Abstract
Background/Objectives
Vision-based speed of processing (VSOP) training is a promising cognitive intervention for older adults. However, it is unknown whether VSOP training can affect cognitive processing in individuals at high risk for dementia. Here, we examined cognitive and neural effects of VSOP training in older adults with amnestic mild cognitive impairment (aMCI) and contrasted those effects with an active control (mental leisure activities; MLA).
Design
A randomized single-blinded controlled pilot trial.
Setting
An academic medical center.
Participants
Twenty-one participants with aMCI.
Intervention
A 6-week computerized VSOP training.
Measurements
Multiple cognitive processing measures, instrumental activities of daily living (IADL), and two key resting state neural networks regulating cognitive processing: central executive network (CEN) and default mode network (DMN).
Results
We found that, compared to MLA control, VSOP training led to significant improvements in trained (processing speed and attention: F1,19 = 6.61, Partial η2 = 0.26, p = .019) and untrained cognitive domains (working memory: F1,19 = 7.33, Partial η2 = 0.28, p = .014; IADL: F1,19 = 5.16, Partial η2 = 0.21, p = .035), and protective maintenance in DMN (F1, 9 = 14.63, Partial η2 = 0.62, p = .004). Additionally, VSOP training, but not MLA, resulted in a significant improvement in CEN connectivity (Z = −2.37, p = .018).
Conclusion
We identified both target and transfer effects of VSOP training and revealed links between VSOP training and two key neural networks associated with aMCI. These findings highlight the potential of VSOP training to slow cognitive decline in aMCI. Further delineation of mechanisms underlying VSOP-induced plasticity is necessary to understand in what populations and conditions such training may be most effective.
Keywords: speed of processing, mild cognitive impairment, central executive network, default mode network
INTRODUCTION
Amnestic mild cognitive impairment (aMCI), especially the multiple-domain subtype, is considered a symptomatic pre-Alzheimer’s disease (AD) phase,1 and reflects a period when the underlying pathobiology may be more receptive to modulation than in AD. Notably, individuals with MCI are highly motivated to engage in activities to maintain cognitive and functional independence.2
One promising intervention is vision-based speed of processing (VSOP) training, a cognitive intervention widely used in community-dwelling older adults free of AD.3, 4 VSOP training primarily addresses visual processing speed and attention, which support most higher-order cognitive functions5 and predict both aMCI incidence and progression to AD.6 A few weeks of VSOP training has been shown to improve multiple cognitive domains and/or everyday function in normal aging,3, 7, 8 HIV,9 and Parkinson’s disease.10 Moreover, individuals with lower baseline cognition are able to experience greater cognitive benefits from training.11 Taken together, these findings suggest that VSOP training might be particularly beneficial for individuals with aMCI. Indeed, a recent study demonstrated beneficial effects of VSOP training on trained domains (i.e., processing speed and attention) across different MCI subtypes.12 It is unknown, however, whether the effects of VSOP training in aMCI transfer on untrained cognitive and functional domains, which is the standard for evaluating the generalizability of improvement in training.
Recent research suggests that neuroplasticity — the brain’s ability to undergo beneficial restructuring or reprogramming in response to environmental stimuli — may be induced in later life, even in aMCI.13 Evidence for neuroplasticity can indicate that the effects of training are not limited to cognitive operations (e.g., increasing task fluency). In healthy older adults, a recent VSOP intervention study showed significant improvement in event-related potential waveforms associated with processing speed and attention.14 Here, we focus on investigating plasticity in neural markers of neurodegeneration as such plasticity might indicate ways to modify AD pathology. Growing evidence conceptualizes AD as a neural connectivity syndrome.15 Central executive network (CEN) and default mode network (DMN) are critical in maintaining visual processing speed and attention, and are susceptible to both normal and abnormal aging processes, including MCI.5, 16 CEN includes the dorsolateral and ventromedial prefrontal cortex, insula, striatum, and the posterior and anterior cingulate gyri. This network directs engagement in tasks with high executive working load and error feedback.17 DMN includes the posterior cingulate cortex, ventromedial prefrontal cortex, lateral occipital cortex, hippocampus, and middle temporal cortex. DMN is related to memory encoding and storage.18 These networks are typically studied using resting state functional connectivity (rsFC), which examines task-independent, spontaneous fluctuations in functional connectivity to reveal brain networks where information is continuously processed and transported between structurally and functionally linked brain regions.19 Recent studies found that, compared to their healthy counterparts, aMCI is associated with weaker connectivity in DMN and stronger connectivity in CEN.17, 20, 21 Similar rsFC changes were also associated with increased beta-amyloid deposition in older adults, further suggesting that DMN and CEN are sensitive to AD pathology.20 To our knowledge, there are no studies examining VSOP training’s effect on rsFC in aMCI.
