Abstract
Objective:
To comparatively assess the antiplaque efficacy of Aloe vera mouthwash and 0.2% chlorhexidine gluconate mouthwash on de novo plaque formation.
Materials and Methods:
This was a randomized, single blind, parallel, controlled clinical study with 90 healthy participants, with mean age of 27.19 ± 12.08 years. After thorough oral prophylaxis, participants were instructed to discontinue mechanical plaque control. Participants were divided randomly into three groups; pure Aloe vera mouthwash was dispensed to the test group; control group received 0.2% chlorhexidine gluconate mouthwash; in Placebo group, flavored distilled water was used as oral rinse twice daily. Effect on 4-day de novo plaque formation was assessed by comparing pre-rinsing Quigley Hein Modified Plaque Scores were analyzed statistically using analysis of variance and Student's t-test.
Results:
Post-rinsing control group showed the least plaque score which was comparable to the test group. Both the control group and test group showed significant difference with the placebo group.
Conclusions:
Herbal mouthwash containing Aloe vera mouthwash has comparable antiplaque efficacy as the gold standard 0.2% chlorhexidine gluconate with fewer side effects and can be considered as an alternative.
Keywords: Aloe vera, antiplaque efficacy, chlorhexidine, plaque index
INTRODUCTION
Periodontal health is a vital and integral component of overall dental health. Epidemiological studies have demonstrated that, when periodontal disease is uncontrolled, it can act as a crucial factor in tooth loss. The mainstay of prevention of periodontal disease is regular and adequate plaque control using personal oral hygiene protocol and periodic professional review and maintenance wherever indicated.[1]
Though mechanical plaque control is the most effective oral hygiene measure, acceptable plaque removal requires time, motivation, and manual dexterity.[2] This in turn has led to a quest for chemotherapeutic agents that would inhibit plaque formation. A variety of products with antiplaque properties are a topic of current research. The mode of delivery for these chemical agents is diverse and includes toothpastes, chewing gum, irrigators, spray, varnishes, and mouthwashes.[3] Mouthwashes have proved to be a simple, safe, and effective delivery system, and in popularity are next only to toothpastes; mouthwashes have proven to play a vital role in plaque reduction.[4]
Chemical agents such as chlorhexidine and triclosan are regularly used as mouth rinses and the later has also been used as an additive in dentifrices for the prevention of plaque formation and thereby gingivitis.[5] Chlorhexidine is a gold standard in preventing dental plaque formation. This broad-spectrum antibiotic, a cationic bis-biguanide is extensively used as an antiplaque agent.
Aloe vera, a cactus-like plant, belongs to the Lilaceae family. The core mucilaginous tissue of the Aloe vera leaf is used as a gel for treatment of multiple conditions such as sunburn, wounds, digestive tract disorders, and as a laxative. Pharmacological actions attributable to Aloe vera include antibacterial, antiviral, antifungal, antioxidant, and anti-inflammatory.[6]
There is no published data available, to the best of authors' knowledge, that assesses antiplaque efficacy of Aloe vera where the study cohort has been recruited from the general population. Hence, this present study was conducted to compare and evaluate efficacy of Aloe vera mouthwash on clinical levels of dental plaque with established antiplaque agent of 0.2% chlorhexidine gluconate and placebo using the approach of 4-day plaque regrowth.
MATERIALS AND METHODS
This study was designed as a single blind, randomized, parallel, controlled clinical trial to comparatively evaluate the antiplaque efficacy of placebo, Aloe vera mouthwash, and 0.2% chlorhexidine gluconate mouthwash. The study recruited 90 participants (45 females and 45 males, with age ranging from 18 to 40 years, and mean age of 27.19 ± 12.08 years). The ethical committee of the institution gave ethical clearance for the study, and the study was carried out according to the ethical principles outlined by the World Medical Association's Declaration of Helsinki (revised in 2002).[7] The signed informed consent was obtained from all the participants.
Inclusion criteria
Systemically healthy participants with (1) 20 natural teeth with 5 teeth per quadrant; (2) deficient supragingival plaque and calculus retentive areas.
