Abstract
An estimated 65% of individuals demonstrate multi-domain cognitive impairment post-stroke, although little is known about the varying role of cognitive risk and protective factors in pre-, peri-, and post-ischemic stroke phases. Longitudinal changes in global cognitive function after ischemic stroke are not well characterized, especially in older adults over age 80. We examined global cognitive function trajectories in these three phases across a mean follow-up of 8.12 (2.30) years in 159 female stroke survivors aged 65–79 at baseline using linear mixed models with change points. In separate models controlling for demographic variables, we tested the interaction of baseline risk and protective factors with stroke phase on global cognitive function. None of the pre-stroke global cognitive function means or trajectories differed significantly. At the time of ischemic stroke, higher body mass index, the presence of hypertension, low optimism, and higher physical function were all associated with significantly greater mean decreases in global cognition (all p’s <.0.0001), but were not significantly different from the contrasting level (all p’s > 0.05). Higher body mass index, the presence of hypertension, low optimism, and higher physical function were in turn protective of global cognitive decline post- ischemic stroke (all contrasting p values <.01). Baseline factors may play either a risk or a protective role in global cognitive function depending on the phase of ischemic stroke.
Keywords: Aging, Stroke, Global Cognitive Function, Ischemic, Longitudinal
While estimates of the incidence of dementia around the time of the stroke are fairly well-characterized (20–25%) (Desmond et al., 2002; Del Ser et al., 2005; Sachdev et al., 2006; Tham et al., 2002), incidence of cognitive impairment without dementia varies depending upon stroke classification and time since stroke (Donovan et al., 2008). Cognitive impairment after ischemic or hemorrhagic stroke typically involves more than one cognitive domain (Donovan et al., 2008; Barker-Collo et al., 2010), and up to 65% of individuals demonstrate multi-domain cognitive impairment after stroke that likely increases caregiver burden and dependence in daily life activities while interfering with functional recovery and the potential benefits of physical rehabilitation (Donovan et al., 2008).
Global cognitive impairment and decline after ischemic stroke are not well characterized, especially in older adults over age 80. Results are mixed and longer-term follow-up is uncommon (Barker-Collo et al., 2010). Most studies assess cognitive function only for 3–6 months after ischemic stroke (Sachdev et al., 2006; Barba et al., 2000; Xu et al., 2008). Moreover, cognitive function prior to ischemic stroke, if assessed, is typically measured indirectly via proxy report (Del Ser et al., 2005; Barba et al., 2000) or IQ test (Sachdev et al., 2006). Improvements in global and domain-specific cognitive function (varying from 10–50% of the cohort) have been found in persons ranging in age from 60–75, with ischemic stroke assessed at 0–6 months and 12–18 months (Tham et al., 2002; Desmond et al., 2002; Ballard et al., 2003). Overall, longitudinal studies of the progression of global cognitive impairment 1–2 years after ischemic stroke, while infrequent, demonstrate heterogeneity of findings - improvement, stability, and decline. Analyses of pre-, peri-, and post-stroke cognitive trajectories that identify individuals with differential cognitive decline may help to clarify the pattern of changes in global cognitive function after ischemic stroke.
Protective factors for maintaining and improving cognitive function after ischemic stroke need to be better identified in order to account for heterogeneous findings. Increased age (Del Ser et al., 2005; Barba et al., 2000; Xu et al., 2008), female gender (Xu et al., 2008), less education (Del Ser et al., 2005; Sachdev et al., 2006; Xu et al., 2008) and higher depression scores (Xu et al., 2008; Bushnell, 2011; Hackett et al., 2005) have all been associated with cognitive impairment after stroke. Studies that evaluate the contribution of vascular risk factors to cognitive impairment after ischemic stroke are less conclusive, with some studies finding that hypertension, diabetes, and atrial fibrillation are unrelated to post-stroke cognitive impairment (Del Ser et al., 2005; Sachdev et al., 2006; Barba et al., 2003), whereas others have found the opposite (Desmond et al., 2002). In a related study, Del Ser et al. (2005) examined the relationship between multiple risk factors and cognitive function at 3 and 24 months after ischemic stroke, and found that cognitive function was maintained in 78% of participants, but highly variable in the remainder. Increased age, proxy report of previous cognitive decline, and diastolic blood pressure <80 mm Hg were associated with the progression of post-stroke cognitive impairment.
The objectives of this study were to characterize global cognitive function trajectories pre-, peri-, and post-stroke, across 15 years of longitudinal data collection in the Women’s Health Initiative Memory Study (WHIMS) and to determine the baseline factors associated with better post-stroke cognitive function. WHIMS is a longitudinal cohort study of 7,479 older women aged 65 to 79 at baseline with detailed measures of cognitive risk and protective factors in participants with adjudicated ischemic stroke. Linear mixed models with change points were used to examine global cognitive function trajectories separately for each level of cognitive risk factor, while controlling for other demographic factors. These models provide important information about the interactions between timing surrounding stroke and cognitive risk and protective factors across the stroke continuum that may be translated into better treatment and intervention delivery post-stroke.
Methods
Participants
Ancillary to the Women’s Health Initiative (WHI) hormone therapy trials, the Women’s Health Initiative Memory Study (WHIMS) is a longitudinal study that began enrollment in 1996 and is now in its second 5-year extension (2010–2015) designed to examine the cognitive effects of conjugated equine estrogen-based HT among 7,479 older postmenopausal women aged 65–79 at baseline (Shumaker et al., 1998; 2003; 2004). WHIMS participants underwent in-person screenings of global cognitive function at enrollment and annually until 2008, after which it transitioned to telephone-based testing, in order to adjudicate the primary outcome of cognitive impairment - no dementia, mild cognitive impairment, or probable dementia. The WHIMS study protocol was approved by the Wake Forest University Institutional Review Board.
