Abstract
Background:
No randomized controlled trial demonstrates the efficacy of erythromycin or metoclopramide in patients with traumatic brain injury (TBI). This study was conducted to determine the efficacy of metoclopramide and erythromycin for improving gastric aspirate volume (GAV) in patients with TBI.
Materials and Methods:
Patients with Glasgow coma score more than 5 admitted to trauma Intensive Care Unit within 72 h of head injury were assessed for eligibility. 115 patients were prospectively randomized to receive metoclopramide, erythromycin, or placebo eighth hourly. Gastric feeding intolerance was defined as GAV more than 150 ml with abdominal symptoms. Two consecutive high GAV was defined as feeding failure. Feeding failure was treated by increasing the frequency of dose to 6 hourly in metoclopramide and erythromycin group. Combination therapy with both drugs was given as rescue in the placebo group.
Results:
Incidence of high GAV was as high as 60.5% in placebo group. Use of erythromycin was associated with a decrease in the incidence of feeding intolerance to 28.9% (P = 0.006). Although feed intolerance decreased to 43.6% in metoclopramide group, values did not reach statistical significance. The proportion of patients not having high GAV at different days were significantly higher in erythromycin group (P = 0.027, log-rank test). There was no difference in the proportion of patients not having feeding failure in three groups with increasing number of days.
Conclusion:
There was a significant decrease in the incidence of high GAV with the use of erythromycin when compared to metoclopramide and placebo.
Keywords: Erythromycin, gastric feeding intolerance, traumatic brain injury
Introduction
Traumatic brain injury (TBI) triggers hypercatabolism and a resultant increase in consumption of nutritional reserves. Depletion of body mass and impairment of immune mechanism associated with this result in a poor clinical outcome. Nutritional support, thus, becomes vital in improving the clinical outcome in critically injured head injury patients. Enteral nutrition (EN) protects gut barrier, modulates systemic immune responses, and hence helps to attenuate disease severity. However, more than 80% of the patients with severe TBI have prolonged and abnormal gastric emptying.[1,2] This may be attributed to factors such as raised intracranial pressure, sympathetic stimulation, hyperglycemia, and concurrent use of opioids.[3,4]
Various strategies such as early administration of enteral feed, standardized individual feeding protocol, and use of prokinetic agents like metoclopramide and erythromycin, elevation of head and glycemic control have been used to improve EN nutrition in severe head injury (SHI).
Only a few studies have evaluated the effect of prokinetic agents in patients with SHI. There was no significant effect of metoclopramide on gastric emptying time in two small studies conducted in patients with head injury.[5,6] Better gastric emptying and motility with a resultant improvement in food tolerance has been reported with use of erythromycin in critically ill trauma patients.[7,8] In a single study conducted in head injury patients, erythromycin was administered orally as a suspension through an orogastric tube in patients who had feed intolerance despite the use of 10 mg intravenous metoclopramide.[9] Authors found that gastric intolerance resolved in all these patients. In a retrospective analysis, Dickerson et al. found the combination of erythromycin and metoclopramide to be more effective than metoclopramide alone decreasing gastric feeding intolerance in trauma patients with TBI.[10] No prospective randomized, double-blind trial has been conducted comparing the efficacy of these two drugs in terms of improving feed tolerance in head injury patients.
The primary aim of this study was to compare the incidence of high gastric aspirate volume (GAV) with the use of either metoclopramide or erythromycin in a patient with TBI. The incidence of feeding failure, the percentage of target calorie requirement met, and association of severity of head injury with feeding intolerance were the secondary outcomes studied.
Materials and Methods
This prospective, randomized, double-blinded, and placebo-controlled trial was conducted over a period of 1½ year (January 2013-June 2014). Following approval of protocol by Ethics Committee of the institute (Chairperson; Prof Kartar Singh under reference number NK/840/MD/3322-23) TBI patients with Glasgow coma score (GCS) of more than 5 admitted to trauma intensive care within 72 h of injury were assessed for eligibility. A total of 122 patients who met the inclusion criteria were enrolled into the study. A written informed consent was obtained from the guardian. Patient with blunt trauma abdomen, severe thoracic injury with associated hemothorax, allergy to macrolide or metoclopramide, abnormal liver function, and renal dysfunction were excluded.