Exclusion criteria
Participants with (1) probing pocket depth ≥3 mm, (2) antimicrobial therapy 1 month prior to the study; (3) using dentifrices or mouth rinses with anti-inflammatory properties; (4) smokers and tobacco consumers; (5) pregnant women; (6) with orthodontic appliances; (7) severely misaligned teeth; (8) fully crowned teeth and removable partial dentures; (9) pharmacological and medical history that could undermine the outcomes of the study.
Study design
In all the selected participants, oral hygiene status assessment was carried out by plaque index (PI) Quigley and Hein (1962),[8] modified first by Turesky et al. (1970)[9] and later by Lobene et al. (1982).[10] 1% erythrosine solution was used to disclose plaque, and the values were recorded on a 5-point scale at six sites per tooth.
Loe and Silness Gingival index (GI) was also evaluated, and only patients with GI scores of <1 at 40% of sites were included. All recordings were assessed by the same examiner. Thorough professional scaling and polishing were carried out 3 weeks prior to the initiation of trial. Participants were instructed to follow a meticulous oral hygiene protocol consisting of use of toothbrush and interdental cleaning. Participants were recalled at 1-week intervals for oral hygiene reinforcement and professional scaling where indicated. Baseline value of plaque score was recorded before the administration of mouthwash.
The enrolled participants were randomly allocated by computer generated random table method to either test group (Aloe vera mouth wash user, n = 30), control group (2% chlorhexidine gluconate mouth wash user, n = 30), placebo group (flavored distilled water).
The sample size of 90 participants of mouthwash study was calculated on the basis of following statistical evaluation: n = (Zα/2 + Zβ)2× (p1 (1 − p1) + p2 (1 − p2))/(p1 − p2)2, where Zα/2 is the critical value of the Normal distribution at α/2 (for a confidence level of 95%, α is 0.05 and the critical value is 1.96), Zβ is the critical value of the normal distribution at β (for a power of 80%, β is 0.2 and the critical value is 0.84), and p1 (0.2% chlorhexidine gluconate mouthwash) and p2 (Aloe vera mouthwash) are the expected sample proportions of the two groups.
Aloe vera mouthwash consisted of pure Aloe vera Juice (Aloe vera Juice, Patanjali Ayurved Ltd, India). Composition of each 10 ml: 99.6% (w/v) Aloe vera juice;0.02% (w/v) citric acid crystal; 0.02% (w/v) sodium benzoate crystal (preservative); Orange flavor (Q.S). 0.2% hexidine mouthwash (ICPA, Health Products, India).
Participants were instructed to refrain from regular oral hygiene practices i.e., tooth brushing or flossing, and instructed to use mouthwash only for next 4 days. Bottles of identical appearance with a volume of 150 ml of solution were dispensed to each participant who was blinded to the mouthwash received. A measure of 10 ml measure to ensure accuracy in dosage was given. All participants were given instructions for rinsing with 10 ml of the solution twice a day for duration of 1 min, once on rising, and once before bedtime. Ingestion of liquids or solids was permitted only after 2 h of rinsing.
This regimen was followed by each group for 4 days. PI scores were evaluated by the same trained examiner on the 5th day.
Mouthwashes were evaluated for side effects by subjective and objective criteria. Subjective criteria included: (1) Taste disturbance; (2) burning sensation; (3) dryness/soreness; (4) pruritis/itchiness; marked as 0 - absent; 1 - present. Objective criteria included (1) ulcer; (2) staining of teeth; (3) staining of tongue; (4) allergy; marked as 0 - absent; 1 - present.
Statistical analysis
The means and standard deviation (mean ± SD) values were determined for all PI scores. Intragroup comparison of plaque indices were done by analysis of variance (ANOVA) and post-hoc least significant difference (LSD) test. Side effects of the mouthwashes were assessed by subjective and objective criteria using a questionnaire. P value of 0.05 was considered to be significant.