Stroke Adjudication. The parent study (WHI) continues to centrally adjudicate incident stroke in WHIMS participants, using established protocols as described below and in Wassertheil-Smoller et al., 2003. Stroke diagnosis requiring or occurring during hospitalization was based on rapid onset of neurological deficit attributable to obstruction or rupture of an arterial vessel system. The deficit was not known to be secondary to brain trauma, tumor, infection, or other cause and must have lasted 24 hours unless death occurred or a lesion compatible with acute stroke was evident on computed tomography (CT) or magnetic resonance imaging (MRI) scan. Strokes were classified as ischemic or hemorrhagic based on the review of reports of brain imaging studies. One of three stroke neurologists centrally adjudicated locally determined strokes, TIA, and self-reported stroke that had not been confirmed by local adjudicators after review of the medical records. Central adjudicators confirmed 94.5% of locally adjudicated strokes. Ischemic stroke, defined as occlusion of cerebral or peri-cerebral arteries with infarction not resulting from a procedure (cerebral thrombosis, cerebral embolism, lacunar infarction) is the focus of this manuscript. Data on arterial distribution of ischemic stroke was not available for this study.
Analytic Sample. The inclusion criteria for this retrospective data analysis was an adjudication of ischemic type stroke with at least one annual in-person global cognitive function assessment following their incident (N=159). Extensive detail on participant characteristics is provided in Results.
Measures
Global cognitive function
The Modified Mini-Mental State Examination (3MSE) is a measure of global cognitive function that has 15 parts comprising 46 items that contribute to a total score ranging from 0 to 100, with higher scores indicating better cognitive function (Teng et al., 1987; Folstein, Folstein, & McHugh, 1975). Test items measure temporal and spatial orientation, immediate and delayed recall, executive function (mental reversal, 3-stage commands), naming, verbal fluency, abstract reasoning (similarities), praxis (obeying commands, sentence writing), writing, and visuo-constructional abilities (copying). The 3MSE was found to have high interrater reliability (intraclass correlation coefficient=0.98) and internal consistency reliability (coefficient alpha=0.91). In addition, it had moderate test-retest reliability over 3 years (.78) and was found to be correlated with the MMSE (.95) (Bassuk & Murphy, 2003). One study of 3,255 older, community-dwelling adults measured at baseline, 5, and 10 years found that a change of ≥ 5 points represented a clinically meaningful difference between groups, although a change of ≥ 1 point is clinically detectable (Andrew & Rockwood, 2008).
Body mass index
Body mass index (BMI) was calculated as weight (kg) divided by the square of measured height (m2), using baseline weight and height measurements. Participants were divided into BMI categories < or ≥25 kg/m2.
Depressive symptomatology
Depressive symptomatology was assessed at baseline using the Burnam 8-item depression screening instrument, which comprises 6 items from the Center for Epidemiologic Studies Depression Scale (CES-D) about the frequency of depressive symptoms during the past week and 2 items from the National Institute of Mental Health’s Diagnostic Interview Schedule (DIS) about the duration of symptoms (Burnam et al, 1988; Tuunainen et al., 2001). The scoring algorithm, using a prediction equation developed by Burnam et al., gives a composite score between 0 and 1 which represents the probability of having depression, using a standard cut point of ≥ 0.06 to dichotomize the scale. Note that severe depression at baseline was an exclusion for the WHI observational study (OS) and clinical trials (CT).
Hypertension
History of hypertension was ascertained by self-report on baseline questionnaires. Participants with measured resting systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg at the initial clinic visit were also classified as hypertensive.
Optimism
The Optimism construct from the Life Orientation Test-Revised (Glaesmer et al., 2012) is the sum of 6 items: ‘In unclear times, I usually expect the best’; ‘If something can go wrong for me, it will’; ‘I’m always hopeful about my future’; ‘I hardly ever expect things to go my way’; ‘I rarely count on good things happening to me’; ‘Overall, I expect more good things to happen to me’ coded from 1=strongly disagree to 5=strongly agree. It has a summary score ranging from 6 to 30, with a higher score indicating greater optimism. Participants were divided into high and low levels based on a cutoff of 25.
Physical function
Questionnaires administered to participants at baseline included the RAND SF-36 (physical functioning sub-scale), a well-validated measure of self-reported physical function (Hays, Sherbourne, & Mazel, 1993; Ware & Sherbourne, 1992). Participants are asked if their health now limits them in the following activities, and, if so, how much, on a 3-point limitation scale (no, not limited at all; yes, limited a little; yes, limited a lot). It includes the following ten items: ‘Vigorous activities, such as running, lifting heavy objects, or strenuous sports’; ‘Moderate activities, such as moving a table, vacuuming, bowling, or golfing’; ‘Lifting or carrying groceries’; ‘Climbing several flights of stairs’; ‘Climbing one flight of stairs’; ‘Bending, kneeling, or stooping’; ‘Walking more than a mile’; ‘Walking several blocks’; ‘Walking one block’; ‘Bathing or dressing yourself’. Values on the physical functioning subscale range from 0 to 100 with a higher score indicating better self-reported performance.
Analyses
Linear mixed models with change points were used to examine the trajectory of global cognitive function surrounding the incidence of ischemic stroke as a function of follow-up time (Hall et al., 2000), including risk and protective factors hypothesized to influence the post-stroke cognitive trajectories. The change-point models provided 5 parameters: pre-stroke means, pre-stroke slopes, peri-stroke decrements, post-stroke means, and post-stroke slopes, each of which we associated with risk and protective factors. We fit these mixed models including linear terms separately for each hypothesized baseline risk and protective factor, including covariates (age, education, hormone therapy use, race & ethnicity) as random effects in each model to identify pre-stroke markers of health status that could influence the post-stroke cognitive trajectories. Risk and protective factors described previously included body mass index (BMI), depressive symptomatology, hypertension (HTN), optimism, and physical function. All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). All tests were two-sided and significance levels were set at an alpha level of 0.05.
