Abstract
The amygdala controls emotional and social behavior and regulates instinctive reflexes such as defense and reproduction by way of descending projections to the hypothalamus and brainstem. The descending amygdalar projections are suggested to show a cortico-striato-pallidal organization similar to that of the basal ganglia (Swanson [2000] Brain Res 886:113–164). To test this model we investigated the embryological origin and molecular properties of the mouse centromedial and extended amygdalar subdivisions, which constitute major sources of descending projections. We analyzed the distribution of key regulatory genes that show restricted expression patterns within the subpallium (Dlx5, Nkx2.1, Lhx6, Lhx7/8, Lhx9, Shh, and Gbx1), as well as genes considered markers for specific subpallial neuronal subpopulations. Our results indicate that most of the centromedial and extended amygdala is formed by cells derived from multiple subpallial subdivisions. Contrary to a previous suggestion, only the central—but not the medial—amygdala derives from the lateral ganglionic eminence and has striatal-like features. The medial amygdala and a large part of the extended amygdala (including the bed nucleus of the stria terminalis) consist of subdivisions or cell groups that derive from subpallial, pallial (ventral pallium), or extratelencephalic progenitor domains. The subpallial part includes derivatives from the medial ganglionic eminence, the anterior peduncular area, and possibly a novel subdivision, called here commissural preoptic area, located at the base of the septum and related to the anterior commissure. Our study provides a molecular and morphological foundation for understanding the complex embryonic origins and adult organization of the centromedial and extended amygdala.
Indexing terms: subpallial amygdala, ventral pallial amygdala, bed nucleus of the stria terminalis, preoptic region, septum, anterior entopeduncular area, transcription factors
The amygdala is a complex telencephalic structure composed of multiple nuclei that regulate emotion, social behavior, and hypothalamic function (Aggleton, 2000; Davis, 2000; Gallagher, 2000; LeDoux, 1992, 2000; Zald, 2003). It is a key component of two distinct (but interrelated) networks: 1) a circuit comprised primarily of subcortical structures, which regulates autonomic and neuroendocrine systems by way of descending projections to the hypothalamus and brainstem; this circuit participates in unconscious, reflex-like (instinctive) behavior such as defense, ingestion, and reproduction; 2) a circuit involving connections with the cerebral cortex, which participates in conscious recognition of emotion (such as facial recognition of emotion) and in decision-making based on the context and previous experience and memory (Swanson, 2000; Stefanacci and Amaral, 2002; de Gelder, 2005).
It has been suggested that the amygdalar circuitry involved in autonomic and neuroendocrine behavior is organized similarly to striato-pallidal pathways that control somatomotor behavior (Turner and Zimmer, 1984; Alheid and Heimer, 1988; Alheid et al., 1995; Swanson, 2000; Alheid, 2003). In particular, Swanson and colleagues argue that the amygdala should be considered an integral part of a cortico-striato-pallidal pathway that projects to effector centers in the hypothalamus and brainstem (Swanson and Petrovich, 1998; Swanson, 2000). To understand how the amygdala integrates within such functional pathways it is essential to elucidate its organization, including how its divisions are related to the basic organization of the telencephalon.
Developmental and neurochemical studies have demonstrated so far that the amygdala consists of pallial and subpallial components, which are essentially comparable to either the claustrum and cerebral cortex (pallium) or the basal ganglia (subpallium), respectively (Holmgren, 1925; Turner and Zimmer, 1984; Alheid and Heimer, 1988; Swanson and Petrovich, 1998; Puelles et al., 2000). Data on the expression of transcription factors have proven to be very useful for elucidating the fundamental organization of the developing telencephalon and, in particular, the amygdala (Puelles et al., 2000; Stenman et al., 2003; Medina et al., 2004; Remedios et al., 2004; Tole et al., 2005). These data indicated that the centromedial and extended amygdalar nuclei (including the bed nucleus of the stria terminalis or BST), which constitute the major output centers projecting to the hypothalamus, derive—at least partially—from striatal and pallidal divisions of the subpallium (Puelles et al., 2000; Stenman et al., 2003; Medina et al., 2004; Tole et al., 2005), corroborating previous insights by Holmgren (1925) and Swanson and Petrovich (1998). However, the subpallium includes at least three major histogenetic subdivisions (striatal, pallidal, and anterior entopeduncular subdivisions), in addition to the anterior, subpallial part of the preoptic area (Bulfone et al., 1993; Puelles et al., 2000, 2004; Marín and Rubenstein, 2002), but there persist uncertainties over what specific parts of the amygdala derive from each subdivision. For example, while neurochemical evidence points to a striatal nature of both central and medial amygdalar nuclei (Swanson and Petrovich, 1988), expression of transcription factors—such as Pax6 and Dlx5—suggests that possibly only the central amygdalar nucleus is striatal-like (Puelles et al., 2000; Medina et al., 2004; Tole et al., 2005). The exact origin and nature of the medial amygdalar nuclear complex is unclear, as it shows some expression of both the subpallial transcription factor Nkx2.1 (a pallido-entopeduncular marker) and the pallial transcription factor Tbr1 (Puelles et al., 2000). Moreover, it has been suggested that a posterior part of the BST may have an extratelencephalic origin (Puelles et al., 2000, 2004). Furthermore, it remains unknown whether any part of the amygdala proper or the extended amygdala (including the BST) originates in the anterior entopeduncular area (AEP).
To address uncertainties about the origin and molecular nature of the centromedial and extended subdivisions of the amygdala, we used in situ hybridization and immunohistochemistry to analyze the expression of genes encoding transcription factors and signaling proteins. In particular, we examined the expression of genes that distinguish striatal (Dlx5) versus pallido-entopeduncular subdivisions (Nkx2.1, Lhx6, Lhx7/8), and genes that distinguish the entopeduncular-preoptic region (Gbx1, Sonic hedgehog). We supplemented this analysis by examining the expression of neuropeptides (somatostatin, neuropeptide Y), choline acetyltransferase, or calbindin. Moreover, we examined the expression of the gene Lhx9, a marker of the ventral pallium (Tole et al., 2005), to help distinguish subpallial versus pallial parts of the amygdala. Our analysis enabled us to suggest that the centromedial and extended amygdala (including the BST) include distinct cell groups that originate from striatal, pallidal, and AEP subpallial subdivisions, as well as from a novel median (septal) subpallial subdivision that we termed the commissural preoptic area (in addition to some extratelencephalic cells). In addition, the medial amygdala appears to contain cells of extratelencephalic origin, and our data suggest that a specific part of it derives from the ventral pallium.
MATERIALS AND METHODS
Mouse embryos of 12.5 days postcoitum (E12.5) through birth (P0), and postnatal mice through day 7 (P7) were used in the present study. All animals were treated according to the regulations and laws of the European Union (86/609/EEC) and the Spanish Government (Royal Decree 223/1998; more recently Royal Decree 1021/2005) for care and handling of animals in research. Pregnant female mice were deeply anesthetized with ethyl ether and the embryos removed by cesarean section. Embryos from E12.5 through E15.5 were fixed by immersion in phosphate-buffered 4% paraformaldehyde (pH 7.4). Older animals (E16.5–E18.5 and P0–P7) were first anesthetized by cold or with ethyl ether, then perfused transcardially with 0.6% NaCl saline solution, followed by phosphate-buffered 4% paraformaldehyde (pH 7.4). The brains were dissected, postfixed overnight at 4°C, and embedded in 4% agarose for sectioning. The brains were sectioned at 125–150 µm-thick (for hybridization) or 70 µm-thick (for immunohistochemistry) using a vibratome in either frontal, horizontal, or sagittal planes.
Brain sections were processed for in situ hybridization following a standard procedure using digoxigenin-labeled riboprobes (Pérez-Villegas et al., 1999; Medina et al., 2004). The genes analyzed were Dlx5 (Eisenstat et al., 1999), Nkx2.1 (Sussel et al., 1999), Lhx6 (Grigoriu et al., 1998), Lhx7/8 (Grigoriu et al., 1998), Sonic hedgehog (Shimamura and Rubenstein, 1997), Gbx1 (Rhinn et al., 2004), and Lhx9 (Retaux et al., 1999). For in situ hybridization we synthesized antisense digoxigenin-labeled riboprobes for the genes mentioned above. Following washes with phosphate-buffered (PB) saline (PBS; pH 7.4, 1×) containing 0.1% Tween-20 (PBT 1×), sections were treated with proteinase K (10 µg/mL; Roche Diagnostics, Mannheim, Germany) in PBT for 5 minutes. After that, sections were abundantly washed and postfixed with PB 4% paraformaldehyde containing 0.1% glutaraldehyde for 20 minutes. The basic solution for hybridization contained 25 mL (50%) of deionized formamide, 3.25 mL of 20 standard saline citrate (pH 5), 0.5 mL of 0.5M ethylene-diaminetetraacetic acid (pH 8.0; Sigma-Aldrich, Steinheim, Germany), 250 µL of yeast tRNA (Sigma-Aldrich), 1 mL of 10% Tween-20, 100 µL of heparin (50 mg/mL; Sigma-Aldrich), completed to 50 mL with water (free of RNAase and DNAase; Sigma-Aldrich). Sections were prehybridized in this solution (diluted 1:1 in PBT 1×) at 58°C for 90 minutes. Thereafter, sections were hybridized overnight at 58°C in the hybridization solution containing 1 µL/mL of riboprobe. After hybridization, all sections were washed, incubated in an alkaline phosphatase-coupled anti-digoxigenin antiserum (diluted 1:3,500; Roche Diagnostics), and stained with BM Purple (Roche Diagnostics).
To better understand the localization of the genes and their relation to specific cell groups, some series of parallel sections and some of the hybridized sections were immunostained for calbindin (CALB), choline acetyltransferase (CHAT), neuropeptide Y (NPY), somatostatin (SOM), or the transcription factor NKX2.1 (TTF-1; protein) following a standard avidin-biotin procedure described previously (Legaz et al., 2005a). Series of parallel sections were also stained for thionin.
For immunostaining we used the following primary antisera. The anti-CALB was raised in rabbit against recombinant rat calbindin D-28k (Swant, Bellinzoa, Switzerland; Cat. No. CB-38a; Lot No. 9.03 and 18F; used at a 1:2,000 dilution). Staining with this antiserum is colocalized with the mRNA distribution of the same protein using in situ hybridization histochemistry (Sequier et al., 1990). The anti-CHAT was generated in goat against the recombinant human placental enzyme (whole enzyme) (Chemicon International, Germany; Cat. No. AB144-P; Lot No. 220900336; used at a 1:100 dilution). The specificity of this antiserum has been checked in rat by Western blot (Brunelli et al., 2005), and the staining with it is colocalized with the mRNA distribution of the same enzyme using in situ hybridization histochemistry (Oh et al., 1992). The anti-NPY was raised in rabbit against synthetic porcine neuropeptide Y (whole molecule) conjugated to bovine serum albumin (BSA) (DiaSorin (Stillwater, MN; Cat. No. 22940; Lot No. 208001; used at a 1:2,000 dilution). Staining with this antiserum was abolished when preincubated with the immunizing peptide (manufacturer’s technical information). Moreover, staining with it is colocalized with the mRNA distribution of the same peptide using in situ hybridization (Gehlert et al., 1987). The anti-SOM was generated in rabbit against the synthetic human peptide (whole molecule) conjugated to BSA (Chemicon International; Cat. No. AB5494; Lot No. 23110299 and 24041363; used at a 1:1,000 dilution). Staining with this antiserum is colocalized with the mRNA distribution of the same peptide using in situ hybridization histochemistry (Fizpatrick-McElligott et al., 1988, 1991). Finally, the anti-NKX2.1 (anti-thyroid transcription factor 1 or TTF-1) was raised in rabbit against a synthetic peptide derived from rat TTF-1 (110–122 amino acids at the amino terminus) (Biopat Immunotechnologies, Italy; Cat. No. PA 0100; used at a 1:1,000 dilution). Staining with this antiserum is colocalized with the mRNA distribution of the same protein using in situ hybridization histochemistry (Lazzaro et al., 1991; Marín et al., 2000, 2002; present results).
The primary antisera were diluted in PBS containing 0.3% Triton X-100 and the tissue was incubated for 2 days at 4°C under constant and gentle agitation. Following this incubation and standard washes in PBS-Triton the sections were incubated in a secondary antiserum for 1 hour at room temperature. The secondary antisera used were either biotinylated goat antirabbit IgG (for CALB, NPY, SOM, and TTF-1) or biotinylated rabbit antigoat IgG (for CHAT). Both secondary antisera were purchased from Vector (Burlingame, CA) and were diluted at 1:200. After washing the sections were incubated in the avidin-biotin complex (ABC kit; Vector; 0,003% dilution) for 1 hour at room temperature. The immunolabeling was revealed by 0.05% diaminobenzidine (DAB; Sigma-Aldrich) in 0.05 M Tris (pH 7.6) containing 0.03% H2O2. To check the specificity of our secondary antisera, some sections were processed omitting the primary antiserum.
For identification of telencephalic cell masses during development, we used known atlases of developing mouse (Jacobowitz and Abbot, 1997) and rat (Paxinos et al., 1994; Foster, 1998) brain, as well as our own publications on the subject. For BST and amygdalar subdivisions we followed the brain atlas of Paxinos et al. (1999) and Paxinos and Franklin (2004), which followed the scheme of Alheid et al. (1995).
Finally, digital photographs were taken on a Leica microscope (DMR HC) equipped with a Zeiss Axiovision digital camera. Digital images were adjusted for brightness/contrast using Adobe Photoshop and figures were mounted and labeled using FreeHand 10.
RESULTS
The combined expression of Dlx5, Nkx2.1, Lhx6, Lhx7/8, Gbx1, Shh, SOM, NPY, CALB, and CHAT has enabled us to distinguish striatal, pallidal, and septo-penduncular subpallial progenitor subdomains and their derivatives, which contribute cells to the amygdala proper, the extended amygdala (including the BST), and the septum. These results have also provided evidence for a novel commissural preoptic subdivision, located at the base of the septum.
The septo-peduncular subdivision corresponds to a domain that extends rostrocaudally from the pallidal septum to the AEP; the AEP is located across the telencephalic stalk and is related to (contains) the cholinergic corticopetal cells groups of the basal telencephalon (see below) and to the internal capsule/cerebral peduncle when entering/exiting the telencephalon (Bulfone et al., 1993; Marin and Rubenstein, 2002; Puelles et al., 2004). Since the AEP is associated with the peduncular region but is not restricted to cells inside the peduncle (as the term “entopeduncular” mistakenly suggests), here we prefer to refer to it as the “anterior peduncular area.”
Below we describe our results on genes expressed in the subpallium in two developmental periods: 1) early development (E12.5–E14.5), when all subpallial progenitor domains are distinguishable and some of their derivatives are already migrating into the mantle (Figs. 1–5); 2) intermediate-late development (E16.5–P7), when most of the derivatives of each subpallial subdivision are in their final (or nearly final) positions in the mantle (Figs. 6–13). Finally, we also provide data on Lhx9 expression, which suggests that the ventral pallium contributes some cells to the medial amygdala (Fig. 14).
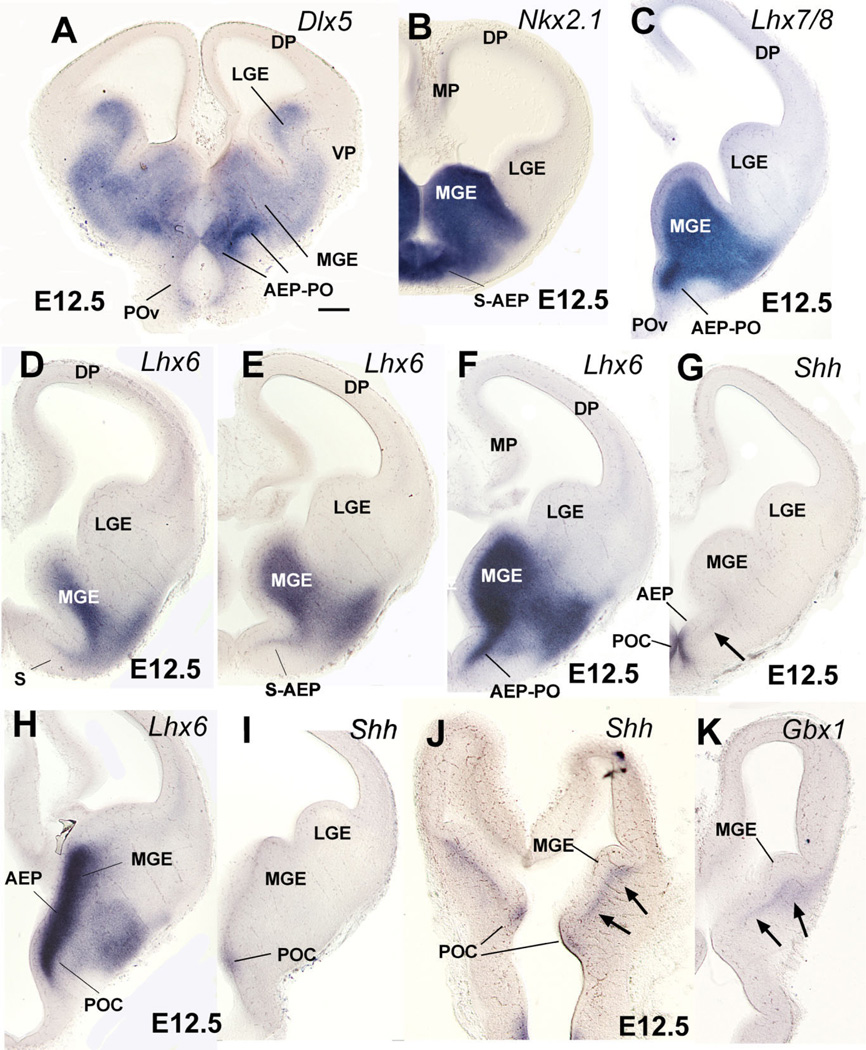
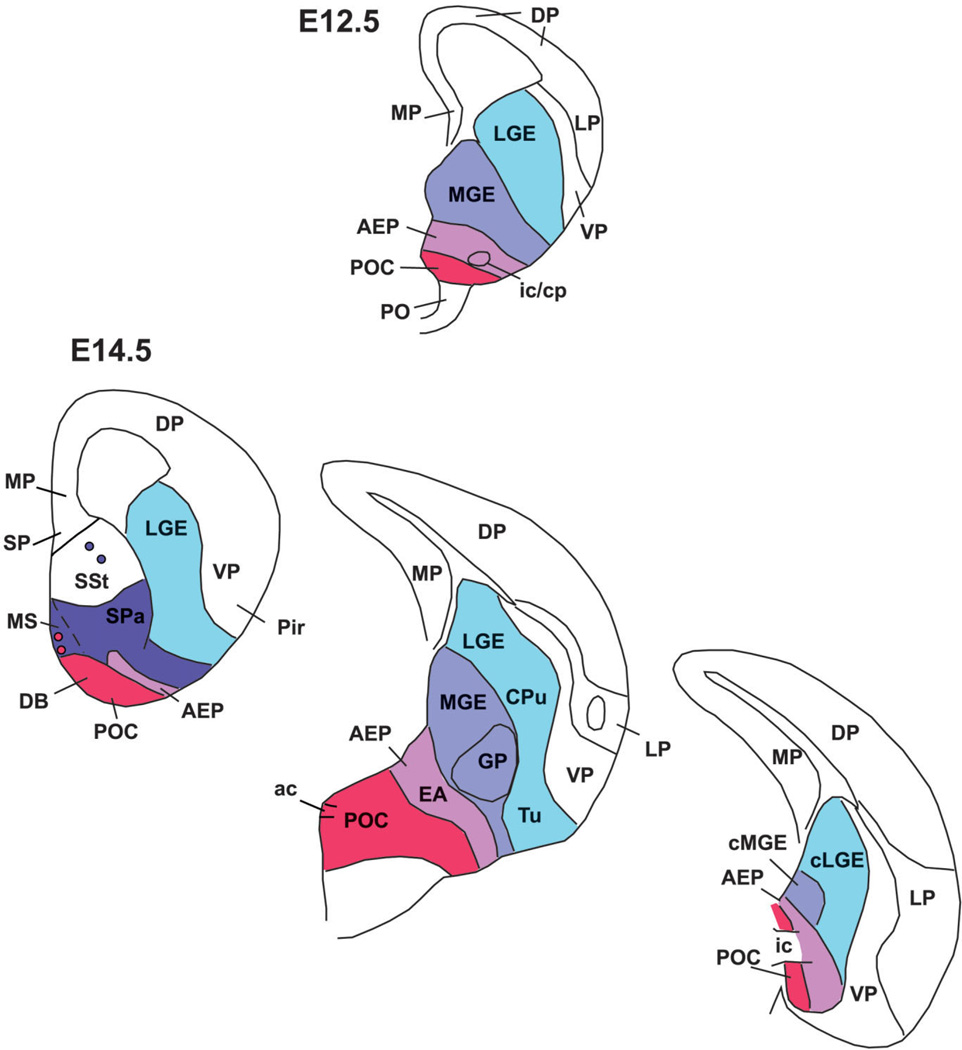
Fig. 1.
