Second, Li et al,36 did not quantify the association between warfarin and mortality outcomes. All of the reviews did not discuss specific stroke risks (ischaemic/haemorrhagic stroke) due to limited data. Third, we performed more comprehensive analyses than the other studies to estimate the effects of adjusted and unadjusted models.
Strengths and limitations
The strength of this meta-analysis consisted of more expansive and up to date evidences, which reflect real-world practices, than previous studies. The analyses have been driven by comprehensively reviewed and rigorous statistical approaches. Robustly, the main findings were consistent between pooled adjusted and unadjusted models. Furthermore, we also evaluated the SOE to further support guideline development.
There are several potential limitations inherent in our evidence that should be mentioned. First, the multifactors for stroke/bleeding risks, dialysis modality and imbalances in comorbidities are the major sources of bias. Using study level data rather than individual patient data (IPD) may limit analysis in certain groups of patients. Access to IPD would help to clarify these questions and provided more reliable evidence to balance risk–benefit of warfarin therapy for stroke prevention in dialysis patients.
Second, this study is observational in nature and mostly relies on medical claim data, which could be prone to information bias and might affect the association between warfarin therapy and the outcomes. The associations revealed could not be causative owing to residual confounding. Misclassification can be noticed due to a lack of a standardised protocol for AF diagnosis and detecting the outcomes. As warfarin prescription was taken at baseline or from a prescription claim database; adherence over time could not be ascertained. Although sophisticated analyses were performed, confounding by indication may not be totally excluded. In addition, it is expected that outcomes maybe underestimated because of reporting bias. To address all these bias, we conducted several sensitivity analyses and applied the GRADE system to define the certainty of the evidence.
Third, the difference due to studies and patients' characteristics appeared to be substantial sources for explaining such heterogeneity. We, therefore, performed random-effect models. However, unmeasured variables still cannot be ruled out.
Fourth, genetic factors, INR/TTR values, various types of comparator agents such as novel oral anticoagulants, and a subset of race/ethnicity cannot be indicated. In addition, comedication such as heparin used to prevent clotting during dialysis cannot be identified. Theoretically, heparin may have interaction with warfarin resulting in the decrease of warfarin effect or increase risk of bleeding. Thus, an interpretation needs to be performed with caution.
Last, publication bias was detected in the major bleeding outcome, which may be explained by the variation in the definition of bleeding. After calibration with trim-and-fill analysis, the direction of findings was unchanged. Moreover, either contour-enhanced plot or Begg's and Egger's test may be underpowered to detect the publication bias due to the small number of studies being analysed.
Implications and future research
Despite some inconsistence and limitations, our findings may have implications for clinical decision-making: (1) the routine use of warfarin for stroke prevention in dialysis patients may not be recommended due to a lack of benefit, particularly in patients with a history of previous life-threatening haemorrhage, high risk of bleeding or frail patients due to concerns related to dementia and due to risk of falls. However, for prior embolic stroke, known atrial thrombus or valvular/rheumatic heart disease, warfarin therapy may be reasonable for secondary stroke prevention with shared decision-making between patients and clinicians. If initiated, more frequent INR monitoring is required; (2) an alternative treatment or novel non-pharmacological approach, such as the left atrial occlude devices may be considered for lowering the risk of stroke in dialysis patients but this also requires further, well-controlled studies.
Critically, given the knowledge gaps in regard to the role of warfarin for stroke prevention in patients with AF undergoing dialysis observed in this review, there is an urgent need for future research focusing on: (1) The elucidation of the complex interrelationship between the pathophysiology and outcomes of stroke in these patients that might favour the expansion of effective strategies. (2) The risk scoring scheme of stroke/bleeding risk, to define and quantify those at risk. (3) Well-designed RCTs are needed to explore the risk–benefit of warfarin therapy in this special population; however, the possibility of such study may be limited by very small treatment effects size leading to very large number of sample sizes. From our data such a study may be powered for non-inferiority of warfarin versus non-anticoagulant treatment. Our suggestion is to develop the collaboration research networks for AF registries in patients undergoing dialysis using IPD to identify the risk–benefit of anticoagulant therapy, and obtain rich data to help guide an appropriate treatment approach. (4) Further studies should be comparison of warfarin versus pharmacological and non-pharmacological treatments such as dual antiplatelet therapy and other novel treatments.