Results
Of the N=361 total WHIMS participants who had an ischemic type stroke, N=159 (44%) had at least one global cognitive function assessment after their incident. The women included in the data analytic sample, compared to those excluded from analyses due to ceased follow-up, had similar baseline characteristics for all factors included in Table 1 (all p’s>0.10) and also similar baseline 3MSE scores: mean (standard error) 95.5 (4.4) versus 96.0 (3.8), p=0.26. Overall mean (SD) years of follow-up for women included in the data analytic sample was 8.12 (2.30) years: pre-stroke=3.35 (1.87) years; post-stroke 3.69 (1.56) years. Their mean (SD) age at ischemic stroke onset was 76.72 (4.96) years (see Table 1). The frequency (%) of the sample distribution was N=100 (62.89) women ≥70 years of age; N=1 (0.63) Asian or Pacific Islander, N=10 (6.29%) Black or African American, N=4 (2.52%) Hispanic or Latino, N=143 (89.94%) White, N=1 (0.63%) Other; N=108 (67.92) had greater than a high school education; N=116 (72.96) had BMI ≥25/kg/m2; N=133 (85.81) had little or no depressive symptomatology; N=85 (53.46) were in the active trial group; N=100 (62.89) had hypertension; N=56 (36.13) had optimism scores ≥25/30; and N=118 (76.13) had physical functioning ≥80/100.
Table 1.
Baseline characteristics of women with ischemic stroke (N=159)
| Characteristics | N (%) |
|---|---|
|
| |
| Age at stroke, Mean (SD) | 76.72 (4.96) |
|
| |
| Age Group | |
| < 70 years | 59 (37.11) |
| ≥ 70 years | 100 (62.89) |
|
| |
| Education | |
| Low ≤ High School | 51 (32.08) |
| High > High School | 108 (67.92) |
|
| |
| Race/Ethnicity | |
| Asian or Pacific Islander | 1 (0.63) |
| Black or African-American | 10 (6.29) |
| Hispanic/Latino | 4 (2.52) |
| White (not of Hispanic origin) | 143 (89.94) |
| Other | 1 (0.63) |
|
| |
| Body Mass Index | |
| Low <25/kg/m2 | 43 (27.04) |
| High ≥25/kg/m2 | 116 (72.96) |
|
| |
| Depressive Symptoms (Burnam Index) (Missing=4) | |
| Low <.06 | 133 (85.81) |
| High ≥.06 | 22 (14.19) |
|
| |
| Hormone Therapy Arm | |
| Active | 85 (53.46) |
| Placebo | 74 (46.54) |
|
| |
| Hypertension | |
| No | 59 (37.11) |
| Yes | 100 (62.89) |
|
| |
| Optimism (Missing=3) | |
| Low<25 | 99 (63.87) |
| High≥25 | 56 (36.13) |
|
| |
| Physical Function (SF-36) (Missing=4) | |
| Low<80 | 37 (23.87) |
| High≥80 | 118 (76.13) |
Pre-, Peri-, and Post-Stroke Cognitive Trajectories
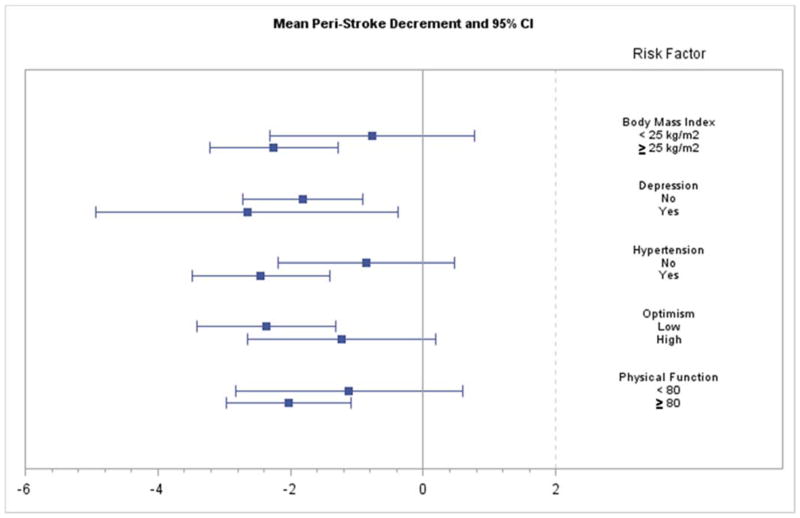
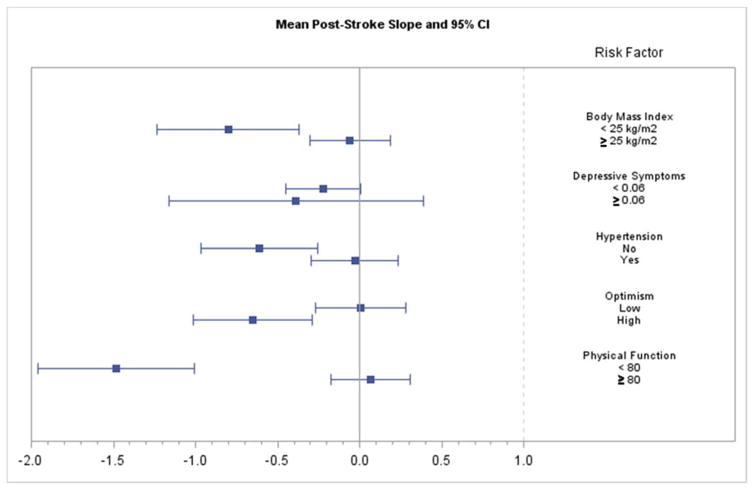
We examined the global cognitive function trajectory across the N=159 cases of ischemic stroke that occurred during the follow-up of WHIMS participants, separately for each level of cognitive risk factor, controlling for age, education, race and HT assignment (active or placebo). Using linear mixed models with change points, we analyzed the mean levels and the slopes prior to ischemic stroke, the mean deficit at ischemic stroke, and the mean levels and slopes after ischemic stroke, fitted to time points demarcating years from ischemic stroke (See Table 2 and Figures 1–2). Table 2 shows the peri- stroke mean (SE) and post-stroke (SE) decreases in global cognitive function, with associated p values. None of the pre-stroke global cognitive function means or slopes differed (all p’s >.20). Additionally, figures 3 and 4 plot 95% Confidence Intervals (CIs) for each level of each factor.