Patients were randomly allocated into one of the three groups using computer generated a random number for 5 days: Group erythromycin-tablet erythromycin 250 mg, group metoclopramide-tablet metoclopramide 10 mg, and group placebo-tablet placebo. Tablet of Vitamin C was given a placebo in group placebo. The blinded nursing staff who had been assigned the job of taking care of the patient during the time administered crushed drug through Ryle's tube every 8 h for 5 days from day of admission to trauma Intensive Care Unit (ICU). Another blinded investigator collected and recorded the data.
Feeding protocol
16 F orogastric tube was inserted on admission to trauma ICU. The distal tip of the orogastric tube was confirmed to be below the gastroesophageal junction and in the stomach using an abdominal X-ray. 10 ml of air was injected through the orogastric tube and auscultated over stomach before giving each feed to confirm the position of the orogastric tube. Gastric feeding regimens for each patient were according to the standard dietary formula supplied from our hospital. Daily requirement of feed was based on patient's body weight to meet energy and protein requirement of 35 kcal/kg/day and 1.5 g/kg/day, respectively. Feed was given in the form of intermittent boluses through an orogastric tube using a 50 ml syringe. Each feed was given over 10-15 min every 4 hourly. The plunger of the syringe was removed, and the syringe was hung up to allow gravity feeding. The target amount of each bolus feed was determined by dividing the 24 h nutrition requirement by 6. Feed tolerance was defined as GAV <150 ml. Intolerance to feed defined as GAV more than 150 ml.
Patients were nursed in a semi-recumbent position. The blinded bedside nursing staff aspirated orogastric tube and measured the GAV before initiation of feed and before each intermittent feeding using a 50 ml syringe. Initial GAV was measured and discarded. If initial GAV was <150 ml, feeding was initiated with a 100 ml bolus feed along with the initial dose of assigned drug. If GAV was more than 150 ml, only assigned oral drug was administered diluted in 20 ml saline. Before the due next feed, orogastric tube aspiration was performed, volume recorded, and discarded. If the feed was tolerated (GAV <150 ml), calculated target volume was administered. Thereafter, this target volume of bolus was repeated at 4 hourly intervals. In case GAV was more than 150 ml, the aspirate was discarded, feeding was withheld for next 4 h and restarted after 4 h. If the second GAV after this feed was also more than 150 ml, it was labeled as enteral feed failure. The gastric aspirate was discarded and feed withheld for next 8 h. It was then restarted as per the initial protocol. During this period, intravenous 5% dextrose at 100 ml/h was administered as rescue therapy. In case of control group, along with intravenous 5% dextrose, oral prokinetic combination (oral metoclopramide 10 mg + oral erythromycin 250 mg) was started 6th hourly through an orogastric tube for 3 days. However, in case of the group erythromycin and metoclopramide, respective drugs were given at an interval of 6 h. Figure 1 shows a flowchart tube depicting methodology of the study.
Figure 1.
