At the basic level, we know the genetic cause of cystic fibrosis: it is an autosomal recessive disease caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) 1,2. At the clinical level, we know that chronic bacterial airway infection, prominent neutrophilic inflammation, mucus-obstructed airways, and progressive bronchiectasis characterize advanced cystic fibrosis lung disease, which causes most cystic fibrosis morbidity and mortality 2. Between those two extremes, how loss of CFTR-mediated chloride and bicarbonate transport leads to chronic airway infection has remained uncertain.
Over the past two decades, investigators have studied people with cystic fibrosis, who have disease causing CFTR mutations, at progressively earlier time points. We have learned that, by 3 years of age, bronchiectasis is present in nearly one in three children with cystic fibrosis3, although the host defense defects that trigger infection continue to be debated 4-10. Even before symptom onset, pulmonary inflammation and infection are often present, although which comes first has been uncertain 11-13. As early as 3 months of age, most babies with cystic fibrosis have abnormal chest X-ray computed tomography (CT) 14, although the relative contribution of inflammation, airway remodeling or other factors remains undefined. Moving to even earlier time points might reveal the origins of cystic fibrosis lung disease and thereby change clinical practice.
Indeed, simply knowing that disease begins before symptoms has been a factor driving cystic fibrosis centers to intervene early, and the outcomes have been encouraging 15. Understanding the initial host defense defects in cystic fibrosis airways could suggest novel preventions and treatments and the means to assess disease status and efficacy of therapeutics. Implementation of universal newborn cystic fibrosis screening and potential new therapeutics that target CFTR 16-18, further emphasize the need to elucidate the origins of this disease. However, access to newborn organs and tissue and the invasive in vivo and ex vivo experimental interventions required to elucidate the pathogenesis are impossible in humans.
Lack of an animal model that mirrors human cystic fibrosis has hindered progress in discovering the origins of cystic fibrosis lung disease 19. Mice with CFTR mutations fail to develop respiratory disease like that in humans. In contrast, recently generated animal models develop lung disease that mimics human cystic fibrosis. In this review, we focus primarily on the newborn time period because this time window is key to discovering origins of cystic fibrosis airway disease.
New animal models mirror human cystic fibrosis
To circumvent limitations of studying cystic fibrosis mice and humans, investigators developed new animal models of cystic fibrosis, including pigs, ferrets and rats 20-22. We focused on pigs because their anatomy, physiology, biochemistry, size, life span, and genetics are more similar to humans than are mice 23. Because embryonic stem cells that can contribute to the germ line had been developed only for mice, a different approach was required. We and our colleagues modified the CFTR gene in porcine fetal fibroblasts and then used them for somatic cell nuclear transfer (à la Dolly, the cloned sheep) to produce the first mammal, other than mice, with a targeted gene modification to generate a disease model 20.
Pigs lacking CFTR manifest features typically observed in people with cystic fibrosis, including meconium ileus, exocrine pancreatic destruction, focal biliary cirrhosis, vas deferens atresia, microgallbladder and abnormal glucose homeostasis (early cystic fibrosis-related diabetes mellitus) 20,24-26. Within weeks to months after birth, cystic fibrosis pigs spontaneously develop airway and nasal sinus disease with hallmark cystic fibrosis features: infection, inflammation, tissue remodeling, mucus accumulation, and obstruction (Fig. 1) 27-29. As in humans, the appearance of airway disease is heterogeneous, both within and between pigs 27-30. Pigs bearing the common cystic fibrosis-associated mutation, CFTR-△F508, also mirror human cystic fibrosis 28. A similar gene targeting strategy was used to produce ferrets lacking CFTR 21. They develop characteristic features of human cystic fibrosis including intestinal, pancreatic, and airway disease, and they may be particularly valuable for studying cystic fibrosis-related diabetes mellitus 21,31-34. Cystic fibrosis rats, recently produced using zinc finger endonuclease technology, develop intestinal, airway and reproductive features consistent with human disease 22.
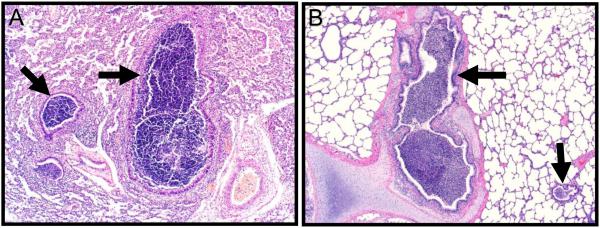
Figure 1. Early Cystic Fibrosis Airway Disease in Humans and Pigs.
Histological images from the lungs of a cystic fibrosis infant (Panel A, 3 months age) and a cystic fibrosis pig (Panel B, 2 months age). Airways-centric neutrophilic inflammation (arrows) obstructs airway lumens. Sections stained with hematoxylin and eosin.
Loss of CFTR function causes congenital airway abnormalities
Airway obstruction occurs early in the lives of babies with cystic fibrosis 14,35,36, which raises questions of whether obstruction might, in part, be congenital. A similar question has been asked for cystic fibrosis nasal sinuses, where hypoplasia is well-described 37. CFTR is expressed early during development 38, so in utero alterations are plausible. Indeed, studies of cystic fibrosis mice, pigs and rats shortly after birth reveal structural tracheal abnormalities including narrowed proximal airways with assorted alterations in airway cartilage, hypoplastic submucosal glands and prominent airway smooth muscle bundles 22,28,39,40 (Fig. 2). Presence of this congenital defect in cystic fibrosis mice, which lack other cystic fibrosis respiratory abnormalities, suggests a distinct mechanism for this defect. Hypoplastic nasal sinuses are also present at birth in cystic fibrosis piglets (Fig. 2) 29, suggesting that a primary cystic fibrosis defect contributes to these congenital changes. These abnormalities may have physiological significance because newborn cystic fibrosis piglets exhibit airflow obstruction and air trapping in the absence of inflammation or mucus obstruction (Fig. 2) 41.
