Abstract
PURPOSE:
The gut barrier is altered in certain pathologic conditions (shock, trauma, or surgical stress), resulting in bacterial and/or endotoxin translocation from the gut lumen into the systemic circulation. In this prospective randomized study, we investigated the effect of surgery on intestinal permeability (IP) and endotoxemia in patients undergoing elective colectomy for colon cancer by comparing the laparoscopic with the open approach.
PATIENTS AND METHODS:
A hundred twenty-three consecutive patients underwent colectomy for colon cancer: 61 cases were open resection (OR) and 62 cases were laparoscopic resection (LR). IP was measured preoperatively and at days 1 and 3 after surgery. Serial venous blood sample were taken at 0, 30, 60, 90, 120, and 180 min, and at 12, 24, and 48 h after surgery for endotoxin measurement.
RESULTS:
IP was significantly increased in the open and closed group at day 1 compared with the preoperative level (P < 0.05), but no difference was found between laparoscopic and open surgery group. The concentration endotoxin systemic increased significantly in the both groups during the course of surgery and returned to baseline levels at the second day. No difference was found between laparoscopic and open surgery. A significant correlation was observed between the maximum systemic endotoxin concentration and IP measured at day 1 in the open group and in the laparoscopic group.
CONCLUSION:
An increase in IP, and systemic endotoxemia were observed during the open and laparoscopic resection for colon cancer, without significant statistically difference between the two groups.
Keywords: Colon cancer, endotoxemia, Gut barrier function, laparoscopic surgery
INTRODUCTION
Laparoscopic surgery is as safe as open colorectal surgery, and laparoscopic colorectal cancer surgery is possible but should be performed within clinical studies and at surgical centers that have sufficient experience with laparoscopic colorectal surgery for benign diseases.[1] In addition to the ongoing discussion about the oncologic safety of this approach, it is not yet clear whether the laparoscopic approach offers significant immunological advantages over the conventional approach.[1] Some have suggested that the differences in cytokine and immune response noted after laparoscopic colorectal surgery are related to the extent of abdominal wall trauma, and thus one would anticipate less marked differences following laparoscopic-assisted colectomy.[1]
The intestinal epithelium undergoes a process of continuous renewal and consist of cells in various stages of differentiation. The integrity of the intestinal epithelium is critical to health[2] and any damage to the cells can influence the proliferation and differentiation, leading to altered cell population and functional changes in the intestine. The intestine acts as a barrier to the luminal contents, which include bacteria and endotoxins. The gut barrier is altered in some pathological conditions such as shock, trauma, or surgical stress, resulting in bacterial and/or endotoxin translocation from the gut lumen into the systemic circulation.[3] This has been implicated in postoperative complications, such as systemic inflammatory response syndrome (SIRS) and multiorgan failure[4] (MOF).
As laparoscopic colorectal surgery is recognized as a “mini-invasive” surgery; in this prospective randomized study, we investigated the effect of surgical intervention on intestinal permeability (IP), and endotoxemia in patients undergoing elective colectomy for colon cancer comparing laparoscopic with open approach.
PATIENTS AND METHODS
From March 2007 to February 2015, we studied, in a prospective randomized study, 123 patients (pts) consecutively (73 men and 47 women; mean age, 69.4 years) with colon cancer [Table 1]. Patients were randomly assigned to be treated with laparoscopic or open approach, according to a computer-generated table of random numbers. Randomization was performed by an independent computer consultant. The patient and the surgeon were informed about the type of approach just before the intervention. The Ethical Committee of Faculty of Medicine of the University of L'Aquila approved the study protocol, and informed consent was obtained for every patient.
Table 1.