In sum, this pilot trial addressed two unresolved questions in the VSOP training literature in relation to dementia prevention: (1) whether VSOP training in aMCI would transfer to untrained cognitive domains and (2) whether VSOP training could be linked to resting state neural networks.3, 4 These questions are important for establishing clinical relevance of VSOP training and better understanding VSOP-induced neuroplasticity. We hypothesized that, compared to MLA, VSOP training would lead to greater and broader cognitive improvements and more efficient rsFC (i.e., decreased CEN and increased DMN connectivity).
METHODS
Participants
We conducted a randomized controlled single-blinded trial. Participants with aMCI were recruited from University of Rochester Memory Care Program (MCP) using the clinical diagnosis of “mild cognitive impairment due to Alzheimer’s disease.”1 All participants had deficits in memory and executive function based on a comprehensive neuropsychological battery but intact basic activities of daily living, and absence of dementia using NIA-AA criteria per assessments at MCP. Other inclusion criteria included stable use of AD medication, capacity to give consent based on clinician assessment, age ≥60 years, English-speaking, adequate visual acuity for testing, and being community-dwelling. Exclusion criteria included participation in another cognitive intervention study, and active treatment with antidepressants or anxiolytics.
The study was approved by the University of Rochester Research Subject Review Board. Twenty-four participants were enrolled and randomly assigned to the VSOP or MLA group after informed consent and baseline assessment. Cognitive function and rsFC were assessed at baseline and post-training. Interviewers were blinded to the participants’ group assignment. Three participants (2 from the VSOP group) withdrew after baseline assessment due to health issues unrelated to the current study. The baseline characteristics of the remaining 21 participants did not significantly differ between the two groups (see Table 1).
Table 1.
Baseline Sample Characteristics.
| VSOP training (n = 10) | MLA control (n = 11) | Independent t or X2 test (p value) | |
|---|---|---|---|
| Demographic and health information | |||
| Age, Mean (SD) | 72.90 (8.23) | 73.09 (9.60) | −0.05 (.96) |
| Male, n (%) | 5 (50.05) | 6 (54.5%) | 0.04 (1.00) |
| Education: HS or lower, n (%) | 1 (10.0%) | 5 (45.5%) | 3.23 (.15) |
| White, n (%) | 7 (70.0%) | 10 (90.9%) | 1.49 (.31) |
| 15-item Geriatric Depressive Scale, Mean (SD) | 2.30 (1.89) | 3.64 (0.71) | −1.39 (.18) |
| History of engaging in mental leisure activities, Mean (SD) | 3.83 (0.71) | 4.44 (1.05) | −1.56 (.14) |
| Montreal Cognitive Assessment, Mean (SD) | 24.44 (2.60) | 25.63 (1.63) | −1.25 (.23) |
Intervention
VSOP training used the INSIGHT online program (Posit Science) which included five training tasks: Eye for detail, Peripheral challenge, Visual sweeps, Double decision, and Target tracker.14 Across tasks, participants responded either by identifying what object they saw or where they saw it on the screen. The training automatically adjusted the task difficulty and speed based on the participant’s performance, ensuring that the participants always operated near their optimal capacity. The completion percentage and score of each task were recorded. Training performance was calculated relative to the normative data in Posit Science database that have completed these same configurations and expressed as a percentile. As expected, VSOP training resulted in significant performance increases (pre-training mean and standard deviation: 34.4% (13.2%); post-training: 52.2% (16.5%); Wilcoxon test: Z = −2.81, p = .005).
MLA control activities were chosen to: 1) control for computer/online experience, and amount of time; 2) simulate participants’ everyday mental activities; and 3) entertain participants to prevent dropping out. Online crossword, Sudoku, and solitaire games were used.3 Participants could choose to practice any combination of these games.
Both groups were asked to practice 1 hour/day, 4 days/week, for 6 weeks in their homes. Hours spent on training tasks were recorded in both groups; no significant differences were found (VSOP: 15.44 hours (6.56); MLA: 19.27 (8.11), t20 = −1.14, p = .27). There were no correlations between training duration and training effects reported below in the entire sample (all p > .10). In VSOP training studies of healthy older adults, typical training duration is around 10 hours.3, 11, 14 Thus, even with a portion of participants that did not complete all of our training, we ended up with a favorably comparable amount of training (~15h).