RESULTS
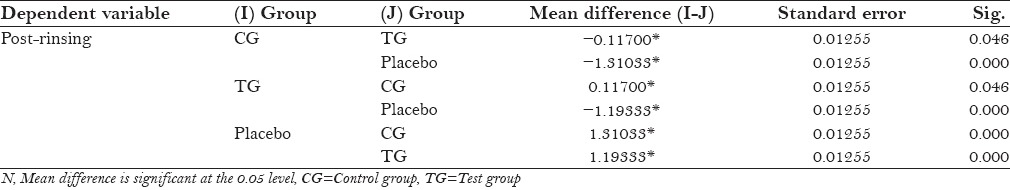
The protocol of the study was to be strictly followed by the participants, with no reported systemic side-effects. All the three groups mean plaque scores at baseline was 2.69 ± 0.055 for control group and 2.58 ± 0.034 for placebo, and after 4th day 1.48 ± 0.061 for control group and 2.79 ± 0.037 for placebo [Table 1]. Table 2 shows statistically significant values (P = 0.000) in mean difference between pre and post-rinsing with t valve of −24.63 of the placebo group. Table 3 shows ANOVA test for difference between pre-rinsing PI scores between the three groups with an f value of 0.286 with no significant difference (P = 0.073). Statistically significant valve (P = 0.000) with an f value of 6.676 were recorded in post-rinsing ANOVA test [Table 4]. Post-hoc LSD was used for multiple comparisons for post-rinsing PI score [Table 5]. Statistically significant difference was seen between control group and test group with placebo (P = 0.000), although comparison between control group and test group was non-significant (P = 0.052).
Table 1.
Mean valve of plaque index score at baseline and after 4th day in different groups
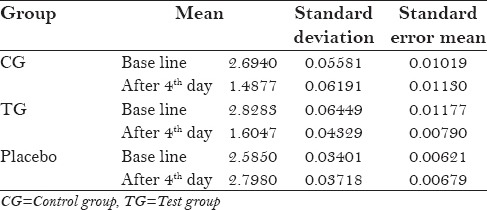
Table 2.
t-values for pre and post-rinsing plaque index scores of the groups
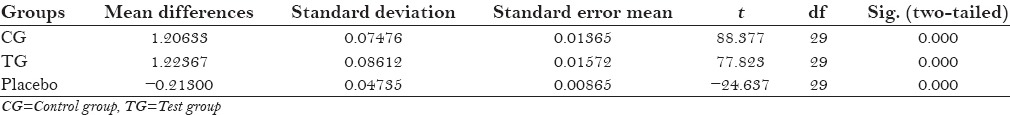
Table 3.
Analysis of variance for pre-rinsing plaque index scores of different groups
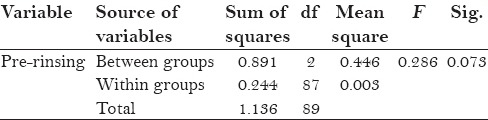
Table 4.
Analysis of variance for post-rinsing plaque index scores of different groups
Table 5.
Post-hoc for post-rinsing plaque index scores of different groups
Subjective and objective evaluation of participants was done after 4 days of mouthwash usage. In the control group, staining was seen in 40%, taste in disturbance 25%, and burning sensation in 2% of subjects. Test group taste disturbance was seen in 4% of subjects. No staining was seen in the test group. There were no reported side effects in the placebo group.
DISCUSSION
Periodontal health can be maintained by regular tooth-brushing, flossing, and rinsing with antibacterial agents containing mouthwashes. 0.2% chlorhexidine gluconate is commonly used in mouthwashes and irrigation. Stained tongue and teeth and alteration of taste sensation are common side effects which limit their use for prolonged period. Chlorhexidine also has documented effects on vital tissues such as cytotoxicity to periodontal ligament (PDL) cells, altered mitochondrial activity, and inhibition of protein synthesis.[11]
In recent times, there has been an increase in demand for alternative medicine.[12,13] Medicinal plants, such as Aloe vera, have natural phytochemicals and have proven to be better than synthetic drugs.[14,15] Studies by Khalessi et al.[16] and Parwani et al.[17] had suggested the better efficacy of herbal mouthwashes in maintaining plaque and gingival status. In vitro study on antimicrobial efficacy exposing supragingival plaque have found to have maximum effect at 100% concentration of Aloe vera.[18] In our study, 100% concentration of Aloe vera was used.