Colon cancer: Open (group1) and Laparoscopic resection (group 2)
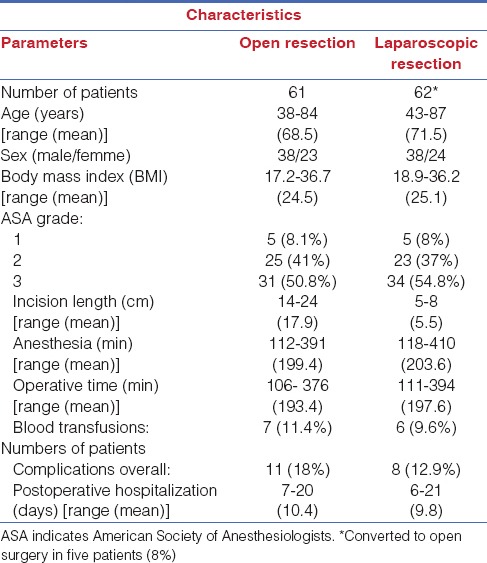
Patients with a known immune dysfunction (advanced liver disease, human immunodeficiency virus (HIV) infection, hepatitis C virus infection) and cardiac or pulmonary insufficiency were excluded for the study. Patients were also excluded if they had evidence of intraperitoneal sepsis or peritoneal contamination and if they had received antibiotics whitin 2 weeks prior to surgery. During hospitalization, patients were not given antispastic drugs, steroids, or nonsteroidal anti-inflammatory drugs (NSAIDs). The patients were classified according to the American Society of Anesthesiologists (ASA) grading system[5] as grade I, II, or III [Table 1]. Thirty-six patients had primary adenocarcinoma of the colon and 50 of the sigmoid colon.
Open resection (OR) was performed on 61 patients, while laparoscopic resection (LR) was adopted for 62 patients [Table 1].
Minimally-invasive colorectal surgery was performed as a laparoscopic-assisted procedure with removal of the resected specimen method by a horizontal minilaparotomy (mean 5.8 cm: Range 5-8 cm) just above the mons pubis. Laparoscopic surgery was performed using a 4-trocar technique with 1 trocar (10 mm) inserted through a paraumbilical incision (camera port). Two additional (5- or 10 mm) trocars were inserted in the right and left of lower abdomen, and one trocar (12 mm) was inserted in the midline just above the mons pubis (the site of the minilaparotomy). After removal of the resected specimen and preparation of the stapler anastomosis, we closed the minilaparotomy and reintroduced the pneumoperitoneum.
Conventional colorectal surgery was performed through a vertical midline incision ranging from 5 to 10 cm above the umbilicus to the mons pubis. After removing the resected specimen, we performed a stapler anastomosis.
All operations were performed by a single surgeon (Gianfranco Amicucci, M.D.).
The TNM system is the most widely used means for classifying the extent of cancer spread: 40 patients, stage I; 50 patients, stage II; 30 patients, stage III; and 3 patients, stage IV. All these patients did not receive any neoadjuvant therapy.
As shown in Table 1, age, sex, ASA grades, body mass index (BMI), blood transfusions, time of anesthesia, and operation were comparable in the two groups, except, obviously, for incision length.
Anesthesia was performed in both groups using the same procedure. Pre-anesthesia was accomplished using atropine (0.01 mg/kg) plus promethazine (0.5 mg/kg), with the addition of sodium thiopental (5 mg/kg) and atracurium (0.5 mg/kg) and tracheal intubation and assisted ventilation using nitrogen dioxide (NO2)/oxygen (O) 2:1. After intubation, anesthesia was maintained with oxygen in air, sevoflurane and remifentanil (0.25 mg/kg/min).
Blood Sampling
Serial venous blood samples were taken at pre-induction (0), intraoperatively (30, 60, 90, 120, and 180 min) and at 12, 24, and 48 h after surgery. Blood was stored in pyrogen-free tubes an centrifuged at 4 °C and 2000 rpm for 10 min and divided into sterile cryotubes (NUNC 36341, Intermed, Denmark) and stored at −80 °C until analysis for subsequent determination of endotoxin.