Outcome Measures
Cognitive measures
The Useful Field of View (UFOV) is a computerized test assessing visual processing speed and attention. Visual and attentional demands of UFOV are similar (although not identical) to the task demands in VSOP training 22. A composite score of UFOV was developed by averaging the reaction times across three individual tasks (processing speed, selective attention, and divided attention). The use of the composite score is consistent with the approach used in other clinical trials.3, 4
The EXAMINER is a computerized test designed for clinical trials, measuring three executive function domains: cognitive control (set shifting and flanker tasks), verbal fluency (phonemic and categorical fluency), and working memory (dot counting and 1-back). This three-domain model was determined using confirmatory factor analysis and the generation of composite scores was based on item response theory methods (for a detailed description see Chapter 11 in the User Manual 23). EXAMINER uses several comparable assessment packages to avoid using identical tests at different assessment points.24
TIADL objectively measures performance speed and accuracy on multiple IADL domains. It is more sensitive than the traditional self-report instruments in detecting subtle decline in everyday function in persons with MCI.25 Time spent on each task was recorded with adjustment on whether an individual accurately completed each task. Detailed description of the scoring process is available in a previous study.26 Average completion time across the tasks was used as the outcome measure.
Neuroimaging data were acquired on a Siemens TimTrio 3T MRI system (Siemens, Erlangen, Germany) using a 32-channel head coil. High-resolution T1 weighted structural images were acquired using MPRAGE (TI = 950 ms, TE/TR = 3.87 ms/1,620 ms, 1mm3 resolution). A 2D axial fast Gradient-Recalled Echo pulse sequence was used to generate field maps, which was used to correct for field inhomogeneity distortions in echo-planar imaging sequences. Two 5-min BOLD functional scans were acquired for each assessment period, using a gradient echo-planar imaging sequence (TR = 2s, TE = 30ms, 4mm3 resolution, 30 axial slices). Participants were instructed to relax with their eyes open without falling asleep.
rsFC data were analyzed using the FSL software (FMRIB Software Library; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). Data preprocessing consisted of motion correction, slice-timing correction, non-brain signal removal and Gaussian spatial smoothing (5mm FWHM). Nuisance parameters (global, white matter and CSF signals, motion) were removed through linear regression. Non-neuronal contributions were reduced with temporal filtering (0.01–0.08 Hz). MELODIC algorithm was used to generate resting state networks. DMN and CEN networks were identified based on previous literature.27 Network specific regions of interest (ROI) were selected using the Harvard-Oxford Atlas. Correlation of time courses between all possible pairs of within-network ROIs were computed and Fisher Z-transformed, with the average correlation coefficient representing the strength of the network.
Other data analysis was conducted using SPSS 21.0. To examine group differences at baseline, independent t-test or χ2 test was conducted for continuous and categorical variables, respectively. To examine within-group effects of training, Wilcoxon test was conducted. Baseline cognitive and neural outcomes did not significantly differ between two groups except that VSOP training had worse working memory (p = .028). To examine between-groups effects of training, repeated measure general linear model was conducted; the main and interacted terms of time and group was examined when controlling for baseline differences. For reported p- values, false-discovery rate was used to address for multiple comparisons across outcomes.
We based our sample size on a previous VSOP training study of multiple-domain amnestic MCI, which reported an effect size (η2) = 0.37 when comparing post-training UFOV with a no-contact control group.12 From this result, we estimated that the minimum total sample size would be 14 (based on: α = .05, power = .80, 2 groups, 2 repeated measures, and .50 correlation between repeated measures). This compares favorably with our total sample size of 21.
RESULTS
Training effects on trained and transferred cognitive outcomes
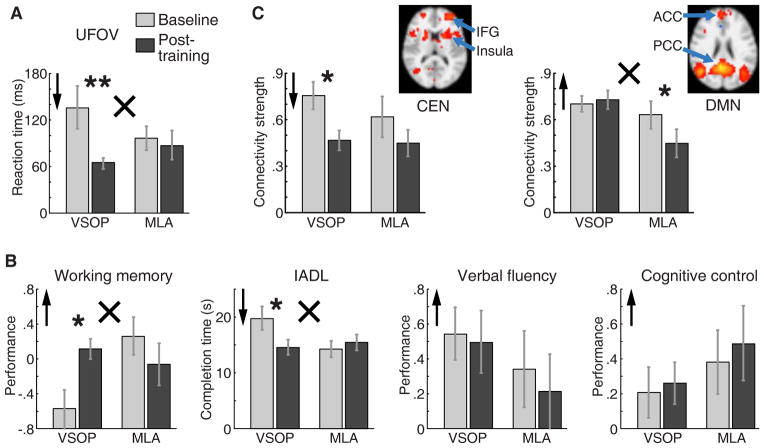
We first examined within-group cognitive changes (Figure 1 A&B, Table 2), contrasting baseline and post-training outcomes. For the VSOP group, we found significant improvements in the trained domain (UFOV, Z = −2.70, p = .007) and two transfer domains (working memory: Z = 2.31, p = .021, and IADL: Z = −2.29, p = .022), but no significant changes in two other transfer domains (verbal fluency and cognitive control). For the MLA group, there were no significant improvements (all p > .10).