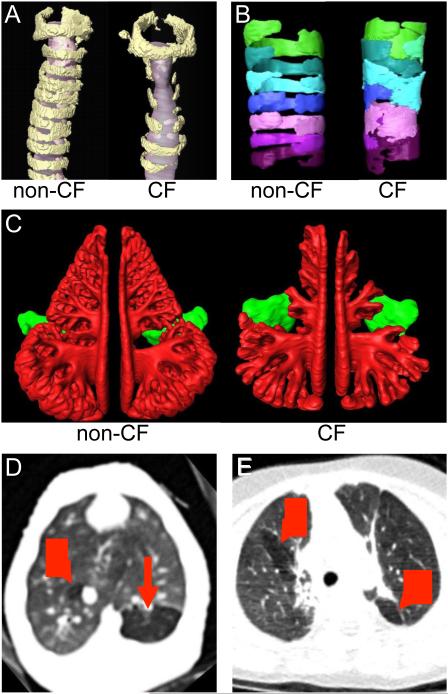
Figure 2. Structural Airway Abnormalities in Cystic Fibrosis.
Panel A shows three-dimensional reconstructions from micro-computed tomography images of the laryngeal and upper tracheal region of non-cystic fibrosis (non-CF) and cystic fibrosis (CF) mice (6-8 weeks old). Cartilage ring structure (yellow) is disrupted, and the tracheal lumen (gray) is narrowed in cystic fibrosis mice. Panel B shows three-dimensional reconstructions from optical coherence tomography (OCT) images of tracheal cartilage rings in non-cystic fibrosis and cystic fibrosis newborn pigs. Individual cartilage rings are highlighted by different colors. Panel C shows three-dimensional reconstructions from computed tomography images of ethmoid (red) and maxillary (green) sinuses in newborn non-cystic fibrosis and cystic fibrosis pigs. Cystic fibrosis pigs have hypoplastic ethmoid sinuses. Panel D shows chest CT image of a cystic fibrosis piglet on the day of birth and before airway infection, inflammation, and mucus obstruction. Air trapping (red arrows), a sign of airway obstruction, is already present. For comparison, Panel E shows air trapping on chest CT image from a 14-month-old human with cystic fibrosis. Murine tracheas were provided by Drs. Craig Hodges and Mitch Drumm (Case Western Reserve University) and analyzed by Ryan Adam (University of Iowa). OCT image acquisition and analysis were performed by Drs. Melissa Suter (Massachusetts General Hospital) and Eman Namati (University of Iowa). Sinus image analysis was performed by Dr. Eugene Chang and Tanner Wallen (University of Iowa).
Congenital abnormalities in three species suggest that humans might also have altered airway development. A reappraisal of published autopsy data 42 from tracheas of infants less than two weeks of age showed that babies with cystic fibrosis had narrowed tracheas similar to that seen in newborn animal models 39. Further, a recent study found that 15% of young children with cystic fibrosis (median age 16 months) had bronchoscopic evidence of tracheomalacia, which was associated with worse airway disease 43. Thus, the airway obstruction and air trapping observed in infants with cystic fibrosis as early as 2-3 months of age 35,36, might, at least in part, be congenital in nature.
Cystic fibrosis airways manifest defective chloride transport but not sodium hyperabsorption in newborns
Cystic fibrosis alters the electrophysiological properties across airway epithelia, and measures of nasal voltage have been used to aid diagnosis and assess effectiveness of interventions 2,16. Two processes determine the bulk of the electrophysiological characteristics, CFTR-mediated anion (chloride and bicarbonate) secretion and epithelial Na+ channel (ENaC)-mediated sodium absorption 2. Alterations in either might change electrophysiological properties.
Newborn porcine cystic fibrosis airway epithelia, extending from nose to bronchi, lack cAMP-stimulated chloride secretion 44. Those results were expected because CFTR is an apical membrane anion channel regulated by phosphorylation with cAMP-dependent protein kinase. These findings correspond with studies in the cystic fibrosis ferret 21,32, rat 22, and human 2 airway epithelia, which consistently demonstrate a loss of anion permeability. In addition, the salt concentration in airway surface liquid was similar between newborn non-cystic fibrosis and cystic fibrosis pigs 45.