Intestinal Permeability
Intestinal permeability was assessed preoperatively and at day 1 and 3 after surgery, using the lactulose/mannitol differential absorption test.[6] This test is nontoxic, non-invasive, simple to perform, relatively inexpensive, and reproducible. It has been accepted as a reliable method for assessing small intestinal permeability. A pretest sample of urine was collected after 6 h of fasting to measured the baseline sugar in the urinary tract. After the pretest sample was obtained, patients were given 10 g of lactulose and 5 g of mannitol dissolved in 100 ml of water orally or via nasogastric tube. Urine was collected for 6 h, divided, and stored at -20° C until assayed. Urinary lactulose and mannitol concentrations were determined by an enzymatic technique.[6] Mannitol excretion was corrected by subtraction of baseline values determined in the pre-test samples, and the lactulose/mannitol excretion ratios (L/M ratio) were calculated.
Entotoxin Measurement
Endotoxin was quantified in duplicates using a modified chromogenic Limus amoebocyte lysate assay (Quadratech, Epsom, UK.). Plasma samples and standard test were diluted in 1:10 pyrogen-free water and heated to 75° C for 10 min to remove plasma inhibitors. The concentration of endotoxin in the sample was taken as the average of the duplicates calculated from standard curve. The assay has a sensitive of 8 pg/ml and is linear in the range 8-100 pg/ml. Each aliquot was assayed for endotoxin only once. If the assay gave poor duplicates or very high values, indicating a possible contamination, a fresh portion of the same sample was retested.
Statistical Analysis
Results were expressed as mean ± standard error of mean (SEM). Statistical analysis was performed using the Mann-Whitney U test or Spearman's rank-correlation coefficient (rs) where appropriate with revelance taken at the 5% level.
RESULTS
The LR required almost the same operating time of the OR [Table 1]. Hospitalization was shorter for LR, but was not statistically significant [Table 1]. In the laparoscopic group, conversion was required in three patients (8%) [Table 1]. The intention-to-treat analysis was performed, and these 5 patients were included in the analysis: 3 patients with left colon cancer and 2 patients with right colon cancer were converted to the open procedure.
Intestinal Permeability
No difference was observed in the preoperative L/M ratios in the two groups of patients [Figure 1].
Figure 1.
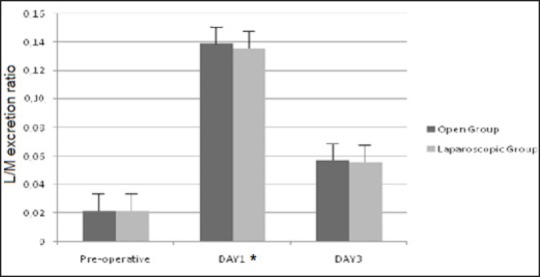
Intestinal permeability measured by lactulose/mannitol excretion ratio (L:M ratio), *P < 0.05 versus pre-operative. No difference between open and laparoscopic group
The L/M ratio was significantly increased in the open and closed group the first day (0.130 ± 0.004 and 0.128 ± 0.004, respectively) compared with the preoperative level (0.022 ± 0.006; P < 0.05), but no differences were found between laparoscopic and open surgery group [Figure 1].
Endotoxin
The systemic endotoxin concentration rose significantly, in both groups, during the course of surgery and returned to near baseline by day 2. No differences were found between laparoscopic and open surgery [Figure 2]. A significant correlation was observed between the maximum systemic endotoxin concentration and intestinal permeability measured at D1 (rs = 0.917; P = 0.001) in the open group [Figure 3a] and in the laparoscopic group (rs = 0.926; P = 0.001); [Figure 3b].
Figure 2.
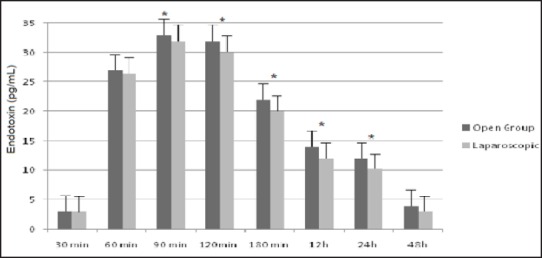
Systemic endotoxin concentration (mean ± SEM), *P <0.05 versus preoperative. No difference between open and laparoscopic group
Figure 3a.