Table 2.
Post-stroke (SE) slope and mean (SE) peri-stroke decrement in global cognitive function, by level of factor (N=159)
| Factor | N | Mean (SE) Peri-Stroke Decrement | p value | Post-Stroke Slope (SE) | p value |
|---|---|---|---|---|---|
|
| |||||
| Body Mass Index | |||||
| Low < 25/kg/m2 | 43 | 0.772 (0.788) | 0.33 | −0.801 (0.220) | 0.0003 |
| High ≥ 25/kg/m2 | 116 | 2.254 (0.492) | <0.0001 | −0.059 (0.125) | 0.64 |
| p=0.11 | p=0.003 | ||||
|
| |||||
| Depressive symptomatology | |||||
| Low <. 06 | 133 | 1.821 (0.460) | 0.0001 | −0.225 (0.116) | 0.052 |
| High ≥. 06 | 22 | 2.653 (1.161) | 0.024 | −0.388 (0.396) | 0.327 |
| p =0.51 | p =0.69 | ||||
|
| |||||
| Hypertension | |||||
| No | 59 | 0.862 (0.679) | 0.21 | −0.610 (0.182) | 0.001 |
| Yes | 100 | 2.446 (0.531) | <0.0001 | −0.031 (0.136) | 0.82 |
| p =0.068 | p =0.011 | ||||
|
| |||||
| Optimism | |||||
| Low < 25 | 99 | 2.361 (0.532) | <0.0001 | 0.006 (0.139) | 0.97 |
| High ≥ 25 | 56 | 1.230 (0.721) | 0.09 | −0.650 (0.184) | 0.0004 |
| p =0.21 | p =0.005 | ||||
|
| |||||
| Physical Function | |||||
| Low < 80 | 37 | 1.119 (0.875) | 0.20 | −1.483 (0.243) | <0.0001 |
| High ≥ 80 | 118 | 2.033 (0.481) | <0.0001 | 0.066 (0.124) | 0.59 |
| p =0.36 | p <0.0001 | ||||
Note. Mean (SE) Peri-Stroke Decrement=difference of Least Squares means. Row p values represent whether factor levels differ significantly from zero. Column p values represent whether factor levels differ significantly from each other.
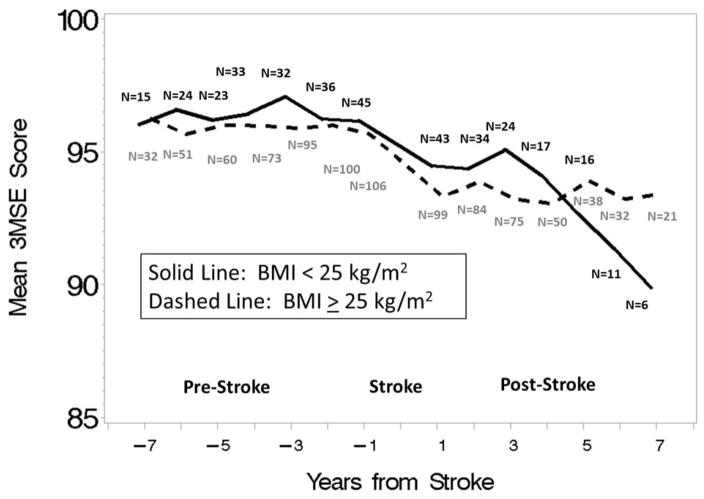
Figure 1.
Global cognitive function scores by years from stroke in low (< 25 kg/m2) and high (≥ 25 kg/m2) levels of body mass index (BMI). Note. BMI=Body Mass Index; 3MSE= Modified Mini-Mental State Examination.
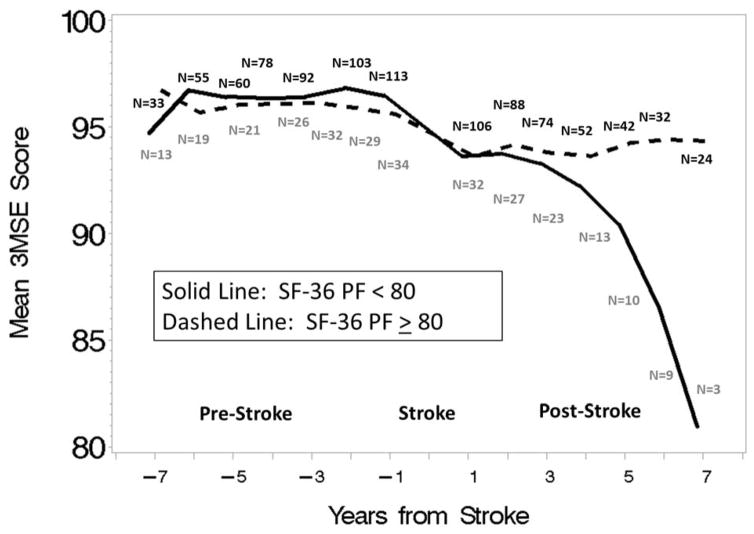
Figure 2.
Mean global cognitive function scores by years from stroke in low (< 80) and high (≥ 80) levels of physical function. Note. SF-36 PF=Physical Function; 3MSE= Modified Mini-Mental State Examination.
Figure 3.
Mean peri-stroke decrement and 95% Confidence Interval (CI) for each level of each risk factor.
Figure 4.
Mean post-stroke slope and 95% Confidence Interval (CI) for each level of each risk factor.
