Abstract
Purpose of review
The method for identification of alveolopleural fistulae (APF) by visual inspection of air bubbles in the chest drainage system has several limitations and suffers from poor accuracy. Here we discuss the use of a novel technique of pleural gas analysis in the identification and management of APF.
Recent findings
We found that pleural gas analysis has higher sensitivity and specificity than visual identification in identifying APF. Additionally, we demonstrated that intrapleural gas milieu impacts lung healing and reduction of intrapleural carbon dioxide can promote resolution of APF.
Summary
Pleural gas analysis is a novel technique to identify and manage APF. Integration of gas analysis in chest drainage systems would provide a more objective method for managing chest tubes and providing a favorable pleural gas environment for lung healing.
Keywords: alveolopleural fistula, pleural hypercarbia, lung healing
Introduction
Air leaks (AL) have a reported incidence of up to 60% following anatomic and nonanatomic lung resections. (1–3). In patients with emphysema, AL can affect over 90% of patients in the immediate post-operative period. (4) Air leaks are assumed to represent alveolopleural or bronchopleural fistulae and in many cases these terms are used interchangeably. All patients undergoing lung surgery have a tube placed in the chest cavity that is connected to a drainage system. The function of the chest tube is to drain the inflammatory pleural fluid and air escaping the cut surface of the lung. Since the inflammatory fluid usually ceases before lung parenchyma heals, AL remain the predominant reason for inability to remove chest tubes in patients undergoing lung surgery.
Prolonged air leak (PAL), defined by Society of Thoracic Surgeons as lasting more than five days after surgery (2), has a significant impact on clinical outcomes. (5–8) Depending on the series, the incidence of PAL can be up to 35%. (4) In fact, the National Emphysema Treatment Trial (NETT) reported the incidence of PAL in emphysema patients to be over 50%. (4) PAL is associated with increased infectious complications, with a reported 8.2–10.4% incidence of empyema, and is known to be the major determinant of morbidity and mortality in patients undergoing lung resection. (2, 6, 9, 10) Therefore, not surprisingly, PAL is associated with increased length of hospitalization and overall cost. (2, 11–14)
The traditional method of chest drainage using the three-bottle principle (Figure 1) can over-estimate the incidence of AL and PAL. It is based on elucidation of air bubbles in the water column that can arise from mechanisms other than air escaping the lung surface. There is a need for better systems to determine whether AL is due to an APF (True AL) or a mechanical error (False AL). We recently demonstrated that analyzing the gas content of AL would better differentiate True and False AL (15, 16). Additionally, we found that the pleural gas milieu impacts lung healing and can be modulated to promote resolution of APF.
Figure 1.
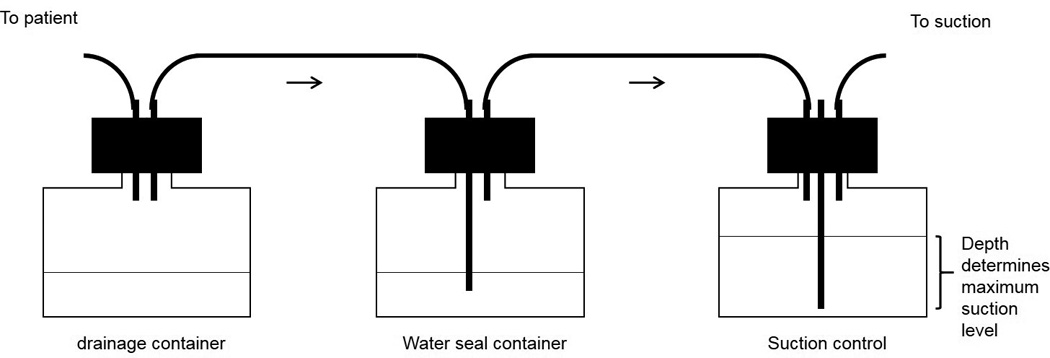
The “three-bottle system”. The chest tube is connected to the first bottle that serves to collect the fluid draining from the patient. The first bottle is connected to the second bottle that has a rigid tube immersed about 2 cm in a water column. Air escaping the patient can bubble through this water column but cannot re-enter when patient inspires. The third tube has a vent through which the air leaves the system and ability to be connected to suction. The water level in the third tube regulates the amount of suction.