Conclusions
This study has shown that warfarin therapy in patients with AF, who are undergoing dialysis, was not associated with mortality and stroke/thromboembolism, but significantly increased the risk of major bleeding. Until more data are obtained, clinicians should be aware of the risks associated with warfarin use in these patients, and the clinical decision to prescribe warfarin should comprise an individualised approach that takes into account the risk of stroke and the haemorrhagic complications. Indisputably, more rigorous studies are needed to settle the optimal preventive strategies and therapeutic modalities in these vulnerable populations.
Footnotes
Contributors: SN, CR and RA had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. SN, CR and RA were involved in the study concept and design. All authors involved in the acquisition, analysis or interpretation of data. SN and CR were involved in the drafting of the manuscript. PD, KN and AP were involved in the critical revision of the manuscript for important intellectual content. SN and CR were involved in the statistical analysis. RA and CR were involved in the administrative, technical or material support. RA and CR were involved in the study supervision.
Funding: This work was supported by the Faculty of Pharmacy, Chiang Mai University.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Goldstein BA, Arce CM, Hlatky MA et al. Trends in the incidence of atrial fibrillation in older patients initiating dialysis in the United States. Circulation 2012;126:2293–301. 10.1161/CIRCULATIONAHA.112.099606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wetmore JB, Ellerbeck EF, Mahnken JD et al. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol 2013;23:112–18. 10.1016/j.annepidem.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–5. 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- 4.Krijthe BP, Kunst A, Benjamin EJ et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–51. 10.1093/eurheartj/eht280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol 2014;11:639–54. 10.1038/nrcardio.2014.118 [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman D, Sood MM, Rigatto C et al. Systematic review and meta-analysis of incidence, prevalence and outcomes of atrial fibrillation in patients on dialysis. Nephrol Dial Transplant 2012;27:3816–22. 10.1093/ndt/gfs416 [DOI] [PubMed] [Google Scholar]
- 7.Genovesi S, Pogliani D, Faini A et al. Prevalence of atrial fibrillation and associated factors in a population of long-term hemodialysis patients. Am J Kidney Dis 2005;46:897–902. 10.1053/j.ajkd.2005.07.044 [DOI] [PubMed] [Google Scholar]
- 8.Wetmore JB, Mahnken JD, Rigler SK et al. The prevalence of and factors associated with chronic atrial fibrillation in Medicare/Medicaid-eligible dialysis patients. Kidney Int 2012;81: 469–76. 10.1038/ki.2011.416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wizemann V, Tong L, Satayathum S et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int 2010;77:1098–106. 10.1038/ki.2009.477 [DOI] [PubMed] [Google Scholar]
- 10.Winkelmayer WC, Patrick AR, Liu J et al. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 2011;22:349–57. 10.1681/ASN.2010050459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genovesi S, Vincenti A, Rossi E et al. Atrial fibrillation and morbidity and mortality in a cohort of long-term hemodialysis patients. Am J Kidney Dis 2008;51:255–62. 10.1053/j.ajkd.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 12.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. 10.7326/0003-4819-146-12-200706190-00007 [DOI] [PubMed] [Google Scholar]
- 13.Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev 2007;(3):CD006186. [DOI] [PubMed] [Google Scholar]
- 14.Wang IK, Cheng YK, Lin CL et al. Comparison of subdural hematoma risk between hemodialysis and peritoneal dialysis patients with ESRD. Clin J Am Soc Nephrol 2015;10:994–1001. 10.2215/CJN.08140814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang JY, Lee TC, Montez-Rath ME et al. Trends in acute nonvariceal upper gastrointestinal bleeding in dialysis patients. J Am Soc Nephrol 2012;23:495–506. 10.1681/ASN.2011070658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Power A, Hamady M, Singh S et al. High but stable incidence of subdural haematoma in haemodialysis—a single-centre study. Nephrol Dial Transplant 2010;25:2272–5. 10.1093/ndt/gfq013 [DOI] [PubMed] [Google Scholar]
- 17.Sood MM, Larkina M, Thumma JR et al. Major bleeding events and risk stratification of antithrombotic agents in hemodialysis: results from the DOPPS. Kidney Int 2013;84:600–8. 10.1038/ki.2013.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi M, Takamatsu I, Kanno Y et al. A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant 2012;27:1580–4. 10.1093/ndt/gfr658 [DOI] [PubMed] [Google Scholar]