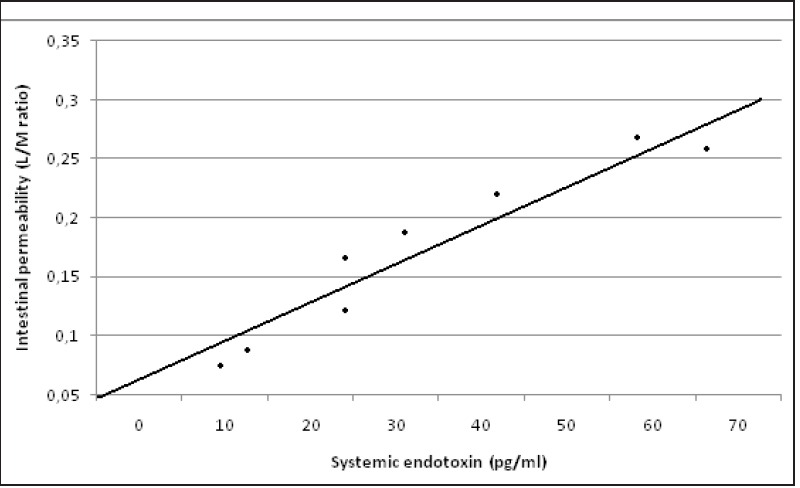
Correlation between systemic endotoxin concentration and intestinal permeability measured as lactulose/mannitol excretion ratio (L/M ratio) in the open group. (rs = 0.917; P = 0.001)
Figure 3b.
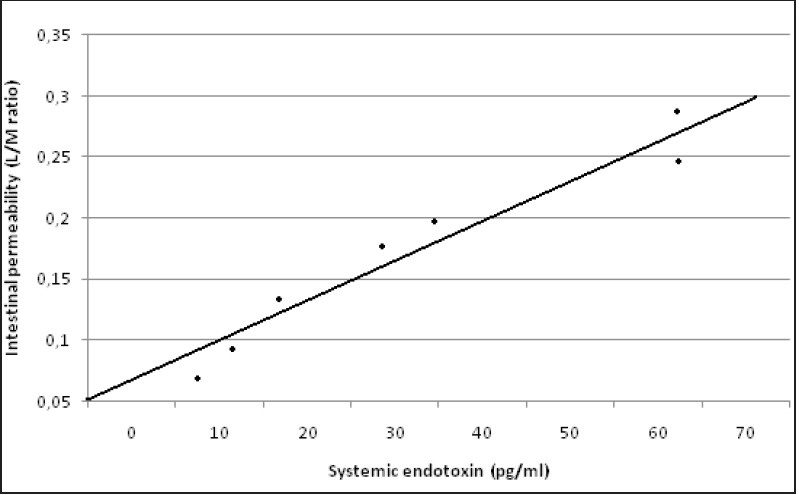
Correlation between systemic endotoxin concentration and intestinal permeability measured as lactulose/mannitol excretion ratio (L/M ratio) in the laparoscopic group. (rs = 0.926; P = 0.001)
Conversion
Five patients (8%) underwent conversion from laparoscopic to open procedure [Table 1]. These patients had intestinal permeability parameters, and systemic endotoxin concentration value similar in the open and closed group.
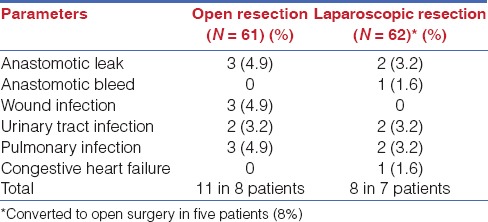
Complications
Complications are reported in Tables 1 and 2. For these patients, intestinal permeability parameters, and systemic endotoxin concentration value are similar to the group they belong to.