Current chest drainage systems
The current chest drainage system dates back to 1875 with Dr. Bulau’s closed water-seal drainage and there have been some advancements in this technology since that time. (17)
Analogue Systems
The current analogue systems are based on the three-bottle concept (Figure 1). The first bottle serves to collect pleural fluid. This is connected to a middle bottle that provides a water seal and has a rigid tube immersed within 2 cm of a water column. The second bottle is connected to a final bottle that allows controlled application of suction. The amount of suction pressure can be adjusted by the depth of immersion of the rigid tube in the third bottle. With this system, air can escape from the patient by bubbling through the water seal column in the second bottle and then through the vent in the third bottle. The water seal column in the second bottle prevents air from re-entering the patient during inspiration, similar to the use of straw for drinking a beverage. The current systems combine the three bottles into one container.
The standard for detection of AL is by visual inspection (VI) of air bubbles within the water seal column of chest drainage container. (3) Bubbles in the water column are believed to represent the egress of air from an alveolopleural or bronchopleural fistula. (3) The bubbles are either spontaneously leaking through the water column or elicited following a maneuver that increases intrathoracic pressure such as coughing or valsalva. It is assumed that the entire system, including the chest tube and the drainage system, is a closed circuit in which external air cannot get introduced. Hence, observed air bubbles are speculated to be coming from the lung.
Digital Drainage Systems
The water seal in the chest drainage systems is an essential component. However, to avoid the problem of spillage of the water column, dry seal systems have been developed that contain a one-way valve where the water column would be. The obvious disadvantage of the dry seal systems is the inability to monitor for AL.
Digital systems have been developed using the concept of dry seal in which an air-flow meter analyzes the air exiting the chest tube (18). Air-flow data are presented on a digital display in numeric and graphic form in real time. The main benefit to these devices is improved inter-observer reliability in the detection and characterization of air leaks. (19–25) However, this has not helped timely removal of chest tubes. (26, 27) There also remains a lack of consensus in the definition of a clinically significant AL, with an allowable leak range from zero - 30 ml/min. (21, 22, 25)
Limitations of current drainage systems
The elicitation of air bubbles in analogue systems or detection of air through air-flow meter in digital systems is dependent on patient effort, tube position, and the presence of fluid or clots in the tube, among other factors. (28) There are a number of circumstances that can produce False AL. After resection there might be air in the pleural space that is evacuated over time which can create a False AL. (3) There can be introduction of air into the system from the tube exit site that can simulate AL. There is also an entity called airflow reversal, whereby deep inspiration can introduce the existing air in the tubing and the first chamber back into the chest cavity that on subsequent cough or forceful expiration produces bubbles. (29) Yet another false AL is termed momentum leak, whereby the momentum of the fluid column generates bubbles in patients who are able to produce a strong cough. (1) It is essential to distinguish True and False AL because the chest tube should not be removed if the AL is truly coming from the lung as that would lead to pneumothorax. Conversely, a False AL would incorrectly indicate keeping the chest tube in the patient. Unfortunately, both analogue and digital system cannot distinguish False AL from causes such as these.
Gas analysis for detection of alveolopleural fistulae
Hypothesis
In order to better distinguish True and False AL, we hypothesized that analyzing the carbon dioxide (CO2) and oxygen (O2) levels in the air leaking out of the chest cavity would have better accuracy. Under normal physiologic conditions, the partial pressure of alveolar carbon dioxide (CO2) is ~ 40 mmHg. This corresponds to 5.6% CO2 at sea level with atmospheric pressure of 760 mmHg. By comparison, ambient air is comprised of 0.04% CO2. In the presence of an APF, alveolar gas containing CO2 escapes into the pleural cavity. Therefore, elevated CO2 in gas collected from the pleural drainage system can indicate an APF. However, CO2 can remain in the pleural cavity even after resolution of an APF, for instance, if the remaining lung fails to completely fill the space left behind after lung resection. In such circumstances, an increase in pleural O2 concentration following nasally administered supplemental O2 could differentiate a persistent APF.
