Abstract
Background:
Renal injury is a common cause of morbidity and mortality after elective abdominal aortic aneurysm (AAA) repair. Propofol has been reported to protect several organs from ischemia/reperfusion (I/R) induced injury. We performed a randomized clinical trial to compare propofol and sevoflurane for their effects on renal I/R injury in patients undergoing elective AAA repair.
Materials and Methods:
Fifty patients scheduled for elective AAA repair were randomized to receive propofol anesthesia in group I or sevoflurane anesthesia in group II. Urinary specific kidney proteins (N-acetyl-beta-glucosamidase, alpha-1-microglobulin, glutathione transferase [GST]-pi, GST-alpha) were measured within 5 min of starting anesthesia as a base line (T0), at the end of surgery (T1), 8 h after surgery (T2), 16 h after surgery (T3), and 24 h postoperatively (T4). Serum pro-inflammatory cytokines (tumor necrosis factor-α and interleukin 1-β) were measured at the same time points. In addition, serum creatinine and cystatin C were measured before starting surgery as a baseline and at days 1, 3, and 6 after surgery.
Results:
Postoperative urinary concentrations of all measured kidney specific proteins and serum pro-inflammatory cytokines were significantly lower in the propofol group. In addition, the serum creatinine and cystatin C were significantly lower in the propofol group compared with the sevoflurane group.
Conclusion:
Propofol significantly reduced renal injury after elective open AAA repair and this could have clinical implications in situations of expected renal I/R injury.
Keywords: Propofol, renal ischemia-reperfusion injury, sevoflurane
Introduction
Renal injury, a result of the hemodynamic changes after aortic cross-clamping and ischemia/reperfusion injury (I/R) after declamping, is a common cause of morbidity and mortality after elective abdominal aortic aneurysm (AAA) repair.[1,2] In one series, acute renal failure was reported in 6.7% of patients after elective open AAA repair[3] and is an independent predictor of death.[1]
Multiple factors are involved in the etiology of renal injury during infrarenal AAA procedures. Aortic cross-clamping below the kidney triggers renal vasoconstriction that is associated with a redistribution of blood flow from the medullary to the cortical compartment.[4] This renal vasoconstriction may be due to changes in the humoral and neurogenic factors that regulate renal blood flow or may be triggered by aortic cross-clamping induced turbulence in blood flow inside the aorta at the level of the kidney. In addition, increase in renin activity may have been induced by aortic cross-clamping.[5,6] The subsequent reduction in renal blood flow may expose the cells of the renal tubules to ischemic injury.[7] Furthermore, the renal tubular cells suffer I/R injury after release of the clamping that is accompanied with a neutrophil-mediated systemic response.[7] The pathophysiology of renal I/R injury is complex. Triggering of lipid peroxidation and the formation of free radicals has been shown to be major factors.[8,9]
Several studies have proven that propofol increases antioxidant capacity in different tissues.[10,11] There is some laboratory evidence to suggest that propofol may provide protection to the kidney through modulation of the systemic inflammatory response.[12,13]
Sevoflurane has been reported to be nephrotoxic in rats[14] while some recent studies suggested that sevoflurane is protective against renal I/R injury in mice.[15,16,17] Sevoflurane toxicity in humans has been tackled by several investigations.[18,19,20,21] Some human studies have reported that sevoflurane anesthesia resulted in increase in the excretion of markers of renal injury indicating potential nephrotoxicity,[18,19] whereas other studies reported no effect.[20,21]
The purpose of this investigation was to compare the renal effect of intravenous (IV) anesthesia with propofol against inhalation anesthesia with sevoflurane in patients undergoing AAA repair.
Materials and Methods
This prospective randomized blinded study was performed on 50 American Society of Anesthesiologists class II or III patients scheduled for elective infrarenal AAA repair. The study was carried out between February, 2012 and April, 2014. Written informed consent was obtained from the patients and Institutional Review Board of Minoufiya Faculty of Medicine has approved the study (Ref: 11/A213/352). The study was registered with PACTR201505001095139.
All the operations were performed by the same surgical team through a mini-laparotomy approach. Thorough clinical evaluation, electrocardiogram, echocardiography, and laboratory investigations were performed as a routine diagnostic check-up.