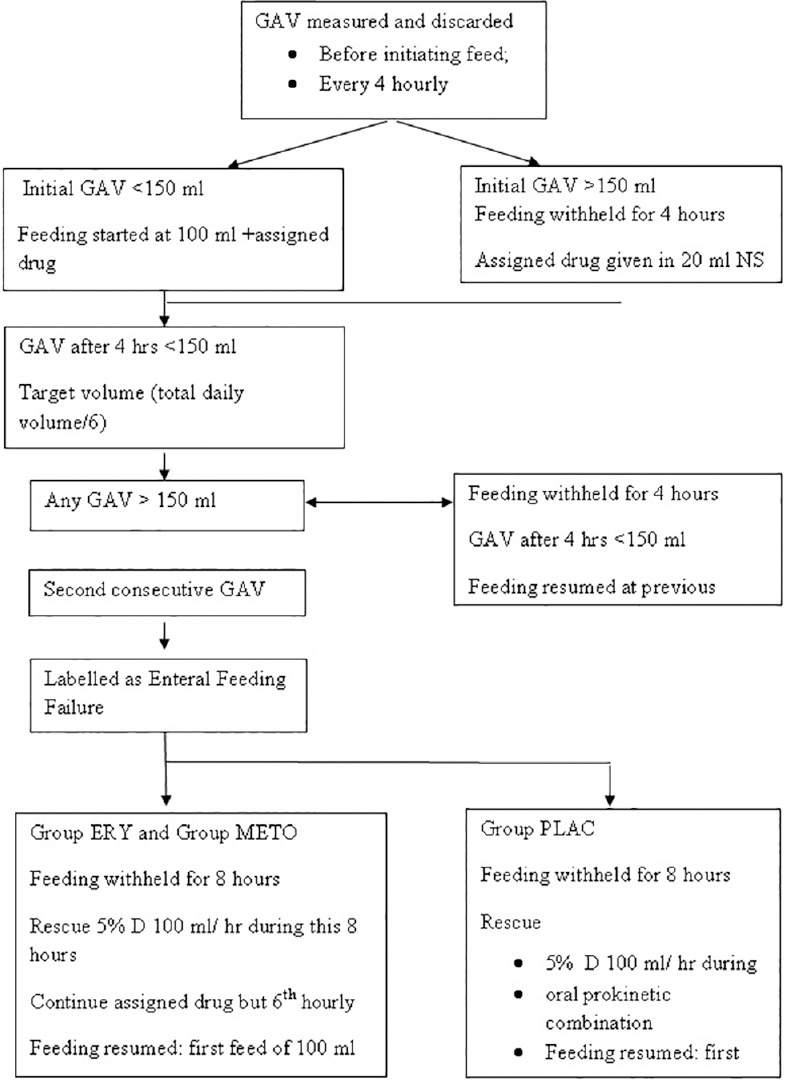
Flow diagram showing feeding protocol
The data were recorded on the ICU flow sheets by nursing staff involved in the care of the patient. Complications of EN were also recorded. Diarrhea was defined as more than three loose or liquid stools per day. Total midazolam and morphine used over the study period were recorded. The use of agents affecting gastric motility such as dopamine and opioids was also recorded. Blood glucose concentration was monitored a twice daily. Target concentration was maintained in between 60 and 200 mg/dl. In case of blood sugar more than 200 mg/dl, intravenous insulin was administered based on sliding scale or continuous infusion. Serum K+ value was maintained at more than 4 mEq/L. In case serum, K+ persisted between 3 and 4 mEq/L, 40 mEq of KCl, and if below 3 mEq/L, 80 mEq of KCl was given intravenously in divided doses.
Daily volume of feed administered, GAV 4 hourly, daily gastric volume emptied (daily volume of feed administered-total GAV discarded daily), enteral feed failure, episodes of diarrhea, and abdominal distension were recorded.
Statistical analysis
The incidence feed intolerance in head injury patients has been reported to be as high as 62.5%.[9] We assumed that supplementation of prokinetic agents would decrease the incidence by 50%. Thirty-eight patients were needed in each group to detect a clinically significant difference with a power of 80% and an alpha error of 0.05. We planned to include 40 patients in each group to include for possible dropouts.
Parametric data are presented as means ± standard deviation, ordinal data as medians and frequency are presented as numbers (percentages). The data were evaluated for normality of the distribution using the Kolmogorov-Smirnov normality test. Interval data were compared using Student's t-test for paired or unpaired variable or the Mann-Whitney U-test with post hoc when comparison appropriate. Among the groups comparison was done using one-way ANOVA followed by post hoc test for normally distributed data. Differences between groups with nominal data were assessed using Chi-square or Fisher's exact analysis. Differences in the success of prokinetic drug therapy over time between the groups were analyzed using Kaplan-Meier survival curves with the log-rank test. A P = 0.05 was considered significant on outcome analysis. The statistical analysis was carried out using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, USA, version 15.0 for windows).