Clinical Validation
We first determined pleural levels of CO2 and O2 in patients with and without a known APF. (15) The APF-positive group consisted of patients who had undergone a lung decortication and had an APF observed in the operating room while the APF-negative group consisted of patients who had a chest tube placed for pleural effusion. We used a Datex analyzer (GE Healthcare, Inc., OK, USA) connected to the sampling port of the pleural drainage system (Atrium, Inc. NH, USA) to measure pleural gases. The mean pleural CO2 in the APF-negative group was 0.9% and in the APF-positive group 4.9%. Supplemental O2 of 5L/min produced an increase in the pleural O2 of 2% or more in the AFP-positive group. The AFP-negative group did not have a demonstrable change in the pleural O2 concentration despite up to 10 L/min of supplemental O2. We therefore set the parameters of an APF as a CO2 concentration >1% and an increase in O2 of 2% or more with 5 L/min of supplemental O2. A CO2 above 1% but an increase in O2 below 2% would suggest recently resolved APF.
We then prospectively compared VI with gas analysis (GA) in 50 patients undergoing surgical lobectomy. There was concordance with both techniques in 36 patients (72%): 19 patients (38%) had positive results for an APF, while 17(34%) had negative results. Notably, there were 12 patients (24%) who were suggested to have an APF based on VI but had a negative result on GA. These patients had their chest tubes removed without development of a pneumothorax, supporting the contention of high incidence of False AL on VI. Out of the 19 patients deemed to have no APF with VI, 2 had had a GA result indicative of an APF. Chest tube removal in both patients resulted in a large pneumothorax. We can conclude that VI was not sensitive enough to detect the APF in these 2 patients. Of the 29 patients without detectable APF by GA, only one went on to develop a pneumothorax after tube removal. VI did not reveal APF in this patient as well. This patient likely had a dysfunctional tube as the GA on replacement of the chest tube was positive for APF.
In all, the incidence of APF was 44% in our study, which is similar to what is reported in the literature (1, 2). As shown in Table 1, GA had better sensitivity (95.5% vs 86.4%), specificity (100% vs 57.1%), positive predictive value (100% vs 61.3%), and negative predictive value (96.6% vs 84.2%) than VI for identifying an APF. These results suggest that a significant proportion of patients, 24% in our study, may have false AL based on VI. This overestimation of APF by VI has significant implications in the post-operative management of lung resection. False AL result in an extended and unnecessary duration of chest tubes, prolonging patient pain and discomfort, increasing complications and length of stay, and producing higher cost of care. More accurate identification of APF with pleural GA has the potential to improve care.
Table 1.
Comparison of pleural gas analysis with the standard visual inspection technique for detection of alveolopleural fistulae.
| Visual inspection | Gas Analysis | |
|---|---|---|
| Sensitivity | 86.40% | 95.50% |
| Specificity | 57.10% | 100% |
| PPV | 61.30% | 100% |
| NPV | 84.20% | 96.60% |
Intrapleural gas milieu and lung healing
The etiology of persistent APF remains unclear. (4, 6, 10, 30–33) It is known that lung repair involves type II pneumocytes. (34–36) These alveolar epithelial cells (AEC) retain the ability to differentiate into and replenish type I pneumocytes. (34) It has been recently shown that hypercarbia impairs alveolar epithelial cell proliferation and lung healing. (37–39) Various mechanisms have been proposed including micro RNA mediated suppression of mitochondrial enzymes resulting in impaired oxygen consumption and ATP production in AEC. (37, 38, 40, 41) There is also evidence that hypercarbia impairs macrophage function which also impacts alveolar epithelium regeneration after injury. (37, 40) Similarly, there is evidence that hypoxia impairs lung healing. (42–45) Using the technique of pleural GA, we provided evidence that the intrapleural gas milieu can affect the rate of lung healing and resolution of APF (15, 16)
In a prospective study of 116 consecutive lung resections there were 55 (47%) anatomic lobectomies and 61 (53%) wedge or sublobar resections. On the morning of post-operative day 1, we performed pleural GA. Poisson regression analysis revealed that every one-unit increase in CO2 was associated with delay in lung healing by 9 hours (p<0.001). Logistic regression demonstrated that every one-unit increase in CO2 level was associated with a 10 times greater odds of PAL (p=0.003). We, therefore, reasoned that a decrease in the intrapleural CO2% would result in faster lung repair and AL resolution. On post-operative day 1, patients with an intrapleural CO2>6% were subjected to either the intervention or standard therapy groups. The intervention consisted of pleural suction up to -40 cm H2O and supplemental oxygen via nasal cannula up to 8L/min, titrated to achieve intrapleural CO2 <5%. The standard treatment was to place the chest tube to water seal and wean supplemental O2 to keep saturations >88%.