Frontal (A–I) or horizontal (J,K) sections through the embryonic telencephalon of different mouse embryos (E12.5) hybridized for Dlx5, Nkx2.1, Lhx6, Lhx7/8, Shh, or Gbx1. Sections shown in A–C,F,G are at similar frontal levels to facilitate comparison of expression patterns. Similarly, sections shown in H,I, and sections of J,K are from similar either frontal (H,I, at a more caudal level) or horizontal levels (J,K). The expression patterns are restricted to the entire subpallium (Dlx5) or to specific subpallial subdivisions or cell groups. For example, expression of Nkx2.1, Lhx6, and Lhx7/8 is restricted to the medial ganglionic eminence (MGE), plus the septo-preoptic ridge. The latter includes two subdivisions based on differential expression of Shh: the AEP (anterior peduncular area), which lacks ventricular expression of Shh, and a novel subdivision called here “commissural preoptic area” or POC, which shows ventricular expression of Shh. Note the Shh-expressing cell corridor that extends tangentially from POC into other subpallial subdivisions (arrows in G,J). The Shh-expressing cell corridor resembles a similar one expressing Gbx1 (arrows in K). See text for more details. For abbreviations, see list. Scale bar = 200 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
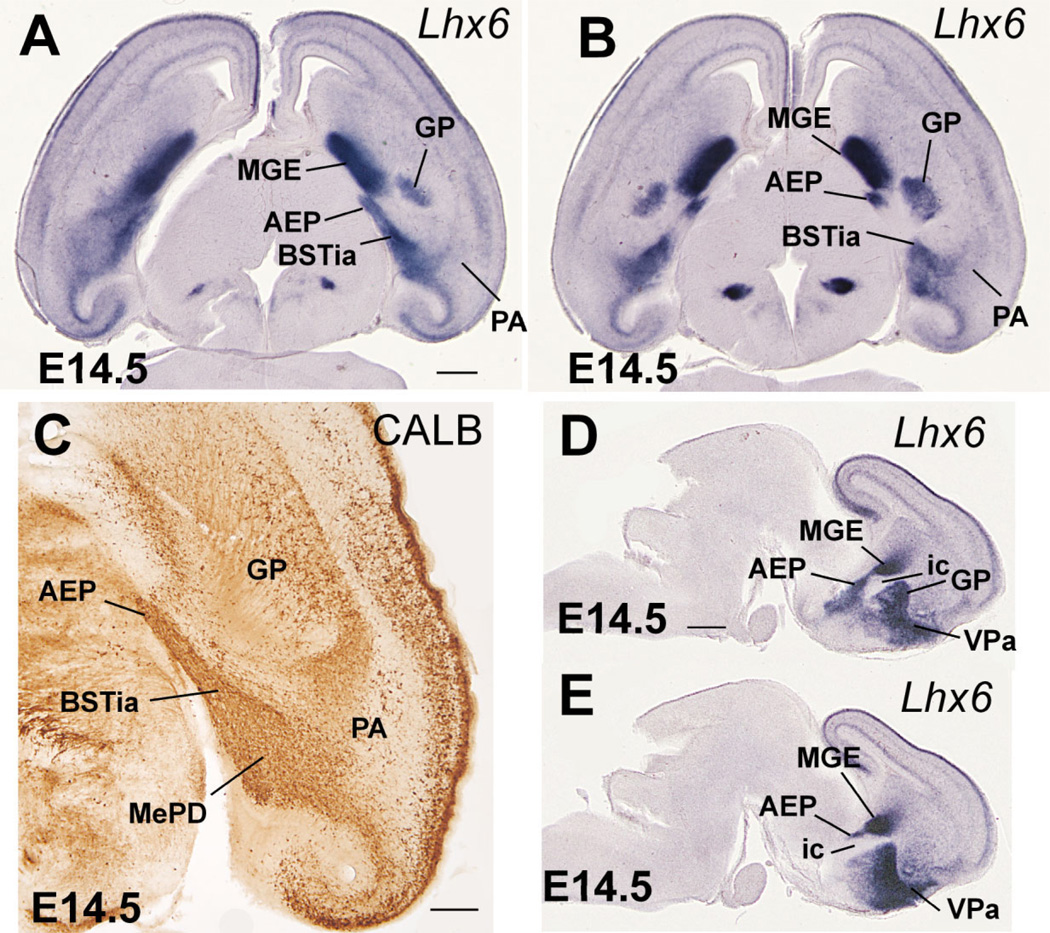
Fig. 5.
Horizontal (A–C) or sagittal (D,E) sections through the embryonic telencephalon of different mouse embryos (E14.5) hybridized for Lhx6, or immunostained for calbindin (CALB). Sections in A,C are at approximately the same level. While MGE is correlated to the globus pallidus (GP), the AEP is radially related to the cell corridor that expresses Lhx6 and contains abundant CALB+ cells, that extends into the posterior part of the medial amygdala (A,C). This cell corridor gives rise to part of the bed nucleus of the stria terminalis (BST), and it will be followed by the fibers of the stria terminalis. Sections in D,E show the relation of AEP with the internal capsule. See text for more details. For abbreviations, see list. Scale bars = 400 µm in A (applies to B); 200 µm in C; 500 µm in D (applies to E). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
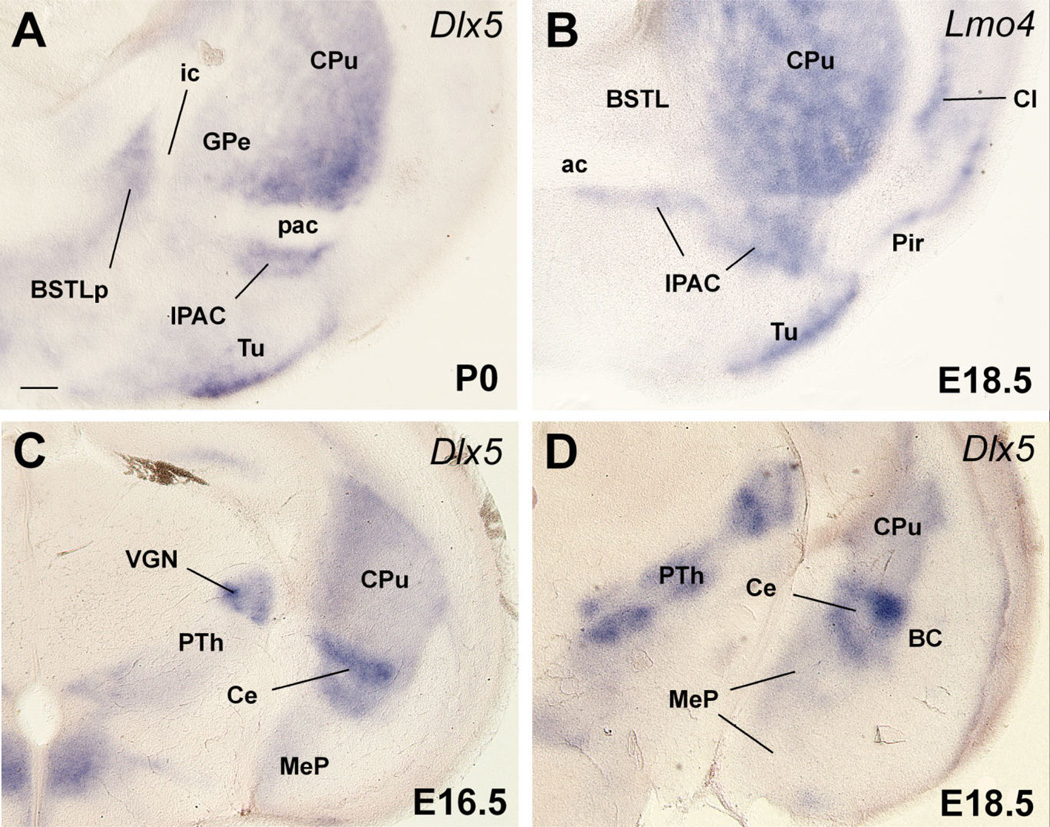
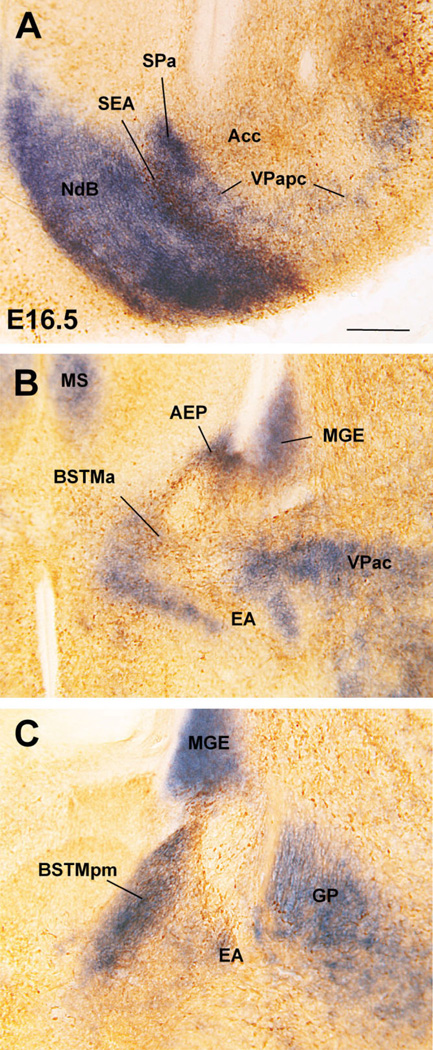
Fig. 6.
Frontal sections through the embryonic telencephalon of different mouse embryos (E16.5, E18.5) or a neonate (P0) hybridized for Dlx5 or Lmo4. Sections in A,B are at about the level of the posterior limb of the anterior commissure (pac), and the nucleus of the pac (IPAC). Sections in C,D are at the level of the posterior medial amygdala and the central amygdala. During early development, Dlx5 is expressed in the entire subpallium, but after E16.5 its expression becomes restricted to LGE derivatives. Many striatal derivatives also differ from other subpallial areas by their specific expression of Lmo4. Based on these criteria, the IPAC and central amygdala, but not the medial amygdala, are striatal (LGE) derivatives. The lateral part of BST (BSTL), which primarily derives from MGE, also shows Dlx5 expression during late development, suggesting that it contains a subpopulation of neurons that originate in LGE. This is consistent with the suggestion that part of BSTL originates in the caudal ganglionic eminence (Nery et al., 2002). See text for more details. For abbreviations, see list. Scale bar = 200 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
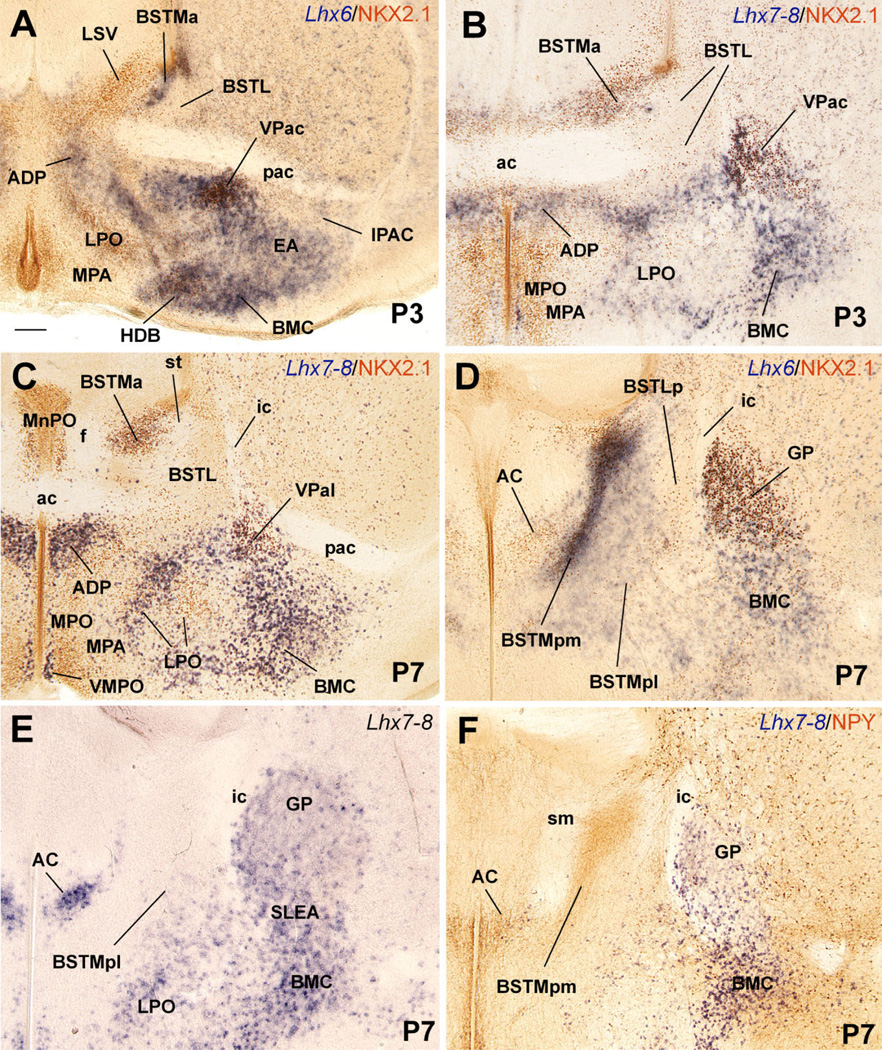
Fig. 13.
Frontal sections through the postnatal telencephalon of different mice (at P3 or P7), at the level of the anterior commissure (A–C) or the globus pallidus (D–F), hybridized for Lhx6 or Lhx7/8 (blue), and/or immunostained for NKX2.1 or NPY (brown). All sections shown, except that in E, are double-labeled. See text for more details. For abbreviations, see list. Scale bar = 200 µm.
Fig. 14.