Table 2.
Complications
DISCUSSION
Until now, it has been reported that the degree of post-operative inflammation is reduced after laparoscopic surgery.[7] Other groups also observed significantly better preservation of lymphocytes subpopulations, neutrophil function, and cell-mediated immunity after laparoscopic versus open colorectal surgery.[8] Furthermore, it has been observed that cell-mediated immunity, as assessed by delayed-type hypersensitivity testing in humans, is better preserved after laparoscopic vs open colorectal resection.[9] This lesser degree of operative stress was also confirmed by experimental animal studies by Kuntz et al.[10] One group of investigators, in single center randomized trials, have reported that patients with colon carcinoma (stage III) undergoing laparoscopic colectomy had a significantly higher disease-free survival rate with mean follow-up of 43 months than patients treated by conventional open approaches.[11] Laurent et al also reported a better survival rate in patients with stage III tumors.[12] in a RCT trial in patients with mid and low rectal cancer and by Morino et al.[13] in a prospective comparative study which focused on patients with extraperitoneal rectal cancer treated with laparoscopic or open surgery. More recently, Law et al.[14] reported in a comparative monocenter series with a median follow-up of 34 months in patients with stage II and III rectal cancer, a 5-year actuarial survival of 71% in the laparoscopic group compared to 59% survival rate in the open-group, also identifying laparoscopy as one of the independent significant factors associated with better survival at the multivariate analysis.
The positive impact of the laparoscopic approach on survival is still unclear. Supporting evidence of the beneficial oncological role of laparoscopy includes its impact on surgical stress response, cellular immunity, cytokine relase, intraoperative tumor manipulation, and blood transfusion. Moreover, during the early postoperative period, laparoscopic patients seem to display decreased levels of pro-inflammatory and vascular endothelial growth factor (VEGF) compared to open.[15]
However, in additional to ongoing discussion about the oncologic safety of laparoscopic approach it is still not completely clear whether this approach offers significant immunological advantages over the conventional open approach.[16] Han et al. reported[17] in a prospective comparative study that in patients with stage III colorectal cancer, the cellular immune factors were significantly decreased; however there was no significant difference between the laparoscopic and open groups except for the mHLA-DR: monocyte human leukocyte antigen-DR that was affected less by the laparoscopic group compared to the open group.
The intestinal epithelium is a self-renewing arising from stem cell located at or near the base of the crypts.[18] Anup et al.,[19] using a rat model, showed that laparotomy with mild intestinal handling can result in permeability alterations and oxidative stress in the enterocytes, mainly as a results of activation of xanthine oxidase, and the damage is reversible with time. We have proved the[20] change of intestinal permeability only in patients undergoing open cholecystectomy, with no change occurring in the laparoscopic group. In the open technique, the peritoneum is manipulated, the small intestine handled, and the mesentery placed under traction, while in the laparoscopic cholecystectomy, these are absent or minimized.
Intestinal permeability
In this study (gut barrier function, and systemic endotoxemia after laparotomic or laparoscopic resection for colon cancer), a change of intestinal permeability was demonstrated both in patients undergoing open colorectal surgery and in patients undergoing laparoscopic colorectal surgery. The L:M ratio was significantly increased in the both groups at day 1 compared with the preoperative level, but no difference were found between laparoscopic and open surgery group. Since the since groups were similar, the greatest factor in difference between the groups of patients may be effect of surgical approach on intestinal manipulation. These factors (manipulation of peritoneum, etc.), which are integral parts of the open approach, evidently are also involved in laparoscopic colo-rectal approach. Laparoscopic-assisted colorectal resections, on the other hand, require an incision substantially larger than that typically required for other advanced laparoscopic procedures, such as gastric bypass or antireflux procedures. In our opinion, no difference, in intestinal permeability, noted after laparoscopic operations, as regards to the open group, is related to the extent of abdominal wall trauma. Furthermore, in regards to colectomy, there is a wide variation in the size of the incision, needed to extract the specimen and facilitate the anastomosis, dependent on body habitus, the size of the specimen and the surgeon. In our study, the size of the minilaparotomy changed from 5 to 8 cm (mean 5.8 cm). Unfortunately, a substantial proportion of colectomy studies do not provide detailed information regarding incision length. In addition, there is no consensus amongst surgeons as to what constitutes a laparoscopic-assisted procedure or conversion. Other variables that may impact the results of intestinal permeability studies include blood transfusion and the extent and location (right versus left) of the bowel resection performed.[21] Therefore, when evaluating the intestinal permeability, the colectomy population is much more heterogenous in regards to procedure performed and abdominal wall trauma induced than other advanced procedures.