Body mass index
The mean (SE) decrement in global cognitive function surrounding stroke was significant from zero for BMI ≥25kg/m2: 2.254 (0.492) (p<0.0001), but not BMI < 25kg/m2: 0.772 (0.788) (p=0.33), and they did not differ from each other (p=0.11). The slope decrease after stroke was significant from zero for BMI < 25kg/m2: -0.801 (0.220) (p=0.0003), but not BMI ≥25kg/m2: −0.059 (0.125) (p=0.64), and they differed significantly from each other (p=0.003). While women with BMI ≥25kg/m2 had a non-significantly higher mean decrement in global cognitive function surrounding stroke (p=0.11), the opposite pattern was seen after stroke, where women with BMI <25/kg/m2 had a significantly greater slope decrease in global cognitive function (p=0.003).
Depressive symptomatology
The mean (SE) decrement in global cognitive function surrounding stroke was significantly different from zero for scores <.06: 1.798 (0.460) (p=.0001) and ≥.06: 2.653 (1.161) (p=0.024), and they did not differ from each other (p=0.51). Women with scores <.06 had a marginally significant slope decrease in global cognitive function after stroke: −0.225 (0.116) (p=0.052) but women with scores ≥.06 did not: −0.388 (0.396) (p=0.327), and they did not differ from each other (p=0.69). Results for women with depressive symptomatology were in the expected direction (greater mean decrements in global cognitive function surrounding stroke, and greater slope decreases in global cognitive function after stroke), but non-significantly different from women without depressive symptomatology (all p’s>0.50).
Hypertension
The mean (SE) decrement in global cognitive function surrounding stroke was significantly different from zero for women with hypertension: 2.446 (0.531), p<0.0001, but not without hypertension: 0.862 (0.679), p=0.21, and they did not differ significantly from each other (p=0.07). Women without hypertension at baseline had a significant slope decrease in global cognitive function after stroke: −0.610 (0.182), p=0.001, and women with hypertension at baseline did not: −0.031 (0.136), p=0.82, and they differed significantly from each other (p=0.011). Whereas women with baseline hypertension had non-significantly greater mean decrements in global cognitive function surrounding stroke than women without (p=0.068), the opposite pattern was seen after stroke, where women without hypertension at baseline had greater slope decreases in global cognitive function than women with hypertension at baseline (p=0.011).
Optimism
The mean (SE) decrement in global cognitive function surrounding stroke was significantly different from zero for women with low optimism: 2.361 (0.532) (p<0.0001), but not for women with high optimism: 1.230 (0.721) (p=0.09), and they did not differ from each other (p=0.21). In contrast, women with low optimism did not demonstrate a significant slope decrease in global cognitive function after stroke: 0.006 (0.139) (p=0.97), whereas women with high optimism did: −0.650 (0.184) (p=0.0004), and they differed significantly from each other (p=0.005). Whereas women with low optimism had a non-significantly greater mean decrement in global cognitive function surrounding stroke than women with high optimism (p=0.21), a different pattern emerged after stroke, where women with high optimism experienced a significantly greater slope decrease in global cognitive function than women with low optimism (p=0.005).
Physical function
The mean (SE) decrement in global cognitive function surrounding stroke was significantly different from zero for women with physical functioning ≥80: 2.033 (0.481) (p<.0001), but not for women with physical functioning <80: 1.119 (0.875) (p=0.20), and they did not differ significantly from each other (p=0.36). Women with physical functioning <80 had a significant slope decrease in global cognitive function after stroke: −1.483 (0.243) (p<0.0001), whereas women with physical functioning ≥80 did not: 0.066 (0.124) (p=0.59), and the slope decrease in global cognitive function after stroke was significantly greater for the women with physical functioning <80 (p<0.0001). Whereas women with high physical functioning had a non-significantly higher mean decrement in global cognitive function surrounding stroke than women with low physical functioning (p=0.36), the opposite pattern was seen after stroke, where women with low physical functioning had significantly greater slope decreases in global cognitive function than women with high physical functioning (p<0.0001).
Discussion
The factors we examined played either a risk or a protective role in global cognitive function (hereafter global cognition) depending on the phase of ischemic stroke. When we examined trajectories of global cognition pre-, peri-, and post- ischemic stroke, for each level of each factor, as well as when we compared the two levels of each factor, none of the pre-stroke global cognition means or trajectories differed. At the time of stroke, however, higher BMI, the presence of HTN, low optimism, and high physical function were all significantly associated with mean decrements in global cognition, but not significantly different from the contrasting level. Factors that signified greater mean decrements in global cognition at the time of the stroke were protective post-stroke. Post- ischemic stroke, higher BMI, the presence of HTN, low optimism and high physical function were protective of further declines in global cognition. In other words, post- ischemic stroke, there was significantly less slope decline in global cognition for higher BMI, the presence of HTN, low optimism and high physical function, compared to the contrasting level.
When we examined BMI and global cognitive decline post-stroke, we found significantly greater cognitive declines for women with low BMI, whereas at the time of stroke low BMI was protective. In addition, women with low BMI post-stroke had greater cognitive declines than women with high BMI. Although the notion of an ‘obesity paradox’, where an inverse relationship between obesity and clinical outcomes such as neurological severity in stroke (Kim et al., 2014) and mortality (Vemmos et al., 2011; Bell et al., 2013), has been the topic of recent attention in patients with CVD, few if any studies have examined the relationship between BMI and global cognition surrounding ischemic stroke. In the Women’s Health Initiative (WHI), BMI had an inverse cross-sectional association with global cognition after adjusting for age, education, and various disease risk factors (Kerwin et al., 2010), and underweight pre-stroke BMI was associated with increased post-stroke mortality but was not associated with short-term post-hospitalization recovery (Bell 2013). In a representative sample of older women, no inverse longitudinal relationship was found between weight gain and cognitive performance favoring higher BMI (Driscoll et al., 2011), and the latter findings were in-line with those of the Health, Aging, and Body Composition (ABC) study, among others (Kanaya et al., 2009; Wolf et al., 2007). Our findings demonstrate a more complex picture based on the timing of interest, where higher BMI may be protective of cognitive decline after ischemic stroke, not surrounding it, and this is a worthy topic for further research.