Figure 1.
Effects of VSOP training and MLA control training on a range of cognitive and neural domains. A. Effects of training on UFOV, the trained domain for VSOP training; B. Effects of training on transfer domains: working memory, IADL, verbal fluency, and cognitive control; C. Effects of training on neural domains: resting state neural connectivity for CEN and DMN; inserts show horizontal brain slices that include key ROIs for each network (IFG = inferior frontal gyrus, ACC = anterior cingulate cortex, PCC = posterior cingulate cortex).
Note.
 Higher scores indicated better outcome;
Higher scores indicated better outcome;
 Lower scores indicated better outcome. Within-group (Baseline vs. Post-training) comparison: * p < .05, ** p < .01. Group (VSOP vs. MLA) by time (Baseline vs. Post-training) comparison: × p < .05.
Lower scores indicated better outcome. Within-group (Baseline vs. Post-training) comparison: * p < .05, ** p < .01. Group (VSOP vs. MLA) by time (Baseline vs. Post-training) comparison: × p < .05.
Table 2.
Baseline and Post-Training Cognitive and Neural Scores by Group
| VSOP training (n = 10) | MLA control (n = 11) | Group × Time interaction d | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Baseline M (SD) | Post-training M (SD) | Z (p value) c | Baseline M (SD) | Post-training M (SD) | Z (p value) e | F (p value) | Partial η2 | |
| UFOV a | 136.35 (87.42) | 63.96 (22.22) | −2.70 (.007)* | 96.63 (48.67) | 87.65 (59.53) | −1.33 (.18) | 6.61 (.019)* | 0.26 |
| Working memory b | −0.58 (0.71) | 0.11 (0.37) | 2.31 (.021)* | 0.26 (0.68) | −0.06 (0.76) | −.98 (.33) | 7.33 (.014)* | 0.28 |
| Verbal fluency b | 0.55 (0.48) | 0.50 (0.57) | −0.46 (.65) | 0.34 (0.69) | 0.21 (0.70) | −0.18 (.86) | 0.09 (.77) | 0.005 |
| Cognitive control b | 0.21 (0.46) | 0.26 (0.38) | 0.68 (.50) | 0.38 (0.58) | 0.49 (0.68) | 1.26 (.21) | 0.14 (.71) | 0.008 |
| IADL a | 19.80 (6.61) | 14.60 (4.25) | −2.29 (.022)* | 14.25 (4.65) | 15.44 (4.49) | 0.71 (.48) | 5.16 (.035)* | 0.21 |
| CEN a | 0.77 (0.23) | 0.47 (0.17) | −2.37 (.018)* | 0.62 (0.26) | 0.45 (0.17) | −1.46 (.14) | 2.03 (.19) | 0.04 |
| DMN b | 0.70 (0.14) | 0.73 (0.16) | 1.04 (.31) | 0.63 (0.18) | 0.45 (0.18) | −1.83 (.068) | 14.63 (.004)* | 0.62 |
Note.
higher is worse;
performance, lower is worse;
Within-group comparison with Wilcoxon’s test;
Between-group comparison with repeated measure general linear model controlled for group and time’s main effects.
Significant level remained after false discovery rate adjustment.
The same pattern of results was evident in between-group comparisons (Figure 1 A&B, Table 2). Namely, compared to MLA, the VSOP group exhibited significant improvements in UFOV (group-by-time interaction F1,19 = 6.61, Partial η2 = 0.26, p = .019), working memory (group-by-time interaction F1, 19 = 7.33, Partial η2 = 0.28, p = .014), and IADL (group-by-time interaction F1, 19 = 5.16, Partial η2 = 0.21, p = .035).