- 19.Nigwekar SU, Bhan I, Turchin A et al. Statin use and calcific uremic arteriolopathy: a matched case-control study. Am J Nephrol 2013;37:325–32. 10.1159/000348806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delanaye P, Krzesinski JM, Warling X et al. Dephosphorylated-uncarboxylated Matrix Gla protein concentration is predictive of vitamin K status and is correlated with vascular calcification in a cohort of hemodialysis patients. BMC Nephrol 2014;15:145 10.1186/1471-2369-15-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holden RM, Sanfilippo AS, Hopman WM et al. Warfarin and aortic valve calcification in hemodialysis patients. J Nephrol 2007;20:417–22. [PubMed] [Google Scholar]
- 22.Danziger J. Vitamin K-dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol 2008;3:1504–10. 10.2215/CJN.00770208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott KC, Trespalacios FC, Taylor AJ et al. Atrial fibrillation in chronic dialysis patients in the United States: risk factors for hospitalization and mortality. BMC Nephrol 2003;4:1 10.1186/1471-2369-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan KE, Lazarus JM, Thadhani R et al. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol 2009;20:2223–33. 10.1681/ASN.2009030319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winkelmayer WC, Liu J, Setoguchi S et al. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol 2011;6:2662–8. 10.2215/CJN.04550511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonde AN, Lip GY, Kamper AL et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study. J Am Coll Cardiol 2014;64:2471–82. 10.1016/j.jacc.2014.09.051 [DOI] [PubMed] [Google Scholar]
- 27.Shah M, Avgil Tsadok M, Jackevicius CA et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation 2014;129:1196–203. 10.1161/CIRCULATIONAHA.113.004777 [DOI] [PubMed] [Google Scholar]
- 28.Wakasugi M, Kazama JJ, Tokumoto A et al. Association between warfarin use and incidence of ischemic stroke in Japanese hemodialysis patients with chronic sustained atrial fibrillation: a prospective cohort study. Clin Exp Nephrol 2014;18:662–9. 10.1007/s10157-013-0885-6 [DOI] [PubMed] [Google Scholar]
- 29.Chan KE, Edelman ER, Wenger JB et al. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation 2015;131:972–9. 10.1161/CIRCULATIONAHA.114.014113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan PH, Huang D, Yip PS et al. Ischaemic stroke in patients with atrial fibrillation with chronic kidney disease undergoing peritoneal dialysis. Europace 2016;18:665–71. [DOI] [PubMed] [Google Scholar]
- 31.Genovesi S, Rossi E, Gallieni M et al. Warfarin use, mortality, bleeding and stroke in haemodialysis patients with atrial fibrillation. Nephrol Dial Transplant 2015;30:491–8. 10.1093/ndt/gfu334 [DOI] [PubMed] [Google Scholar]
- 32.Mitsuma W, Matsubara T, Hatada K et al. Clinical characteristics of hemodialysis patients with atrial fibrillation: the RAKUEN (Registry of atrial fibrillation in chronic kidney disease under hemodialysis from Niigata) study. J Cardiol Published Online First: 30 Oct 2015. 10.1016/j.jjcc.2015.08.023 10.1016/j.jjcc.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 33.Shen JI, Montez-Rath ME, Lenihan CR et al. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis 2015;66:677–88. 10.1053/j.ajkd.2015.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang TK, Sathananthan J, Marshall M et al. Relationships between anticoagulation, risk scores and adverse outcomes in dialysis patients with atrial fibrillation. Heart Lung Circ 2016;25:243–9. 10.1016/j.hlc.2015.08.012 [DOI] [PubMed] [Google Scholar]
- 35.Yodogawa K, Mii A, Fukui M et al. Warfarin use and incidence of stroke in Japanese hemodialysis patients with atrial fibrillation. Heart Vessels Published Online First: 8 Dec 2015. 10.1007/s00380-015-0777-7 10.1007/s00380-015-0777-7 [DOI] [PubMed] [Google Scholar]
- 36.Li J, Wang L, Hu J et al. Warfarin use and the risks of stroke and bleeding in hemodialysis patients with atrial fibrillation: a systematic review and a meta-analysis. Nutr Metab Cardiovasc Dis 2015;25:706–13. 10.1016/j.numecd.2015.03.013 [DOI] [PubMed] [Google Scholar]
- 37.Dahal K, Kunwar S, Rijal J et al. Stroke, major bleeding and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest 2016;149:951–9. 10.1378/chest.15-1719 [DOI] [PubMed] [Google Scholar]
- 38.Liu G, Long M, Hu X et al. Effectiveness and safety of warfarin in dialysis patients with atrial fibrillation: a meta-analysis of observational studies. Medicine (Baltimore) 2015;94:e2233 10.1097/MD.0000000000002233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Methods guide for effectiveness and comparative effectiveness reviews. AHRQ Publication No. 10(14)-EHC063-EF. Rockville, MD: Agency for Healthcare Research and Quality (US), January 2014. http://www.effectivehealthcare.ahrq.gov [PubMed] [Google Scholar]
- 40.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 41.Wells G, Shea B, O'Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 14 Oct 2015).