Patients were excluded from the study if they needed concomitant procedures other than AAA repair, had experienced an acute coronary syndrome within 3 months, or were >85 years of age.
Bisoprolol was prescribed preoperatively in a dose of 5 mg daily in the absence of contra-indications (heart rate below 60 bpm or systolic blood pressure <100 mm Hg). Cardiac medications were continued up to the day of surgery.
The patients were randomly allocated to receive propofol (n = 25) or sevoflurane (n = 25) anesthesia using a random number table generated by Microsoft Excel. An independent statistician was assigned to perform central randomization to ensure proper concealment of the study management from the patients and investigators until the release of the final statistical results.
In the propofol group, general anesthesia was induced with propofol 1.5-2 mg/kg and fentanyl 3 μg/kg. Tracheal intubation was facilitated by administration of cis-atracurium 0.1 mg/kg. Anesthesia was maintained with a continuous infusion of propofol 4-6 mg/kg/h, and cis-atracurium 2 μg/kg/min. In the sevoflurane group, anesthesia was induced as above but maintained with sevoflurane 1 MAC and cis-atracurium 2 μg/kg/min. The bispectral index electrode (BIS-Sensor, Aspect Medical Systems, USA) was positioned on the patient's forehead to monitor depth of anesthesia where BIS value was kept between 45 and 55 in both groups through modulating propofol infusion rate or sevoflurane concentration. In the operating room, a radial arterial catheter and multiple peripheral IV catheters were inserted. Heart rate, arterial blood pressure and oxygen saturation were continuously monitored during the whole procedure. Fluid loading was performed with 1.0 L of 6% 130/0.4 hydroxyethyl starch (Voluven) infusion. Fluid and blood replacements were adjusted to maintain patient hematocrit value above 30%. Norepinephrine and nicardipine were used if required (if mean arterial blood pressure changed by more than 20%) to maintain hemodynamic stability. Normothermia was maintained with fluid warming and forced air warming (Bair-Hugger). Blood glucose level was kept normoglycemic (3.9-8.3 mmol/L). One analyst was blinded in respect to the drug under study during the procedure by covering the lines, infusion pump, gas analyzer, and by numeric codes during the whole process of data evaluation. Furthermore, physicians who were charged for postoperative care of patients and for their discharges from intensive care unit (ICU) and hospital were effectively blinded to the study design. The patients stayed in the ICU till return to their preoperative physiological homeostasis including stable hemodynamics, adequate ventilation, normothermia, and satisfactory pain control. Hospital discharge was guided by the ability to ambulate independently and to tolerate oral feeding.
Epidural analgesia was performed before starting anesthesia at the T8-T10 level by inserting an epidural catheter (Braun perifix 18 ba and a microporous filter).
A test dose of 4 ml 1% lidocaine with epinephrine 5 μg/ml was used for testing intrathecal or intravascular injection, respectively. Epidural block activation was performed by injecting 12 ml of bupivacaine hydrochloride 0.25%. Furthermore, 4 ml was injected 2 h later as a maintenance dose and every hour thereafter for postoperative epidural analgesia. Acetaminophen IV was also used postoperatively if needed.
Assessment of kidney function
All patients had a bladder catheter. The following assays were performed on urine specimens, taken within 5 min of starting anesthesia as a base line (T0), at the end of surgery (T1), 8h after surgery (T2), 16h after surgery (T3), and 24 h postoperatively (T4): N-acetyl-beta-dglucosamidase (beta-NAG) analyzed by a spectrophotometric method, normal value 0-7 U/L, intra- and inter-assay coefficients of variation 3.9%; alpha-1-microglobulin (alpha-1-M) assessed by immunonephelometry, normal value <14 mg/L, intra- and inter-assay coefficients of variation 3.7%; glutathione transferase-pi (GST-pi) measured by enzyme immunoassay, normal value 12-15 μg/L, intra- and inter-assay coefficients of variation 4.6%; and GST-alpha measured by enzyme immunoassay, normal value 3.5-11 μg/L, intra- and inter-assay coefficients of variation 3.5%.[19] In addition, the plasma pro-inflammatory cytokines, (tumor necrosis factor α [TNF-α] and interleukin-1β [IL-1β]) were measured at the same time points. Blood samples were immediately centrifuged and the serum separated, divided into aliquots, and placed in Eppendorf tubes and frozen at -80°C until assay. Commercial kits were used for the determination of TNF-α and IL-1β (enzyme-linked immunosorbent assay [ELISA] Kit; Biomed, Diepenbeek, Belgium) based on ELISA. Recordings were carried out on a plate reader (GEST, General ELISA System Technology, Menarini Labs, Badalona, Spain) for the automatic ELISA technique in triplicate. The lower limit of detection of the assay for TNF-α and IL-1β were 10.7 and 4.2 pg/ml, respectively. Intra- and inter-assay coefficients of variation for TNF-α and IL-1β were below 8%.