A widely held hypothesis is that CFTR inhibits ENaC, and loss of that effect causes amiloride-inhibitable sodium hyperabsorption, which dehydrates airways, reduces the height of periciliary liquid, and disrupts mucociliary clearance 9,46,47. Studies of cultured human airway epithelia, as well as mouse fibroblast and dog kidney cell lines expressing recombinant CFTR and ENaC suggest that without CFTR, ENaC-mediated sodium transport increases. In addition, mice overexpressing ENaC show decreased airway surface liquid height and reduced mucociliary clearance 9,46,47, suggesting that increased ENaC activity can alter airway surface liquid. In contrast to the hypothesis that sodium hyperabsorption initiates disease, newborn porcine cystic fibrosis airway epithelia do not hyperabsorb sodium 44. Studies of neonatal cystic fibrosis ferrets 32,33, 3-6 week-old cystic fibrosis rats 22, and some studies of human cystic fibrosis airway epithelia 48 also showed no evidence of increased sodium absorption. In addition, two other human tissues that express both CFTR and ENaC, sweat gland ducts and submucosal glands, do not hyperabsorb sodium in cystic fibrosis 4,49,50. Secondary changes in airways might increase sodium absorption as the disease progresses, but these data suggest that loss of CFTR does not directly increase ENaC activity at the genesis of disease. Nevertheless, compared to controls, in both human and porcine cystic fibrosis nasal epithelia, amiloride inhibits a greater fraction of the transepithelial voltage and short-circuit current, which is sometimes taken to indicate increased sodium absorption. Sweat gland ducts show similar changes without hyperabsorbing sodium. How is this apparent paradox explained? The CFTR chloride conductance and the ENaC sodium conductance sit in parallel in the apical membrane, and elimination of the chloride conductance (a shunt pathway, in part) magnifies sodium-dependent electrophysiological properties without increasing sodium absorption 44.
Loss of CFTR reduces the pH of airway surface liquid
CFTR conducts bicarbonate 51, and loss of CFTR eliminates bicarbonate secretion by porcine airway epithelia 44. As a result, the airway surface liquid of newborn cystic fibrosis piglets has a reduced pH when measured in vivo, ex vivo, and in cultured epithelia (Fig. 3A) 45. These findings are in accord with earlier reports that cultured human cystic fibrosis airway epithelia lack bicarbonate secretion 52 and have an acidic airway surface liquid pH 53 and that secretions from human cystic fibrosis submucosal glands are abnormally acidic 54. A small study of infants with cystic fibrosis also found a reduced nasal airway surface liquid pH compared to non-cystic fibrosis 55. However, in older children and adults, genotype-dependent differences in pH of nasal airway surface liquid may be more variable 55-57. The reasons for this variability remain to be determined.
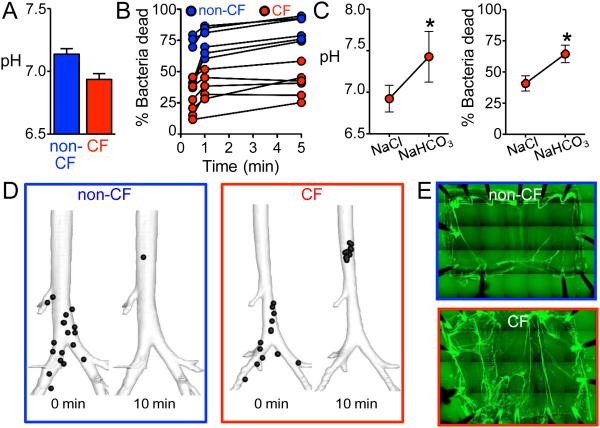
Figure 3. Host Defense Defects in Newborn Cystic Fibrosis Pigs.
Panel A. At birth, newborn cystic fibrosis (CF) pigs have a more acidic airway surface liquid pH, than non-cystic fibrosis (non-CF) littermates. Panel B. Airway surface liquid in newborn non-cystic fibrosis piglets quickly killed most S. aureus applied to the airway surface. In contrast, killing was reduced by about half in cystic fibrosis piglets. Each set of connected points represents data from an individual piglet. Panel C. Increasing airway surface liquid pH by aerosolizing sodium bicarbonate (NaHCO3) onto airways of cystic fibrosis piglets rescued the cystic fibrosis bacterial killing defect, compared to treatment with saline (NaCl) alone. Panels A-C are from Pezzulo et al. 45. Panel D. Images from a computed-tomography based assay of mucociliary transport that tracked radio-opaque microdisks. Images are three-dimensional reconstructions of the airway tree from newborn non-cystic fibrosis and cystic fibrosis piglets taken at the beginning and end of the tracking period (10 min duration). Positions of microdisks are represented by black circles (larger than actual microdisks). Following in vivo cholinergic stimulation to stimulate mucus secretion from submucosal glands, most microdisks cleared the airway tree in non-cystic fibrosis piglets. In cystic fibrosis piglets, some microdisks move normally, but some became stuck and failed to clear the viewing field. Panel D is from Hoegger et al. 67. Panel E. Mucus strands were visualized with fluorescent nanospheres that bound to mucus in excised tracheas submerged in saline. Tracheas were studied ex vivo following in vivo cholinergic stimulation. In non-cystic fibrosis tracheas, most of the mucus (green) accumulated around the tracheal edges, and very few mucus strands were observed. In contrast, in cystic fibrosis tracheas, numerous mucus strands failed to detach from submucosal gland ducts. These tethered mucus strands contribute to impaired mucociliary transport.
Airway infection precedes inflammation in cystic fibrosis lungs
The chicken and egg conundrum about infection and inflammation has long vexed the field 6,10-13,58. During the first hours after birth, airways of cystic fibrosis piglets show no evidence of inflammation on histopathology, cell counts, cytokines or transcript analysis 20,27,28,39. Yet, after a pulmonary challenge with S. aureus, they fail to eradicate bacteria as well as controls 27. Moreover, newborn cystic fibrosis piglets and neonatal ferrets harbor more bacteria than non-cystic fibrosis littermates 27,33. Species isolated include a wide variety of gram-positive and gram-negative organisms including S. aureus. While P. aeruginosa is rare in young cystic fibrosis pigs, it infects older cystic fibrosis pigs with clinical disease 29,30. A similar pattern occurs in human cystic fibrosis; during the initial months to years of life, a wide variety of bacteria are recovered from cystic fibrosis lungs 15,59. With time, the human cystic fibrosis lung becomes chronically colonized with a more restricted number of species, most notably P. aeruginosa 15.