- 42.Berkman ND, Lohr KN, Ansari MT et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. J Clin Epidemiol 2015;68:1312–24. [DOI] [PubMed] [Google Scholar]
- 43.Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 44.Dundar Y, Dodd S, Dickson R et al. Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies. Health Technol Assess 2006;10:iii–iv, ix–145 10.3310/hta10050 [DOI] [PubMed] [Google Scholar]
- 45.Deeks JJ, Dinnes J, D'Amico R et al. Evaluating non-randomised intervention studies. Health Technol Assess 2003;7:iii–x, 1–173 10.3310/hta7270 [DOI] [PubMed] [Google Scholar]
- 46.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815–34. [DOI] [PubMed] [Google Scholar]
- 47.Tierney JF, Stewart LA, Ghersi D et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guevara JP, Berlin JA, Wolf FM. Meta-analytic methods for pooling rates when follow-up duration varies: a case study. BMC Med Res Methodol 2004;4:17 10.1186/1471-2288-4-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 50.Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Costa BR, Juni P. Systematic reviews and meta-analyses of randomized trials: principles and pitfalls. Eur Heart J 2014;35:3336–45. 10.1093/eurheartj/ehu424 [DOI] [PubMed] [Google Scholar]
- 52.Peters JL, Sutton AJ, Jones DR et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008;61:991–6. 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 53.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 54.Egger M, Davey Smith G, Schneider M et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 56.Olesen JB, Lip GY, Kamper AL et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012;367:625–35. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 57.Herrington W, Haynes R, Staplin N et al. Evidence for the prevention and treatment of stroke in dialysis patients. Semin Dial 2015;28:35–47. 10.1111/sdi.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lutz J, Menke J, Sollinger D et al. Haemostasis in chronic kidney disease. Nephrol Dial Transplant 2014;29:29–40. 10.1093/ndt/gft209 [DOI] [PubMed] [Google Scholar]
- 59.Wetmore JB, Phadnis MA, Mahnken JD et al. Race, ethnicity, and state-by-state geographic variation in hemorrhagic stroke in dialysis patients. Clin J Am Soc Nephrol 2014;9:756–63. 10.2215/CJN.06980713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.January CT, Wann LS, Alpert JS et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–267. 10.1161/CIR.0000000000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.You JJ, Singer DE, Howard PA et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141(Suppl):e531S–575S. 10.1378/chest.11-2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Camm AJ, Lip GY, De Caterina R et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. [DOI] [PubMed] [Google Scholar]
- 63.Agarwal S, Hachamovitch R, Menon V. Current trial-associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta-analysis. Arch Intern Med 2012;172:623–31; discussion 631–3. [DOI] [PubMed] [Google Scholar]
- 64.Chao TF, Liu CJ, Wang KL et al. Incidence and prediction of ischemic stroke among atrial fibrillation patients with end-stage renal disease requiring dialysis. Heart Rhythm 2014;11:1752–9. 10.1016/j.hrthm.2014.06.021 [DOI] [PubMed] [Google Scholar]
- 65.Herzog CA, Asinger RW, Berger AK et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011;80: 572–86. [DOI] [PubMed] [Google Scholar]
- 66.Verma A, Cairns JA, Mitchell LB et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol 2014;30:1114–30. 10.1016/j.cjca.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 67.Wang AY, Brimble KS, Brunier G et al. ISPD cardiovascular and metabolic guidelines in adult peritoneal dialysis patients Part II—management of various cardiovascular complications. Perit Dial Int 2015;35:388–96. 10.3747/pdi.2014.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Providência R, Marijon E, Boveda S et al. Meta-analysis of the influence of chronic kidney disease on the risk of thromboembolism among patients with nonvalvular atrial fibrillation. Am J Cardiol 2014;114:646–53. 10.3747/pdi.2014.00278 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2016-000441supp.pdf (2.1MB, pdf)