Systemic endotoxemia
The surgical approach on intestinal manipulation may lead to the production of vasoactive agents that can cause both local and systemic hemodynamic changes.[22] There is evidence to support the role of prostaglandins is causing these hemodynamic responses, even though the exact mechanism leading to their generation is not fully understood.[23] The mucosa of the intestine and the endothelium of blood vessels contain enzymes capable of synthesizing prostaglandins, production of which may be initiated by neural, ischemic, toxic, or mechanical stimuli.[24] The generation of these vasoactive prostaglandins can therefore induce splanchnic ischemia with subsequent disruption of mucosal integrity and increased intestinal permeability. Therefore, intestinal manipulation may lead to the endotoxemia detected in the systemic circulation, in patients undergoing colorectal surgery. The results of the present study concur with this suggestion, as endotoxine was found in the systemic circulation in the open and laparoscopic groups of patients. The systemic endotoxin concentration rose significantly, in the both groups, during the course of surgery but returned to near baseline by day 2 and no difference were found between laparoscopic an open surgery group. This implies that mere manipulation of the intestine may have a deleterious effect on intestinal mucosa. Regardless of the etiology of impaired mucosal barrier function, permeation of luminal contents across the bowel wall does occur and results in the endotoxemia.[25] Endotoxin is a potent stimulator of release of cytokines, such as interleukin-6 and tumor necrosis factor. These inflammatory mediators play an important role in the pathogenesis of systemic inflammatory response syndrome and multiple-organ dysfunction syndrome.[26,27] However, the significance of the correlation between systemic endotoxin concentration and increased intestinal permeability remains unclear. In our study, the endotoxemia was detected mainly during the intraoperative period, but the increased intestinal permeability was observed only 24 h after surgery. Although it would be easy to suggest that endotoxemia was due to an increase in bowel permeability, one may develop independently of the other. Indeed, endotoxemia is known to reduce splanchnic blood flow and cause disruption of mucosal barrier function.[28] Furthermore, exposing the peritoneal cavity to the atmosphere may in it self lead to endotoxemia.[29]
In some cases, intestinal permeability, and endotoxemia was detected somewhat higher in the laparoscopic than the open surgery, although such data are not statistically significant. In ten patients including five with rectosigmoid cancer, and five from the splenic flexure cancer, the operation went beyond 4 h; three of these cases were part of the initial laparoscopic experience.
This study confirms our preliminary data,[30] but further prospective randomized studies are required, especially in advanced laparoscopic procedures (carcinoma of the middle and low rectum), also considering whether the surgeon's experience provides an additional biological and metabolic advantage for the patients.
CONCLUSION
In conclusion, during laparoscopic colorectal procedure:
A wide variation in the size of the incision was needed to extract the specimen and facilitate anastomosis.
Subsequent manipulation of peritoneum and the intestine handled.