The present study is in compliance with the ideal mouth rinse protocol requirements given by Barnett.[19] There was a significant decrease in PI scores at the 4th day in the control and test group. Chlorhexidine gluconate is effective against assorted microorganisms, such as Gram-positive organisms, Gram-negative organisms, yeasts, viruses, and fungi is known. However, there are few studies, mainly in vivo studies on microorganisms such as Streptococcus, Actinomyces viscosus, and Candida albicans to suggest antimicrobial effect of Aloe vera.[20] Thus, the low plaque score in our study shows antimicrobial efficacy of Aloe vera, as seen in the study by Chandrahas et al.[21]
Aloe vera contains various anti-inflammatory agents such as carboxypeptidase, which reduce prostaglandin synthesis, magnesium lactate, which inhibits histidine decarboxylase preventing mast cell activity, sterols, and lupeol as pain modulators.[22] Aloe vera also reduces edema by inhibiting matrix metalloproteinases blocking polymorphonuclear leucocyte (PMNs) release, cyclooxygenase, and lipo-oxygenase pathways. These activated PMNs in turn inhibit free oxygen radicals.[23] These suggest potential anti-inflammatory action of Aloe vera, which showed a decrease in the GI.[15,24]
Both the control and test groups showed significant reductions in plaque scores (chlorhexidine 46%, Aloe vera, 44%) which are consistent with previous studies. Aloe vera use was not associated with any side-effect, and post-rinsing PI scores between control group and test group were also not statistically significant. This suggests that Aloe vera has a similar potential as chlorhexidine as far as antiplaque activity is concerned.[25]
Shortcomings of this study are:
A 4-day plaque regrowth model is used for the first time which is insufficient to evaluate gingival scores
A cross-over design with wash-out period would have been more authenticating because it eliminates the bias of variable host response.
Therefore, future studies can be directed toward testing Aloe vera-based herbal mouthwash antiplaque and anti-gingivitis spectrum with a prolonged usage period.
CONCLUSIONS
Within the limitations of this study, it can be concluded that Aloe vera mouth wash has an efficacy which is comparable to the antiplaque agent 0.2% chlorhexidine gluconate mouthwash. Considering the side effects associated with chlorhexidine, Aloe vera mouthwash can be considered as a viable alternative. However, studies (with a long-term rinsing period) should be carried out done to delineate the advantages and disadvantages associated with this herbal mouthwash.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wu CD, Savitt ED. Evaluation of the safety and efficacy of over the counter oral hygiene products for the reduction and control of plaque and gingivitis. Periodontol 2000. 2002;28:91–105. doi: 10.1034/j.1600-0757.2002.280105.x. [DOI] [PubMed] [Google Scholar]
- 2.DePaola LG, Overholser CD, Meiller TF, Minah GE, Niehaus C. Chemotherapeutic inhibition of supragingival dental plaque and gingivitis development. J Clin Periodontol. 1989;16:311–5. doi: 10.1111/j.1600-051x.1989.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 3.Addy M. The use of antiseptics in periodontal therapy. In: Lindhe J, Karring T, Lang NP, editors. Clinical Periodontology and Implant Dentistry. 4th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2003. p. 464. [Google Scholar]
- 4.Paraskevas S. Randomized controlled clinical trials on agents used for chemical plaque control. Int J Dent Hyg. 2005;3:162–78. doi: 10.1111/j.1601-5037.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 5.Yates R, Jenkins S, Newcombe RG, Wade WG, Moran J, Addy M. A 6-month home usage trial of 1% chlorhexidine toothpaste (1).Effects on plaque, gingivitis, calculus and toothstaining. J Clin Periodontol. 1993;20:130–8. doi: 10.1111/j.1600-051x.1993.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 6.Vogler BK, Ernst E. Aloe vera: A systemic review of its clinical effectiveness. Br J Gen Pract. 1999;49:823–8. [PMC free article] [PubMed] [Google Scholar]