The interventions lead to a decrease in mean intrapleural CO2 from 6.6±0.7 to 3.1±0.5%, while increasing intrapleural O2 from 15.4±2.0 to 27.1±3.2%. There were no significant differences in baseline intrapleural CO2% or O2% or the size of AL at baseline between the two groups. We found that the intervention group had significantly shorter AL duration: 3.4±1.1 vs. 6.0±1.2 days. These results suggest that the intrapleural gas milieu can impact lung healing and GA might offer interventions to modulate pleural gas and promote lung healing.
Conclusions
APF are a common problem after lung surgery and their management remains an intensely debated topic. In this review, we have summarized a novel technique for the identification of APF that is more accurate than the current standard. We also described the application of this technique to provide a simple therapeutic intervention that can promote lung healing. Hence, we believe that pleural gas analysis has the potential to produce a paradigm shift in the management of APF, significantly impacting the morbidity of lung surgery and reducing its attendant costs.
Key Points.
Pleural gas analysis is more accurate than the current standard of visual inspection in identifying alveolopleural fistulae.
Pleural hypercarbia impairs lung healing and prolongs APF resolution.
Pleural gas analysis allows for more effective management of chest tubes and can promote APF resolution.
Real-time pleural gas analysis can expedite chest tube removal, decrease post-operative morbidity, and reduce length of stay and overall costs after lung surgery.
Acknowledgments
We would like to thank Ms. Elena Susan for administrative assistance in manuscript preparation and submission.
Financial support and sponsorship
A.B. is supported by NIH Grant #K08 HL125940 and matching funds from Thoracic Surgery Foundation.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Burt BM, Shrager JB. The Prevention and Management of Air Leaks Following Pulmonary Resection. Thorac Surg Clin. 2015;25(4):411–419. doi: 10.1016/j.thorsurg.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Singhal S, Ferraris VA, Bridges CR, Clough ER, Mitchell JD, Fernando HC, et al. Management of alveolar air leaks after pulmonary resection. Ann Thorac Surg. 2010;89(4):1327–1335. doi: 10.1016/j.athoracsur.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Mueller MR, Marzluf BA. The anticipation and management of air leaks and residual spaces post lung resection. J Thorac Dis. 2014;6(3):271–284. doi: 10.3978/j.issn.2072-1439.2013.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeCamp MM, Blackstone EH, Naunheim KS, Krasna MJ, Wood DE, Meli YM, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg. 2006;82(1):197–206. doi: 10.1016/j.athoracsur.2006.02.050. discussion -7. [DOI] [PubMed] [Google Scholar]
- 5.Abolhoda A, Liu D, Brooks A, Burt M. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest. 1998;113(6):1507–1510. doi: 10.1378/chest.113.6.1507. [DOI] [PubMed] [Google Scholar]
- 6.Okereke I, Murthy SC, Alster JM, Blackstone EH, Rice TW. Characterization and importance of air leak after lobectomy. Ann Thorac Surg. 2005;79(4):1167–1173. doi: 10.1016/j.athoracsur.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 7.Stephan F, Boucheseiche S, Hollande J, Flahault A, Cheffi A, Bazelly B, et al. Pulmonary complications following lung resection: a comprehensive analysis of incidence and possible risk factors. Chest. 2000;118(5):1263–1270. doi: 10.1378/chest.118.5.1263. [DOI] [PubMed] [Google Scholar]
- 8.Elsayed H, McShane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: is it a patient or surgeon related problem? Ann R Coll Surg Engl. 2012;94(6):422–427. doi: 10.1308/003588412X13171221592258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunelli A, Xiume F, Al Refai M, Salati M, Marasco R, Sabbatini A. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest. 2006;130(4):1150–1156. doi: 10.1378/chest.130.4.1150. [DOI] [PubMed] [Google Scholar]
- 10.Cerfolio RJ, Tummala RP, Holman WL, Zorn GL, Kirklin JK, McGiffin DC, et al. A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg. 1998;66(5):1726–1731. doi: 10.1016/s0003-4975(98)00958-8. [DOI] [PubMed] [Google Scholar]