Although psychological factors such as depressive symptomatology and optimism are not formally established risk factors for stroke, there is evidence to support the relationship between depression and stroke, and psychosocial stressors and stroke (O’Donnell et al., 2010). In the present study, decreases in global cognition surrounding ischemic stroke were significant and higher for women with depressive symptomatology, although not significantly higher than those women without depressive symptomatology. Women with depressive symptomatology, however, did not demonstrate a significant decrease in global cognition post-stroke, as might be expected. One possible explanation for this incongruent finding is that severe depression was an exclusion criterion in the parent study (WHI), and our sample had low rates of depressive symptomatology. While recent meta-analyses of depression and stroke incidence (using different endpoints) demonstrate a link between the two (Pan et al., 2011; Dong et al., 2012), several prospective studies have found mixed results, prompting recent studies to focus on age and sex differences in this relationship (Jackson et al., 2013; Kohler et al., 2013; Seifert et al., 2012). Seifert et al. examined the influence of depressive symptomatology on incidence of ischemic stroke in 3852 participants older than 55 years over 6 years, stratified by age group (<65 or > 65 years) and sex. Multivariate analyses for sex subgroups adjusted for age and established risk factors showed a significantly higher stroke risk in women (HR 1.62, 95% CI 1.02–2.57, p=0.043) with depressive symptoms, but not men, and an elevated risk of ischemic stroke in participants <65 years with depressive symptomatology at baseline (HR=2.84, 95% CI 1.11–7.29, p=0.03) overall. There is some support that the role of depressive symptomatology in cognitive decline after ischemic stroke deserves greater attention in larger samples, especially in women.
Similar to the pattern of results for depressive symptomatology, women with low optimism also had a significant mean decrease in global cognition at the time of ischemic stroke that was non-significantly greater than women with high optimism. In contrast, after ischemic stroke, women with high optimism had a markedly and significantly greater decrease in global cognition than women with low optimism. Although one population-based prospective analysis of 6044 older adults in the Health and Retirement Study who were free of stroke at baseline found that higher optimism (also measured by the LOT) was associated with a lower risk of self-reported stroke incidence of any type after 2 years of follow-up (Kim, Park, & Peterson, 2011), the relationship between optimism and global cognition surrounding ischemic stroke has not been reported. In fact, a recent review of the influence of psychological factors on health-related quality of life after stroke concluded that further research is needed to determine how psychological factors should be modified and the optimal timing for intervening after stroke (van Mierlo et al., 2013). Taken together, our findings point to a protective role of optimism on global cognition peri-ischemic stroke, but not post-ischemic stroke, and suggest that optimism could play an important role in cognitive decline after ischemic stroke.
We examined the relationship between baseline HTN and longitudinal global cognition after ischemic stroke, and found notable differences in the timing of stroke-related cognitive decline based on the presence or absence of this risk factor. As expected, women who had HTN at baseline had a somewhat greater mean decrement in global cognition at the time of the stroke than women without HTN. However, post-stroke, women with HTN at baseline had significantly less decline in global cognition than women without HTN at baseline. Other measures of arterial stiffness such as central systolic blood pressure (sBP), central pulse pressure (PP), and the aortic augmentation index (AIx) have also been associated with increased cardiovascular risk and risk of stroke (Hatanaka et al., 2011; Kim et al., 2014; Selwaness et al., 2013), and mortality following ischemic stroke (Tziomalos et al., 2014; Wohlfahrt et al., 2014), but they have not been associated with global cognition after stroke, and we did not find significant differences in global cognition at the time of stroke or post-stroke between high vs. low levels of sBP and narrow vs. normal PP in additional follow-up analyses. The HTN findings bear replication in light of the fact that unknown treatment factors related to blood pressure control may be influential in the peri- and post-stroke phases.
Higher physical function at baseline was also protective of global cognitive decline post-stroke, but did not show the same pattern at the time of stroke. Whereas women with high physical function had a non-significantly higher mean decrement in global cognition at the time of ischemic stroke than women with low physical functioning, the opposite pattern was seen post-ischemic stroke, where women with low physical functioning had markedly and significantly greater decreases in global cognition than women with high physical functioning. Although risk of stroke and post-stroke mortality have been shown to be increased in women with low physical activity and low physical function (Bell et al., 2013; Chomistek et al., 2013), this study adds to the stroke literature by additionally demonstrating that women with low physical function have significantly greater cognitive declines than their counterparts after ischemic stroke.
Strengths of this study are that it augments previous studies which lacked data on global cognition and baseline risk factors before stroke and includes extended longitudinal follow-up of global cognition after stroke. While some variables in the dataset that relate to our outcome of interest were lacking, such as rehabilitation after stroke, this is a common limitation of retrospective analyses of large datasets, and the broadly well-characterized WHIMS cohort offered the opportunity to examine many important risk and protective factors of interest in the literature that have not been examined systematically. It is possible that differential survival and follow-up may have influenced our findings; however the stroke survivors included in our analyses have reasonably similar risk factor profiles to WHIMS women excluded from our analyses due to no post-stroke follow-up. In addition, while the non-representativeness of this type of sample is likely to increase across follow-up due to mortality, percent drop-out after 2 years was similar across all of the factors we examined. Finally, because stroke severity at hospital discharge could be confounded with the first global cognitive function assessment after the incident, we conducted a series of follow-up analyses in our sample showing no differences between the levels of each predictor on a proxy for stroke severity, the Glasgow Outcomes Scale score (used to assess the prognosis for post-stroke functional recovery: Teasdale & Jennett, 1974; Albanese et al., 1994) (F’s ranging from .04–2.62; all p’s >.10), demonstrating that the distribution of severity scores in our ischemic stroke sample was remarkably similar across the levels of each predictor.