These findings indicate that within hours of birth, cystic fibrosis lungs exhibit an “equal opportunity” host defense defect that impairs eradication of many different bacteria. That abnormality can initiate a cascade of airway inflammation and airway remodeling. Later in life, the infection narrows to a few predominant species, likely due to an interplay between a changing host and bacterial genetic adaptations. In addition, although infection precedes inflammation, subsequent inflammatory responses, resolution of inflammation and/or adaptive immune responses might be abnormal 10.
Acidic airway surface liquid impairs bacterial killing in cystic fibrosis
Airways employ multiple mechanisms to protect lungs against infection. One important defense is the complex soup of antimicrobial peptides, proteins and lipids in airway surface liquid. Alexander Fleming was the first to identify one of these – lysozyme – after he noticed that sneeze droplets killed bacteria on his culture dish 60. Since then, more factors have been identified, including lactoferrin, defensins, cathelicidins, secretory leukocyte peptidase inhibitor, and others 61. Many of these exhibit individual as well as synergistic effects that rapidly permeabilize bacteria 62.
In wild-type piglets, airway surface liquid very quickly kills most S. aureus (Fig. 3B) 45. In contrast, loss of CFTR reduces acute bacterial killing by about half. This is not due to a decreased abundance of airway surface liquid antimicrobials. Rather, the reduced pH of cystic fibrosis airway surface liquid inhibits their antimicrobial activity. Increasing airway surface liquid pH by aerosolizing sodium bicarbonate onto airways of cystic fibrosis piglets rescues the bacterial killing defect (Fig. 3C). Conversely, making airway surface liquid acidic diminishes killing in wild-type piglets.
These findings directly link loss of CFTR function to a host defense defect; without CFTR-dependent bicarbonate secretion, airway surface liquid pH falls and impairs antibacterial activity. The reduced bacterial killing may be one of the critical first steps in a downward spiral from a sterile newborn lung to one that is chronically colonized.
Cystic fibrosis mucus fails to detach from submucosal gland ducts
Another important airway defense is mucociliary transport. Mucociliary transport guards the lung by trapping invading pathogens and particulates in mucus, which is then propelled up the airways by cilia 63,64. Although people with advanced cystic fibrosis can exhibit slowed mucociliary transport 64, whether mucociliary transport is impaired at the disease’s origin has been unknown 64,65.
Mucociliary transport, assayed using an X-ray computed tomography-based approach to track discrete airway particles, appears similar in newborn cystic fibrosis and non-cystic fibrosis piglets under basal conditions 66,67. However, after cholinergic stimulation, which elicits copious mucus secretion from submucosal glands, many particles move normally in cystic fibrosis piglets, but some become stuck and fail to move up the airways (Fig. 3D). Subsequent mechanistic investigations using excised airways revealed that cystic fibrosis submucosal glands secrete strands and blobs of mucus that sometimes do not break free after emerging and remain tethered to the gland ducts, hindering mucociliary transport (Fig. 3E). The mucociliary transport defect is not attributable to periciliary liquid depletion, because it persists when the airway surface is submerged in saline. Importantly, inhibiting anion secretion in wild-type airways replicates the cystic fibrosis abnormalities. These results were predicted by earlier analyses from the Wine laboratory and studies from the Ballard laboratory of wild-type pig airways treated with agents that inhibit anion secretion 5,68. These data are consistent with findings of slowed mucociliary clearance in excised trachea of 3-8 month-old ferrets 33. They are also in concert with findings showing that slowed tracheal mucociliary transport in excised cystic fibrosis pig trachea was not related to reduced periciliary liquid depth 69.
These findings directly link impaired mucociliary transport to loss of CFTR anion transport, indicating that defective mucociliary transport is a primary abnormality not dependent on infection, inflammation or remodeling. Nevertheless, advancing infection and bronchiectasis might further disrupt mucociliary transport to fuel disease progression. The data also suggest that the submucosal gland environment into which mucus is initially secreted likely alters its properties causing abnormal detachment. Further research is needed to determine whether defective bicarbonate secretion, liquid secretion, or a combination is the key requirement for abnormal mucociliary transport 67,68,70-72.
Finding that mucus abnormalities are a problem in cystic fibrosis lungs has parallels in other organs 71,72. Quinton has emphasized the contribution of defective bicarbonate and mucus secretion, referring back to one of the early names for the disease, mucoviscidosis 71. Indeed, there is a rogues’ gallery of mucus abnormalities in multiple organs including airways, intestine, pancreas and gallbladder of cystic fibrosis pigs, ferrets, and humans (Fig. 4).
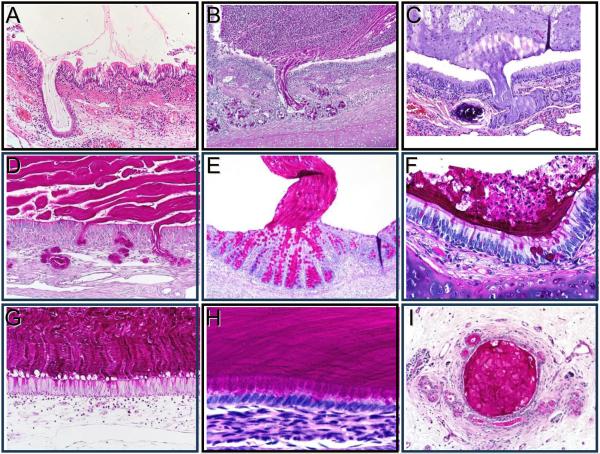
Figure 4. Accumulation of Mucus in Humans with Cystic Fibrosis and Animal Models of Cystic Fibrosis.