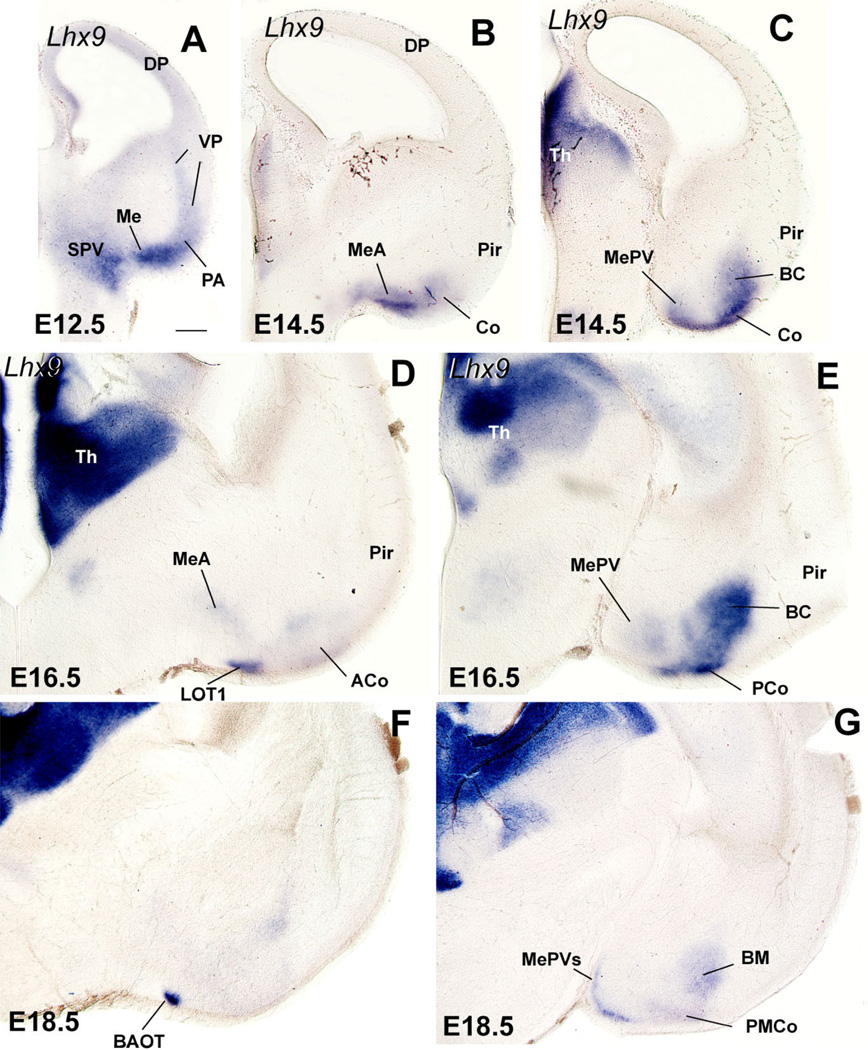
Frontal sections through the embryonic telencephalon of four different mouse embryos (at E12.5, E14.5, E16.5, or E18.5), hybridized for Lhx9. Within the telencephalon this gene is specifically expressed in derivatives of the ventral pallium, and particularly in amygdalar derivatives. Note that the expression spreads into the medial amygdala, suggesting that the ventral pallium gives rise to a cell subpopulation in the anterior medial amygdala (MeA) and to a superficial subdivision of the posteroventral medial amygdala (MePV). See text for more details. For abbreviations, see list. Scale bar = 200 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Early subpallial development: distinction of striatal, pallidal, septo-peduncular, and commissural preoptic subdivisions
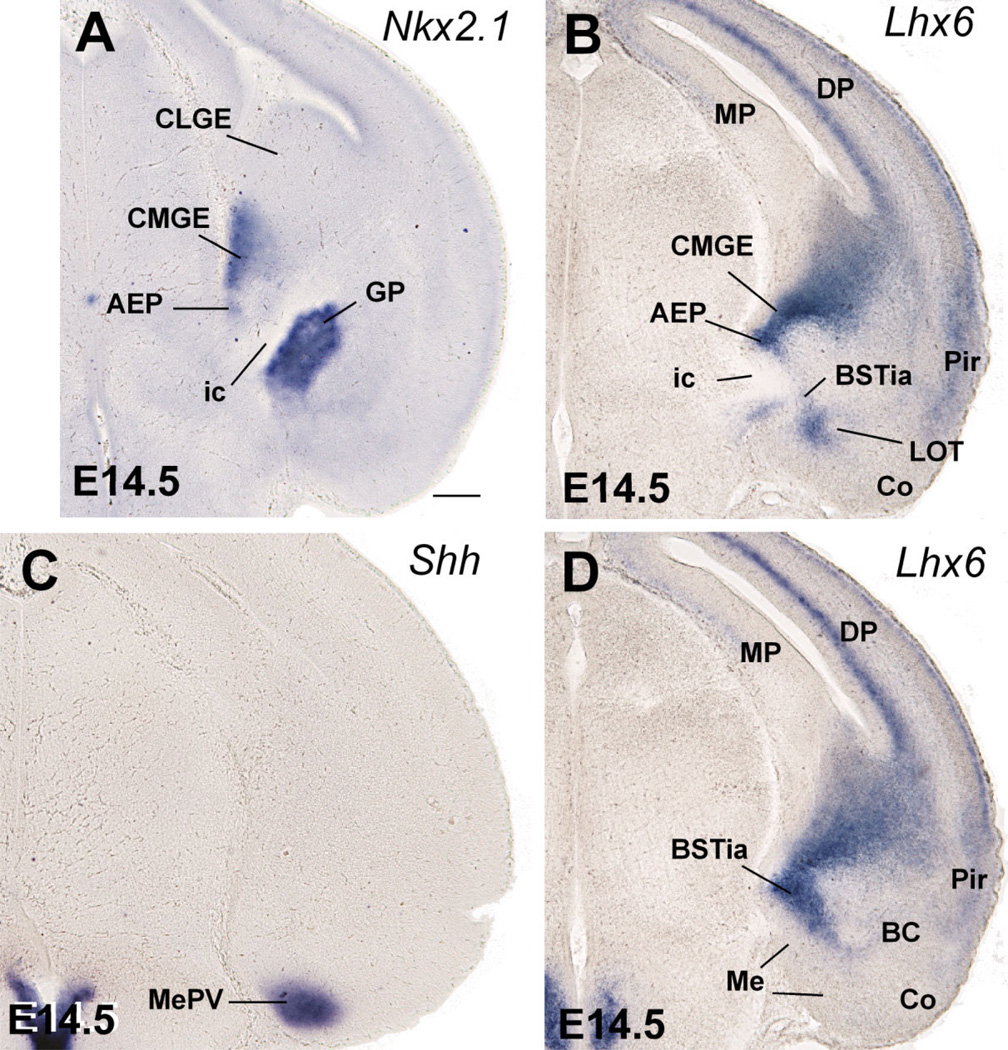
During early mouse development (E10.5–E14.5), Dlx5 expression appears to mark the entire subpallium (Eisenstat et al., 1999), whereas expression of Nkx2.1, Lhx6, and Lhx7/8 in progenitor zones is restricted to the pallidal (MGE), septo-peduncular, and preoptic regions (Fig. 1; Table 1A,B) (Grigoriu et al., 1998; Marin et al., 2000; Asbreuk et al., 2002; Marin and Rubenstein, 2002). At E12.5 the lateral ganglionic eminence (LGE; corresponding to the striatal subdivision) shows Dlx5 expression, but lacks expression of Nkx2.1, Lhx6, and Lhx7/8, except in tangentially migrating interneurons destined for the striatum and pallium (Fig. 1A–F). On the contrary, all of these genes (Dlx5, Nkx2.1, Lhx6, and Lhx7/8) are expressed in the medial ganglionic eminence (MGE; corresponding to the pallidal subdivision) plus an additional ventricular ridge at the junction between the lateral and third ventricle, corresponding to the anterior peduncularpreoptic ridge. The latter ridge is continuous rostromedially with the septum (Fig. 1D–F). In contrast to the MGE, at E12.5 the peduncular-preoptic ridge does not show mantle zone expression of Dlx5, Lhx6, and Lhx7/8, whereas it exhibits ventricular zone expression of Shh (Fig. 1A–I). However, only part of the peduncular-preoptic ridge expresses Shh in the ventricular zone (Fig. 1F–I). Analysis at E14.5 indicates that this Shh-expressing ventricular zone domain is restricted to a median (septal) region of the subpallial preoptic area where the anterior commissure crosses the midline (Fig. 2A,C,E). For this reason, we called this Shh-expressing domain the commissural preoptic area (POC). Comparison of Shh and Lhx6 expression indicates that the POC represents a distinct histogenetic subdivision of the preoptic subpallium, which is intercalated between the AEP and more rostroventral parts of the preoptic area (Fig. 2B,C,E).
TABLE 1.
Genetic and Cellular Features of Mouse Telencephalic Subdivisions at E12.5–E16.5
| A: E12.5 | |||||||
|---|---|---|---|---|---|---|---|
| Lhx9 | Dlx5 | Nkx2.1 | Lhx6 | Lhx7/8 | Shh | Gbx1 | |
| VP | 3 svz, m | 0 | 0 | 0 | 0 | 0 | 0 |
| LGE | 0 | 3 svz, m | 0 | 0 | 0 | 0 | 0 |
| MGE | 0 | 3 svz, m | 3 vz, svz, m | 3 svz, m | 3 svz, m | 11 svz | 11 svz, m |
| Septo-AEP | 0 | 3 svz, m | 3 vz, svz, m | 3 svz | 3 svz | 11 svz | 11 svz, m |
| POC | 0 | 3 svz, m | 3 vz, svz, m | 3 svz | 3 svz | 3 vz, svz | 2 svz, m |
| B: E14.5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lhx9 | Dlx5 | Nkx2.1 | Lhx6 | Lhx7/8 | Shh | Gbx1 | SOM | CALB | CHAT | |
| VP | 3 | 0 | 0 | 11 | 0 | 0 | 0 | 0–11 | 1–21 | 0 |
| LGE | 0 | 3 | 11 | 11 | 11 | 0 | 0 | 0–11 | 1–21 | 0 |
| MGE | 0 | 3 | 3 | 3 | 3 | 11 | 11 | 0 | 1–2 | 0 |
| Septo-AEP | 0 | 3 | 3 | 3 | 3 | 11 | 11 | 3 | 3 | 11 |
| POC | 0 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 1–2 | 3 |
| C: E16.5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lhx9 | Dlx5 | Nkx2.1 | Lhx6 | Lhx7/8 | Shh | SOM | CALB | CHAT | |
| VP | 3 | 0 | 0 | 11 | 0 | 0 | 11 | 21,2 | 0 |
| LGE | 0 | 3 | 11 | 11 | 11 | 0 | 11 | 1–21,2 | 11 |
| MGE | 0 | 1 | 3 | 3 | 1–21 | 11 | 0 | 1–21,3 | 11 |
| Pallidal Septum | 0 | 1–2 | 2–3 | 1–3 | 2–3 | 1 1 | 0 | 1–23 | 11 |
| AEP | 1 | 1–2 | 3 | 1–2 1 | 11 | 3 | 34 | 11 | |
| POC | 0 | 1 | 2 | 2 | 3 | 3 | 0 | 1–2 | 3 |
m, mantle; svz, subventricular zone; v, ventricular zone.
0, no expression; 1, weak expression; 2, moderate expression; 3, strong expression.
Expression is related completely or partially to immigrant cells.
Only mantle.
Mostly periventricular.
Periventricular + mantle.
Fig. 2.
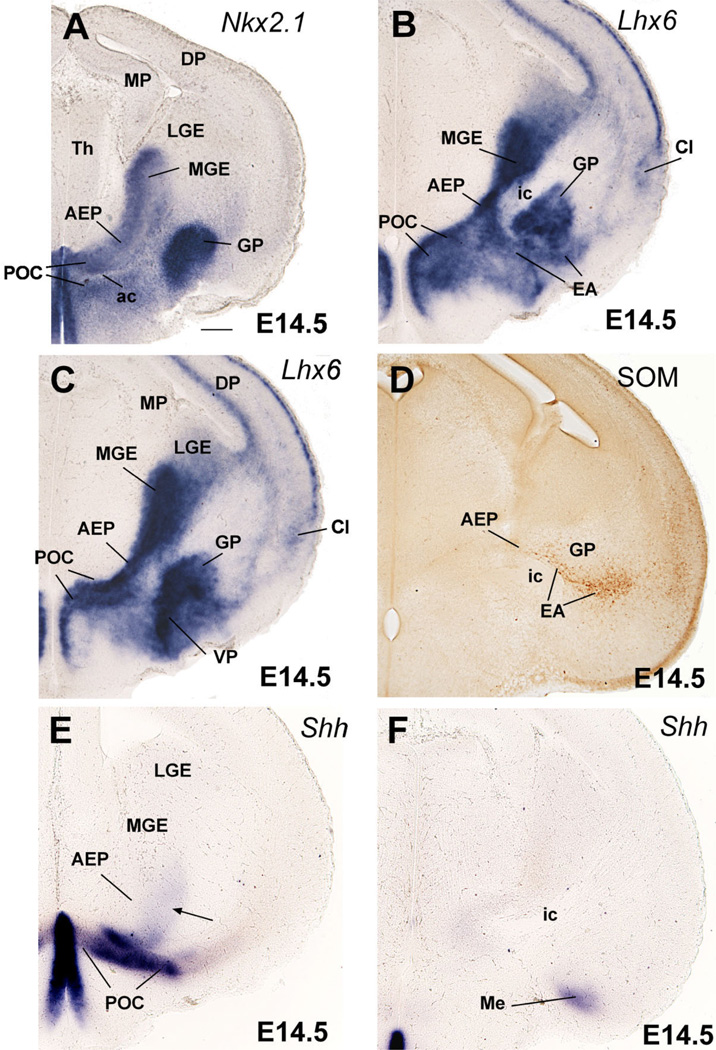
Frontal sections through the embryonic telencephalon of different mouse embryos (E14.5) hybridized for Nkx2.1, Lhx6, or Shh, or immunostained for the neuropeptide somatostatin (SOM). Sections shown in A,C,E are at similar frontal levels (at about the level of the anterior commissure; ac), to facilitate comparison of expression patterns. Section shown in B is just caudal to the anterior commissure. Sections shown in D,F are at a more caudal level, when the internal capsule (ic) enters/exists the telencephalic vesicle. Lhx6 expression shows three distinct radial subdivisions in the subpallium: MGE, AEP, and POC. Only the POC shows strong expression of Shh in the ventricular zone (E), which extends laterally, invading the dorsolateral preoptic area and the medial amygdala (Me; F). A band of weak Shh expression extends from the POC domain into AEP/MGE, and may represent tangentially migrating cells (arrow in E). MGE is radially correlated with the globus pallidus (GP). The AEP is sandwiched between MGE and POC. It consists of a thick radial band of Lhx6 expression extending from the subventricular zone to the surface, below the GP. It appears to give rise to the sublenticular part of the extended amygdala (EA). It is also related to a corridor of SOM+ cells (D), which appear to originate in AEP. At this age, Lhx6 is also expressed in cells that migrate tangentially to the cortical/pallial regions (including the cerebral cortex and claustrum). See text for more details. For abbreviations, see list. Scale bar = 200 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
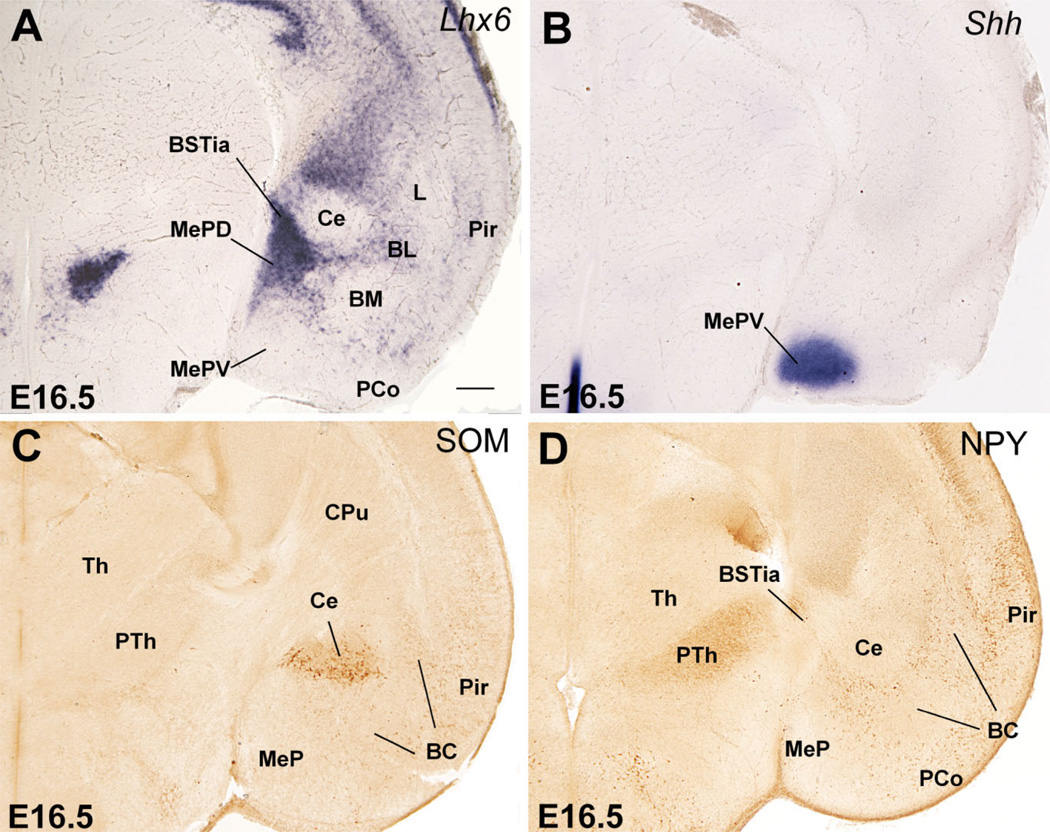
At E12.5 a band of Shh-expressing cells extends tangentially from the POC ventricular zone into the subventricular zone of the AEP and MGE (best seen in horizontal section; arrows in Fig. 1J). POC derivatives identified by their Shh expression are observed more clearly at E14.5, and include the dorsolateral part of the preoptic area (Figs. 2E,F; 3). Shh expression extends lateralward to invade the primordium of the medial amygdalar nucleus (Me), reaching its anterior and posteroventral subdivisions (MePV) (Figs. 2F, 3C). This suggests that at least a subset of cells in these medial amygdalar subnuclei originate in POC (see discussion). As previously, at E14.5 Shh signal still extends into the subventricular zone of the MGE, AEP (Fig. 2E), and pallidal part of the septum (not shown).
Fig. 3.
Frontal sections through the caudal embryonic telencephalon of different mouse embryos (E14.5) hybridized for Nkx2.1, Lhx6, or Shh. Sections in A,B are at about the level when internal capsule enters/exits the telencephalon. Sections in C,D are at the level of the posterior medial amygdala (Me). AEP continues to be observed as a distinct domain (different from MGE). While MGE is related to the globus pallidus (GP), the AEP is related to a band of Lhx6 expression that extends into the amygdala following the future course of the stria terminalis. It appears to produce part of the bed nucleus of the stria terminalis (BST). Note the strong Shh expression in the primordium of the posteroventral part of the medial amygdala (MePV), suggesting at least a partial origin in POC. See text for more details. For abbreviations, see list. Scale bar = 200 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
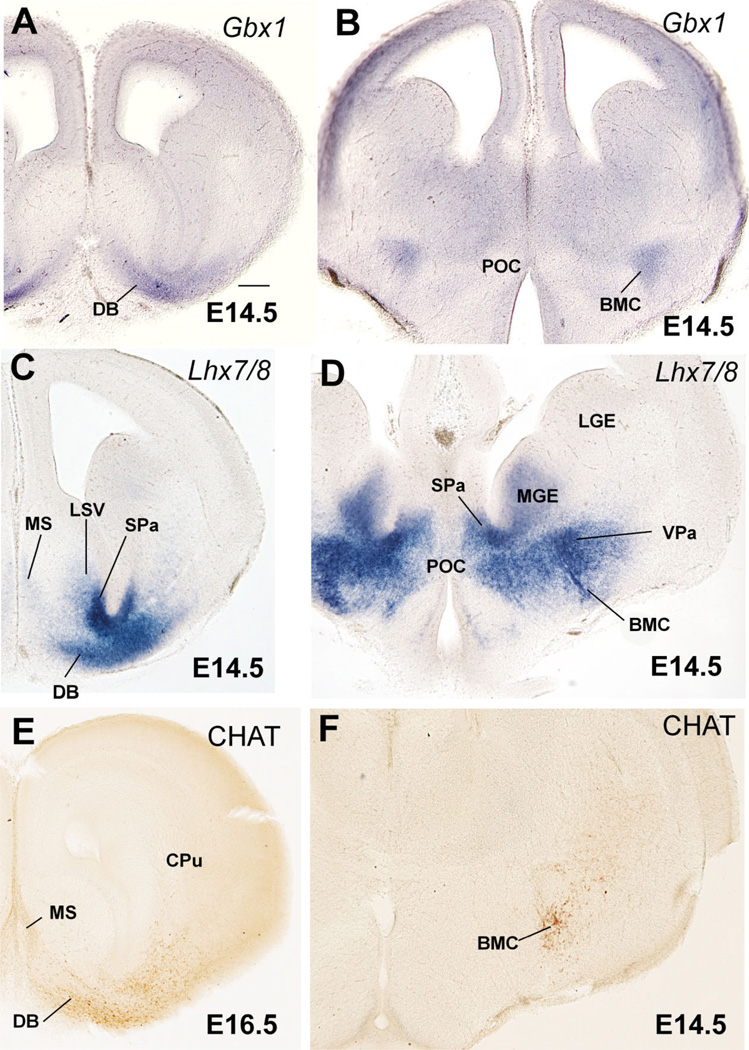
Comparison of Shh and Gbx1 suggests that some POC-derived cells apparently express Gbx1 or overlap with cells doing so (Fig. 1J,K). Correlation of Gbx1 and CHAT (a marker of cholinergic neurons) suggests that the Gbx1-expressing cells include the corticopetal cholinergic neurons of the basal telencephalon, such as those of the diagonal band nucleus and medial septum, as well as those of the basal magnocellular complex (Fig. 4A,B,E,F). As previously observed (Asbreuk et al., 2002; Zhao et al., 2003), these corticopetal cholinergic cell groups also express the LIM-homeodomain gene Lhx7/8 (Fig. 4C,D).