Serum creatinine and cystatin C levels (markers of renal glomerular function)[20,21,22,23] were measured before starting surgery as a baseline, and at days 1, 3, and 6 after surgery. Serum creatinine was measured using Jaffe reaction (sensitivity, 8.6 μm/L; intra- and inter-assay coefficients of variance, <2.6%; normal range for men, 60-106 μm/L; normal range for women, 42-80 μm/L) while serum cystatin C was determined by an immunonephelometric assay (N-Latex cystatin C; DADE-Behring Marburg GmbH, Marburg, Germany) using the Behring Nephelometer BNII (sensitivity, 0.25 mg/L; intra- and inter-assay coefficients of variance, <3.7%; normal range, 0.75-1.50 mg/L).
Statistical analysis
Continuous variables are expressed as mean (standard deviation) and categorical variables are reported as percentages. Statistical analyses were performed using statistical for windows version 10.0 software. A preliminary study had demonstrated that for patients scheduled for elective AAA repair at our hospital, the mean value of urinary beta-NAG was 2.5 (0.5) U/L, alpha-1-M was 4.9 (1.4) mg/L, GST-pi was 13.7 (3.4) μg/L and GST-alpha was 4.9 (1.3) μg/L. With a two-sided type I error of 5 % and study power at 80%, a mean sample size of 25 patients in each group was found sufficient to demonstrate a difference in the urinary specific kidney proteins (0.6 U/L for beta-NAG, 1.5 mg/L for alpha-1-M, 3.6 μg/L for GST-pi, and 1.5 μg/L for GST-alpha). The Kolmogorov-Smirnov test was used to verify normal distribution of data. Distribution of residuals testing was performed to confirm that analysis of variance (ANOVA) was appropriate to our data. Data were analyzed on an intention to treat basis using two-way ANOVA for repeated-measures. This was followed by Student-Newman-Keuls test, if a difference between groups had been detected. Changes over time in nonnormally distributed data sets were analyzed by Friedman repeated-measures ANOVA on ranks. P < 0.05 was considered statistically significant (SigmaStat, Systat Software, Richmond, USA).
Results
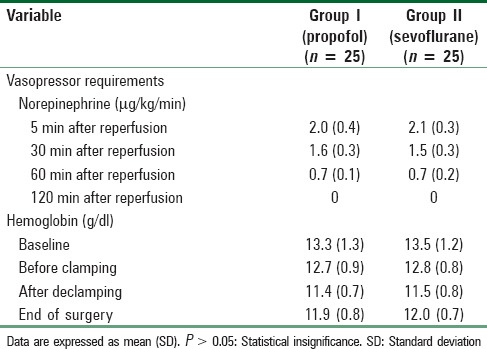
Baseline characteristics and operative characteristics including cross-clamp time, operating time [Table 1], vasopressor requirements, and hemoglobin concentration changes [Table 2] were comparable in both groups.
Table 1.