Panels A-E. “Stringy” appearance of mucus arising from glands. Mucus secreted from submucosal glands in pulmonary airways remained in the gland duct in a 7-month-old baby with cystic fibrosis (A), a 2-month-old cystic fibrosis pig (B), and an 8-month-old cystic fibrosis ferret (C). Mucus also emerged from submucosal glands in ethmoid sinus olfactory epithelium that did not contain goblet cells (D, 1-month-old cystic fibrosis pig). Similar to mucus from submucosal glands, mucus arising from colonic crypts of newborn cystic fibrosis pigs can show a stringy appearance and adherence to the site of origin (E, newborn cystic fibrosis pig). Panels F-I. Lamellar appearance of mucus along epithelia. In affected intrapulmonary airways, mucus can have a lamellar appearance lying along airway walls (F, 2-month-old cystic fibrosis pig). A similar pattern of mucus arising from goblet cells can occur in ethmoid sinuses where respiratory epithelium lacks submucosal glands, and mucus can sometimes be traced back to the cells of origin (G, 1-month-old cystic fibrosis pig). Likewise, mucus can have a lamellar appearance and be traced back to the cell of origin in microgallbladder (H, newborn cystic fibrosis pig). Pancreatic ducts also can show obstruction by mucus (I, 6-month-old cystic fibrosis pig). The authors thank Drs. Marcus Nashelsky, and Morris Dailey (University of Iowa, Department of Pathology) for assistance with archival autopsy data.
Additional implications and speculations
Discoveries from these new animal models raise more questions for future research and have implications for care of people with cystic fibrosis, although any therapeutic implications will require assessment in humans. In addition, whether defects that are key at the origins of cystic fibrosis retain pathophysiological importance later in the disease remains uncertain. What are some of the take-home points?
Treat early
We suspect that like in cystic fibrosis piglets, host defense defects begin on the day babies with cystic fibrosis are born. That timing suggests immediate institution of preventive measures. Cystic fibrosis clinics already exhibit substantial momentum toward earlier intervention, and these data support that trend.
Loss of CFTR delivers multiple “hits.”
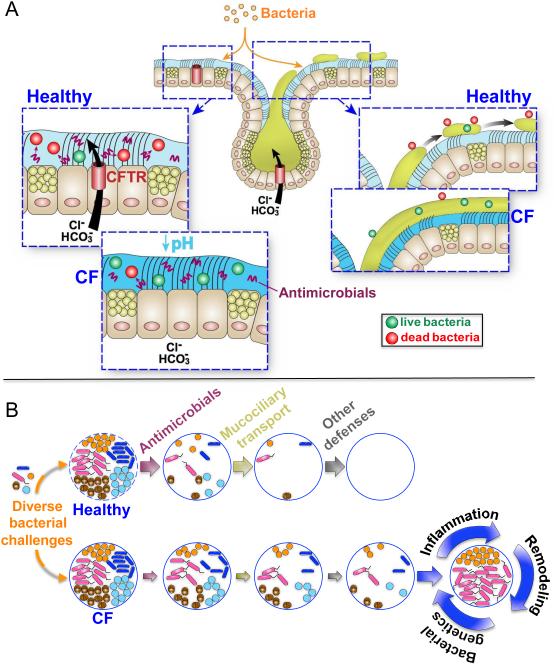
Loss of CFTR does not completely eliminate any single defense, instead it reduces effectiveness of at least two defenses, mucociliary transport and antimicrobial activity 45,67, and other defenses may also be degraded 6,10 (Fig. 5). Compromising one host defense mechanism places a greater burden on other defenses. If those are also impaired, problems may ensue. For example, without robust antimicrobial activity to rapidly kill bacteria, increased numbers of viable organisms might prompt submucosal gland secretion, impairing mucociliary transport. Likewise, failure of mucus detachment might allow bacteria to grow under conditions that promote resistance to antibacterial defenses that are already diminished by cystic fibrosis 45,73. Thus, partially disrupting two or more defenses may elicit a vicious cycle of disease.
Figure 5. Model of Cystic Fibrosis Airway Host Defense Defects.
Panel A. Cystic fibrosis (CF) airways exhibit two host defense defects at the genesis of the disease. On the left, loss of CFTR channels that conduct chloride (Cl−) and bicarbonate (HCO3−) onto the airway surface causes the airway surface liquid pH to fall, and the acidic airway surface liquid inhibits the activity of antimicrobials. On the right, loss of CFTR channels in submucosal glands causes mucus to develop abnormal properties so that it does not break free after emerging and remains tethered to the gland ducts. Panel B. When bacteria enter non-cystic fibrosis airways (top) they are killed by airway surface liquid antimicrobials, mucociliary transport sweeps them out of the lung, and other defenses including phagocytic cells eradicate them to maintain sterile lungs. In cystic fibrosis (bottom) antimicrobial activity and mucociliary transport are less effective than in non-cystic fibrosis and other defenses may also be impaired. Eventually, the host defenses are overwhelmed, and bacteria proliferate, with inflammation, remodeling, immunity, and genetic changes in the bacteria influencing the species that will dominate. In addition, the resulting inflammation and airway remodeling may further enhance or impair host defense mechanisms. Airway insults will also affect host defenses. The authors thank Dr. Mahmoud Abou Alaiwa and Mr. Shawn Roach for assistance with graphics.