This might explain why no differences were observed, in regards to the intestinal permeability and endotoxin concentration versus the open procedure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wichmann MW, Hüttl TP, Winter H, Spelsberg F, Angle MK, Heiss MM, et al. Immunological effects of laparoscopic vs open colorectal surgery: A prospective clinical study. Arch Surg. 2005;140:692–7. doi: 10.1001/archsurg.140.7.692. [DOI] [PubMed] [Google Scholar]
- 2.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–7. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 3.Marshall JC, Christo NV, Meakins JL. The gastrointestinal tract: The “undrained abscess” of multiple organ failure. Ann Surg. 1993;218:111–9. doi: 10.1097/00000658-199308000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: What is important in human beings? Surgery. 2002;131:241–4. doi: 10.1067/msy.2002.116408. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Anesthesiologists. New Classification of Physiology Status. Anesthesiology. 1963;24:111. [Google Scholar]
- 6.Behrens RH, Docherty H, Elia M, Neale G. A simple enzymatic method for the assay of urinary lactulose. Clin Chim Acta. 1984;137:361–7. doi: 10.1016/0009-8981(84)90125-6. [DOI] [PubMed] [Google Scholar]
- 7.Schwenk W, Jacobi C, Mansmann U, Böhm B, Müller JM. Inflammatory response after laparoscopic and conventional colorectal resection results of a prospective randomized trial. Langenbecks Arch Surg. 2000;385:2–9. doi: 10.1007/s004230050002. [DOI] [PubMed] [Google Scholar]
- 8.Bolla G, Tuzzato G. Immunologic postoperative competence after laparotomy vs laparotomy. Surg Endosc. 2003;17:1247–50. doi: 10.1007/s00464-002-9135-9. [DOI] [PubMed] [Google Scholar]
- 9.Whelan RL, Franklin M, Holubar SD, Donahue J, Fowler R, Munger C, et al. Postoperative cell mediated immune response is better preserved after laparoscopic vs open colorectal resection in humans. Surg Endosc. 2003;17:972–8. doi: 10.1007/s00464-001-8263-y. [DOI] [PubMed] [Google Scholar]
- 10.Kuntz C, Wunsch A, Bay F, Windeler J, Glaser F, Herfarth C. Postoperative randomized study of stress and immune responses in laparoscopic vs conventional colonic resection. Surg Endosc. 1998;12:963–7. doi: 10.1007/s004649900757. [DOI] [PubMed] [Google Scholar]
- 11.Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: A randomised trial. Lancet. 2002;359:2224–9. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 12.Laurent C, Leblanc F, Wütrich P, Scheffler M, Rullier E. Laparoscopic versus open surgery for rectal cancer: Long-term oncologic results. Ann Surg. 2009;250:54–61. doi: 10.1097/SLA.0b013e3181ad6511. [DOI] [PubMed] [Google Scholar]
- 13.Morino M, Allaix ME, Giraudo G, Corno F, Garrone C. Laparoscopic versus open surgery for extraperitoneal rectal cancer: A prospective comparative study. Surg Endosc. 2005;19:1460–7. doi: 10.1007/s00464-004-2001-1. [DOI] [PubMed] [Google Scholar]
- 14.Law WL, Poon JT, Fan JK, Lo SH. Comparison of outcome of open and laparoscopic resection for stage II and stage III rectal cancer. Ann Surg Oncol. 2009;16:1488–93. doi: 10.1245/s10434-009-0418-4. [DOI] [PubMed] [Google Scholar]
- 15.Belizon A, Balik E, Feingold DL, Bessler M, Arnell TD, Forde KA, et al. Major abdominal surgery increases plasma levels of vascular endothelial growth factor: Open more so than minimally invasive methods. Ann Surg. 2006;244:792–8. doi: 10.1097/01.sla.0000225272.52313.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wichmann MW, Meyer G, Angele MK, Schildberg FW, Rau HG. Recent advances in minimally ivasive colorectal cancer surgery. Onkologie. 2002;25:318–23. doi: 10.1159/000066048. [DOI] [PubMed] [Google Scholar]