Results
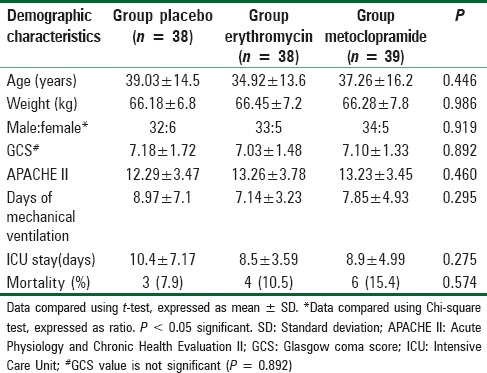
Figure 2 shows the flow diagram of the patients. Of the patients admitted to trauma ICU during the study period, a total of 292 patients with TBI were assessed for eligibility. One hundred twenty-two patients met the study criteria and were enrolled and randomized in this trial. Study protocol could not be followed in seven patients. Data were analyzed for 115 patients. Demographic data were comparable in all the three groups. 84.3% of the patients had SHI (GCS [3-8]), 15.7% had moderate HI (GCS [9-13]), and one had mild HI (GCS [14-15], P = 0.546) [Table 1].
Figure 2.
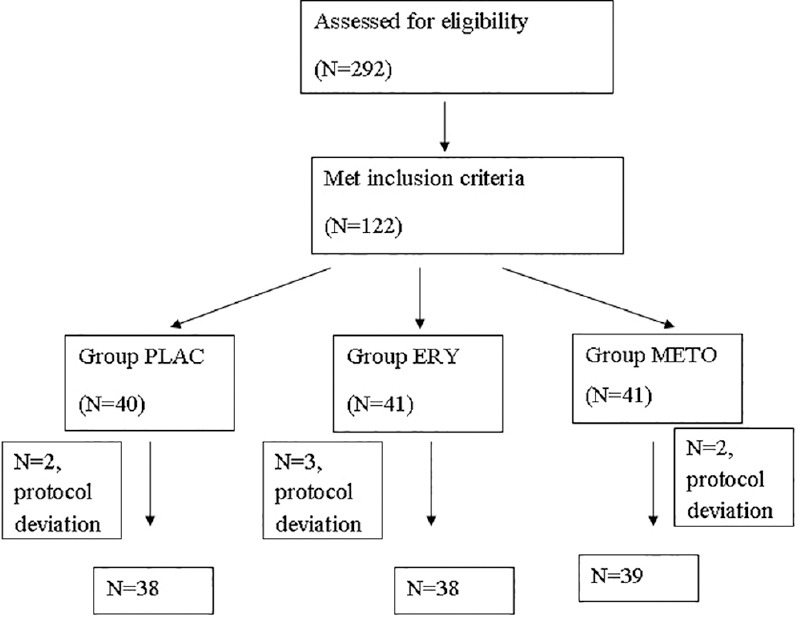
Study flow diagram
Table 1.
Demographic characteristic of the three groups
The incidence of high GAV was significantly different in all three groups (Chi-square test, P = 0.021). Incidence was significantly reduced with the use of erythromycin when compared to placebo (P = 0.006). The incidence of high GAV was comparable between the group erythromycin and metoclopramide. There was no difference in the incidence of high GAV between the metoclopramide and placebo group also [Table 2].
Table 2.
Incidence of high GAV and feeding failure in the three groups
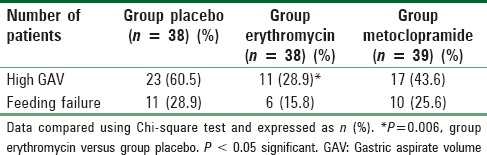
Figure 3 shows the Kaplan-Meier analysis curve of a number of patients having high GAV. The proportion of patients not having high GAV at different days were significantly higher in group erythromycin (P = 0.027, log-rank test). Twenty-seven patients were censored in group erythromycin as compared to 22 in group metoclopramide and 15 in group placebo. There was no difference in the proportion of patients not having feeding failure between the three group with increasing number of days [Figure 4, P = 0.412, log-rank test].
Figure 3.