Patients’ baseline data, operative characteristics, and postoperative outcomes
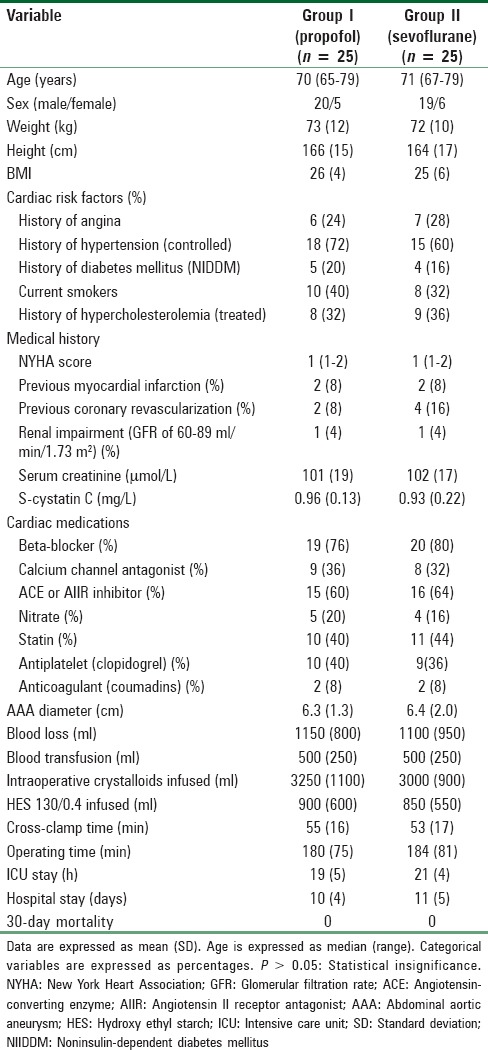
Table 2.
Vasopressor requirements and hemoglobin concentration changes in both groups
Patients in the propofol group had lower urinary concentrations of all measured kidney specific proteins [Table 3] and lower serum creatinine and cystatin C [Table 4], in addition to lower serum pro-inflammatory cytokines [Table 5] as follows.
Table 3.
Changes in kidney specific urinary proteins
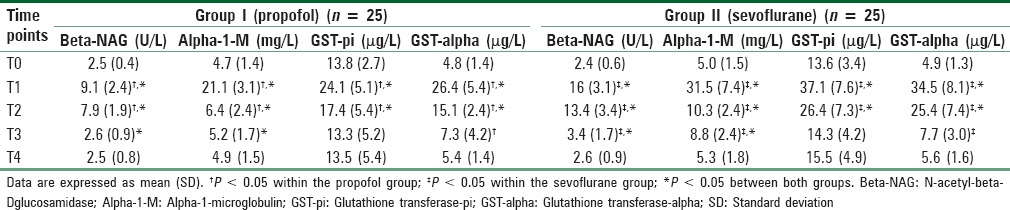
Table 4.
Changes of serum creatinine and cystatin C
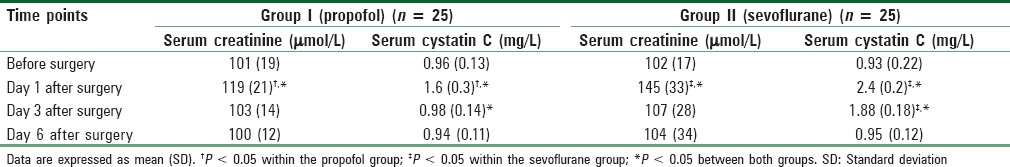
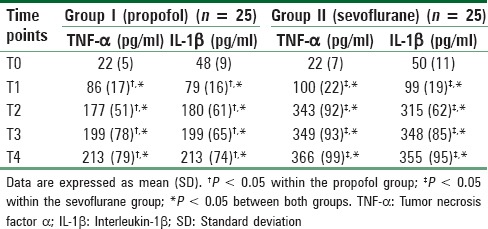
Table 5.
Changes in serum pro-inflammatory cytokines
Both beta-NAG and alpha-1-M increased significantly in both groups but their concentrations were significantly different between the two groups at a number of time points. For beta-NAG the overall for the two-way repeated-measures ANOVA for the comparing different time points and the propofol and sevoflurane groups was significant (F = 25.04 and P = 0.001 at end of surgery, F = 22.77 and P = 0.001 8h after surgery, F = 12.76 and P = 0.002 16 h after surgery). For alpha-1-M the overall for the two-way repeated-measures ANOVA for the comparing different time points and the propofol and sevoflurane groups was also significant (F = 17.49 and P = 0.001 at end of surgery, F = 13.11 and P = 0.001 8 h after surgery, F = 19.45 and P = 0.001 16 h after surgery). Both started to increase at the end of surgery in both groups and continued to be higher than baseline values at 8 h after surgery in the propofol group and at 16 h after surgery in the sevoflurane group.