- 7.World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 8.Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc. 1962;65:26–9. doi: 10.14219/jada.archive.1962.0184. [DOI] [PubMed] [Google Scholar]
- 9.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41:41–3. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 10.Lobene RR, Soparkar PM, Newman MB. Use of dental floss. Effect on plaque and gingivitis. Clin Prev Dent. 1982;4:5–8. [PubMed] [Google Scholar]
- 11.Chang YC, Huang FM, Tai KW, Chou MY. The effect of sodium hypochlorite and chlorhexidine on cultured human periodontal ligament cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:446–50. doi: 10.1067/moe.2001.116812. [DOI] [PubMed] [Google Scholar]
- 12.Gupta DA, Bhaskar DJ, Gupta RK, Karim B, Jain A, Dalai DR. Green tea: A review on its natural anti-oxidant therapy and cariostatic benefits. Issues Biol Sci Pharm Res. 2014;2:008–12. [Google Scholar]
- 13.Gupta D, Bhaskar DJ, Gupta RK, Jain A, Yadav P, Dalai DR, et al. Is complementary and alternative medicine effective in job satisfaction among dentists with musculoskeletal disorders? A cross sectional study. Med Pr. 2014;65:317–23. [PubMed] [Google Scholar]
- 14.Gupta DA, Bhaskar DJ, Gupta RK. Contemporary and Alternative Dentistry: Ayurveda in Dentistry. Lap Lambert Academic Publishing. 2013 [Google Scholar]
- 15.Sahebjamee M, Mansourian A, Mohammad MH, Zadeh MT, Bekhradi R, Kazemian A, et al. Comparative efficacy of Aloe Vera and benzidamine mouthwashes on radiation-induced oral mucositis: A triple-blind, randomized, controlled clinical trial. Oral Health Prev Dent. 2015;13:309–15. doi: 10.3290/j.ohpd.a33091. [DOI] [PubMed] [Google Scholar]
- 16.Khalessi AM, Pack AR, Thomson WM, Tompkins GR. An in vivo study of the plaque control efficacy of Persica: A commercially available herbal mouthwash containing extracts of Salvadora persica. Int Dent J. 2004;54:279–83. doi: 10.1111/j.1875-595x.2004.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 17.Parwani SR, Parwani RN, Chitnis PJ, Dadlani HP, Sai Prasad SV. Comparative evaluation of anti-plaque efficacy of herbal and 0.2% chlorhexidine gluconate mouthwash in a 4-day plaque re-growth study. J Indian Soc Periodontol. 2013;17:72–7. doi: 10.4103/0972-124X.107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karim B, Bhaskar JD, Agali C, Gupta D, Gupta RK, Jain A, et al. Effect of Aloe vera mouthwash on periodontal health: Triple blind randomized control trial. Oral Health Dent Manag. 2014;13:14–9. [PubMed] [Google Scholar]
- 19.Barnett ML. The role of therapeutic antimicrobial mouth rinses in clinical practice: Control of supragingival plaque and gingivitis. J Am Dent Assoc. 2003;134:699–704. doi: 10.14219/jada.archive.2003.0255. [DOI] [PubMed] [Google Scholar]
- 20.Lee SS, Zhang W, Li Y. The antimicrobial potential of 14 natural herbal dentifrices: Results of an in vitro diffusion method study. J Am Dent Assoc. 2004;135:1133–41. doi: 10.14219/jada.archive.2004.0372. [DOI] [PubMed] [Google Scholar]
- 21.Chandrahas B, Jayakumar A, Naveen A, Butchibabu K, Reddy PK, Muralikrishna T. A randomized, double-blind clinical study to assess the antiplaque and antigingivitis efficacy of Aloe vera mouth rinse. J Indian Soc Periodontol. 2012;16:543–8. doi: 10.4103/0972-124X.106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Prasad S, Reuter S, Kannappan R, Yadev VR, Park B, et al. Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases: “Reverse pharmacology” and “bedside to bench” approach. Curr Drug Targets. 2011;12:1595–653. doi: 10.2174/138945011798109464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ajmera N, Chatterjee A, Goyal V. Aloe vera: It's effect on gingivitis. J Indian Soc Periodontol. 2013;17:435–8. doi: 10.4103/0972-124X.118312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta RK, Gupta D, Bhaskar DJ, Yadav A, Obaid K, Mishra S. Preliminary antiplaque efficacy of aloe vera mouthwash on 4 day plaque re-growth model: Randomized control trial. Ethiop J Health Sci. 2014;24:139–44. doi: 10.4314/ejhs.v24i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]