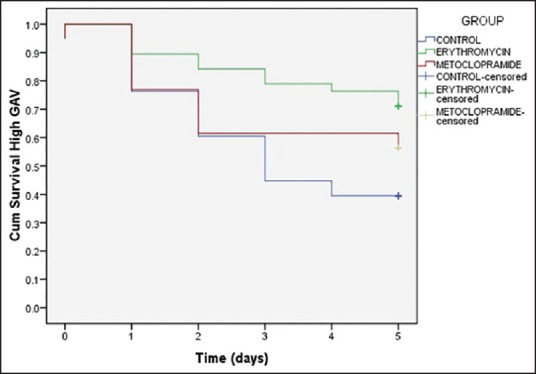
Kaplan-Meier analysis curve showing proportion of patients having high gastric aspirate volume. A significant difference in proportion of patients not having high gastric aspirate volume at different days was found with group erythromycin (P= 0.027, log-rank test)
Figure 4.
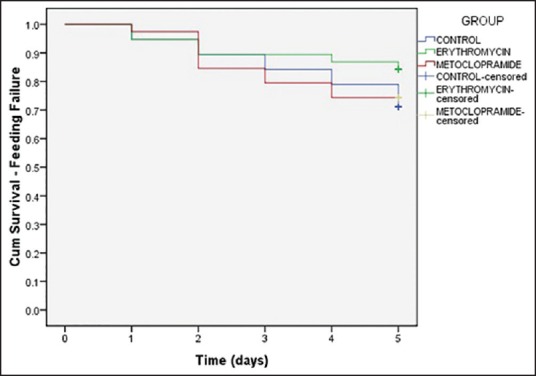
Kaplan-Meier analysis curve showing proportion of patients having feeding failure. No significant difference was found in proportion of patients not having feeding failure between the three group with increasing number of days
Twenty-seven patients out of 115 patients showed feeding failure (two consecutive high GAV). There was no statistical significant difference in the occurrence of feeding failure among the three groups (P = 0.371 Chi-square test) [Table 2]. After giving respective rescue drugs, 3 patients had subsequent high aspirates in the placebo group. 3/10 patients had recurrent high GAV after the escalation of metoclopramide, and 2/6 patients had recurrent high aspirate after the escalation of erythromycin dose.
Target calories met was calculated using the formula: Target calorie met = Target calorie accepted/target calorie intended ×100. Significantly, greater percentage of target calories was met in erythromycin group (90.13 ± 15.88%) as compared to metoclopramide and placebo group (P = 0.046, one-way ANOVA). Abdominal distension was seen in nine patients in group placebo versus 4 in group erythromycin and 3 in group metoclopramide. A number of patients who developed diarrhea were comparable among the three groups (2 vs. 4 vs. 4 in groups placebo, erythromycin and metoclopramide, respectively).
The baseline and 5th day serum albumin were comparable with no statistical different among the groups. No statistical significant intergroup difference of RBS was observed.
Discussion
Severe TBI causes hypermetabolism and hypercatabolism. This results in depletion of nutritional reserves and unfavorable effects on immune function and morbidity.[11] An appropriate amount of early nutritional support enhances immunity, decreases infectious morbidity and is associated with short hospitalization.[12] Though EN is the preferred route of feeding in these patients, most patients with severe TBI do not tolerate EN during first 2 weeks after the injury.[2,13]
EN intolerance manifests as increased gastric residual volume, vomiting, abdominal distention and diarrhea. These complications result in poor enteral feeding as well as increase the risk of aspiration pneumonia. All this leads to prolonged ICU stay and increased mortality rates.[14] Hence, it becomes utmost important to solve the problem of feeding failure in patients with TBI.
American Society for Parenteral and EN and European Society for Parenteral and EN recommend the use of metoclopramide or erythromycin in critically ill patients with intolerance to EN.[1,15] Canadian Clinical Practice Guidelines recommend metoclopramide because of bacterial resistance associated with the use of erythromycin in critically ill patients.[16] However, there is limited data regarding the use of these prokinetic drugs in patients with TBI.