Fig. 4.
Frontal sections through the embryonic telencephalon of different mouse embryos (E14.5, E16.5) hybridized for Gbx1 or Lhx7/8, or immunostained for the enzyme choline acetyltransferase (CHAT, a marker of cholinergic neurons). Sections in A,C,E are at a similar level, where the primordium of the diagonal band nucleus (DB) is observed. Sections in B,D,E are at a similar level behind the anterior commissure, where the primordium of the basal magnocellular nucleus (BMC) is present. Note the correlation between Gbx1 expression and the cholinergic cells of the developing BMC and DB. See text for more details. For abbreviations, see list. Scale bar = 200 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In contrast to the POC, the AEP lacks ventricular zone expression of Shh and is topographically related to the locus where the internal capsule/cerebral peduncle enters/exits the telencephalon (Figs. 1G, 2C,E,F, 3A,B; Table 1A,B). During early development the AEP resembles the MGE in its molecular profile: expression of Dlx5, Nkx2.1, Lhx6, and Lhx7/8, and lack of ventricular zone expression of Shh (Figs. 1A–G, 2A–C,E). However, analysis of Lhx6 and SOM at E14.5 indicates that the AEP corresponds to a distinct radial domain, sandwiched between the MGE (pallidum proper) and the POC, which produces specific cell groups such as SOM+ cells (Fig. 2B,D). This suggests that the AEP might represent a pallidal-like domain distinct from MGE, and we might talk about a main or principal pallidum (MGE; pallidum proper) and a peduncular or AEP pallidum. The AEP is rostrally continuous with the pallidal part of the septum, which shows a similar molecular profile (Fig. 1D–F). However, while the septal and AEP subdivisions of the pallidal-like septo-peduncular domain share common molecular features, they also have distinct properties. For example, at E14.5 the AEP (but not the pallidal septum) is specifically related to a stream of SOM-immunostained cells that spread radially from the ventricular zone to the brain surface (Fig. 2D). These SOM-immunostained cells spread below the developing globus pallidus, across the developing sublenticular* extended amygdala. At caudal telencephalic levels, AEP derivatives—expressing Lhx6 and including SOM+ cells—appear to enter the amygdala proper and are related to the primordium of the intraamygdaloid part of the BST (BSTia; Figs. 2D, 3B,D). The connection between the AEP ventricular zone and the amygdala proper is also evident in horizontal sections (Fig. 5). In this view the AEP appears as a distinct Lhx6-expressing domain extending from the ventricular zone, through the path of the stria terminalis, into the BSTia and MePD regions of the amygdala, which correlates with a cell corridor of CALB+ cells following a similar trajectory (Fig. 5A–C).
Thus, the AEP is a distinct histogenetic subdivision of the subpallium, related to the internal capsule / cerebral peduncle, and appears to produce SOM+ and CALB+ cells that spread into specific parts of the amygdala primordium. All of these features make the AEP clearly distinct from the pallidal septum, MGE, and POC (Figs. 2B,D, 5A–E).
Intermediate–late stages of subpallial development: amygdalar derivatives of the striatal, pallidal, pallidoseptal, peduncular, and commissural preoptic subdivisions
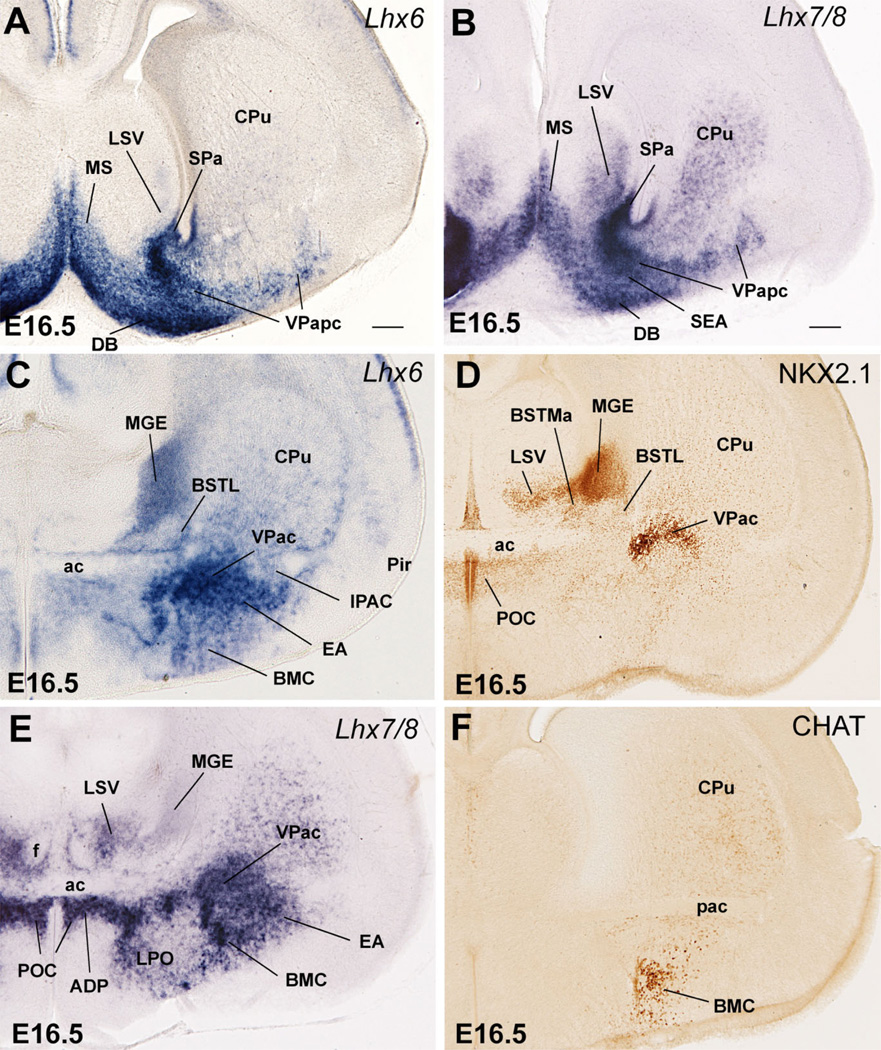
At E16.5 the mouse telencephalon and, in particular, the amygdala proper and the extended amygdala (including the BST) begin to show a mature morphological aspect (Figs. 6C, 7–10). All major amygdalar nuclei/areas are distinguishable and most of them appear located at final (or nearly final) positions. The expression of Dlx5, Nkx2.1, and Lhx6 at E16.5 is—in general—similar to that at E14.5, allowing distinction of striatal derivatives versus derivatives from the MGE, septo-AEP, and POC subdivisions (Figs. 6, 7; Tables 1C, 2). In addition to the strong Lhx6 expression in the pallido-septo-AEP-POC territory, this gene is also expressed in immature interneurons that emigrate tangentially into the striatum and pallium/cerebral cortex (including the claustrum and pallial amygdala; Marín et al., 2000; Alifragis et al., 2004; Legaz et al., 2005b) (Figs. 2B,C, 7C).
Fig. 7.
Frontal sections through the embryonic telencephalon of different mouse embryos (E16.5), at similar precommissural (A,B) or anterior commissural (C–F) levels, hybridized for Lhx6 or Lhx7/8, or immunostained for NKX2.1 or CHAT. Note the difference in expression intensity of NKX2.1, Lhx6, and Lhx7/8 between pallidoseptal, MGE, AEP, and POC derivatives. For example, MGE derivatives generally show the strongest NKX2.1 expression (except BSTL), whereas POC derivatives show the strongest Lhx7/8 expression. The pallidoseptal domain appears to give rise to a precommissural part of the ventral pallidum (VPapc), whereas a caudal, commissural part of the ventral pallidum appears to derive from MGE. See text for more details. For abbreviations, see list. Scale bars = 250 µm in A; 200 µm in B (applies to C–F). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Fig. 10.
Frontal sections through the embryonic telencephalon of different mouse embryos (E16.5), at the level of the posterior medial amygdala (MePD, MePV), hybridized for Lhx6 or Shh, or immunostained for NPY or SOM. Based on the distinct gene expression profile, MePD and MePV appear to originate in different parts of the subpallium. The MePD shows strong expression of Lhx6 but lacks Shh expression, and appears to be an AEP derivative. On the other hand, the MePV (its central part) is poor in Lhx6 but shows strong expression of Shh, and appears to derive at least partially from the POC (although another subset of cells in this nucleus may derive from the diencephalon/hypothalamus). The superficial part of MePV does not express subpallial marker genes, and apparently originate in the ventral pallium. Note the group of SOM+ cells (possibly immigrant cells of AEP origin) in the central amygdala. See text for more details. For abbreviations, see list. Scale bar = 200 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
TABLE 2.
Proposed Amygdalar Derivatives of Each Telencephalic Subdivision
| Derivatives | |
|---|---|
| VP | L1, BM, ACo, PMCo, MePVs, LOT1 |
| LGE | Str, Ce, IM, IPAC, AAd |
| MGE | GP, VPac, BSTL2 |
| Pallidal septum | LSv, MS, VPapc |
| AEP | SEA, SLEA, BSTMa, BSTMpm, BSTia, AAv, MePD, MeA, LOT3 |
| POC | ADP, LPO, AC, BMC, NdB, MS, MePVc, MeA |
Based on data from previous publications (Puelles et al., 2000; Medina et al., 2004).
BSTL also appears to include a subpopulation of cells of striatal origin.
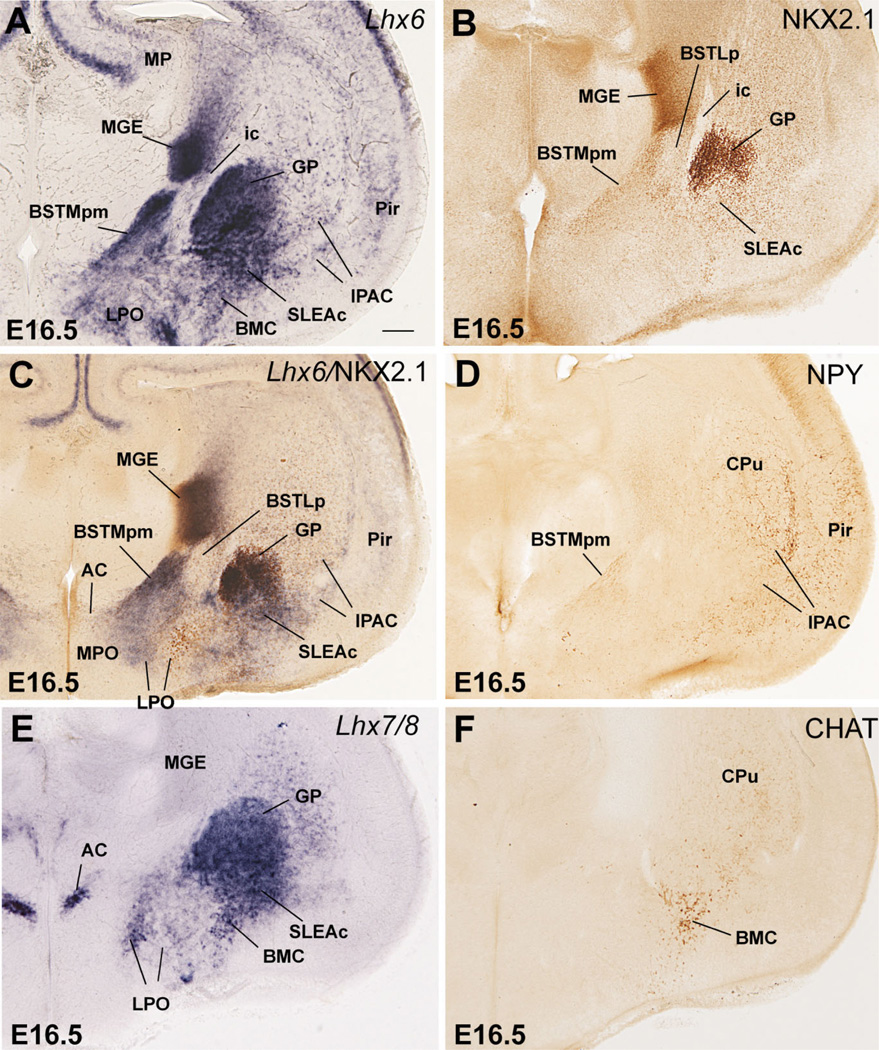
Differences in the expression of Nkx2.1, Lhx7/8, and Shh, together with CALB, CHAT, NPY, and SOM, help distinguish MGE, pallidoseptal, AEP, and POC derivatives at E16.5. Nkx2.1 expression (mRNA and protein) is very strong in MGE derivatives, but is clearly weaker in pallidoseptal, AEP, and POC derivatives (Figs. 7D, 8B,C; here the NKX2.1 protein is detected by immunohistochemistry, which closely resembles Nkx2.1 mRNA expression; Table 1C). In addition, by E16.5 Lhx7/8 expression is reduced in the progenitor zones of MGE and AEP and becomes primarily restricted to the globus pallidus, pallidal septum, and POC, plus some derivatives such as the diagonal band nuclei and dorsolateral preoptic area (Figs. 7B,E, 8E). Moreover, at E16.5 Shh continues to be specifically expressed in POC derivatives in the mantle (Figs. 9C, 10B).
Fig. 8.
Frontal sections through the embryonic telencephalon of different mouse embryos (E16.5), at the level of the globus pallidus and medial BST (BSTM), hybridized for Lhx6 or Lhx7/8, or immunostained for NKX2.1, CHAT or NPY. Panel C shows a double-labeled section, hybridized for Lhx6 (blue) and immunostained for NKX2.1 (brown). At this age Lhx6 is strongly expressed in derivatives of MGE, AEP, and POC. However, NKX2.1 expression remains very strong only in MGE derivatives (such as globus pallidus). BSTL is an exception to this general rule, which is possibly due to the presence of abundant immigrant cells of striatal origin. In contrast, NKX2.1 is generally moderate or light in AEP derivatives (such as posteromedial BSTM [BSTMpm] and sublenticular extended amygdala [SLEA]). On the other hand, POC derivatives (including the cholinergic cells of the basal magnocellular complex or BMC) show the strongest Lhx7/8 expression, but this gene shows a trend to be downregulated in the periventricular parts of MGE and AEP. See text for more details. For abbreviations, see list. Scale bar = 200 µm.
Fig. 9.
Frontal sections through the embryonic telencephalon of different mouse embryos (E16.5), at the level of the nucleus of the lateral olfactory tract (LOT) and anterior medial amygdala (MeA), hybridized for Lhx6, Lhx7/8, or Shh, or immunostained for NPY or SOM. Note the band of Lhx6 expression extending from the subventricular zone of AEP into the extended amygdala, including the posteromedial BSTM, the sublenticular extended amygdala (SLEA), and the anterior medial amygdala (MeA). This suggests that all of these areas/nuclei primarily derive from AEP. The MeA also shows weak expression of Shh, suggesting that it contains cells that originate in POC. The AEP also gives rise to peptidergic cells (containing SOM or NPY). Some of these appear to migrate tangentially into striatal and pallial (cortical) territories. A striking example of SOM+ cells that emigrate from AEP into the central amygdala (Ce, a striatal derivative) is shown in D. See text for more details. For abbreviations, see list. Scale bar = 200 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
On the other hand, while most AEP derivatives contain a subpopulation of NPY- or SOM-immunostained cells, derivatives from MGE, pallidal septum, or POC generally show very little or no trace of NPY or SOM immunoreactivity at E16.5 (for example, Fig. 8D; see below for details). Furthermore, although CALB+ neurons are numerous in many parts of the telencephalon at E16.5, the AEP continues to be particularly rich in CALB+ cells. Double labeling of Lhx6 and CALB indicates that the CALB-rich AEP domain is physically connected to (is continuous with) the ventricular zone of the caudal septum, and can be followed more rostrally into a narrow cell band (Fig. 11), which appears related to a rostral and septal part of the extended amygdala. Thus, several features, such as the high density of CALB+ cells, distinguish the AEP (including its narrow septal extension) from the pallidal septum.
Fig. 11.
Details of double-labeled frontal sections through the embryonic telencephalon of mouse (at E16.5), at different rostrocaudal levels, hybridized for Lhx6 and immunostained for calbindin (CALB). Note the correlation of AEP derivatives and densely grouped CALB+ cells. See text for more details. For abbreviations, see list. Scale bar = 200 µm.
Therefore, the combined analysis of the expression of Dlx5, Nkx2.1, Lhx6, Lhx7/8, Shh, and several markers of specific neuronal subpopulations (including CALB, NPY, SOM, and CHAT) at E16.5 and during later development distinguishes striatal, pallidal, pallidoseptal, AEP, and POC subpallial derivatives, and are particularly relevant for those in the centromedial and extended amygdala. Below we describe how these patterns evolve from E16.5 through P7; these results are summarized in Tables 1C and 2 and Figures 15 and 16.
