Abstract
Background
Higher cognitive reserve is reported to reduce risk of dementia. However, its role in reducing delirium risk is not well established.
Objectives
To examine the role of cognitive reserve on reducing delirium incidence and severity in older surgical patients.
Design
Prospective cohort study.
Setting and Participants
142 older adults (mean age 71.2 y, 65% women) undergoing elective orthopedic surgery.
Measurements
Incidence (assessed by Confusion Assessment Method) and severity (Memorial Delirium Assessment Scale) of postoperative delirium were the primary outcomes. Predictors included early life (literacy) and late life (cognitive activities) proxies for cognitive reserve.
Results
45 participants (32%) developed delirium. Higher cognitive activity participation was associated with lower incidence (odds ratio 0.92 corresponding to increase of 1 activity per week, 95% CI 0.86 to 0.98, p = 0.006) and lower severity of delirium (B = −0.06, 95% CI −0.11 to −0.01, p = 0.019) after adjustment for age, sex, medical illnesses and baseline cognition. Higher literacy was not associated with decreased delirium incidence and severity. Among the individual leisure activities, reading books, using electronic mail, singing and computer games were associated with lower dementia incidence and severity.
Conclusion
Higher late life cognitive reserve was associated with a reduced delirium incidence and severity in older surgical patients. Interventions to enhance cognitive reserve by initiating or increasing participation in cognitive activities may be explored as a delirium prophylaxis strategy.
Keywords: Delirium, cognitive reserve, epidemiology
INTRODUCTION
Delirium is a major health problem. Older patients who experience delirium are at an increased risk of functional decline, dementia, and mortality.1-3 The severity of delirium also affects outcomes; individuals with more severe delirium have a greater risk of institutionalization and death.4 The concept of ‘cognitive reserve’ postulates that some individual characteristics helps maintain cognitive function in the face of accumulating brain pathology,5 and lowers risk of dementia.6, 7 Since dementia predisposes an individual to delirium, it is posited that increased cognitive reserve may reduce risk of developing delirium.8
While improving cognitive reserve may be a potential delirium preventive strategy,9 the link between cognitive reserve and delirium needs to be clarified. Findings from previous studies are mixed; some show an inverse relationship between education and delirium incidence,10-12 whereas others reported negative results.8, 13-16 The limited studies have mainly focused on early life cognitive reserve proxies such as literacy and education years. Very few have prospectively examined the role of later life cognitive reserve proxies (such as cognitive activities) in reducing risk of delirium.
To address these gaps in knowledge regarding the role of cognitive reserve in delirium, we conducted a prospective cohort study in 142 older adults undergoing elective orthopedic surgery. Older surgical patients are a high risk group,1 with postoperative delirium incidence ranging from 12% to 51%.3 We examined literacy as a measure of cognitive reserve built up earlier in life and participation in cognitive activities as a late life cognitive reserve measure. We hypothesized that older adults with higher cognitive reserve (measured using either literacy or cognitive activities as proxies) will have decreased incidence and severity of postoperative delirium. Based on cognitive reserve studies in dementia,17 we predicted that the late life cognitive reserve measure would be more strongly associated with delirium incidence and severity than the early life measure. Unlike early life cognitive reserve activities, activities that influence late life cognitive reserve can be enhanced and encouraged. Establishing the role of late life cognitive activities on incidence and severity of delirium can not only help to improve delirium risk assessments but also guide development of new preventive strategies.
METHODS
Study population
We conducted a prospective cohort study to examine the role of cognitive reserve in delirium. Potential participants were recruited from patients attending the Montefiore Medical Center Orthopedics outpatient clinics (Bronx, NY) of three surgeons for preoperative evaluations for elective hip, knee or spine surgeries from October 2011 to August 2014. This elective surgery population was selected as preoperative and postoperative schedules and management as well as postoperative mobilization procedures were standardized for the individual surgeries, and independent of pre-operative cognitive reserve status. Inclusion criteria were age 60 or older and English-speaking. Exclusion criteria included delirium at baseline, severe auditory or visual deficits, or physician diagnosis of dementia. Patients with Mini Mental State Examination (MMSE) scores below 24 were also excluded. The local Institutional Review Board approved the study design and waived written consent as the study was deemed to be of ‘no to minimal risk’. Verbal informed consent was obtained from participants.
Cognitive reserve
Research assistants obtained baseline measures of cognitive reserve 5, 17 and other covariates at the preoperative clinic visit. They also did the Confusion Assessment Method (CAM), described later, to confirm absence of delirium at enrollment.