Cystic fibrosis initially causes an “equal opportunity” host defense defect that may serve as a gateway for infection with typical cystic fibrosis-associated bacteria
The mix of many different bacterial species so early in the disease could elicit inflammatory, remodeling, and structural changes that become irreversible and predispose to more intractable infections with typical cystic fibrosis pathogens. We speculate that preventive interventions and antibacterial treatments should not wait for the appearance of P. aeruginosa or “typical” cystic fibrosis pathogens.
Correcting even one host defense defect might be beneficial
As an example, treating cystic fibrosis with antibiotics improves clinical status, but does not address mucus abnormalities. In another example, primary ciliary dyskinesia completely obliterates one defense, mucociliary transport, yet causes less severe lung disease than cystic fibrosis 74. Primary ciliary dyskinesia might be less severe because other defenses (e.g., antimicrobials) are intact, although differences in how these diseases impair mucociliary transport might also contribute.
Environmental insults may trigger airway disease in cystic fibrosis lungs
Another “hit” to cystic fibrosis airways might come from infections and/or environmental injuries. Such insults trigger protective responses, including submucosal gland mucus secretion. But in cystic fibrosis, what would normally be a protective reflex might further cripple mucociliary transport.
Cystic fibrosis lung disease may begin in large and small airways
It is often said that cystic fibrosis airway disease begins in small airways based on histopathology from infants dying within weeks to months of birth. However, rapidly advancing inflammation and remodeling confound interpretation about the initiating location. Histopathological studies of older cystic fibrosis pigs identify disease in both large and small airways 27,28,30. Large airways exhibit antibacterial and mucociliary transport defects at birth, which suggests that they are a susceptible site for disease onset. However, small airways also express CFTR 75, probably have defective antibacterial activity, and goblet cell-derived mucus might impair mucociliary transport there, suggesting that they may also be an initial site. Another consideration is that the total area of small airways is much greater than that of large airways, and thus if physiological defects in both airways were equal on a per square meter basis, small airways would be overrepresented.
Infants with cystic fibrosis may have congenital airway defects
The airway and nasal sinus defects might impact disease progression. They could also complicate assessments. For example, if air trapping is, in part, due to a congenital defect instead of solely inflammation and abnormal mucus, attempting to “treat” based on its appearance might not be entirely appropriate.
We need better assays of early cystic fibrosis airway disease
Sensitive assays could potentially identify and quantify early host defense defects and track disease progression and therapeutic interventions. Studies in animal models suggest that assays of airway surface liquid pH, antimicrobial activity, or mucociliary transport could be informative, especially if sensitive. For example, developing methods for humans that assay mucociliary transport with the data granularity achieved in pigs, could transform pulmonary imaging of mucociliary transport in cystic fibrosis and possibly other airway diseases.
Implications for other diseases
These discoveries in cystic fibrosis may also have implications for other diseases. First, they emphasize the value of an animal model that replicates human disease. Second, they highlight the importance of investigating disease at its genesis and before the onset of secondary manifestations. Manifestations of advanced disease may not reflect the initiating events, and without such knowledge, treatments and preventions may not be optimal. Pulmonary fibrosis is perhaps another respiratory disease where investigation before clinical manifestations could be revealing. Third, multiple, partial, perhaps even subtle impairments, or “hits”, can have a profound impact. That concept is likely the case for more common pulmonary diseases such as asthma and chronic obstructive pulmonary disease, as well as for non-respiratory diseases.
The origins and initiating factors in cystic fibrosis lung disease likely determine the progression, severity and disease burden later in life. Understanding the origins, quantifying the initial defects, and intervening early could make a big difference for afflicted people.
ACKNOWLEDGEMENTS
The University of Iowa Research Foundation has submitted patent applications for CF pigs and has licensed materials and technologies to Exemplar Genetics. MJW is a co-founder and holds equity in Exemplar Genetics, and the University of Iowa Research Foundation shares licensing revenue with D.A.S. and M.J.W.