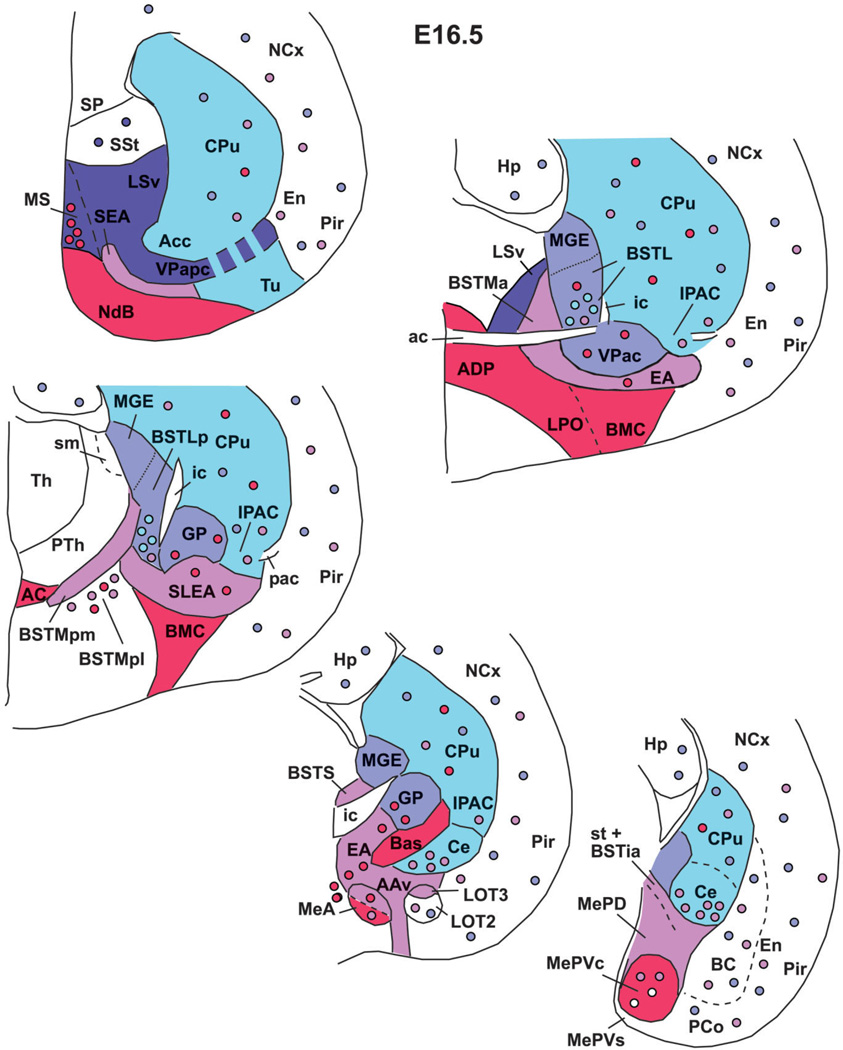
Fig. 15.
Schematic drawings of frontal sections through the embryonic mouse telencephalon (at E12.5 or E14.5), representing the major histogenetic subdivisions of the subpallium. LGE produces the striatal components of the telencephalon. MGE is the pallidum proper and produces the globus pallidus. AEP is related to the peduncle/internal capsule, and produces many sublenticular components of the extended amygdala. POC is related to the anterior commissure, shows specific expression of Shh in the ventricular zone, and produces the cholinergic cells of the basal telencephalon and the dorsolateral preoptic region. The septum also contains striatal- and pallidal-like subdivisions. Note that AEP and POC extend into the posterior medial amygdala and into the septum. See text for more details. For abbreviations, see list.
Fig. 16.
Schematic drawings of frontal sections through the embryonic mouse telencephalon (at E16.5), representing the derivatives of the major histogenetic subdivisions of the subpallium (each one with a different solid color, except the striatal-like septum, which was not studied here and is color-free). Colored circles represent immigrant cells (i.e., tangentially migrated cells) that originate in the subpallial subdivision showing the same color. Briefly, the IPAC, intercalated amygdalar masses, and central amygdala derive from the striatal subdivision (LGE). The BSTL is primarily a MGE derivative, but apparently contains immigrant cells of striatal origin (among cells of other origins). The BSTMa, BSTMpm, BSTia, sublenticular extended amygdala (SLEA), and part of the medial amygdala (MeA and MePD) appear to derive from AEP. The POC gives rise, among other groups, to a central part of MePV (MePVc) and also contributes cells to MeA. Note that the ventral pallium extends into the superficial part of MePV (and also produces a cell subpopulation of MeA; not shown in the scheme). See text for more details. For abbreviations, see list.
Striatal derivatives (from LGE)
These are defined by strong, nearly homogeneous expression of Dlx5 and scattered expression of Nkx2.1 or Lhx6 in interneurons produced in the pallido-peduncular region (Marin et al., 2000). During late development, Dlx5 expression (previously in the entire subpallium) becomes restricted to LGE and its derivatives, and appears to be downregulated in MGE and AEP. Moreover, most striatal derivatives (except at caudal levels) can be distinguished from those of other subpallial subdivisions by their specific expression of the LIM-only gene Lmo4 (Fig. 6B). Based on these criteria, the following nuclei of the amygdala and extended amygdala have striatal-like molecular properties: 1) the interstitial nucleus of the posterior limb of the anterior commissure, or IPAC (previously called striatal fundus; a medial portion of IPAC is considered to be part of the extended amygdala; Alheid et al., 1995; Shammah-Lagnado et al., 2001); 2) the central amygdalar nucleus (Fig. 6A–D); 3) the main intercalated amygdalar masses; and 4) the dorsal part of the anterior amygdala (not shown; Medina et al., 2004; Tole et al., 2005). In contrast, no part of the medial amygdala meets these criteria. Curiously, the BSTL, which shows expression of pallidal marker genes such as Nkx2.1 and Lhx6, and appears to primarily derive from MGE (see below) (Fig. 7C,D), also shows expression of Dlx5 during intermediate–late development (Fig. 6A). This suggests that BSTL includes a subset of neurons that originate LGE (see below).
Pallidal derivatives (from MGE or pallidum proper)
These are defined by very strong expression of Nkx2.1 (both mRNA and protein) and Lhx6 and include the globus pallidus and part of the ventral pallidum (these centers represent the major parts of the pallidum proper; Figs. 7C,D, 8A–C). However, based on NKX2.1 expression and topography, only a caudal, commissural part of the ventral pallidum (at the level of the posterior limb of the anterior commissure) appears to derive from MGE (Fig. 7D). In contrast, the rostral (precommissural) part of the ventral pallidum only shows moderate NKX2.1 expression (not shown) and appears directly connected to (and to derive from) the pallidal septum (see below; Fig. 7A,B). Furthermore, the MGE and some of its periventricular derivatives show only negligible Lhx7/8 signal at E16.5 (Fig. 7E); this trend for Lhx7/8 downregulation becomes more pronounced later in development.
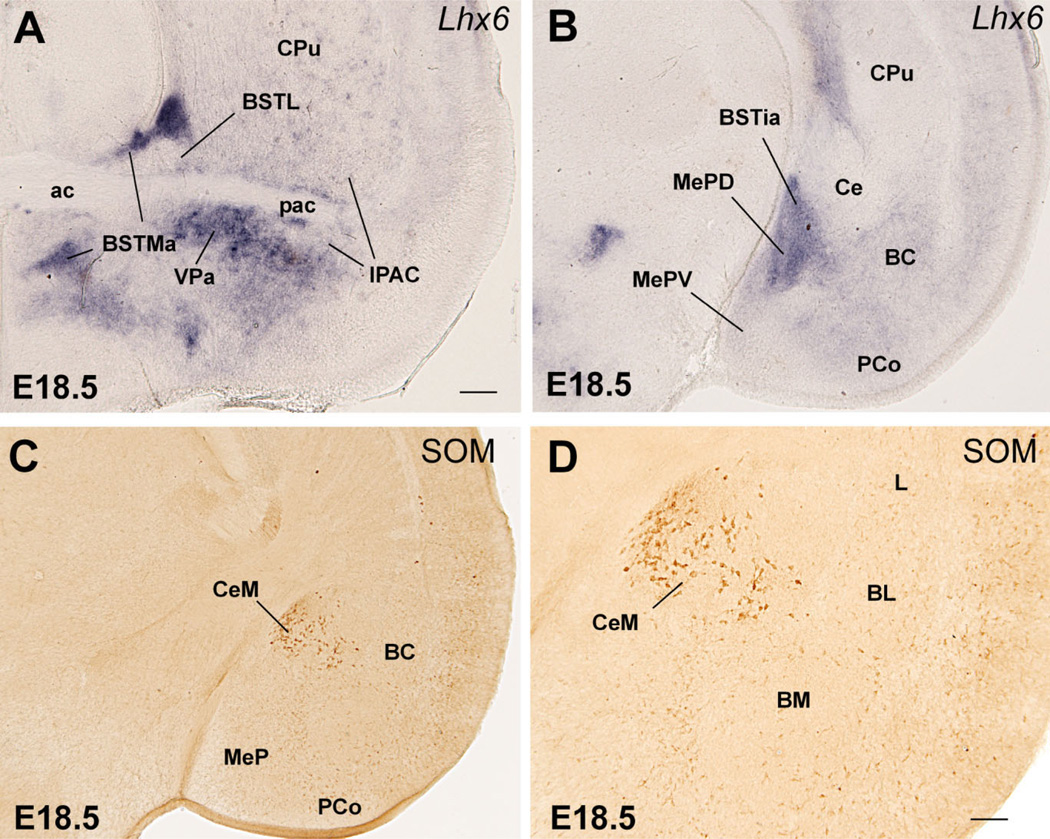
The lateral part of the developing BST (BSTL; which includes the primordium of the oval subnucleus, as identified by Ju and Swanson, 1989; this is called dorsal subnucleus in the atlas by Paxinos and colleagues) lies immediately adjacent to the proliferative zone of MGE, intercalated between this and the pallidum (ventral pallidum; globus pallidus; Figs. 7, 8). It shows Nkx2.1 and Lhx6 expression, but only negligible Lhx7/8 expression (Figs. 7C–E, 8A–C). Based on its position and gene expression profile during early development, the BSTL primordium may primarily derive from MGE. However, the expression of NKX2.1 protein and Lhx6 in the BSTL is only moderate to light at E16.5 (Figs. 7C,D, 8A–C), and becomes generally weak during subsequent development. During the first postnatal week the BSTL only contains scattered cells showing nuclear expression of NKX2.1 (Fig. 13). This is in sharp contrast with other MGE derivatives, and raises question as to the origin of BSTL cells that do not express NKX2.1 (see Discussion). As noted above, the BSTL also shows expression of Dlx5 during late development (Fig. 6A), suggesting that it contains a subset of striatal cells (possibly immigrant cells).
In general, the expression patterns described in MGE and derivatives at E16.5 are still observed at E18.5 and during the first postnatal week (Fig. 13A–D). At P7, most MGE derivatives (including the globus pallidus) lack or show relatively low Lhx7/8 signal (Fig. 13E,F). In the globus pallidus, strong expression of Lhx7/8 has become mostly restricted to a subpopulation of cells (Fig. 13E,F), which—based on their somatic size and number—could correspond to the cholinergic neurons.
Septopallidal and AEP derivatives
At E16.5 and later the molecular features of the septopallidal and AEP subdivisions and their derivatives are, in general, similar to those of the pallidum proper (MGE) and derivatives, with slight differences in expression intensity of Nkx2.1, Lhx6, and Lhx7/8 (Figs. 7–11; Table 1C). In general, Nkx2.1 expression is strongest in the MGE, intermediate in the pallidal septum, and weak in the AEP (Table 1C). Pallidoseptal and AEP derivatives generally show moderate-to-strong Lhx6 expression (except part of the pallidal septum, such as the lateroventral septal nucleus, which shows very low Lhx6 signal). On the other hand, Lhx7/8 expression is moderate to strong in derivatives of the pallidal septum, but most AEP derivatives generally show weak expression of Lhx7/8.
Two additional features are going to distinguish the pallidal septum from the AEP and MGE: 1) the topographic position in the septum or the morphological evidence that cells derive directly from it; 2) the relation with subpopulations of peptidergic (SOM+, NPY+) or CALB+ cells, which are typical in AEP derivatives (in fact, the AEP appears to be an important source of these types of cells in the telencephalon, although additional sources appear to exist). In contrast to AEP derivatives, the pallidal septum is generally poor in these cell types.
Based on these features the lateroventral septal nucleus and a rostromedial (precommissural) part of the ventral pallidum appear to derive from the pallidal septum (Figs. 4C, 7B–E, 16). The medial septal nucleus, or part of it, may also be a derivative of the pallidal septum (Fig. 7B, 16). However, the medial septum also shows Gbx1 expression correlated with a subpopulation of cholinergic cells that may derive from POC (see below).
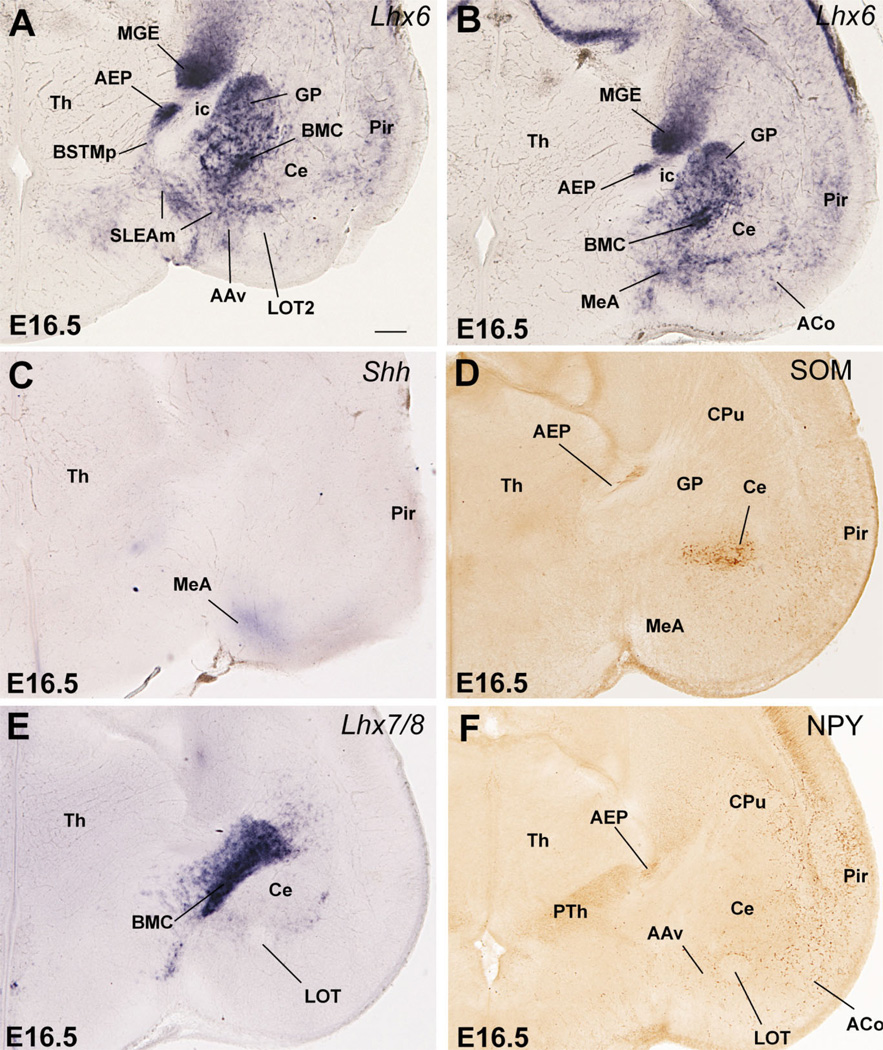
On the other hand, most AEP derivatives are located at the level of the anterior commissure, or more caudally, generally show strong Lhx6 expression but moderate to light Nkx2.1 and Lhx7/8 expression, and have densely grouped CALB+ cells or dispersed NPY+ or SOM+ cells. Based on this, the anterior part of the medial BST (BSTMa), part of the posterior BSTM (mainly its posteromedial part or BSTMpm) and the intramygdaloid BST (BSTia) appear to derive from the AEP (Figs. 7C–E, 8A–C,E, 10A, 16). Further, the ventral part of the anterior amygdala (AAv), the posterodorsal part of the medial amygdala (MePD), and at least part of the anterior medial amygdalar nucleus (MeA) also appear to derive from the AEP (Figs. 8A–C, 9A,B, 10A, 12B, 16). However, the MeA also shows Shh expression (Fig. 9C), suggesting that MeA also may contain cells derived from POC (see below and Discussion).
Fig. 12.
Frontal sections through the embryonic telencephalon of different mouse embryos (E18.5), at the level of the anterior commissure (A) or the posterior medial amygdala (B–D), hybridized for Lhx6, or immunostained for SOM. AEP derivatives (including most parts of BSTM and MePD) continue to express Lhx6 at E18.5. AEP also produces peptidergic neurons (containing NPY or SOM). A striking subpopulation of SOM+ cells that emigrate from AEP into the central amygdala is shown in C,D (D is a detail of those cells in the medial central amygdala or CeM). See text for more details. For abbreviations, see list. Scale bars = 200 µm A (applies to B,C); 100 µm in D. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Most of the extended amygdala also appears to derive from the AEP division. This includes its sublenticular part (SLEA, below the globus pallidus), extending from the periventricular part of BSTM to the MeA and BSTia/MePD (Figs. 8A–C, 9A). In addition, a rostromedial extension of the extended amygdala (in the septum) that is rich in CALB+ cells also appears to derive from AEP (Figs. 11, 16). This rostral extension abuts the precommissural part of the ventral pallidum (sandwiched between it and the diagonal band nucleus); this region is sometimes referred to in the literature as the anterior part of the substantia innominata (Gritti et al., 1993, 2003).
In addition to the numerous NPY+ or SOM+ cells present in many AEP derivatives (except its extension into the septum), a subpopulation of dispersed NPY+ or SOM+ cells is also present in striatal and pallial (cortical) derivatives, including striatal and pallial parts of the amygdala (Figs. 8D, 9F, 10C,D, 12E,F). These peptidergic cells observed in striatal and various pallial regions may represent immigrant cells of AEP origin. Among them, there is a striking group of large SOM-containing cells located in part of the central amygdala at E16.5 (Figs. 9D, 10C,D). Later in development, this specific cell group appears to represent a specific subpopulation of neurons located in the medial part of the central amygdala (CeM; Fig. 12E,F).
POC derivatives
At E16.5 and later, POC and some of its derivatives continue to show strong expression of Shh and/or Lhx7/8. Based on this, the so-called anterodorsal preoptic nucleus (ADP, adjacent to the anterior commissure) and the lateral preoptic area appear to derive from the POC (Figs. 2E, 7E, 9E, 13B,C). Further, the paramedian preoptic nucleus (not shown) and ventromedial preoptic nucleus (Fig. 13C) express either Shh or Lhx7/8, respectively, suggesting that they may originate in POC. As previously (E14.5), its strong Shh expression suggests that the posteroventral part of the medial amygdalar nucleus (MePV), particularly a central part of it, may at least partially derive from POC (Fig. 10B). In addition, based on its weak Shh signal, the MeA may receive a minor cell contribution from the POC (Fig. 9C). However, both MePV and MeA may also include cells from hypothalamic origin (see Discussion). Finally, the correlation between Gbx1, Lhx7/8, and CHAT (also observed at E14.5) suggests that the corticopetal cholinergic cell groups (CHAT-immunoreactive) of the basal telencephalon derive from POC, including the cholinergic neurons of the basal magnocellular complex, plus those of the diagonal band nucleus and medial septum (Figs. 4A–E, 7E,F, 8E,F).