Training effects on resting state neural networks
For the VSOP group, we found a significant improvement in CEN connectivity (Z = −2.37, p = .018, as indexed by reduced connectivity strength) and no change in DMN (Figure 1C, Table 2). The MLA group showed no CEN changes and trend for worsening of DMN (Z = 1.83, p = .068, as indexed by reduced strength of connectivity). Between-group analysis (Figure 1C, Table 2) showed that, compared to MLA control, VSOP training resulted in significant improvements (indexed by increased connectivity) of DMN (group-by-time interaction F1, 9 = 14.63, Partial η2 = 0.62, p = .004), but not in CEN.
Summary of the results is presented in Table 2.
DISCUSSION
The present study shows that, in addition to the improvement in the trained domain, VSOP training led to improvements in working memory and IADL. The results also link VSOP training with the maintenance of DMN connectivity strength and decreased CEN connectivity.
The transfer of VSOP training to untrained cognitive and functional domains is of likely clinical significance. There may be several non-exclusive explanations of this transfer effect. First, as individuals with MCI have low baseline cognitive capacity, they have more room for improvement both in the trained and untrained domains. Second, VSOP training used here includes a rich combination of visual processing speed and attention tasks (see Methods). This is in contrast to previous studies that usually relied on a single task.7 However, transfer effects of the training still exhibited a certain degree of specificity. For example, we did not find significant changes in verbal fluency, which is likely due to the lack of linguistic stimuli in the training tasks. The specificity of transfer effects across different executive function domains requires further investigation with larger sample size.
The two brain networks examined in the present study provide a possible functional platform for disseminating training effects from one region to another. VSOP training in MCI was linked with reduced CEN connectivity and maintenance of DMN connectivity. One explanation for the reduced CEN connectivity is that VSOP training helped enhance the efficiency of information processing, which reduced the frontal lobe-oriented dependence. Turning to DMN, weakening of DMN connectivity is a consistently identified marker for neurodegeneration.28 Although the VSOP training did not enhance DMN connectivity, the maintenance of DMN connectivity can be viewed as a positive outcome given naturally worsening processes in MCI. Supporting this argument, we found a trend for weakened DMN connectivity in the MLA group. This is not surprising, since a recent cohort study found MLA to be independent from brain pathology. 29
Limitations of the study need to be acknowledged. First, this study was designed to investigate VSOP-training induced changes in various cognitive and neural measures. While our sample size provided sufficient power to examine training-induced changes, it was insufficient to examine correlations among various cognitive domains and indexes of neural changes. Whether the cognitive changes correspond to neural changes is critical in linking the cognitive and neural effects, and needs to be addressed in future studies with larger sample size. Second, although the five tasks within VSOP training share similar visual components, we did not specify training effects of individual tasks (similar to other cognitive training studies 16). This will also require a much larger sample size. Third, although there were no significant differences in the training duration between groups, this does not ensure that the “intensity” of the training is equated. Future research should determine whether, and to what degree, training intensity differences accounted for differences in the effects of VSOP and MLA training.
Acknowledgments
Funding: The study was supported by the University of Rochester CTSA award No. KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health to F.L. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The manuscript preparation was also funded by the Alzheimer’s Association New Investigator Grant (NIRG-14-317353) and NIH R01 grant (NR015452) to F.L.
Sponsor’s Role: University of Rochester CTSA award No. KL2 TR000095 from the National Center for Advancing Translational Sciences of the National Institutes of Health: support the design of the study, and data collection, analysis, and interpretation.
Alzheimer’s Association New Investigator Grant (NIRG-14-317353): support the preparation of the manuscript.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Indicate authors’ role in study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript. (See section on “Authorship and Duplicate Publication”).
Feng Lin: designed the study, collected, analyzed and interpreted the data, and wrote the manuscript.