- 17.Han SA, Lee WY, Park CM, Yun SH, Chun HK. Comparison of immunologic outcomes of laparoscopic versus open approaches in clinical stage III colorectal cancer. Int J Colorectal Dis. 2010;25:631–8. doi: 10.1007/s00384-010-0882-0. [DOI] [PubMed] [Google Scholar]
- 18.Simmy T, Anup R, Prabhu R, Balasubramanian KA. Effect of surgical manipulation of the rat intestine on enterocyte populations. Surgery. 2001;130:479–88. doi: 10.1067/msy.2001.115832. [DOI] [PubMed] [Google Scholar]
- 19.Anup R, Susama P, Balasubramanian KA. Role of xantine oxidase in small bowel mucosal dysfunction after surgical stress. Br J Surg. 2000;87:1094–101. doi: 10.1046/j.1365-2168.2000.01469.x. [DOI] [PubMed] [Google Scholar]
- 20.Schietroma M, Carlei F, Cappelli S, Amicucci G. Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann Surg. 2006;243:359–63. doi: 10.1097/01.sla.0000201455.89037.f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sylla P, Kirman I, Whelan RL. Immunological advantages of advanced laparoscopic. Surg Clin North Am. 2005;85:1–18, vii. doi: 10.1016/j.suc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Kalff JC, Türler A, Schwarz NT, Schraut WH, Lee KK, Tweardy DJ, et al. Intra-abdominal activation of a local inflammatory response within the human muscularis externa during laparotomy. Ann Surg. 2003;237:301–15. doi: 10.1097/01.SLA.0000055742.79045.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seltzer JL, Goldberg ME, Larijani GE, Ritter DE, Starsnic MA, Stahl GL, et al. Prostacyclin mediation of vasodilation following mesenteric traction. Anesthesiology. 1988;68:514–8. doi: 10.1097/00000542-198804000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Hudson JC, Wurm WH, O'Donnel TF, Jr, Shoenfeld NA, Mackey WC, Callow AD, et al. Hemodynamic and prostacyclin release in the early phase of aortic surgery: Comparison of transabdominal and retroperitoneal approaches. J Vasc Surg. 1988;7:190–8. [PubMed] [Google Scholar]
- 25.Soong CV, Blair PH, Halliday MI, McCaigue MD, Campbell GR, Hood JM, et al. Endotoxaemia, the generation of cytokines and their relationship to intramucosal acidosis of the sigmoid colon in elective abdominal aortic aneurysm repair. Eur J Vasc Surg. 1993;7:534–9. doi: 10.1016/s0950-821x(05)80366-4. [DOI] [PubMed] [Google Scholar]
- 26.Schietroma M, Carlei F, Mownah A, Franchi L, Mazzotta C, Sozio A, et al. Changes in the blood coagulation, fibinolysis, and cytokines profile during laparoscopic and open cholecystectomy. Surg Endosc. 2004;18:1090–6. doi: 10.1007/s00464-003-8819-0. [DOI] [PubMed] [Google Scholar]
- 27.Schietroma M, Carlei F, Lezoche E, Agnifili A, Enang GN, Mattucci S, et al. Evaluation of immune response in patients after open or laparoscopic cholecystectomy. Hepatogastroenterology. 2001;48:642–6. [PubMed] [Google Scholar]
- 28.Deitch EA, Berg R, Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch Surg. 1987;122:185–90. doi: 10.1001/archsurg.1987.01400140067008. [DOI] [PubMed] [Google Scholar]
- 29.Watson RW, Redmond HP, McCarthy J, Burke PE, Bouchier-Hayes D. Exposure of the peritoneal cavity to air regulates early inflammatory responses to surgery in murine model. Br J Surg. 1995;82:1060–5. doi: 10.1002/bjs.1800820820. [DOI] [PubMed] [Google Scholar]
- 30.Schietroma M, Pessia B, Carlei F, Cecilia EM, Amicucci G. Intestinal permeability, systemic endotoxemia, and bacterial translocation after open or laparoscopic resection for colon cancer: A prospective randomized study. Int J Colorectal Dis. 2013;28:1651–60. doi: 10.1007/s00384-013-1751-4. [DOI] [PubMed] [Google Scholar]