Ventral pallial contribution to the medial amygdala based on Lhx9 expression
At E12.5 the ventral pallium shows specific expression of the LIM-homeodomain gene Lhx9 (Fig. 14A; Tole et al., 2005; see also Retaux et al., 1999). At this age the in situ hybridization signal is located in the ventral migratory stream plus the ventral pallial part of the amygdala. Interestingly, the expression domain of Lhx9 in the pallial amygdala bends medially near the surface and overlaps partially the medial amygdala primordium (Fig. 14A). This is also observed at E14.5, suggesting that the ventral pallium produces some cells of the medial amygdala. At E14.5–E16.5, Lhx9 expression is specifically observed in amygdalar derivatives of the ventral pallium, including the basomedial amygdalar nucleus, the anterior and posteromedial cortical amygdalar areas, layer 1 of the nucleus of the lateral olfactory tract (LOT1), the bed nucleus of the accessory olfactory tract (BAOT), as well as the anterior and posteroventral parts of the medial amygdala (MeA, MePV; Fig. 14B–E). At E18.5, expression of Lhx9 in MePV becomes restricted to a thin superficial band, coinciding with an area of high cell density of this nucleus (Fig. 14G). This particular superficial area of MePV did not show Shh expression at E16.5, which suggests that MePV contains two distinct subdivisions of different origins: a superficial band, expressing Lhx9, that derives from the ventral pallium; and a central part, expressing Shh, that derives from the POC (and possibly the hypothalamus; see Discussion).
DISCUSSION
Four subpallial histogenetic divisions produce most of the cell masses of the centromedial and extended amygdala
We present evidence, based on the combined analysis of the expression of seven developmental regulatory genes and four phenotypic neuronal markers, indicating that the mouse subpallium contains at least four major histogenetic divisions that give rise to different parts of the centromedial and extended amygdala (including the BST) (Figs. 15, 16). These divisions are the LGE (striatal division), the MGE (the pallidum proper), the AEP pallidum and a novel commissural preoptic division or POC (at the base of the septum, in relation to the anterior commissure and dorsolateral preoptic area). Conventional developmental studies already indicated the contribution of LGE and MGE to parts of the amygdala and the BST (Holmgren, 1925; see also Bayer, 1987; review by Swanson, 2000). More recently, analysis of transcription factors combined with morphological analysis indicated that the subpallium includes four major subdivisions: the LGE (striatal part), the MGE (pallidal part), the so-called anterior entopeduncular area or AEP, and an anterior, subpallial part of the preoptic area (Bulfone et al., 1993; Puelles and Rubenstein, 1993, 2003; Puelles et al., 2000; Marin and Rubenstein, 2002). The AEP was initially related to the cholinergic and other cell groups at the telencephalic stalk, and to the fiber tracts that go through it connecting telencephalon and thalamus/brainstem (internal capsule/cerebral peduncle) or both hemispheres (anterior commissure) (reviewed by Marin and Rubenstein, 2002).
The LGE, MGE, and AEP were previously noted to express different combinations of developmental regulatory genes (encoding transcription factors or signaling proteins), and some of these were used as markers to identify the derivatives of each division. The LGE and its derivatives were characterized by expression of Dlx1/2/5 (Puelles et al., 2000; Medina et al., 2004); the MGE derivatives were characterized by expression of Dlx1/2/5 and Nkx2.1 (Sussel et al., 1999; Puelles et al., 2000); and the AEP was characterized by expression of Dlx1/2/5, Nkx2.1, and Shh (Shimamura and Rubenstein, 1997; reviewed by Marin and Rubenstein, 2002; Puelles et al., 2004). These studies provided evidence that the central amygdala derives from the LGE (striatal subdivision) (Puelles et al., 2000; Medina et al., 2004; Tole et al., 2005), an anterior part of the BST derives from the Nkx2.1-expressing domain (including MGE or pallidal subdivision) (Puelles et al., 2000), whereas the AEP was said to give rise to most cholinergic cells of the basal telencephalon (Marin and Rubenstein, 2002; Zhao et al., 2003) and was also considered a source of telencephalic oligodendrocytes (Olivier et al., 2001).
Our results largely corroborate fundamental aspects of earlier work, but provide novel and more specific data on the amygdalar derivatives of each subpallial subdivision. In addition, we present data indicating that the previously defined AEP consists of two distinct histogenetic subdivisions: a true AEP related to the peduncle (here called the anterior peduncular area; see Results); and a novel commissural preoptic area (or commissural septo-preoptic area; POC), located at the base of the septum and related to the anterior commissure and dorsolateral preoptic area. Notably, only the POC—but not the AEP—shows ventricular zone expression of Shh. Based on Shh expression, we propose that POC derivatives include at least a subpopulation of cells of the medial amygdala. In addition, POC derivatives appear to include the cholinergic corticopetal cell groups of the basal telencephalon, such as the basal magnocellular complex (basal nucleus and preoptic magnocellular nucleus), the diagonal band nuclei, and likely the cholinergic cells of the medial septum, which show expression of Gbx1 as well as Lhx7/8 (Asbreuk et al., 2002; Zhao et al., 2003). In addition, since the POC is the only telencephalic ventricular sector expressing Shh, it is likely that it constitutes the source of telencephalic oligodendrocytes previously attributed to the Shh-expressing AEP (Olivier et al., 2001; Kessaris et al., 2006).
Our results also show a specific and interesting relation between the expression of Shh and the developing anterior commissure. Data in the spinal cord have indicated that—in addition to its known role in dorsoventral patterning during early developmental stages (Briscoe and Ericson, 2001)—Shh has other roles later in development, guiding commissural growing axons (Charron et al., 2003; Bourikas et al., 2005). In particular, in the spinal cord Shh acts (in collaboration with netrin-1) as a chemoattractor of growing axons toward the floor plate where the commissure forms (Charron et al., 2003), or, later, as a repellent factor of postcommissural growing axons, leading them to turn and direct rostrally (Bourikas et al., 2005). The close relationship between Shh expression and the anterior commissure during early development suggests that, in the telencephalon, Shh may also play a role in guiding commissural growing axons.
In contrast to the POC, the AEP lacks ventricular zone expression of Shh. At E14.5 the AEP appears as a distinct histogenetic territory sandwiched between POC and MGE, which expresses both Lhx6 and Nkx2.1, and is related to a distinct cell corridor of peptidergic (SOM+ or NPY+) and calbindin-containing neurons spreading through the extended amygdala into the amygdala proper. The close relationship between the AEP and a corridor of CALB+ cells extending into the amygdala was noted in a previous publication (Legaz et al., 2005a). The AEP is rostromedially continuous with the pallidal septum, and both show a similar molecular profile. However, only the AEP is correlated with the corridor of peptidergic or CALB+ cells and produce cell groups distinct from those of the pallidal septum (see below). Based on their molecular profile similar to that of MGE during early developmental stages—strong expression of Nkx2.1, Lhx6, Lhx7/8 and lack of Shh—the pallidal septum and AEP subdivisions appear to represent additional pallidal-like domains of the telencephalon (septal pallidum and peduncular pallidum), distinct from the MGE (which represents the pallidum proper). As explained in detail in the next section, the MGE and AEP (as well as the POC) produce different parts of the medial and extended amygdala (including the BST).
In addition, a previous study indicated that the septum also includes a striatal-like division that expresses Dlx family genes but lacks Nkx2.1 expression in the proliferative zone (Puelles et al., 2000). In fact, Puelles et al. (2000) already showed that all subpallial subdivisions extend rostromedially into the septum. Our data are consistent with this, and provide additional evidence indicating that the subpallial part of the septum actually includes striatal, pallidal, AEP, and POC subdomains (Fig. 16). Based on expression of Nkx2.1, Lhx6, Lhx7/8, and specially Gbx1, the diagonal band nuclei and perhaps the cholinergic cells of the medial septum appear to derive from POC (Figs. 15, 16). On the other hand, the lateroventral septal nucleus expresses Nkx2.1, Lhx7/8, but not Gbx1, and appears to derive from the pallidal septum. Previous functional studies considered the medial septum and diagonal band as the “pallidal part” of the septum, whereas the lateral septum was considered “striatal,” irrespective of their apparent superposed radial disposition (Swanson, 2000). Our data provide an alternative map of the histogenetic divisions of the septum, which may help in the understanding of some septal connectivity patterns. For example, the lateroventral septal nucleus shows connections similar to those of some derivatives of the peduncular pallidum or AEP (as the anterior BSTM), such as subicular (hippocampal) input and output to the neuroendocrine hypothalamic zone (Swanson, 2000; Dong et al., 2001), and both may represent pallidal-like output stations of the telencephalon.
On the other hand, our data indicate that a large rostral, precommissural part of the ventral pallidum derives from the septal pallidum (or pallidal septum), but the remaining caudal, commissural part of the ventral pallidum derives from MGE (Figs. 15, 16). It would be interesting to analyze in the future whether these two parts differ substantially in their connectivity patterns. Based on its apparent MGE origin, the connections of the commissural ventral pallidum might be similar to those of the globus pallidus (also a MGE derivative), and might be primarily related to somatomotor functions. On the contrary, based on its pallidoseptal origin, the rostral part of the ventral pallidum might be primarily connected to parts of the so-called limbic system (hippocampus, septum, hypothalamus).
Amygdalar and extended amygdalar derivatives of the LGE, MGE, AEP, and POC
Our gene expression results suggest that the subpallial subdivisions LGE, MGE, AEP, and POC give rise to different parts of the centromedial and extended amygdala (including the BST) (Figs. 15, 16).
Striatal derivatives (from LGE)
Based on their strong Dlx5 expression and weak Nkx2.1, Lhx6, and Lhx7/8 expression, the IPAC, the central amygdalar nucleus, and the main intercalated amygdalar masses appear to derive from the LGE (possibly from its caudal extension in the so-called caudal ganglionic eminence), thus representing the striatal parts of the amygdala proper and extended amygdala. This is consistent with the previous suggestion of the striatal nature of the central amygdalar nucleus (Puelles et al., 2000; Medina et al., 2004) and main intercalated masses (Medina et al., 2004) based on expression of Pax6 and/or Dlx2/5, but the absence of pallial marker genes such as Tbr1. Our interpretation is also consistent with the suggestion that the central and intercalated amygdalar nuclei are in part derived from the dorsal part of the LGE (dLGE), a molecular subdomain expressing Pax6 but lacking pallial marker genes such as Lhx2, Lhx9, and Emx2 (Yun et al., 2001; Tole et al., 2005). Similarly, it appears that the dorsal part of the anterior amygdala (AAd) is also a dLGE derivative (Tole et al., 2005). Our results provide novel developmental evidence supporting the striatal nature of IPAC (previously called striatal fundus or fundus striati) (Alheid et al., 1995, 1999; Shammah-Lagnado et al., 2001). In particular, Shammah-Lagnado et al. only considered the lateral part of IPAC as “striatal,” whereas its medial part was considered to be part of the extended amygdala system. According to our data, both parts may be striatal derivatives. Consistent with their LGE (striatal) origin, the central and main intercalated nuclei, as well as the IPAC, show striatal-like chemoarchitecture and connectivity features (Swanson and Petrovich, 1998; Swanson, 2000; Shammah-Lagnado et al., 2001; Gartner et al., 2002).
In addition, the BSTL—which as discussed below appears to primarily derive from MGE—also shows Dlx5 expression, suggesting that although primarily pallidal it may contain a subset of striatal cells (originating in LGE). This is consistent with a previous suggestion that part of BSTL originates in the caudal ganglionic eminence (CGE) (Nery et al., 2002), which includes a major striatal domain. Nery et al. consider the CGE as a histogenetic entity distinct from LGE and MGE, giving rise to distinct subsets of cortical and subcortical neurons, including those of the central amygdala and part of BSTL. However, the CGE includes striatal and pallidal portions (the CGE contains, in fact, a major caudal continuation of LGE and a minor caudal continuation of MGE) and, for this reason, the conclusions of Nery et al. (2002) need to be considered with caution. Nevertheless, it appears that the striatal part of CGE gives rise to a different subset of cortical interneurons (Xu et al., 2004), and includes a dorsal subdomain (apparently an expanded caudal continuation of dLGE) that gives rise to the central amygdala (Tole et al., 2005). Perhaps this striatal dorsal subdomain of CGE giving rise to part of the central extended amygdala also produces a subset of cells that populate part of the BSTL (a structure that, as noted above and below, appears to be primarily pallidal).
MGE, AEP, and POC derivatives
Our results indicate that the MGE, AEP, and POC subpallial subdivisions give rise to different parts of the medial and extended amygdala (including different parts of the BST) (Figs. 15, 16). The three subdivisions show strong expression of Nkx2.1, Lhx6, and Lhx7/8 during early development, and from E16.5 they start to show differences in expression intensity (see details in Results and Table 1C). Moreover, POC differs from the rest because of its expression of Shh, whereas the AEP differs due to its correlation with SOM+, NPY+, and CALB+ cells (Table 1C). Based on the features explained above and summarized in Table 1C, the AEP appears to give rise to MePD, part of MeA, several BST subnuclei (BSTMa, BSTMpm, BSTia), the sublenticular and rostral/septal parts of the extended amygdala (SLEA, SEA), and the ventral part of the anterior amygdala (AAv) (Fig. 16). The posterolateral part of the BSTM (BSTMpl) shows weak expression of Nkx2.1, Lhx6, and Lhx7/8, and apparently includes a minor subpopulation of subpallial cells (perhaps derived from AEP and/or POC). Based on Shh expression, the POC may generate at least a subset of cells of MePV (located in its central part) and MeA, although these nuclei also appear to include cells of diencephalic origin (see next section) (Fig. 16). Based on the expression of Lhx9, some cells of MeA and a superficial part of MePV derive from the ventral pallium. On the other hand, based on its position and gene expression profile, the BSTL primoridum may primarily derive from the MGE (pallidum proper) (Fig. 16). However, as it matures it appears to receive a subpopulation of striatal neurons (based on Dlx5 expression and experimental data provided by Nery et al., 2002; explained above). In addition, BSTL may receive a subset of Shhexpressing cells derived from POC, noted above to migrate tangentially into MGE (Figs. 1J, 2E).
Our results are consistent with the previous suggestions that part of BST derives from the Nkx2.1-expressing domain of the subpallium (Puelles et al., 2000) or from MGE (Bayer, 1987). Our data also confirm previous descriptions of expression of several LIM-homeodomain genes (Lhx6, Lhx9) in specific subnuclei of the medial amygdala during development (Remedios et al., 2004; Tole et al., 2005). Recent data indicate that these transcription factors are also expressed in similar patterns in the amygdala of adult mice (Choi et al., 2005). However, our analysis of the combinatory expression patterns of genes and markers of neuronal subtypes provides relevant information for understanding the exact histogenetic origin of many parts of the centromedial and extended amygdala in either LGE, MGE, AEP, or POC subdivisions of the subpallium, or in the ventral pallium. Moreover, our analysis provides the embryological basis that helps understanding the complex molecular, cellular, and anatomical features of the centromedial and extended amygdala.
Comparison to previous studies on the centromedial and extended amygdala
Medial amygdala: multiple subdivisions and origins
Our analysis is particularly relevant for understanding the medial amygdala, which appears to be formed by components of different origins: MePD from AEP, a central part of MePV from POC, a superficial part of MePV from the ventral pallium, and MeA from AEP, POC, and ventral pallium (Fig. 16). As discussed below, it is likely that MePV and MeA also contain cells of hypothalamic/diencephalic origin. Remarkably, no part of the medial amygdala derives from the pallidum proper or MGE, although some cell groups derive from the peduncular pallidum or AEP (i.e., these could be regarded as pallidal-like based on their similar molecular profile). However, our data disagree with the suggestion of a striatal nature of the medial amygdala (Swanson and Petrovich, 1998; Swanson, 2000). Our data are consistent with a recent study showing that Lhx6- and Lhx9-expressing cells of the medial amygdala represent different subpopulations, most of which occupy different positions either in MePD or MePV, respectively (Choi et al., 2005; see also Remedios et al., 2004; Tole et al., 2005). Our data indicate that these two cell populations originate either in AEP or ventral pallium, respectively. The subpallial origin of Lhx6 cells of MePD is consistent with these cells being GABAergic and having inhibitory projections to the BST and hypothalamus in adult rodents (Choi et al., 2005).
The MePV, on the other hand, appears to have glutamatergic projections to the hypothalamus, although these projections do not originate in Lhx9 cells of MePV (Choi et al., 2005). Our data indicate that Lhx9 cells of MePV concentrate in a superficial part of the nucleus with ventral pallial origin, which is consistent with the previous finding of Tbr1 expression in this superficial area (Puelles et al., 2000; see also Medina et al., 2004). The MePV projections to the ventromedial hypothalamic nucleus reported to be glutamatergic originate in the central part of the nucleus (see fig. 7 in Choi et al., 2005); i.e., the part that shows Shh-expressing cells, putatively derived from POC. The phenotype of the Shh-expressing cells of MePVc is unknown, although these likely are either GABAergic or cholinergic, but not glutamatergic (which would be atypical for the subpallium). It is possible that the glutamatergic cells of the central part of MePV originate in a subdivision other than POC, such as the thalamic eminence or the supraopto-paraventricular hypothalamic domain (Puelles et al., 2000, 2004; Medina et al., 2004). Both extratelencephalic domains express Tbr1 (Puelles et al., 2000, 2004; Medina et al., 2004), a transcription factor involved in differentiation of glutamatergic neurons (Hevner et al., 2001). Moreover, the supraoptic-paraventricular hypothalamic domain shows specific expression of Sim1 and Otp, which extends into (and may contribute cells to) the amygdala (Fan et al., 1996; Wang and Lufkin, 2000).
The MeA also appears to contain cells of different histogenetic origins, including at least the ventral pallium (Lhx9-expressing cells), the AEP (Lhx6-expressing cells), the POC (Shh-expressing cells), as well as extratelencephalic. Interestingly, the MeA also includes a subpopulation of neurons expressing Lhx5 in adult (Choi et al., 2005), as well as developing mice (Abellán and Medina, unpubl. obs.). The Lhx9-, Lhx6-, and Lhx5-expressing cells of the medial amygdala represent nonoverlapping subpopulations (Choi et al., 2005), which is consistent with their distinct embryological origin. As noted above, the Lhx6-expressing cells appear to derive from AEP, whereas the Lhx9 cells are likely to derive from the ventral pallium. It is possible that the Lhx5-expressing cells of the MeA originate in the thalamic eminence and/or the supraoptic-paraventricular hypothalamus, which show specific expression of this transcription factor during mouse development (Sheng et al., 1997; Abellán and Medina, unpubl. obs.).