GST-pi increased significantly in both groups at the end of surgery and 8h postoperatively (F = 18.47 and P = 0.001 at end of surgery, F = 11.84 and P = 0.001 8 h after surgery). On post-hoc testing, there was a significant difference between the two groups at 8 h but not at 16 h.
The two-way ANOVA for changes in GST-alpha was significant (F = 12.0 and P = 0.001 at end of surgery, F = 21.24 and P = 0.001 8 h after surgery). Concentrations were significantly higher than baseline values in both groups at the end of surgery, 8 h and 16 h postoperatively but were significantly lower in the propofol group at the end of surgery and 8 h postoperatively. The results of post-hoc tests for the urinary markers are shown in Table 3.
Serum creatinine at day 1 after surgery was significantly higher than baseline values in both groups but was significantly lower in the propofol group when compared with the sevoflurane group. The overall two-way ANOVA analysis of the groups was significant (F = 8.66 and P = 0.001). Post-hoc test results are shown in Table 4.
The overall two-way ANOVA analysis of serum cystatin C was also significant (F = 22.41 and P = 0.001 at day 1, F = 52.46 and P = 0.001 at day 3). Cystatin C was significantly higher than baseline value in the propofol group at day 1 after surgery and returned to near normal baseline values thereafter whereas it was significantly higher than baseline value in the sevoflurane group at both days 1 and 3 and was higher than comparable values in the propofol group. Again the results of post-hoc tests are shown in Table 4.
Serum pro-inflammatory cytokines (TNF-α and IL-1β) were significantly higher than baseline values in both groups at the end of surgery and at all-time points thereafter but were significantly lower in the propofol group. For TNF-α the Friedman repeated-measures ANOVA on ranks comparing groups and changes over time achieved statistical significance (F = 5.34 and P = 0.002 at the end of surgery, F = 47.31 and P < 0.001 8 h after surgery, F = 27.17 and P < 0.001 16 h after surgery, F = 36.06 and P < 0.001 24 h postoperatively). For IL-1β the Friedman repeated-measures ANOVA on ranks comparing groups and changes over time was also significant (F = 8.53 and P = 0.001 at end of surgery, F = 40.34 and P < 0.001 8 h after surgery, F = 31.89 and P < 0.001 16 h after surgery, F = 18.17 and P < 0.001 24 h postoperatively). The results of post-hoc tests are shown in Table 5.
Discussion
In this randomized trial, propofol reduced the risk of peri-operative renal impairment in patients undergoing AAA repair as manifested by changes in kidney specific proteins, lower serum creatinine, and cystatin C levels.
Several studies have reported that propofol can provide protection against I/R injury while sevoflurane could not provide such protection.[24,25,26,27,28,29] Several possible mechanisms have been proposed. Sαnchez-Conde et al.,[24] compared the abilities of propofol and sevoflurane to modulate inflammation and oxidative stress to the kidney caused by supra-renal aortic cross-clamping. Compared to sevoflurane, propofol administration led to the modulation of markers of inflammation and decreased NF-kappa B expression. Wang et al.[25] demonstrated a protective effect of propofol in renal I/R injury in rats and suggested that this was due to the induction of the heme oxygenase-1 expression. In a study comparing propofol against sevoflurane for their effects on the systemic inflammatory response during aortic surgery in pigs, propofol anesthesia was associated with less neutrophil infiltration, lower plasma pro-inflammatory cytokine levels, lower production of oxygen free radicals, less lipid peroxidation, and reduced inducible nitric oxide synthase activity.[12] Another mechanism for renal protective effect of propofol was reported by a study by Feng et al.,[26] where pretreatment with 5 μg/ml propofol protected human proximal renal tubular epithelial cells against anoxia-reoxygenation injury at clinically relevant concentrations by regulating the expression of apoptosis related genes. Assad et al.,[27] reported that protection by propofol was probably due to a preconditioning effect and was at least in part mediated by KATP channels. Obal et al.,[28] compared the effect of preconditioning with sevoflurane and preconditioning with short episodes of ischemia on renal I/R injury in the rat in vivo. They reported that sevoflurane could not preserve renal function or attenuate cell damage in the rat in vivo. Higuchi et al.,[29] compared the effects of high- and low-flow sevoflurane and isoflurane anesthesia on renal function and on markers of nephrotoxicity in humans. Increased urinary beta-NAG excretion was seen in the low-flow and high-flow sevoflurane groups, but not in the isoflurane group (P < 0.01) but was not associated with any changes in blood urea nitrogen, creatinine, and creatinine clearance.