Limited effect of metoclopramide on gastric emptying has been reported in patients with severe TBI in two small studies.[5,6] Marino et al. administered 10 mg of intravenous metoclopramide every 8thh over a period of 48 h in patients with SHI and studied its effect on gastric emptying. Blood paracetamol absorption assays were performed at baseline and at 48 h. Authors did not find the augmented gastric emptying following administration of metoclopramide. In another study, Nursal et al.[6] administered 10 mg metoclopramide thrice a day for 5 days or an equal volume of saline. Baseline gastric emptying was measured using paracetamol absorption test before administering metoclopramide and then at day 5. Similar to Marino et al., authors did not find any significant difference in gastric emptying parameters among the groups. However, the percentage of paracetamol absorption test parameters increased in metoclopramide group at day 5. We administered 10 mg of metoclopramide 8th hourly and defined feed intolerance as GAV >150 ml. Use of metoclopramide resulted in a decrease in the incidence of feed intolerance to 43.6% as compared to 60.5% in the control group, but values were not statistically significance. We did not come across extrapyramidal symptoms (dystonia, parkinsonism, and tardive dyskinesia) in any of our patients.
On the other hand, use of erythromycin has resulted in improved tolerance to EN in critically ill patients, including those who have suffered from various types of trauma.[7] Critically injured patient with GRV >150 ml during the first 48 h was randomized to receive intravenous erythromycin or placebo by Berne et al. Authors found that there was a significant improvement in the amount of feed tolerated in the erythromycin group as compared to the placebo group (58% vs. 44%, P = 0.0011) during 48 h of therapy. We enrolled patients having TBI only and found a significant decrease in the incidence of feed intolerance with the use of erythromycin (43.6%). We were concerned regarding the consequences associated with prolonged intolerance in patients not responding to initial therapy. So, we increased the dose of metoclopramide and erythromycin in their respective groups in patients who developed feeding failure. With the same purpose, we added a combination of both metoclopramide and erythromycin in the control group. Further, two recent studies had demonstrated that combination therapy with erythromycin and metoclopramide exhibited greater benefits monotherapy with either agent.[8,10] This may account for the lack of difference in the proportion of patients not having feeding failure between the three group with increasing number of days. Also, percentage acceptance of target calories met was higher in the control group than metoclopramide group (83.16 ± 16.25% in metoclopramide group vs. 80.42 ± 19.78% in placebo group) in our study.
One of the concerns with the use of erythromycin is the occurrence of various types of arrhythmias. Our patients were continuously monitored in intensive care, and we did not come across prolonged QT-interval, torsade de pointes, or ventricular dysrhythmias in any of our patients.
The relationship between disease severity and the occurrence of gastrointestinal disorders was controversial in previous studies.[6,9,13,17] Initial studies had shown that intolerance to enteral feeding in TBI patients were associated with the severity of the brain injury.[13,17] However, studies conducted later were not able to demonstrate the relationship between the two.[6,9] This was attributed to the smaller number of patients enrolled in these studies. We also did not find any relationship between the disease severity and the occurrence of high GAV in our study.
One of the limitations of our study is that we used oral preparation. This was unavoidable because of nonavailability of intravenous preparation of erythromycin in India. Another limitation of use of erythromycin is its antibiotic effect. We did not find any differences in the incidence of nosocomial infections amongthe three groups.