Literacy. Literacy was assessed by the American National Adult Reading Test; a 45-word reading test. Due to the variability in the quality of education, literacy may be a better measure than years of schooling.17
Cognitive activities. Increased participation in cognitive activities is associated with reduced risk of dementia;18 and was measured using a previously validated Cognitive Activity Scale.6, 7, 17, 19 Briefly, the Cognitive Activity Scale measured frequency of participation in 20 cognitive activities over the previous week. One point on the Cognitive Activity Scale corresponds to participating in one activity per day per week. We excluded 8 activities done by <5% of our sample to improve reliability and increase generalizability. The remaining 12 activities included reading newspapers, reading books, knitting, playing cards, board games, computer games, crossword puzzles, bingo, electronic mail (email), singing, writing, and group meetings. Participation frequency in the 12 activities was summed to generate an overall score (range 0 to 84 activity days per week).
Covariates
Cognition was evaluated by Mini-Mental state examination (MMSE).3 Mood was assessed via the 15-item geriatric depression scale (GDS).15, 20, 21 The Charlson comorbidity index was used to quantify medical comorbidity.3 Preoperative functional status was assessed by a validated activity of daily living scale (range 0 to 14).22 Other covariates with inconsistent relationship with delirium in prior studies were not significantly associated with delirium incidence in this sample in our preliminary analyses, and are not reported; head size, alcohol intake, medication count and classes, visual loss, current or past smoking history, and preoperative and postoperative levels of pain.3, 23, 24
Outcome Assessment
Primary outcomes were delirium incidence using CAM and delirium severity assessed by the Memorial Delirium Assessment Scale. All participants were examined after surgery by a single investigator (AT) trained in delirium assessment and blinded to baseline assessments. As in other hospital-based delirium studies,25 participants were not evaluated in the first post-operative day to avoid confounding by any residual effects of anesthesia or pain medications on cognition. The initial examination was done in all participants at a median of 22 hours (IQR 19 to 26) after surgery, and the second assessment at a median of 32 hours (IQR 29 to 34) after surgery. In patients who did not meet delirium criteria at either in-person assessment, the investigator conducted a chart review of medical and nursing records for features consistent with confusion, altered mental status or agitation.16 All information was reviewed with a senior neurologist (JV), and no additional cases of delirium were identified beyond the cases identified by AT during the two in-person assessments or chart review.
The CAM is the most widely used method for detecting delirium, and is based on presence of an acute and fluctuating change in the ability to maintain attention plus either disordered thinking or altered level of consciousness.26 A senior neurologist (JV) reviewed CAM interviews in 20 participants; and there was excellent agreement on delirium diagnosis (IRR κ=1.00).
The Memorial Delirium Assessment Scale is a highly reliable scale that rates delirium severity on a 4-point scale on 10 items assessing disturbances in arousal and level of consciousness, attention, memory, cognition, and psychomotor activity (range 0 to 30).27
Data Analysis
All analyses were conducted using Stata Version 11.2. Descriptive statistics were used to compare baseline characteristics of participants who did and did not develop delirium. For delirium incidence, logistic regression was performed with cognitive reserve measures as predictors and reported as odds ratio (OR) with 95% confidence intervals (CI). The two predictors were examined individually as well as entered together in a single model adjusted for all covariates to assess their independent effects on delirium incidence. All models were adjusted for age, sex, comorbidities, and MMSE. Covariates were chosen based on significant associations with delirium in univariate analyses and previous literature. We also explored the association of individual cognitive activities with delirium incidence. Our final sample (n = 142) and observed delirium incidence (32%) provided >90% power to test our main hypotheses. Linear regression was performed to assess the association of cognitive reserve measures with postoperative delirium severity and reported as estimates (B) with 95% CI. The predictors were modeled individually as well as together in the fully adjusted models. The association of individual cognitive activities with delirium severity was examined as an exploratory analysis. Model assumptions were tested statistically and graphically, and were adequately met.
While we excluded cases of dementia using MMSE and clinical information, variability in cognitive status in this sample (including mild dementia cases) is expected. We accounted for baseline cognitive status by adding MMSE scores to main models. Based on published norms,28 we conducted subgroup analyses by stratifying participants into those with MMSE scores of 24 to 27 (minimal cognitively impaired) and scores of 28 to 30 (cognitively normal). Additional sensitivity analyses to account for effects of sex and surgery type as well as influence of individual cognitive activities on delirium risk and severity were also done.
RESULTS
Study Population
One hundred seventy-eight patients were screened; 155 enrolled, 15 declined and 8 excluded. All patients were living at home prior to surgery. Reasons for exclusion included previous dementia diagnosis (2), non-English speaking (2), below age sixty (3), and failed medical clearance (1). Of the 155 enrolled, 8 dropped out prior to their surgery, 2 had surgery cancelled and 3 lost to follow-up. Hence, 142 participants were eligible (mean age 71.2 y, 65% women). Surgeries were done 18 ±15 days after baseline visit; 98 knee, 42 hip, and 2 spine surgeries. Seventy-seven patients received spinal, 60 general, and 52 epidural anesthesia. Delirium rates did not differ by either surgery or anesthesia type (Table 1).
Table 1.