REFERENCES
- 1.Riordan JR, Rommens JM, Kerem BS, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 2.Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic Fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B, editors. The Metabolic and Molecular Basis of Inherited Disease. 8 McGraw-Hill; New York: 2001. pp. 5121–89. [Google Scholar]
- 3.Stick SM, Brennan S, Murray C, et al. Bronchiectasis in infants and preschool children diagnosed with cystic fibrosis after newborn screening. J Pediatr. 2009;155:623–8. doi: 10.1016/j.jpeds.2009.05.005. e1. [DOI] [PubMed] [Google Scholar]
- 4.Quinton P. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79:S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 5.Wine JJ, Joo NS. Submucosal glands and airway defense. Proc Am Thorac Soc. 2004;1:47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 6.Chmiel JF, Davis PB. State of the art: why do the lungs of patients with cystic fibrosis become infected and why can't they clear the infection? Respir Res. 2003;4:8. doi: 10.1186/1465-9921-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verkman AS, Song Y, Thiagarajah JR. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol Cell Physiol. 2003;284:C2–15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- 8.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 9.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–70. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 10.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2012;18:509–19. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–82. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 12.Balough K, McCubbin M, Weinberger M, Smits W, Ahrens R, Fick R. The relationship between infection and inflammation in the early stages of lung disease from cystic fibrosis. Ped Pulmonol. 1995;20:63–70. doi: 10.1002/ppul.1950200203. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong DS, Grimwood K, Carlin JB, et al. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156:1197–204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 14.Sly PD, Brennan S, Gangell C, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–52. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 15.Foundation CF. Cystic Fibrosis Foundation Patient Registry. 2012 Annual Data Report. 2012 [Google Scholar]
- 16.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clancy JP, Jain M. Personalized medicine in cystic fibrosis: dawning of a new era. Am J Respir Crit Care Med. 2012;186:593–7. doi: 10.1164/rccm.201204-0785PP. [DOI] [PubMed] [Google Scholar]
- 19.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 20.Rogers CS, Stoltz DA, Meyerholz DK, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–41. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun X, Sui H, Fisher JT, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–60. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuggle KL, Birket SE, Cui X, et al. Characterization of defects in ion transport and tissue development in cystic fibrosis transmembrane conductance regulator (CFTR)-knockout rats. PLoS One. 2014;9:e91253. doi: 10.1371/journal.pone.0091253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers CS, Abraham WM, Brogden KA, et al. The porcine lung as a potential model for cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;295:L240–63. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176:1377–89. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierucci-Alves F, Akoyev V, Stewart JC, 3rd, Wang LH, Janardhan KS, Schultz BD. Swine models of cystic fibrosis reveal male reproductive tract phenotype at birth. Biol Reprod. 2011;85:442–51. doi: 10.1095/biolreprod.111.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uc A, Olivier AK, Griffin MA, et al. Glycemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clinical science. 2014 doi: 10.1042/CS20140059. doi:10.1042/CS20140059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoltz DA, Meyerholz DK, Pezzulo AA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostedgaard LS, Meyerholz DK, Chen J-H, et al. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3:74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang EH, Pezzulo AA, Meyerholz DK, et al. Sinus hypoplasia precedes sinus infection in a porcine model of cystic fibrosis. Laryngoscope. 2012;122:1898–905. doi: 10.1002/lary.23392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoltz DA, Rokhlina T, Ernst S, et al. Intestinal CFTR expression alleviates meconium ileus in cystic fibrosis pigs. J Clin Invest. 2013;123:2685–93. doi: 10.1172/JCI68867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olivier AK, Yi Y, Sun X, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122:3755–68. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher JT, Tyler SR, Zhang Y, et al. Bioelectric Characterization of Epithelia from Neonatal CFTR Knockout Ferrets. Am J Respir Cell Mol Biol. 2013;49:837–44. doi: 10.1165/rcmb.2012-0433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun X, Olivier AK, Liang B, et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am J Respir Cell Mol Biol. 2014;50:502–12. doi: 10.1165/rcmb.2013-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X, Olivier AK, Yi Y, et al. Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets. Am J Pathol. 2014;184:1309–22. doi: 10.1016/j.ajpath.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall GL, Logie KM, Parsons F, et al. Air trapping on chest CT is associated with worse ventilation distribution in infants with cystic fibrosis diagnosed following newborn screening. PLoS One. 2011;6:e23932. doi: 10.1371/journal.pone.0023932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoo AF, Thia LP, Nguyen TT, et al. Lung function is abnormal in 3-month-old infants with cystic fibrosis diagnosed by newborn screening. Thorax. 2012;67:874–81. doi: 10.1136/thoraxjnl-2012-201747. [DOI] [PubMed] [Google Scholar]
- 37.Woodworth BA, Ahn C, Flume PA, Schlosser RJ. The △ F508 mutation in cystic fibrosis and impact on sinus development. Am J Rhinol. 2007;21:122–7. doi: 10.2500/ajr.2007.21.2905. [DOI] [PubMed] [Google Scholar]
- 38.Trezise AE, Chambers JA, Wardle CJ, Gould S, Harris A. Expression of the cystic fibrosis gene in human foetal tissues. Hum Mol Genet. 1993;2:213–8. doi: 10.1093/hmg/2.3.213. [DOI] [PubMed] [Google Scholar]