- 11.Slade M. Management of pneumothorax and prolonged air leak. Semin Respir Crit Care Med. 2014;35(6):706–714. doi: 10.1055/s-0034-1395502. [DOI] [PubMed] [Google Scholar]
- 12.Varela G, Jimenez MF, Novoa N, Aranda JL. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg. 2005;27(2):329–333. doi: 10.1016/j.ejcts.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Irshad K, Feldman LS, Chu VF, Dorval JF, Baslaim G, Morin JE. Causes of increased length of hospitalization on a general thoracic surgery service: a prospective observational study. Canadian journal of surgery Journal canadien de chirurgie. 2002;45(4):264–268. [PMC free article] [PubMed] [Google Scholar]
- 14.Rivera C, Bernard A, Falcoz PE, Thomas P, Schmidt A, Benard S, et al. Characterization and prediction of prolonged air leak after pulmonary resection: a nationwide study setting up the index of prolonged air leak. Ann Thorac Surg. 2011;92(3):1062–1068. doi: 10.1016/j.athoracsur.2011.04.033. discussion 8. [DOI] [PubMed] [Google Scholar]
- 15. Bharat A, Graf N, Cassidy E, Smith S, Gillespie C, Meyerson S, et al. Pleural Gas Analysis for Detection of Alveolopleural Fistulae. Ann Thorac Surg. 2015;99(6):2179–2182. doi: 10.1016/j.athoracsur.2014.12.074. This is the first reported use of pleural gas analysis for the identification of alveolopleural fistulae
- 16. Bharat A, Graf N, Mullen A, Kanter J, Andrei AC, Sporn PH, et al. Pleural Hypercarbia After Lung Surgery Is Associated With Persistent Alveolopleural Fistulae. Chest. 2016;149(1):220–227. doi: 10.1378/chest.15-1591. This paper applies pleural gas analysis for the characterization of pleural gas milieu in the pathophysiology of alveolopleural fistulae. This paper also describes a method to improve resolution of APF.
- 17.Meyer JA. Gotthard Bulau and closed water-seal drainage for empyema, 1875–1891. Ann Thorac Surg. 1989;48(4):597–599. doi: 10.1016/s0003-4975(10)66876-2. [DOI] [PubMed] [Google Scholar]
- 18.Zisis C, Tsirgogianni K, Lazaridis G, Lampaki S, Baka S, Mpoukovinas I, et al. Chest drainage systems in use. Ann Transl Med. 2015;3(3):43. doi: 10.3978/j.issn.2305-5839.2015.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuire AL, Petrcich W, Maziak DE, Shamji FM, Sundaresan SR, Seely AJ, et al. Digital versus analogue pleural drainage phase 1: prospective evaluation of interobserver reliability in the assessment of pulmonary air leaks. Interact Cardiovasc Thorac Surg. 2015;21(4):403–407. doi: 10.1093/icvts/ivv128. [DOI] [PubMed] [Google Scholar]
- 20.Varela G, Jimenez MF, Novoa NM, Aranda JL. Postoperative chest tube management: measuring air leak using an electronic device decreases variability in the clinical practice. Eur J Cardiothorac Surg. 2009;35(1):28–31. doi: 10.1016/j.ejcts.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Brunelli A, Salati M, Refai M, Di Nunzio L, Xiume F, Sabbatini A. Evaluation of a new chest tube removal protocol using digital air leak monitoring after lobectomy: a prospective randomised trial. Eur J Cardiothorac Surg. 2010;37(1):56–60. doi: 10.1016/j.ejcts.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Pompili C, Detterbeck F, Papagiannopoulos K, Sihoe A, Vachlas K, Maxfield MW, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg. 2014;98(2):490–496. doi: 10.1016/j.athoracsur.2014.03.043. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 23.Cerfolio RJ, Bryant AS. The benefits of continuous and digital air leak assessment after elective pulmonary resection: a prospective study. Ann Thorac Surg. 2008;86(2):396–401. doi: 10.1016/j.athoracsur.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Cerfolio RJ, Varela G, Brunelli A. Digital and smart chest drainage systems to monitor air leaks: the birth of a new era? Thorac Surg Clin. 2010;20(3):413–420. doi: 10.1016/j.thorsurg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Filosso PL, Nigra VA, Lanza G, Costardi L, Bora G, Solidoro P, et al. Digital versus traditional air leak evaluation after elective pulmonary resection: a prospective and comparative mono-institutional study. J Thorac Dis. 2015;7(10):1719–1724. doi: 10.3978/j.issn.2072-1439.2015.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert S, McGuire AL, Maghera S, Sundaresan SR, Seely AJ, Maziak DE, et al. Randomized trial of digital versus analog pleural drainage in patients with or without a pulmonary air leak after lung resection. J Thorac Cardiovasc Surg. 2015;150(5):1243–1249. doi: 10.1016/j.jtcvs.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 27.Lijkendijk M, Licht PB, Neckelmann K. Electronic versus traditional chest tube drainage following lobectomy: a randomized trialdagger. Eur J Cardiothorac Surg. 2015;48(6):893–898. doi: 10.1093/ejcts/ezu535. [DOI] [PubMed] [Google Scholar]