Conclusion
To conclude, the incidence of high GAV in TBI patients was 60.5%, and there was a significant decrease in the incidence of high GAV with the use of erythromycin when compared to metoclopramide and placebo. Since, the combination of erythromycin and metoclopramide was associated with acceptance of greater percentage of target calories in the control group, further studies should be carried out using this combination.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) JPEN J Parenter Enteral Nutr. 2009;33:277–316. doi: 10.1177/0148607109335234. [DOI] [PubMed] [Google Scholar]
- 2.Kao CH, ChangLai SP, Chieng PU, Yen TC. Gastric emptying in head-injured patients. Am J Gastroenterol. 1998;93:1108–12. doi: 10.1111/j.1572-0241.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 3.Ott L, Annis K, Hatton J, McClain M, Young B. Postpyloric enteral feeding costs for patients with severe head injury: Blind placement, endoscopy, and PEG/J versus TPN. J Neurotrauma. 1999;16:233–42. doi: 10.1089/neu.1999.16.233. [DOI] [PubMed] [Google Scholar]
- 4.Kirby DF. As the gut churns: Feeding challenges in the head-injured patient. JPEN J Parenter Enteral Nutr. 1996;20:1–2. doi: 10.1177/014860719602000101. [DOI] [PubMed] [Google Scholar]
- 5.Marino LV, Kiratu EM, French S, Nathoo N. To determine the effect of metoclopramide on gastric emptying in severe head injuries: A prospective, randomized, controlled clinical trial. Br J Neurosurg. 2003;17:24–8. [PubMed] [Google Scholar]
- 6.Nursal TZ, Erdogan B, Noyan T, Cekinmez M, Atalay B, Bilgin N. The effect of metoclopramide on gastric emptying in traumatic brain injury. J Clin Neurosci. 2007;14:344–8. doi: 10.1016/j.jocn.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Berne JD, Norwood SH, McAuley CE, Vallina VL, Villareal D, Weston J, et al. Erythromycin reduces delayed gastric emptying in critically ill trauma patients: A randomized, controlled trial. J Trauma. 2002;53:422–5. doi: 10.1097/00005373-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NQ, Chapman MJ, Fraser RJ, Bryant LK, Holloway RH. Erythromycin is more effective than metoclopramide in the treatment of feed intolerance in critical illness. Crit Care Med. 2007;35:483–9. doi: 10.1097/01.CCM.0000253410.36492.E9. [DOI] [PubMed] [Google Scholar]
- 9.Pinto TF, Rocha R, Paula CA, de Jesus RP. Tolerance to enteral nutrition therapy in traumatic brain injury patients. Brain Inj. 2012;26:1113–7. doi: 10.3109/02699052.2012.666369. [DOI] [PubMed] [Google Scholar]
- 10.Dickerson RN, Mitchell JN, Morgan LM, Maish GO, 3rd, Croce MA, Minard G, et al. Disparate response to metoclopramide therapy for gastric feeding intolerance in trauma patients with and without traumatic brain injury. JPEN J Parenter Enteral Nutr. 2009;33:646–55. doi: 10.1177/0148607109335307. [DOI] [PubMed] [Google Scholar]
- 11.Krakau K, Omne-Pontén M, Karlsson T, Borg J. Metabolism and nutrition in patients with moderate and severe traumatic brain injury: A systematic review. Brain Inj. 2006;20:345–67. doi: 10.1080/02699050500487571. [DOI] [PubMed] [Google Scholar]
- 12.Perel P, Yanagawa T, Bunn F, Roberts I, Wentz R, Pierro A. Nutritional support for head-injured patients. Cochrane Database Syst Rev. 2006;18:CD001530. doi: 10.1002/14651858.CD001530.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norton JA, Ott LG, McClain C, Adams L, Dempsey RJ, Haack D, et al. Intolerance to enteral feeding in the brain-injured patient. J Neurosurg. 1988;68:62–6. doi: 10.3171/jns.1988.68.1.0062. [DOI] [PubMed] [Google Scholar]
- 14.Mentec H, Dupont H, Bocchetti M, Cani P, Ponche F, Bleichner G. Upper digestive intolerance during enteral nutrition in critically ill patients: Frequency, risk factors, and complications. Crit Care Med. 2001;29:1955–61. doi: 10.1097/00003246-200110000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, et al. ESPEN guidelines on enteral nutrition: Intensive care. Clin Nutr. 2006;25:210–23. doi: 10.1016/j.clnu.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P. Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr. 2003;27:355–73. doi: 10.1177/0148607103027005355. [DOI] [PubMed] [Google Scholar]
- 17.Acosta Escribano JA, Carrasco Moreno R, Fernández Vivas M, Navarro Polo JN, Más Serrano P, Sánchez Payá J, et al. Gastric enteral intolerance in mechanically ventilated patients with traumatic cerebral lesion. Nutr Hosp. 2001;16:262–7. [PubMed] [Google Scholar]