- 39.Meyerholz DK, Stoltz DA, Namati E, et al. Loss of CFTR function produces abnormalities in tracheal development in neonatal pigs and young children. Am J Respir Crit Care Med. 2010;182:1251–61. doi: 10.1164/rccm.201004-0643OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonvin E, Le Rouzic P, Bernaudin JF, et al. Congenital tracheal malformation in cystic fibrosis transmembrane conductance regulator-deficient mice. J Physiol. 2008;586:3231–43. doi: 10.1113/jphysiol.2008.150763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adam RJ, Michalski AS, Bauer C, et al. Air trapping and airflow obstruction in newborn cystic fibrosis piglets. Am J Respir Crit Care Med. 2013;188:1434–41. doi: 10.1164/rccm.201307-1268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sturgess J, Imrie J. Quantitative evaluation of the development of tracheal submucosal glands in infants with cystic fibrosis and control infants. Am J Pathol. 1982;106:303–11. [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer AJ, Singh SB, Adam RJ, et al. Tracheomalacia is associated with lower FEV and Pseudomonas acquisition in children with CF. Pediatr Pulmonol. 2013 doi: 10.1002/ppul.22922. doi:10.1002/ppul.22922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J-H, Stoltz DA, Karp PH, et al. Loss of anion transport without increased sodium absorption characterize newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–23. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pezzulo AA, Tang XX, Hoegger MJ, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–13. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 47.Hobbs CA, Da Tan C, Tarran R. Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease? J Physiol. 2013;591:4377–87. doi: 10.1113/jphysiol.2012.240861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itani OA, Chen JH, Karp PH, et al. Human cystic fibrosis airway epithelia have reduced Cl-conductance but not increased Na+ conductance. Proc Natl Acad Sci U S A. 2011;108:10260–5. doi: 10.1073/pnas.1106695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinton PM. Cystic fibrosis: lessons from the sweat gland. Physiology (Bethesda) 2007;22:212–25. doi: 10.1152/physiol.00041.2006. [DOI] [PubMed] [Google Scholar]
- 50.Joo NS, Irokawa T, Robbins RC, Wine JJ. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem. 2006;281:7392–8. doi: 10.1074/jbc.M512766200. [DOI] [PubMed] [Google Scholar]
- 51.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994;91:5340–4. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89:1148–53. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coakley RD, Grubb BR, Paradiso AM, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci U S A. 2003;100:16083–8. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song Y, Salinas D, Nielson DW, Verkman AS. Hyperacidity of secreted fluid from submucosal glands in early cystic fibrosis. Am J Physiol Cell Physiol. 2006;290:C741–9. doi: 10.1152/ajpcell.00379.2005. [DOI] [PubMed] [Google Scholar]
- 55.Abou Alaiwa MH, Beer AM, Pezzulo AA, et al. Neonates with cystic fibrosis have a reduced nasal liquid pH; A small pilot study. J Cyst Fibros. 2014;13:373–7. doi: 10.1016/j.jcf.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McShane D, Davies JC, Davies MG, Bush A, Geddes DM, Alton EW. Airway surface pH in subjects with cystic fibrosis. Eur Respir J. 2003;21:37–42. doi: 10.1183/09031936.03.00027603. [DOI] [PubMed] [Google Scholar]
- 57.Garland AL, Walton WG, Coakley RD, et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci U S A. 2013;110:15973–8. doi: 10.1073/pnas.1311999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tirouvanziam R, de Bentzmann S, Hubeau C, et al. Inflammation and infection in naive human cystic fibrosis airway grafts. Am J Respir Cell Mol Biol. 2000;23:121–7. doi: 10.1165/ajrcmb.23.2.4214. [DOI] [PubMed] [Google Scholar]
- 59.Gangell C, Gard S, Douglas T, et al. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin Infect Dis. 2011;53:425–32. doi: 10.1093/cid/cir399. [DOI] [PubMed] [Google Scholar]
- 60.Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond Biol. 1922;3:306–17. [Google Scholar]
- 61.Travis SM, Singh PK, Welsh MJ. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr Opin Immunol. 2001;13:89–95. doi: 10.1016/s0952-7915(00)00187-4. [DOI] [PubMed] [Google Scholar]
- 62.Singh PK, Tack BF, McCray PB, Jr., Welsh MJ. Synergistic and additive killing by antimicrobial factors found in human airway surface liquid. Am J Physiol. 2000;279:L799–L805. doi: 10.1152/ajplung.2000.279.5.L799. [DOI] [PubMed] [Google Scholar]
- 63.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–47. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robinson M, Bye PT. Mucociliary clearance in cystic fibrosis. Pediatr Pulmonol. 2002;33:293–306. doi: 10.1002/ppul.10079. [DOI] [PubMed] [Google Scholar]
- 65.McShane D, Davies JC, Wodehouse T, Bush A, Geddes D, Alton EW. Normal nasal mucociliary clearance in CF children: evidence against a CFTR-related defect. Eur Respir J. 2004;24:95–100. doi: 10.1183/09031936.04.00097503. [DOI] [PubMed] [Google Scholar]
- 66.Hoegger MJ, Awadalla M, Namati E, et al. Assessing mucociliary transport of single particles in vivo shows variable speed and preference for the ventral trachea in newborn pigs. Proc Natl Acad Sci U S A. 2014;111:2355–1260. doi: 10.1073/pnas.1323633111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoegger MJ, Fischer AJ, McMenimen JD, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–22. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trout L, Gatzy JT, Ballard ST. Acetylcholine-induced liquid secretion by bronchial epithelium: role of Cl- and HCO3- transport. Am J Physiol. 1998;275:L1095–9. doi: 10.1152/ajplung.1998.275.6.L1095. [DOI] [PubMed] [Google Scholar]
- 69.Birket SE, Chu KK, Liu L, et al. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med. 2014;190:421–32. doi: 10.1164/rccm.201404-0670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joo NS, Cho HJ, Khansaheb M, Wine JJ. Hyposecretion of fluid from tracheal submucosal glands of CFTR-deficient pigs. J Clin Invest. 2010;120:3161–6. doi: 10.1172/JCI43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–7. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 72.Gustafsson JK, Ermund A, Ambort D, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263–72. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Staudinger BJ, Muller JF, Halldorsson S, et al. Conditions Associated with the Cystic Fibrosis Defect Promote Chronic Pseudomonas aeruginosa Infection. Am J Respir Crit Care Med. 2014;189:812–24. doi: 10.1164/rccm.201312-2142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cohen-Cymberknoh M, Simanovsky N, Hiller N, Gileles Hillel A, Shoseyov D, Kerem E. Differences in disease expression between primary ciliary dyskinesia and cystic fibrosis with and without pancreatic insufficiency. Chest. 2014;145:738–44. doi: 10.1378/chest.13-1162. [DOI] [PubMed] [Google Scholar]
- 75.Shamsuddin AK, Quinton PM. Surface fluid absorption and secretion in small airways. J Physiol. 2012;590:3561–74. doi: 10.1113/jphysiol.2012.230714. [DOI] [PMC free article] [PubMed] [Google Scholar]