A very interesting observation is that the different origins of MePD and MePV correlate well with, and might explain, their different patterns of projections to the BST and medial hypothalamus and their functional differences (see below). For example, the MePD (an AEP derivative) is specifically activated by sexual olfactory stimuli and projects primarily and heavily to the hypothalamic centers involved in control of reproductive behavior, such as the ventrolateral part of the ventromedial hypothalamic nucleus, or VMH (Petrovich et al., 2001; Choi et al., 2005). This projection is specifically mediated by MePD cells expressing Lhx6, which are GABAergic (Choi et al., 2005). On the other hand, the MePV (mainly a POC derivative) is activated mainly by defensive stimuli, but also by reproductive stimuli, and projects to hypothalamic centers involved in either defensive behavior (such as the dorsomedial part of VMH) or reproductive behavior (the ventrolateral VMH) (Petrovich et al., 2001; Choi et al., 2005). It appears that the MePV cells that project to either part of VMH (reproductive or defensive) are distinct, and both are excitatory (Choi et al., 2005). Interestingly, the MePV cells that project to the reproductive part of VMH are specifically activated by defensive stimuli (but not by reproductive stimuli), offering a double-gate mechanism for control of reproduction: either by way of reproductive stimuli and the inhibitory projection of MePD, or by way of defensive stimuli and the excitatory projection of MePV (Choi et al., 2005) (see Fig. 17).
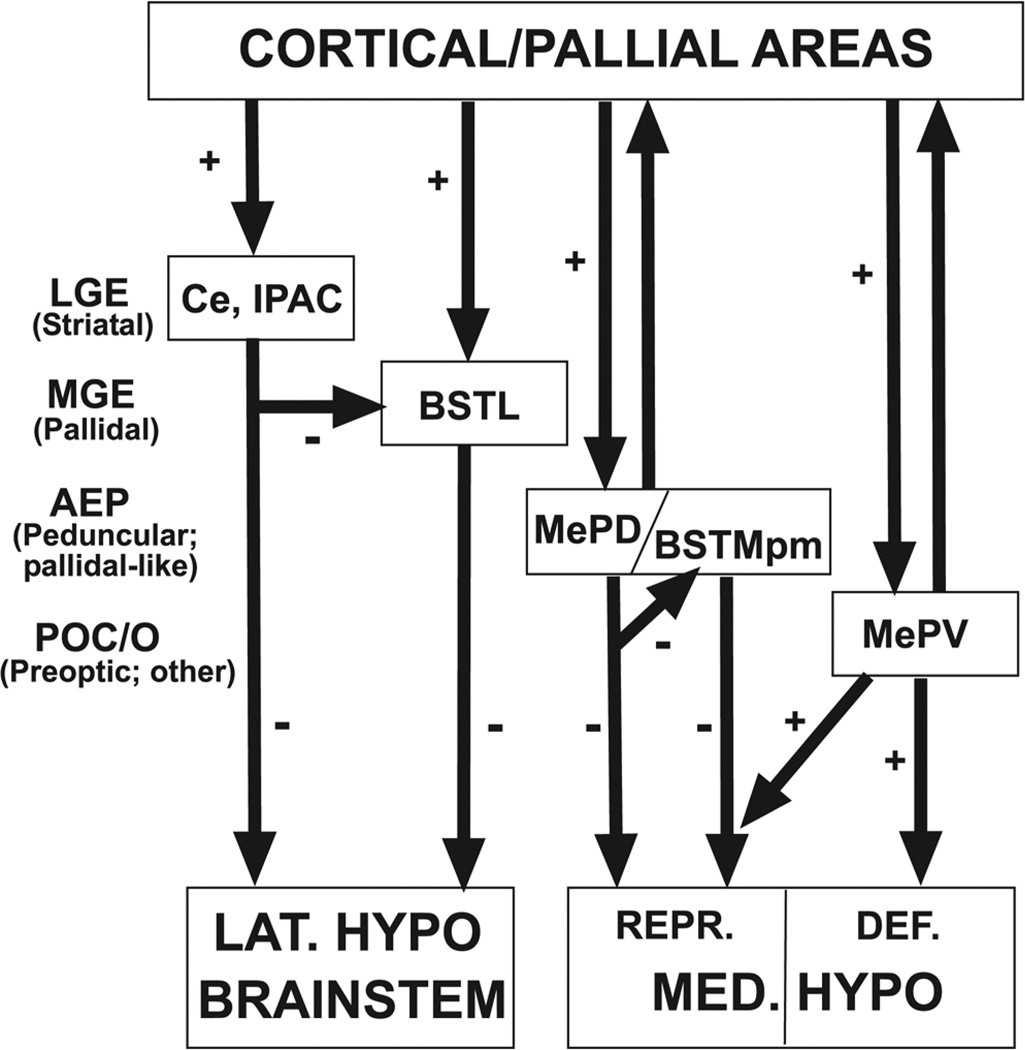
Fig. 17.
Diagram representing the functional organization of the amygdala in the context of the telencephalic ontogenetic units (as evidenced here). The centromedial and extended amygdala represent the output centers for the cerebral control of autonomic and neuroendocrine systems (regulating reflex-like behaviors such as reproduction and defense). There appears to be a correlation between the connections of each amygdalar subdivision and its histogenetic origin. For example, the central amygdala (Ce, derived from LGE and, therefore, striatal-like) projects directly and indirectly to the lateral hypothalamus and brainstem (involved in escape behavior), and the indirect projection primarily relays on the BSTL (derived from MGE or pallidum proper, and possibly containing abundant cells of striatal origin). On the other hand, the posterodorsal medial amygdala (MePD; derived from AEP or peduncular pallidum) projects directly and indirectly to the medial hypothalamus (specifically to centers involved in reproductive behavior), and the indirect projection primarily relays on AEP-derived parts of BST (such as BSTMpm). Finally, the posteroventral medial amygdala (MePV; derived from the POC, pallium, and diencephalon) projects directly and indirectly to the medial hypothalamus (to centers involved in either reproduction or defense), and the primary relay for the indirect projection is a BST part of putative extratelencephalic origin (BSTMpl). While the projections from subpallial parts of the centromedial and extended amygdala are inhibitory (GABAergic), the projections of MePV to the hypothalamus are excitatory (the embryological origin of the projection cells remains unclear). Amygdalar centers with different origins are controlled by different cortical/pallial areas, and may regulate different aspects of behavior (for example, reproduction versus defense) or, as proposed recently (Choi et al., 2005), may provide alternative mechanisms for regulating behavior in answer to different types of stimuli. For example, a sexual stimulus activates the circuits through MePD to the hypothalamus that promote reproductive behavior (perhaps involving disinhibition of hypothalamic targets); however, a defense stimulus may block reproductive behavior by way of the projections from MePV to hypothalamic centers involved in reproduction (perhaps ending presynaptically on inhibitory terminals of MePD/BSTMpm-hypothalamic axons). See text for more details.
BST and the extended amygdala
Our analysis is also helpful for understanding the origin of different subdivisions of the extended amygdala and, particularly, the BST (Fig. 14). According to our data, the BSTL (or at least a subset cells of BSTL) appears to derive from the MGE, whereas the anterior and posteromedial BSTM and the BSTia derive from the AEP. Furthermore, the posterolateral BSTM appears to contain a minor subpopulation of cells derived from the subpallium (perhaps AEP and/or POC). However, most cells of the posterolateral BSTM do not express subpallial marker genes, and may have an extratelencephalic origin. The existence of a posterior part of the BST having an extratelencephalic origin (possibly in the eminentia thalami) was previously noticed by Puelles et al. (2000), based on expression of Tbr1 and Pax6 (eminentia thalami markers), and the lack of the subpallial markers Dlx2 and Nkx2.1. Based on Sim1 expression, the supraoptic-paraventricular hypothalamic domain (which also expresses Tbr1 and Pax6) has also been suggested as a possible contributor of cells to the BST (Fan et al., 1996; Puelles and Rubenstein, 2003; Puelles et al., 2004).
The BST has been divided into anterior and posterior parts based on developmental criteria (Johnston, 1923; Bayer, 1987; see also Ju and Swanson, 1989), a scheme that has been adopted in recent developmental studies including the BST (Puelles et al., 2000). The anterior–posterior divisional scheme was recently modified to anteromedial, anterolateral, and posterior divisions to better account for the complex BST connectivity patterns (Dong et al., 2001). On the contrary, other authors have divided the BST in lateral and medial major components (Alheid et al., 1995; brain atlas by Paxinos et al., 1999; and Paxinos and Franklin, 2004; here we have followed this scheme). Our data indicate that the development of the BST is more complex than previously thought, and apparently contains subdivisions that originate in different subpallial histogenetic territories (MGE and AEP), and may also include an extratelencephalic part. It is not clear whether the nomenclature based on anteroposterior divisions (for example, Dong et al., 2001) is more appropriate than the one based on lateromedial divisions (Paxinos and Franklin, 2004) or vice versa. For example, the posterior BST of Dong et al. (2001) includes derivatives from AEP (our posteromedial BSTM) and possibly from outside the telencephalon (most of the posterolateral BSTM). However, as a first approximation, our data agree with the existence of anterolateral, anteromedial, and posterior parts of the BST derived from different histogenetic divisions. The anterolateral BST (our BSTL) appears to primarily derive from the MGE (apparently with a contribution from LGE). The anteromedial BST derives from the AEP division and includes the BSTMa and BSTMpm, whereas the posterior BST may primarily have an extratelencephalic origin and includes the posterolateral BSTM.
On the other hand, analysis of the BST connections (see excellent review by Dong et al., 2001) in the context of the embryological origin of subdivisions (as evidenced here) shows some interesting correlations (Fig. 17). For example, among the pathways related with the accessory olfactory system, projections from the MePD (an AEP derivative) show a trend to specifically target BST nuclei derived from the AEP division (anterior BSTM and posteromedial BSTM), but avoid BST nuclei derived from MGE or other origins (BSTL, posterolateral BSTM) (see fig. 3 of Dong et al., 2001). On the contrary, MePV (which central part derives from POC) projects very heavily to the posterolateral BSTM (primarily extratelencephalic), but shows only light to moderate projections to AEP derivatives (anterior and posteromedial BSTM), and does not project at all to MGE/LGE derivatives (BSTL). Thus, some of the complex connectivity patterns of the BST with the amygdala are consistent with our proposed embryological origin of their subdivisions; future studies will be needed to further evaluate this suggestion.
On the other hand, our data are partially consistent with previous studies indicating the existence of a continuum of cells connecting the centromedial amygdala with the BST (extended amygdala concept, proposed by Alheid, Heimer, and de Olmos; Alheid and Heimer, 1988; Alheid et al., 1995; Alheid, 2003). Herein, we provide developmental and genetic evidence indicating the existence of a true cell corridor connecting the MePD and the BSTM: both the cell corridor (including the sublenticular part of the extended amygdala or SLEA) and the extremes (MePD/BSTia caudolaterally and BSTMa/BSTMpm medially) derive all from AEP. During early developmental stages (E14 in rat), the corridor connecting the BSTM and MePD appears related to glial fibers (possibly radial glial fibers; Cooke and Simmerly, 2005), supporting that this represents a true radial histogenetic domain. Thus, this continuum constitutes both an embryological unity (according to our data) as well as a functional unity (based on chemoarchitecture and connections; Alheid et al., 1995). Neurochemical and connectivity data support the existence of central and medial divisions of the extended amygdala as proposed by Alheid et al. (1995): i.e., one cell corridor connecting the central amygdala-BSTL and another one connecting the medial amygdala-BSTM (involving central and medial subdivisions of SLEA) (Alheid and Heimer, 1988; Alheid et al., 1995; Shammah-Lagnado et al., 1999, 2001; Alheid, 2003; Bourgeais et al., 2001). The proposed central and medial divisions of the extended amygdala are not clearly distinguished based on embryological data, since the entire SLEA appears to derive from AEP and is pallidal-like. However, it is interesting to note that while all components of the medial extended amygdala derive from AEP, most components of the central extended amygdala (central amygdala, IPAC, BSTL) derive from either LGE or MGE.
Central amygdala: a striatal nucleus with a subpopulation of AEP-derived cells
As noted previously, the central nucleus of the amygdala derives from the caudal part of LGE and is striatal-like (Puelles et al., 2000; Medina et al., 2004; Tole et al., 2005; present results). The caudal part of the LGE would correspond to the main, lateral part of the caudal ganglionic eminence (CGE) described in previous studies. However, our developmental data indicate that the central amygdala includes a subpopulation of immigrant SOM+ cells that originate in the AEP (see Results). This group of immigrant cells is going to occupy the medial part of the central amygdala, and constitutes a remarkable cell group of large, strongly SOM+ cell bodies. It is unknown whether these immigrant neurons of the medial part of the central amygdala are interneurons (as usually occurs in the caudoputamen; Marin et al., 2000) or whether they constitute a subset of ectopic pallidal-like projections neurons, a possibility that has been observed in the striatum of birds (Farries et al., 2005). Supporting the latter suggestion, a subset of projection neurons the mammalian central amygdala co-contain GABA and somatostatin (Batten et al., 2002; Saha et al., 2002) as do some projection neurons of BSTL and other pallidal/MGE-derived structures (Vincent and Brown, 1986; Moga et al., 1989). Interestingly, the medial part of the central amygdala contains a type of projection neuron that receives pallial/cortical input (from the basolateral amygdala), striatal-like input from the capsular/lateral divisions of the central amygdala, and projects to the hypothalamus/brainstem; this pathway is important for modulation of autonomic fear responses (Huber et al., 2005). This resembles a pallidal-like cell in a cortico-striato-pallidal output pathway, similar to those described in other parts of the telencephalon (Swanson, 2000; see discussion on this below). Nevertheless, more studies are needed to know whether the cell type described by Huber et al. (2005) in the medial part of the central amygdala is striatal-like or pallidal-like from an embryological point of view.
Implications for understanding the functional organization of the amygdala
It has been suggested that the amygdala is a central component of a cortico-striato-pallidal output circuit involved in control of neuroendocrine and autonomic systems (Swanson, 2000). This interesting proposal should be reexamined in the context of our current understanding of the embryological origin of the different amygdalar and extended amygdala subdivisions. Our data indicate that, in addition to pallial/cortical parts (Puelles et al., 2000; Medina et al., 2004), the amygdala proper and the extended amygdala include striatal (LGE), pallidal (MGE), peduncular (AEP, pallidal-like), and commissural preoptic (POC) subpallial derivatives, in addition to cells derived from the diencephalon/hypothalamus. Thus, the amygdalar pathways for control of neuroendocrine and autonomic systems need to be reevaluated considering these new data (Fig. 17). For example, the central amygdala—a striatal derivative—receives cortical/pallial input (including, among others, input from gustatory and visceral neocortical areas, and from the lateral and basolateral amygdala) and projects directly to the lateral hypothalamic region involved in autonomic system control (Bourgeais et al., 2001; Petrovich et al., 2001; Alheid, 2003). In addition, the central amygdala projects indirectly to the hypothalamus, primarily by way of the dorsal BSTL (MGE derivative, with LGE contribution), and weakly to moderately by way of the anterior BSTM (AEP derivative) (Dong et al., 2001). These may represent two different pallidal or pallidal-like stations of a cortico-striato-pallidal output pathway for indirect regulation of different aspects of autonomic behavior.
On the other hand, the medial amygdala is known to project to medial parts of the hypothalamus, but the projections differ depending on the embryological origin of the subdivision (explained above; Fig. 17). For example, the MePD (an AEP derivative) projects heavily to the hypothalamic region involved in control of reproductive behavior, and this projection is inhibitory (Petrovich et al., 2001; Choi et al., 2005; see also Coolen and Wood, 1998; Coolen et al., 1998). On the contrary, the MePV (which central part may derive from the POC and diencephalon) projects to hypothalamic centers involved in either reproductive or defensive behavior, and this projection is excitatory (Petrovich et al., 2001; Choi et al., 2005); moreover, the MePV shows the heaviest influence on the neuroendocrine system (Petrovich et al., 2001). In addition, the medial amygdala shows indirect projections to the hypothalamus by way of the BST (Coolen and Wood, 1998). As noted above, each subdivision of the medial amygdala projects preferentially to BST parts of similar embryological origin. Thus, there may be separate indirect pathways for modulation of different aspects of behavior. However, these pathways cannot be explained following the cortico-striato-pallidal model as applied previously (Swanson, 2000), since the medial amygdala does not appear to be striatal. Instead, they appear to represent different AEP or POC (or other) output pathways to the hypothalamus (Swanson, 2000; Dong et al., 2001). The latter is not surprising, since many of the previously recognized cortical projections to the pallidum, such as those to the substantia innominata and most parts of the BST (see review by Swanson, 2000), include targets in the pallidum proper (MGE), AEP, and POC parts of the basal telencephalon. Another interesting observation is that, as many other pallidal, pallidal-like (AEP) or POC structures, all parts of the medial amygdala show direct (reciprocal) projections to the cortex (hippocampal formation) (McDonald et al., 1999; Petrovich et al., 2001). In contrast, the striatal central nucleus shows no direct cortical projections (Petrovich et al., 2001), but projects to corticopetal cell groups of the basal telencephalon (targeting mostly noncholinergic neurons, but also a caudal subset of cholinergic cells, shown here to be POC derivatives) that in turn project to the cerebral cortex, and this indirect pathway to the cortex has been involved in attention during association learning tasks (Gastard et al., 2002; Jolkkonen et al., 2002).
Acknowledgments
Grant sponsor: Spanish Ministry of Science and Technology (DGICYT-FEDER); Grant numbers: BFI2003-06453-C02-02, BFU2006-14804-C02-02/BFI (to L.M.); BFU2005-09378, C01-C02/BFI (to L.P.); Grant sponsor: Health Institute Carlos III-FEDER; Grant number: 01/0057-02 (to L.M.); Grant sponsor: Séneca Foundation; Grant number: PB/50/FS/02 (to L.M.); Grant sponsor: Nina Ireland (to J.L.R.R.); Grant sponsor: National Institute of Mental Health (NIMH); Grant number: K05 MH065670 (to J.L.R.R.).
We thank the investigators who kindly provided us with some of the cDNAs used in the present study (V. Pachnis: Lhx6, Lhx7/8; M. Brand: Gbx1; S. Retaux: Lhx9; A.P. McMahon: Shh). We thank the anonymous reviewers for very useful suggestions for improving this article.