This study examined interactions between stroke risk and protective factors and pre-, peri-, and post-stroke phases, on global cognition in ischemic stroke. Whether factors are risk or protective of global cognition varies significantly by phase in relation to ischemic stroke. Women with high versus low levels of BMI and physical function showed less decline in global cognition after stroke but a greater mean decrease in global cognition at the time of stroke, whereas women with high versus low levels of optimism demonstrated the opposite pattern of greater declines in global cognition after stroke and less mean decrease in global cognition at the time of stroke. Because the timing of cognitive rehabilitative interventions in ischemic stroke is rarely, if ever, addressed in the literature, this study has important clinical implications. These include but are not limited to: improving the design of trials to limit deficits in cognitive function after stroke, identifying optimal modifiable risk and protective factors and their respective intervention and assessment schedules, and identifying individuals who may receive the greatest benefit from potential interventions. These results may guide the future development of interventions to improve the daily life of stroke survivors. In particular, outpatient hospital or community-based cognitive rehabilitative interventions aimed at maintaining cognitive (and presumably daily life function) beyond the usual acute time-course of medical and allied health interventions may extend independent functioning in persons with ischemic stroke.
Acknowledgments
Funding: WHIMS was funded by the National Heart, Lung and Blood Institute, Contract No. HHSN-268-2004-6-4221C through the initial follow-up period, WHIMS-ECHO is funded by the National Institute on Aging, Contract No. HHSN-271-2011-00004C, and the WHI program is funded by the National Heart, Lung and Blood Institute, U.S. Department of Health and Human Services.
Contributor Information
Leslie Vaughan, Email: alvaugha@wakehealth.edu.
Cheryl Bushnell, Email: cbushnel@wakehealth.edu.
Christina L. Bell, Email: bellcl@hawaii.edu.
Mark A. Espeland, Email: mespelan@wakehealth.edu.
References
- Albanese MA, Clarke WR, Adams HP, Woolson RF. Ensuring reliability of outcome measures in multicenter clinical trials of treatments for acute ischemic stroke. The program developed for the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Stroke. 1994;25:1746–51. doi: 10.1161/01.str.25.9.1746. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, … Wassertheil-Smoller S. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Barker-Collo S, Feigin VL, Parag V, Lawes CM, Senior H. Auckland Stroke Outcomes Study. Part 2: Cognition and functional outcomes 5 years post-stroke. Neurology. 2010:1608–1616. doi: 10.1212/WNL.0b013e3181fb44c8. [DOI] [PubMed] [Google Scholar]
- Ballard C, Stephens S, McLaren A, Wesnes K, Kenny R. Mild cognitive impairment and vascular cognitive impairment in stroke patients. International Psychogeriatriatrics. 2003;15:123–126. doi: 10.1017/S1041610203009074. [DOI] [PubMed] [Google Scholar]
- Barba R, Martinez-Espinosa S, Rodriquez-Garcia E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia: clinical features and risk factors. Stroke. 2000;31:1494–1501. doi: 10.1161/01.str.31.7.1494. [DOI] [PubMed] [Google Scholar]
- Bell CL, LaCroix A, Masaki K, Hade EM, Manini T, Mysiw WJ, … Wassertheil-Smoller S. Pre-stroke factors associated with post-stroke mortality and recovery in older women in the Women’s Health Initiative. Journal of the American Geriatric Society. 2013;61:1324–1330. doi: 10.1111/jgs.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depressive disorders. Medical Care. 1988;8:775–789. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- Bushnell C. Depression and the risk of stroke in women: an identification and treatment paradox. Stroke. 2011;42:2718–2719. doi: 10.1161/STROKEAHA.111.626895. [DOI] [PubMed] [Google Scholar]
- Chomistek AK, Manson JE, Stefanick ML, Lu B, Sands-Lincoln M, Going SB, … Eaton CB. Relationship of sedentary behavior and physical activity to incident cardiovascular disease: results from the Women’s Health Initiative. Journal of the American College of Cardiology. 2013;61:2346–2354. doi: 10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Ser T, Barba R, Morin MM, Domingo J, Cemillan C, Pondal M, Vivancos J. Evolution of cognitive impairment after stroke and risk factors for delayed progression. Stroke. 2005;36:2670–2675. doi: 10.1161/01.STR.0000189626.71033.35. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of dementia after ischemic stroke: Results of a longitudinal study. Stroke: A Journal of Cerebral Circulation. 2002;33:2254–2260. doi: 10.1161/01.str.0000028235.91778.95. [DOI] [PubMed] [Google Scholar]
- Dong JY, Zhang YH, Tong J, Qin LQ. Depression and risk of stroke: a meta-analysis of prospective studies. Stroke. 2012;43:32–37. doi: 10.1161/STROKEAHA.111.630871. [DOI] [PubMed] [Google Scholar]
- Donovan NJ, Kendall DL, Heaton SC, Kwon S, Velozo CA, Duncan PW. Conceptualizing functional cognition in stroke. Neurorehabilitation and Neural Repair. 2008;22:122–35. doi: 10.1177/1545968307306239. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Espeland MA, Wassertheil-Smoller S, Gaussoin SA, Ding J, Granek IA, … Resnick SM. Weight change and cognitive function: findings from the Women’s Health Initiative Study of Cognitive Aging. Obesity. 2011;19:1595–1600. doi: 10.1038/oby.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Glaesmer H, Rief W, Martin A, Mewes R, Brähler E, Zenger M, Hinz A. Psychometric properties and population-based norms of the Life Orientation Test Revised (LOT-R) British Journal of Health Psychology. 2012;17:432–45. doi: 10.1111/j.2044-8287.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Stewart WF. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Statistics in Medicine. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Economics. 1993;2:217–227. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- Jackson CA, Mishra GD. Depression and risk of stroke in midaged women: a prospective longitudinal study. Stroke. 2013;44:1555–1560. doi: 10.1161/STROKEAHA.113.001147. [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Lindquist K, Harris TB, Launer L, Rosano C, Satterfield S, Yaffe K. Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Archives of Neurology. 2009;66:329–335. doi: 10.1001/archneurol.2008.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin DR, Zhang Y, Kotchen JM, Espeland MA, Van Horn L, McTigue KM, … Hoffmann R. The cross-sectional relationship between body mass index, waist-hip ratio, and cognitive performance in postmenopausal women enrolled in the Women’s Health Initiative. Journal of the American Geriatric Society. 2010;58:1427–1432. doi: 10.1111/j.1532-5415.2010.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Park N, Peterson C. Dispositional optimism protects older adults from stroke: the Health and Retirement Study. Stroke. 2011;42:2855–2859. doi: 10.1161/STROKEAHA.111.613448. [DOI] [PubMed] [Google Scholar]
- Kim JY, Bushnell CD, Park JH, Han SM, Im JH, Han SW, Baik JS, Park JH. Central aortic pressure and pulsatility index in acute ischemic stroke. Journal of Neuroimaging. 2014 Jul 25; doi: 10.1111/jon.12151. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim CK, Jung S, Yoon BW, Lee SH. Obesity-stroke paradox and initial neurological severity. Journal of Neurological and Neurosurgical Psychiatry. 2014 Sep 10; doi: 10.1136/jnnp-2014-308664. pii: jnnp-2014-308664 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Köhler S, Verhey F, Weyerer S, Wiese B, Heser K, Wagner M, … Maier W. Depression, non-fatal stroke and all-cause mortality in old age: a prospective cohort study of primary care patients. Journal of Affective Disorders. 2013;150:63–69. doi: 10.1016/j.jad.2013.02.020. [DOI] [PubMed] [Google Scholar]
- O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, … Yusuf S. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306:1241–1249. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Lin Y, Geng JL, Li HW, Chen Y, Li YS. The prevalence and risk factors for cognitive impairment following ischemic stroke. Chinese Journal of Internal Medicine. 2008;47:981–984. [PubMed] [Google Scholar]
- Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JC, Berman K, … Zagami AS. Clinical Determinants of Dementia and Mild Cognitive Impairment following Ischaemic Stroke: The Sydney Stroke Study. Dementia and Geriatric Cognitive Disorders. 2006;21:275–283. doi: 10.1159/000091434. [DOI] [PubMed] [Google Scholar]
- Seifert CL, Poppert H, Sander D, Feurer R, Etgen T, Ander KH, … Bickel H. Depressive symptoms and the risk of ischemic stroke in the elderly--influence of age and sex. PLoS One. 2012;7:e50803. doi: 10.1371/journal.pone.0050803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwaness M, van den Bouwhuijsen QJ, Verwoert GC, Dehghan A, Mattace-Raso FU, Vernooij M, … Witteman JC. Blood pressure parameters and carotid intraplaque hemorrhage as measured by magnetic resonance imaging: The Rotterdam Study. Hypertension. 2013;61:76–81. doi: 10.1161/HYPERTENSIONAHA.112.198267. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, … Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, … Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Reboussin BA, Espeland MA, Rapp SR, McBee WL, Dailey M, … Jones BN. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled Clinical Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui H. The Modified Mini-Mental State (3MS) examination. Journal of Clinical Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Teoh V, Sims J, Milgrom J. Psychosocial predictors of quality of life in a sample of community-dwelling stroke survivors: a longitudinal study. Topics in Stroke Rehabilitation. 2009;16:157–166. doi: 10.1310/tsr1602-157. [DOI] [PubMed] [Google Scholar]
- Tham W, Auchus AP, Thong M, Goh ML, Chang HM, Wong MC, Chen CP. Progression of cognitive impairment after stroke: One year results from a longitudinal study of Singaporean stroke patients. Journal of the Neurological Sciences. 2002;203–204:49–52. doi: 10.1016/s0022-510x(02)00260-5. [DOI] [PubMed] [Google Scholar]
- Tuunainen A, Langer RD, Klauber MR, Kripke DF. Short version of the CES-D (Burnam screen) for depression in reference to the structured psychiatric interview. Psychiatry Research. 2001;103:261–70. doi: 10.1016/s0165-1781(01)00278-5. [DOI] [PubMed] [Google Scholar]
- Tziomalos K, Bouziana SD, Spanou M, Giampatzis V, Papadopoulou M, Kazantzidou P, … Hatzitolios AI. Increased augmentation index is paradoxically associated with lower in-hospital mortality in patients with acute ischemic stroke. Atherosclerosis. 2014;236:150–153. doi: 10.1016/j.atherosclerosis.2014.06.028. [DOI] [PubMed] [Google Scholar]
- van Mierlo ML, Schröder C, van Heugten CM, Post MW, de Kort PL, Visser-Meily JM. The influence of psychological factors on health-related quality of life after stroke: a systematic review. International Journal of Stroke. 2014;9:341–348. doi: 10.1111/ijs.12149. [DOI] [PubMed] [Google Scholar]
- Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, … Alevizaki M. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke. 2011;42:30–36. doi: 10.1161/STROKEAHA.110.593434. [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). Conceptual framework and item selection. Medical Care. 1992;30:473–83. [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, … Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–84. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. American Journal of Epidemiology. 1977;106:203–14. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Current Alzheimer’s Research. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- Wohlfahrt P, Krajcoviechova A, Jozifova M, Mayer O, Vanek J, Filipovsky, … Cifkova R. Large artery stiffness carotid flow pulsatility in stroke survivors. Journal of Hypertension. 2014;32:1097–103. doi: 10.1097/HJH.0000000000000137. [DOI] [PubMed] [Google Scholar]