In contrast to our findings, some studies have reported renal protection by sevoflurane.[15,16,30,31] Lee et al.,[15] reported that sevoflurane protects against renal I/R injury in mice via the transforming growth factor-β1 (TGF-β1) pathway and stated that this protection was absent in mice deficient in TGF-β1 signaling, and this fact can explain why sevoflurane protection is controversial in different studies. In another study, Lee et al.,[16] approved sevoflurane protection against renal I/R injury in cultured human proximal tubular cells and reported that this protection was through activation of the TGF-β1signaling pathways. Equipotent doses of volatile anesthetics (desflurane, halothane, isoflurane, or sevoflurane) were compared against injectable anesthetics (pentobarbital or ketamine) in rats subjected to renal I/R.[30] Rats treated with volatile anesthetics had lower plasma creatinine and reduced renal necrosis 24-72 h after injury compared with rats anesthetized with pentobarbital or ketamine. Annecke et al.,[31] compared effects of sevoflurane and propofol on I/R injury after thoracic-aortic occlusion in pigs. Serum markers of cellular injury (lactate dehydrogenase, aspartate transaminase, and alanine aminotransferase) were lower with sevoflurane. However, these markers are not specific to the kidney.
Some studies have reported that hyrdroxy ethyl starch infusion may have negative impact on kidney function.[32,33] In our study, both groups were comparable regarding volume of hyrdroxy ethyl starch infused and this fact can eliminate its effect on our results.
One limitation of the present study is the use of urinary alpha-1-M as a marker of renal tubular dysfunction (since its increase suggests impaired reabsorption by the tubules and hence tubular dysfunction). It should be emphasized that increased tubular dysfunction is not necessarily a bad thing as it suggests temporary dysfunction of the tubules, less renal tubular work, and less tubular oxygen consumption. In fact increased alpha-1-M can be argued as being renoprotective through reducing renal workload and hence oxygen demand at a time of reduced oxygen delivery.
Our study suffers from an additional number of limitations. With the exception of serum creatinine, the renal function measures used are at best subclinical markers of renal dysfunction and injury and the clinical application of this work would require clinically used measures of renal function with greater patient numbers in the study.
Conclusion
We have demonstrated that the use of propofol significantly reduced renal injury after elective open AAA repair and this could have clinical implications in situations of expected renal I/R injury as patients suffering from hemorrhagic, traumatic, or septic shock, and certain surgical procedures including renal transplantation or abdominal aortic surgery.
Financial support and sponsorship
Minoufiya University is funding the research.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We thank the operating room and ICU staff in Minoufiya University Hospital for their support in conducting the study procedures. We pay great gratitude to Clinical Pathology Department, Minoufiya University for the effort in doing the laboratory work.