Abbreviations
- AAd
Anterior amygdala, dorsal part
- AAv
Anterior amygdala, ventral part
- ac
Anterior commissure
- AC
Nucleus of the anterior commissure
- Acc
Nucleus accumbens
- ACo
Anterior cortical amygdalar area
- ADP
Anterodorsal preoptic nucleus
- AEP
Anterior peduncular area (previously called anterior entopeduncular area)
- BAOT
Nucleus of the accessory olfactory tract
- Bas
Basal nucleus (Meynert)
- BC
Basal amygdalar complex
- BM
Basomedial amygdalar nucleus
- BMC
Basal magnocellular complex
- BST
Bed nucleus of the stria terminalis
- BSTia
Bed nucleus of the stria terminalis, intra-amygdaloid part
- BSTL
Bed nucleus of the stria terminalis, lateral part
- BSTLd
Bed nucleus of the stria terminalis, lateral part, dorsal subdivision
- BSTLp
Bed nucleus of the stria terminalis, lateral part, posterior subdivision
- BSTM
Bed nucleus of the stria terminalis, medial part
- BSTMa
Bed nucleus of the stria terminalis, medial part, anterior subdivision
- BSTMpl
Bed nucleus of the stria terminalis, medial part, posterolateral subdivision
- BSTMpm
Bed nucleus of the stria terminalis, medial part, posteromedial subdivision
- Ce
Central amygdala (central amygdalar nucleus)
- CeM
Central amygdala, medial part
- Cl
Claustrum
- cLGE or CLGE
Lateral ganglionic eminence, caudal part (usually included in the caudal ganglionic eminence)
- cMGE or CMGE
Medial ganglionic eminence, caudal part part (usually included in the caudal ganglionic eminence)
- Co
Cortical amygdalar area
- cp
Cerebral peduncle
- CPu
Caudate-putamen
- DB
Nuclei of the diagonal band
- DP
Dorsal pallium
- EA
Extended amygdala
- En
Endopiriform nucleus
- GP
Globus pallidus
- HDB
Nucleus of the diagonal band, horizontal part
- ic
Internal capsule
- IM
Intercalated masses of the amygdala, main part
- IPAC
Interstitial nucleus of the posterior limb of the anterior commissure
- L
Lateral amygdalar nucleus
- LGE
Lateral ganglionic eminence
- LOT
Nucleus of the lateral olfactory tract
- LOT1
Nucleus of the lateral olfactory tract, layer 1
- LOT2
Nucleus of the lateral olfactory tract, layer 2
- LOT3
Nucleus of the lateral olfactory tract, layer 3
- LP
Lateral pallium
- LPO
Lateral preoptic area
- LSv
Lateroventral septal nucleus
- Me
Medial amygdala (medial amygdalar nucleus)
- MeA
Medial amygdala, anterior part
- MeP
Medial amygdala, posterior part
- MePD
Medial amygdala, posterodorsal part
- MePV
Medial amygdala, posteroventral part
- MePVc
Medial amygdala, posteroventral part, central subdivision
- MePVs
Medial amygdala, posteroventral part, superficial subdivision
- MGE
Medial ganglionic eminence
- MP
Medial pallium
- MPA
Medial preoptic area
- MPO
Medial preoptic nucleus
- MS
Medial septal nucleus
- NCx
Neocortex
- PA
Palial amygdala
- pac
Posterior limb of the anterior commissure
- PCo
Posterior cortical amygdalar area
- Pir
Piriform cortex
- PMCo
Posteromedial cortical amygdalar area
- PO
Preoptic area
- POC
Commissural preoptic area (or commissural septo-preoptic area)
- PTh
Prethalamus (ventral thalamus)
- SEA
Extended amygdala, septal part
- SLEA
Extended amygdala, sublenticular part, Stria medullaris
- SP
Pallial septum
- SPa
Pallidal septum (or pallidal-like septum)
- SSt
Striatal-like septum
- st
Stria terminalis
- Str
Striatum
- Th
Thalamus (dorsal thalamus)
- Tu
Olfatory tubercule
- VGN
Lateral ventral geniculate nucleus
- VMPO
Ventromedial preoptic nucleus
- VP
Ventral pallium
- VPa
Ventral pallidum
- VPac
Ventral pallidum, commissural part
- VPapc
Ventral pallidum, precommissural part
Footnotes
The topographic term “sublenticular” refers to the globus pallidus–putamen complex, originally called “lenticular nucleus” in classical anatomical works; “supra,” “retro,” and “sublenticular” are still in use in neuroanatomical and neurological terminology, although “lenticular nucleus” has fallen into disuse, due to its implicit erroneous assumption that the putamen and globus pallidus form a morphological unit.
LITERATURE CITED
- Aggleton JP. The amygdala: a functional analysis. 2nd. Oxford: Oxford University Press; 2000. [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1998;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alheid GF, de Olmos J, Beltramino CA. Amygdala and extended amygdala. In: Paxinos G, editor. The rat nervous system. San Diego: Academic Press; 1995. pp. 495–578. [Google Scholar]
- Alheid GF, Shammah-Lagnado SJ, Beltramino CA. The interstitial nucleus of the posterior limb of the anterior commissure: a novel layer of the central division of extended amygdala. Ann N Y Acad Sci. 1999;877:645–654. doi: 10.1111/j.1749-6632.1999.tb09294.x. [DOI] [PubMed] [Google Scholar]
- Alifragis P, Liapi A, Parnavelas JG. Lhx6 regulates the migration of cortical interneurons from the ventral telencephalon but does not specify their GABA phenotype. J Neurosci. 2004;24:5643–5648. doi: 10.1523/JNEUROSCI.1245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbreuk CHJ, van Schaick HSA, Cox JJ, Kromkamp M, Smidt MP, Burbach JPH. The homeobox genes Lhx7 and Gbx1 are expressed in the basal forebrain cholinergic system. Neuroscience. 2002;109:287–298. doi: 10.1016/s0306-4522(01)00466-3. [DOI] [PubMed] [Google Scholar]
- Batten TF, Gamboa-Esteves FO, Saha S. Evidence for peptide cotransmission in retrograde- and anterograde-labelled central nucleus of amygdala neurones projecting to NTS. Auton Neurosci. 2002;98:28–32. doi: 10.1016/s1566-0702(02)00026-7. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Neurogenetic and morphogenetic heterogeneity in the bed nucleus of the stria terminalis. J Comp Neurol. 1987;265:47–64. doi: 10.1002/cne.902650105. [DOI] [PubMed] [Google Scholar]
- Bourgeais L, Gauriau C, Bernard JF. Projections from the nociceptive area of the central nucleus of the amygdala to the forebrain: a PHA-L study in the rat. Eur J Neurosci. 2001;14:229–255. doi: 10.1046/j.0953-816x.2001.01640.x. [DOI] [PubMed] [Google Scholar]
- Bourikas D, Pekarik V, Baeriswyl T, Grunditz A, Sadhu R, Nardó M, Stoeckli EY. Sonic hedgehog guides commissural axons along the longitudinal axis of the spinal cord. Nat Neurosci. 2005;8:297–304. doi: 10.1038/nn1396. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr Opin Neurobiol. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Brunelli G, Spano PF, Barlati S, Guarneri B, Barbon A, Bresciani R, Pizzi M. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:8752–8757. doi: 10.1073/pnas.0500530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone A, Puelles L, Porteus MH, Frohman MA, Martin GR, Rubenstein JLR. Spatially restricted expression of Dlx-1, Dlx-2 (Tes-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J Neurosci. 1993;13:3155–3172. doi: 10.1523/JNEUROSCI.13-07-03155.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Simerly RB. Ontogeny of bidirectional connections between the medial nucleus of the amygdala and the principal bed nucleus of the stria terminalis in the rat. J Comp Neurol. 2005;489:42–58. doi: 10.1002/cne.20612. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Anatomical interrelationships of the medial preoptic area and other brain regions activated following male sexual behavior: a combined fos and tract-tracing study. J Comp Neurol. 1998;397:421–435. doi: 10.1002/(sici)1096-9861(19980803)397:3<421::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala: a functional analysis. 2nd. Oxford: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- de Gelder B. Towards the neurobiology of emotional body language. Nat Rev Neurosci. 2005;7:242–249. doi: 10.1038/nrn1872. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Rev. 2001;38:192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Eisenstat DD, Liu JK, Mione M, Zhong W, Yu G, Anderson S, Ghatas I, Puelles L, Rubenstein JLR. DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J Comp Neurol. 1999;414:217–237. doi: 10.1002/(sici)1096-9861(19991115)414:2<217::aid-cne6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Fan CM, Kuwana E, Bulfone A, Fletcher CF, Copeland NG, Jenkins NA, Crews S, Martinez S, Puelles L, Rubenstein JLR, Tessier-Lavigne M. Expression patterns of two murine homologs of Drosophila single-minded suggest possible roles in embryonic patterning and in the pathogenesis of Down syndrome. Mol Cell Neurosci. 1996;7:1–16. doi: 10.1006/mcne.1996.0001. [DOI] [PubMed] [Google Scholar]
- Farries MA, Ding L, Perkel DJ. Evidence for “direct” and “indirect” pathways through the song system basal ganglia. J Comp Neurol. 2005;484:93–104. doi: 10.1002/cne.20464. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick-McElligott S, Card JP, Lewis ME, Baldino F., Jr Neuronal localization of prosomatostatin mRNA in the rat with in situ hybridization histochemistry. J Comp Neurol. 1988;273:558–572. doi: 10.1002/cne.902730410. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick-McElligott S, Card JP, O’Kane TM, Baldino F., Jr Ontogeny of somatostatin mRNA-containing perikarya in the rat central nervous system. Synapse. 1991;7:123–134. doi: 10.1002/syn.890070206. [DOI] [PubMed] [Google Scholar]
- Foster GA. A developmental atlas. Oxford: Oxford University Press; 1998. Chemical neuroanatomy of the prenatal rat brain. [Google Scholar]
- Gallagher M. The amygdala and associative learning. In: Aggleton JP, editor. The amygdala: a functional analysis. 2nd. Oxford: Oxford University Press; 2000. pp. 311–329. [Google Scholar]
- Gartner U, Hartig W, Riedel A, Brauer K, Arendt T. Immunocytochemical evidence for the striatal nature of the rat lateral part of interstitial nucleus of the posterior limb of the anterior commissure. J Chem Neuroanat. 2002;24:117–125. doi: 10.1016/s0891-0618(02)00035-2. [DOI] [PubMed] [Google Scholar]
- Gastard M, Jensen SL, Martin JR, 3rd, Williams EA, Zahm DS. The caudal sublenticular region/anterior amygdaloid area is the only part of the rat forebrain and mesopontine tegmentum occupied by magnocellular cholinergic neurons that receives outputs from the central division of extended amygdala. Brain Res. 957:207–222. doi: 10.1016/s0006-8993(02)03513-8. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Chronwall BM, Schafer MP, O’Donohue TL. Localization of neuropeptide Y messenger ribonucleid acid in rat and mouse brain by in situ hybridization. Synapse. 1987;1:25–31. doi: 10.1002/syn.890010106. [DOI] [PubMed] [Google Scholar]
- Grigoriu M, Tucker AS, Sharpe PT, Pachnis V. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development. 1998;125:2063–2074. doi: 10.1242/dev.125.11.2063. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Jones BE. Codistribution of GABA- with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J Comp Neurol. 1993;329:438–457. doi: 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458:11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JLR. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 2001;29:353–366. doi: 10.1016/s0896-6273(01)00211-2. [DOI] [PubMed] [Google Scholar]
- Holmgren N. Points of view concerning forebrain morphology in higher vertebrates. Acta Zool. 1925;6:413–477. [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Jacobowitz DM, Abbott LC. Chemoarchitectonic atlas of the developing mouse brain. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- Johnston JB. Further contributions to the study of the evolution of the forebrain. J Comp Neurol. 1923;35:337–481. [Google Scholar]
- Jolkkonen E, Miettinen R, Pikkarainen M, Pitkanen A. Projections from the amygdaloid complex to the magnocellular cholinergic basal forebrain in rat. Neuroscience. 2002;111:133–149. doi: 10.1016/s0306-4522(01)00578-4. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Kessaris TN, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Brain mechanisms of emotion and emotional learning. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Legaz I, Olmos L, Real MA, Guirado S, Dávila JC, Medina L. Development of neurons and fibers containing calcium-binding proteins in the pallial amygdala of mouse, with special emphasis in those of the basolateral amygdalar complex. J Comp Neurol. 2005a;488:492–513. doi: 10.1002/cne.20608. [DOI] [PubMed] [Google Scholar]
- Legaz I, García-López M, Medina L. Subpallial origin of part of the calbindin-positive neurons of the claustral complex and piriform cortex. Brain Res Bull. 2005b;66:470–474. doi: 10.1016/j.brainresbull.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR. Patterning, regionalization, and cell differentiaton in the forebrain. In: Rossant J, Tam PPL, editors. Mouse development: patterning, morphogenesis, and organogenesis. San Diego: Academic Press; 2002. pp. 75–106. [Google Scholar]
- Marín O, Anderson SA, Rubenstein JLR. Origin and molecular specification of striatal interneurons. J Neurosci. 2000;20:6063–6076. doi: 10.1523/JNEUROSCI.20-16-06063.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin O, Baker J, Puelles L, Rubenstein JLR. Patterning of the basal telencephalon and hypothalamus is essential for guidance of cortical projections. Development. 2002;129:761–773. doi: 10.1242/dev.129.3.761. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Shammah-Lagnado SJ, Shi C, Davis M. Cortical afferents to the extended amygdala. Ann N Y Acad Sci. 1999;877:309–338. doi: 10.1111/j.1749-6632.1999.tb09275.x. [DOI] [PubMed] [Google Scholar]
- Medina L, Legaz I, González G, de Castro F, Rubenstein JLR, Puelles L. Expression of Dbx1, Neurogenin 2, Semaphorin 5A, Cadherin 8, and Emx1 distinguish ventral and lateral pallial histogenetic divisions in the developing claustroamygdaloid complex. J Comp Neurol. 2004;474:504–523. doi: 10.1002/cne.20141. [DOI] [PubMed] [Google Scholar]
- Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989;283:315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1277. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Oh JD, Woolf NJ, Roghani A, Edwards RH, Butcher LL. Cholinergic neurons in the rat central nervous system demonstrated by in situ hybridization of choline acetyltransferase. Neuroscience. 1992;47:807–822. doi: 10.1016/0306-4522(92)90031-v. [DOI] [PubMed] [Google Scholar]
- Olivier C, Cobos I, Perez Villegas EM, Spassky N, Zald B, Martinez S, Thomas JL. Monofocal origin of telencephalic oligodendrocytes in the anterior entopeduncular area of the chick embryo. 2001;128:1757–1769. doi: 10.1242/dev.128.10.1757. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2nd. San Diego: Academic Press; 2004. [Google Scholar]
- Paxinos G, Ashwell KWS, Törk I. Atlas of the developing rat nervous system. 2nd. San Diego: Academic Press; 1994. [Google Scholar]
- Paxinos G, Kus L, Ashwell KWS, Watson C. Chemoarchitectonic atlas of the rat forebrain. San Diego: Academic Press; 1999. [Google Scholar]
- Perez-Villegas EM, Olivier C, Spassky N, Poncet C, Cochard P, Zalc B, Thomas JL, Martinez S. Early specification of oligodendrocytes in the chick embryonic brain. Dev Biol. 1999;216:98–113. doi: 10.1006/dbio.1999.9438. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JLR. Expression patterns of homeobox and other putative regulatory genes in the embryonic mouse forebrain suggest a neuromeric organization. Trends Neurosci. 1993;16:472–479. doi: 10.1016/0166-2236(93)90080-6. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JLR. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JLR. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Martínez S, Martínez-de-la-Torre M, Rubenstein JLR. Gene maps and related histogenetic domains in the forebrain and midbrain. In: Paxinos G, editor. The rat nervous system. 3rd. Amsterdam: Elsevier, Academic Press; 2004. pp. 3–25. [Google Scholar]
- Remedios R, Subramanian L, Tole S. LIM genes parcellate the embryonic amygdala and regulate its development. J Neurosci. 2004;24:6986–6990. doi: 10.1523/JNEUROSCI.0001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retaux S, Rogard M, Bach I, Failli V, Besson MJ. Lhx9: a novel LIM-homeodomain gene expressed in the developing forebrain. J Neurosci. 1999;19:783–793. doi: 10.1523/JNEUROSCI.19-02-00783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M, Lun K, Werner M, Simeone A, Brand M. Isolation and expression of the homeobox gene Gbx1 during mouse development. Dev Dyn. 2004;229:334–339. doi: 10.1002/dvdy.10435. [DOI] [PubMed] [Google Scholar]
- Saha S, Henderson Z, Batten TF. Somatostatin immunoreactivity in axon terminals in rat nucleus tractus solitarii arising from central nucleus of amygdala: coexistence with GABA and postsynaptic expression of sst2A receptor. J Chem Neuroanat. 2002;24:1–13. doi: 10.1016/s0891-0618(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Sequier JM, Hunziker W, Andressen C, Celio MR. Calbindin D-28k protein and mRNA localization in the rat brain. Eur J Neurosci. 1990;2:1118–1126. doi: 10.1111/j.1460-9568.1990.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Afferent connections of the interstitial nucleus of the posterior limb of the anterior commissure and adjacent amygdalostriatal transition area in the rat. Neuroscience. 1999;94:1097–1123. doi: 10.1016/s0306-4522(99)90280-4. [DOI] [PubMed] [Google Scholar]
- Shammah-Lagnado SJ, Alheid GF, Heimer L. Striatal and central extended amygdala parts of the interstitial nucleus of the posterior limb of the anterior commissure: evidence from tract-tracing techniques in the rat. J Comp Neurol. 2001;439:104–126. doi: 10.1002/cne.1999. [DOI] [PubMed] [Google Scholar]
- Sheng HZ, Bertuzzi S, Chiang C, Shawlot W, Taira M, Dawid I, Westphal H. Expression of murine Lhx5 suggests a role in specifying the forebrain. Dev Dyn. 1997;208:266–277. doi: 10.1002/(SICI)1097-0177(199702)208:2<266::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JLR. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Wang B, Campbell K. Tlx controls proliferation and patterning of lateral telencephalic progenitor domains. J Neurosci. 2003;23:10568–10576. doi: 10.1523/JNEUROSCI.23-33-10568.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JLR. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–330. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Tole S, Remedios R, Saha B, Stoykova A. Selective requirement of Pax6, but not Emx2, in the specification and development of several nuclei of the amygdaloid complex. J Neurosci. 2005;25:2753–2760. doi: 10.1523/JNEUROSCI.3014-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BH, Zimmer J. The architecture of some of the interconnections of the rat’s amygdala and lateral periallocortex. J Comp Neurol. 1984;227:540–557. doi: 10.1002/cne.902270406. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Brown JC. Somatostatin immunoreactivity in the entopeduncular projection to the lateral habenula in the rat. Neurosci Lett. 1986;68:160–164. doi: 10.1016/0304-3940(86)90134-5. [DOI] [PubMed] [Google Scholar]
- Wang W, Lufkin T. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev Biol. 2000;227:432–449. doi: 10.1006/dbio.2000.9902. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De la Cruz E, Rubenstein JLR, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JLR. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Marin O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JLR, Westphal H. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci U S A. 2003;100:9005–9010. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]