Kathi L. Heffner: designed the study, wrote the manuscript
Ping Ren: analyzed and interpreted the data and wrote the manuscript
Madalina E. Tivarus: analyzed and interpreted the data and wrote the manuscript
Judith Brasch: collected the data and wrote the manuscript
Ding-Geng Chen: analyzed and interpreted the data and wrote the manuscript
Mark Mapstone: designed the study, collected the data, and wrote the manuscript
Anton P. Porsteinsson: designed the study, collected the data, and wrote the manuscript
Duje Tadin: designed the study, collected the data, and wrote the manuscript
References
- 1.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease:Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin F, Heidrich SM. Role of older adult’s illness schemata in coping with mild cognitive impairment. J Psychosom Res. 2012;72:357–363. doi: 10.1016/j.jpsychores.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Wolinsky FD, Vander Weg MW, Howren MB, et al. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One. 2013;8:e61624. doi: 10.1371/journal.pone.0061624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert MA. Slowing down: Age-related neurobiological predictors of processing speed. Front Neurosci. 2011;5:25. doi: 10.3389/fnins.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sylvain-Roy S, Bherer L, Belleville S. Contribution of temporal preparation and processing speed to simple reaction time in persons with Alzheimer’s disease and mild cognitive impairment. Brain Cogn. 2011;74:255–261. doi: 10.1016/j.bandc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards JD, Valdes EG, Peronto C, et al. The efficacy of insight cognitive training to improve useful field of view performance: A brief report. J Gerontol B Psychol Sci Soc Sci. 2015;70:417–422. doi: 10.1093/geronb/gbt113. [DOI] [PubMed] [Google Scholar]
- 9.Vance DE, Fazeli PL, Ross LA, et al. Speed of processing training with middle-age and older adults with HIV: A pilot study. J Assoc Nurses AIDS Care. 2012;23:500–510. doi: 10.1016/j.jana.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards JD, Hauser RA, O’Connor ML, et al. Randomized trial of cognitive speed of processing training in Parkinson disease. Neurology. 2013;81:1284–1290. doi: 10.1212/WNL.0b013e3182a823ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ball K, Edwards JD, Ross LA. The impact of speed of processing training on cognitive and everyday functions. J Gerontol B Psychol Sci Soc Sci. 2007;62(Spec No 1):19–31. doi: 10.1093/geronb/62.special_issue_1.19. [DOI] [PubMed] [Google Scholar]
- 12.Valdes EG, O’Connor ML, Edwards JD. The effects of cognitive speed of processing training among older adults with psychometrically- defined mild cognitive impairment. Curr Alzheimer Res. 2012;9:999–1009. doi: 10.2174/156720512803568984. [DOI] [PubMed] [Google Scholar]
- 13.Simon SS, Yokomizo JE, Bottino CM. Cognitive intervention in amnestic Mild Cognitive Impairment: A systematic review. Neurosci Biobehav Rev. 2012;36:1163–1178. doi: 10.1016/j.neubiorev.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 14.O’Brien JL, Edwards JD, Maxfield ND, et al. Cognitive training and selective attention in the aging brain: An electrophysiological study. Clin Neurophysiol. 2013;124:2198–2208. doi: 10.1016/j.clinph.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Ramirez J, Wu J. Network-based biomarkers in Alzheimer’s disease: Review and future directions. Front Aging Neurosci. 2014;6:12. doi: 10.3389/fnagi.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeuchi H, Kawashima R. Effects of processing speed training on cognitive functions and neural systems. Rev Neurosci. 2011;23:289–301. doi: 10.1515/revneuro-2012-0035. [DOI] [PubMed] [Google Scholar]
- 17.Agosta F, Pievani M, Geroldi C, et al. Resting state fMRI in Alzheimer’s disease: Beyond the default mode network. Neurobiol Aging. 2012;33:1564–1578. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 19.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Mormino EC, Smiljic A, Hayenga AO, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex. 2011;21:2399–2407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Supekar K, Menon V, Rubin D, et al. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin F, Chen DG, Vance D, Mapstone M. Trajectories of combined laboratory- and real world-based speed of processing in community-dwelling older adults. J Gerontol B Psychol Sci Soc Sci. 2013;68:364–373. doi: 10.1093/geronb/gbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manual U, editor. NINDS: Domain Specific Tasks of Executive Function. Executive Abilities: Measures and Instruments for Neurobehavioral Evaluation and Research (EXAMINER) [Google Scholar]
- 24.Possin KL, Feigenbaum D, Rankin KP, et al. Dissociable executive functions in behavioral variant frontotemporal and Alzheimer dementias. Neurology. 2013;80:2180–2185. doi: 10.1212/WNL.0b013e318296e940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jefferson AL, Byerly LK, Vanderhill S, et al. Characterization of activities of daily living in individuals with mild cognitive impairment. Am J Geriatr Psychiatry. 2008;16:375–383. doi: 10.1097/JGP.0b013e318162f197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owsley C, Sloane M, McGwin G, Jr, et al. Timed instrumental activities of daily living tasks: Relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48:254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- 27.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennis EL, Thompson PM. Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol Rev. 2014;24:49–62. doi: 10.1007/s11065-014-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson RS, Boyle PA, Yu L, et al. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81:314–321. doi: 10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]