References
- 1.Diehl JT, Cali RF, Hertzer NR, Beven EG. Complications of abdominal aortic reconstruction. An analysis of perioperative risk factors in 557 patients. Ann Surg. 1983;197:49–56. [PMC free article] [PubMed] [Google Scholar]
- 2.Brady AR, Fowkes FG, Greenhalgh RM, Powell JT, Ruckley CV, Thompson SG. Risk factors for postoperative death following elective surgical repair of abdominal aortic aneurysm: Results from the UK small aneurysm trial. On behalf of the UK small aneurysm trial participants. Br J Surg. 2000;87:742–9. doi: 10.1046/j.1365-2168.2000.01410.x. [DOI] [PubMed] [Google Scholar]
- 3.Wald R, Waikar SS, Liangos O, Pereira BJ, Chertow GM, Jaber BL. Acute renal failure after endovascular vs open repair of abdominal aortic aneurysm. J Vasc Surg. 2006;43:460–6. doi: 10.1016/j.jvs.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Gamulin Z, Forster A, Morel D, Simonet F, Aymon E, Favre H. Effects of infrarenal aortic cross-clamping on renal hemodynamics in humans. Anesthesiology. 1984;61:394–9. doi: 10.1097/00000542-198410000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Welch M, Knight DG, Carr HM, Smyth JV, Walker MG. Influence of renal artery blood flow on renal function during aortic surgery. Surgery. 1994;115:46–51. [PubMed] [Google Scholar]
- 6.Gamulin Z, Forster A, Simonet F, Aymon E, Favre H. Effects of renal sympathetic blockade on renal hemodynamics in patients undergoing major aortic abdominal surgery. Anesthesiology. 1986;65:688–92. doi: 10.1097/00000542-198612000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Cherr GS, Hansen KJ. Renal complications with aortic surgery. Semin Vasc Surg. 2001;14:245–54. doi: 10.1053/svas.2001.27877. [DOI] [PubMed] [Google Scholar]
- 8.De La Cruz JP, Sedeño G, Carmona JA, Sánchez de la Cuesta F. The in vitro effects of propofol on tissular oxidative stress in the rat. Anesth Analg. 1998;87:1141–6. doi: 10.1097/00000539-199811000-00031. [DOI] [PubMed] [Google Scholar]
- 9.Ichikawa I, Hoyer JR, Seiler MW, Brenner BM. Mechanism of glomerulotubular balance in the setting of heterogeneous glomerular injury. Preservation of a close functional linkage between individual nephrons and surrounding microvasculature. J Clin Invest. 1982;69:185–98. doi: 10.1172/JCI110430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathy-Hartert M, Mouithys-Mickalad A, Kohnen S, Deby-Dupont G, Lamy M, Hans P. Effects of propofol on endothelial cells subjected to a peroxynitrite donor (SIN-1) Anesthesia. 2000;55:1066–71. doi: 10.1046/j.1365-2044.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 11.Runzer TD, Ansley DM, Godin DV, Chambers GK. Tissue antioxidant capacity during anesthesia: Propofol enhances in vivo red cell and tissue antioxidant capacity in a rat model. Anesth Analg. 2002;94:89–93. doi: 10.1097/00000539-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-López JM, Sánchez-Conde P, Lozano FS, Nicolás JL, García-Criado FJ, Cascajo C, et al. Laboratory investigation: Effects of propofol on the systemic inflammatory response during aortic surgery. Can J Anesth. 2006;53:701–10. doi: 10.1007/BF03021629. [DOI] [PubMed] [Google Scholar]
- 13.Cróinín DF, Shorten GD. Anesthesia and renal disease. Curr Opin Anesthesiol. 2002;15:359–63. doi: 10.1097/00001503-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Kharasch ED, Schroeder JL, Sheffels P, Liggitt HD. Influence of sevoflurane on the metabolism and renal effects of compound A in rats. Anesthesiology. 2005;103:1183–8. doi: 10.1097/00000542-200512000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Lee HT, Chen SW, Doetschman TC, Deng C, D’Agati VD, Kim M. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway. Am J Physiol Renal Physiol. 2008;295:F128–36. doi: 10.1152/ajprenal.00577.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HT, Kim M, Song JH, Chen SW, Gubitosa G, Emala CW. Sevoflurane-mediated TGF-beta1 signaling in renal proximal tubule cells. Am J Physiol Renal Physiol. 2008;294:F371–8. doi: 10.1152/ajprenal.00277.2007. [DOI] [PubMed] [Google Scholar]