- 28.Varela G, Jimenez MF, Novoa N. Portable chest drainage systems and outpatient chest tube management. Thorac Surg Clin. 2010;20(3):421–426. doi: 10.1016/j.thorsurg.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Stouby A, Neckelmann K, Licht PB. Reverse airflow in certain chest drains may be misinterpreted as prolonged air leakage. World J Surg. 2011;35(3):596–599. doi: 10.1007/s00268-010-0943-0. [DOI] [PubMed] [Google Scholar]
- 30.Kanter JA, Sun H, Chiu S, DeCamp MM, Sporn PH, Sznajder JI, et al. Decreased CXCL12 is associated with impaired alveolar epithelial cell migration and poor lung healing after lung resection. Surgery. 2015;158(4):1073–1080. doi: 10.1016/j.surg.2015.04.051. discussion 80-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciccone AM, Meyers BF, Guthrie TJ, Davis GE, Yusen RD, Lefrak SS, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg. 2003;125(3):513–525. doi: 10.1067/mtc.2003.147. [DOI] [PubMed] [Google Scholar]
- 32.Keller CA. Lasers, staples, bovine pericardium, talc, glue and…suction cylinders? Tools of the trade to avoid air leaks in lung volume reduction surgery. Chest. 2004;125(2):361–363. doi: 10.1378/chest.125.2.361. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Caro A, Calvo MJ, Lanzas JT, Chau R, Cascales P, Parrilla P. The approach of fused fissures with fissureless technique decreases the incidence of persistent air leak after lobectomy. Eur J Cardiothorac Surg. 2007;31(2):203–208. doi: 10.1016/j.ejcts.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, et al. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003;92(4):453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 36.Berthiaume Y, Lesur O, Dagenais A. Treatment of adult respiratory distress syndrome: plea for rescue therapy of the alveolar epithelium. Thorax. 1999;54(2):150–160. doi: 10.1136/thx.54.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vadasz I, Hubmayr RD, Nin N, Sporn PH, Sznajder JI. Hypercapnia: a nonpermissive environment for the lung. Am J Respir Cell Mol Biol. 2012;46(4):417–421. doi: 10.1165/rcmb.2011-0395PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vohwinkel CU, Lecuona E, Sun H, Sommer N, Vadasz I, Chandel NS, et al. Elevated CO(2) levels cause mitochondrial dysfunction and impair cell proliferation. J Biol Chem. 2011;286(43):37067–37076. doi: 10.1074/jbc.M111.290056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu SKJ, Sun H, Bharat A, Sporn PHS, Bharat A. Effects of Hypercapnia in Lung TIssue Repair and Transplant. Curr Transpl Rep. 2015;2(1):98–103. [Google Scholar]
- 40.Wang N, Gates KL, Trejo H, Favoreto S, Jr, Schleimer RP, Sznajder JI, et al. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 2010;24(7):2178–2190. doi: 10.1096/fj.09-136895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helenius IT, Krupinski T, Turnbull DW, Gruenbaum Y, Silverman N, Johnson EA, et al. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18710–18715. doi: 10.1073/pnas.0905925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, et al. The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum. 2005;48(7):1460–1470. doi: 10.1007/s10350-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol. 2003;284(6):L964–L971. doi: 10.1152/ajplung.00421.2002. [DOI] [PubMed] [Google Scholar]
- 44.Sano H, Ichioka S, Sekiya N. Influence of oxygen on wound healing dynamics: assessment in a novel wound mouse model under a variable oxygen environment. PLoS One. 2012;7(11):e50212. doi: 10.1371/journal.pone.0050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17(1):1–18. doi: 10.1111/j.1524-475X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