- 17.Lee HT, Kim M, Kim J, Kim N, Emala CW. TGF-beta1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol. 2007;27:416–24. doi: 10.1159/000105124. [DOI] [PubMed] [Google Scholar]
- 18.Eger EI, 2nd, Koblin DD, Bowland T, Ionescu P, Laster MJ, Fang Z, et al. Nephrotoxicity of sevoflurane versus desflurane anesthesia in volunteers. Anesth Analg. 1997;84:160–8. doi: 10.1097/00000539-199701000-00029. [DOI] [PubMed] [Google Scholar]
- 19.Eger EI, 2nd, Gong D, Koblin DD, Bowland T, Ionescu P, Laster MJ, et al. Dose-related biochemical markers of renal injury after sevoflurane versus desflurane anesthesia in volunteers. Anesth Analg. 1997;85:1154–63. doi: 10.1097/00000539-199711000-00036. [DOI] [PubMed] [Google Scholar]
- 20.Ebert TJ, Frink EJ, Jr, Kharasch ED. Absence of biochemical evidence for renal and hepatic dysfunction after 8 hours of 1.25 minimum alveolar concentration sevoflurane anesthesia in volunteers. Anesthesiology. 1998;88:601–10. doi: 10.1097/00000542-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Kharasch ED, Frink EJ, Jr, Zager R, Bowdle TA, Artru A, Nogami WM. Assessment of low-flow sevoflurane and isoflurane effects on renal function using sensitive markers of tubular toxicity. Anesthesiology. 1997;86:1238–53. doi: 10.1097/00000542-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Boldt J, Brenner T, Lehmann A, Lang J, Kumle B, Werling C. Influence of two different volume replacement regimens on renal function in elderly patients undergoing cardiac surgery: Comparison of a new starch preparation with gelatin. Intensive Care Med. 2003;29:763–9. doi: 10.1007/s00134-003-1702-6. [DOI] [PubMed] [Google Scholar]
- 23.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez-Conde P, Rodríguez-López JM, Nicolás JL, Lozano FS, García-Criado FJ, Cascajo C, et al. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth Analg. 2008;106:371–8. doi: 10.1213/ane.0b013e318160580b. [DOI] [PubMed] [Google Scholar]
- 25.Wang HH, Zhou HY, Chen CC, Zhang XL, Cheng G. Propofol attenuation of renal ischemia/reperfusion injury involves heme oxygenase-1. Acta Pharmacol Sin. 2007;28:1175–80. doi: 10.1111/j.1745-7254.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 26.Feng Y, Bai T, Ma H, Wang JK. Propofol attenuates human proximal renal tubular epithelial cell injury induced by anoxia-reoxygenation. Lab Med. 2008;39:356–60. [Google Scholar]
- 27.Assad AR, Delou JM, Fonseca LM, Villela NR, Nascimento JH, Verçosa N, et al. The role of KATP channels on propofol preconditioning in a cellular model of renal ischemia-reperfusion. Anesth Analg. 2009;109:1486–92. doi: 10.1213/ANE.0b013e3181b76396. [DOI] [PubMed] [Google Scholar]
- 28.Obal D, Dettwiler S, Favoccia C, Rascher K, Preckel B, Schlack W. Effect of sevoflurane preconditioning on ischaemia/reperfusion injury in the rat kidney in vivo. Eur J Anesthesiol. 2006;23:319–26. doi: 10.1017/S0265021505002000. [DOI] [PubMed] [Google Scholar]
- 29.Higuchi H, Sumita S, Wada H, Ura T, Ikemoto T, Nakai T, et al. Effects of sevoflurane and isoflurane on renal function and on possible markers of nephrotoxicity. Anesthesiology. 1998;89:307–22. doi: 10.1097/00000542-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology. 2004;101:1313–24. doi: 10.1097/00000542-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Annecke T, Kubitz JC, Kahr S, Hilberath JM, Langer K, Kemming GI, et al. Effects of sevoflurane and propofol on ischaemia-reperfusion injury after thoracic-aortic occlusion in pigs. Br J Anesth. 2007;98:581–90. doi: 10.1093/bja/aem049. [DOI] [PubMed] [Google Scholar]
- 32.Wiedermann CJ. Systematic review of randomized clinical trials on the use of hydroxyethyl starch for fluid management in sepsis. BMC Emerg Med. 2008;8:1. doi: 10.1186/1471-227X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamal R, Ghannoum M, Naud JF, Turgeon PP, Leblanc M. Permanent renal failure induced by pentastarch. NDT Plus. 2008;1:322–5. doi: 10.1093/ndtplus/sfn075. [DOI] [PMC free article] [PubMed] [Google Scholar]