Baseline Characteristics of Study Population. All values are means with standard deviation unless otherwise indicated.
| Variable (range) | Overall | No delirium N=97 |
Delirium N=45 | P value |
|---|---|---|---|---|
| Age, years | 71.2(7.5) | 70.9 (7.8) | 71.8 (7.0) | 0.37 |
| Female, N (%) | 92 (65%) | 64 (66%) | 28 (62%) | 0.71 |
| High school level education, N (%) | 106 (75%) | 78 (81%) | 28 (62%) | 0.02 |
| General anesthesia, N (%) | 60 (38%) | 40 (41%) | 18 (40%) | 0.89 |
| Regional anesthesia, N (%) | 92 (65%) | 65 (67%) | 27 (60%) | 0.42 |
| Hip replacement surgery, N (%) | 41 (29%) | 31 (32%) | 10 (23%) | 0.25 |
| Surgery length, minutes, mean (SD) | 96.2 (27.9) | 95.9 (27.0) | 96.8 (31.2) | 0.87 |
| Peri-operative opiate use, N (%) | 26 (18%) | 18 (19%) | 8 (18%) | 0.92 |
| ADL scale, Median (IQR) (range 0-14) | 1 (0-2) | 1 (0-2) | 1 (0-2) | 0.48 |
| Geriatric Depression Scale, Median (IQR) (range 0-15) |
2 (1-4) | 2. (1-4) | 2 (1-4) | 0.45 |
| Charlson Comorbidity Index >0, N (%) | 73 (57%) | 50 (56%) | 23 (59%) | 0.72 |
| Body mass index, kg/m2 | 32.5 (6.5) | 32.8 (6.9) | 31.9 (5.8) | 0.44 |
| Preoperative Pain score at rest (range 0-10) | 4.3 (3.0) | 4.3 (3.0) | 4.2 (3.2) | 0.78 |
| Postoperative Pain at 24 hours (range 0-10) | 8.3 (2.1) | 8.3 (2.1) | 8.5(2.1) | 0.48 |
| MMSE, Median (IQR) (range 0-30) | 29.0 (26.0-30.0) | 29.0 (27.5-30.0) | 27.0 (25.0-29.0) | <0.001 |
| Cognitive reserve proxies | ||||
| AMNART errors (range 0-45) | 21.2 (12.3) | 18.4 (10.9) | 27.0 (13.0) | <0.001 |
| Cognitive activity scale, (range 0-84), | 13.9 (9.7) | 16.5 (9.7) | 8.5 (7.2) | <0.001 |
IQR, Interquartile Range 25-75 percentiles; ADL, Activities of Daily Living; AMNART, American National Adult Reading Test
None of the participants were taking cholinesterase inhibitors, anxiolytics or antipsychotic medications pre-operatively or were started on these medications during their hospital stay. No participant was on narcotics pre-operatively. No patients were comatose, developed strokes or seizures, or required intensive care during or following surgery.
Forty-five participants (32%) developed postoperative delirium; 31 detected on the first assessment and 14 more on the second assessment. The mean hospital stay was three days; 82% of patients were discharged by post-operative day 4 and 90% by post-operative day 5. Age, gender, comorbidity, depression score, functional status, preoperative pain scores, and postoperative pain scores did not differ by final delirium outcome status (Table 1). Those who developed delirium had lower literacy and less participation in cognitive activities (Table 1).
Delirium incidence
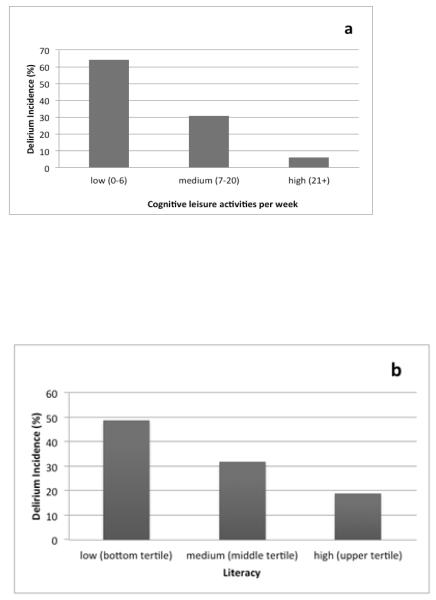
The Cognitive Activity Scale but not literacy scores predicted delirium incidence when examined individually (Table 2). In a model with both cognitive reserve measures entered simultaneously, higher levels of cognitive activities (OR for each additional activity per week 0.92, 95% CI 0.86 to 0.98) but not literacy (OR for each 1 point increase in literacy score 0.99, 95% CI 0.95 to 1.04) predicted delirium incidence. Figure 1 illustrates incidence of delirium based on cognitive reserve levels; for both cognitive activities and literacy, those with lower levels had higher incidence rates of delirium.
Table 2.
Logistic regression model of delirium risk with cognitive reserve measures. Literacy and cognitive activities were examined individually (see Methods).
| Variables | OR (95% CI), P value | OR (95% CI), P value |
|---|---|---|
| Literacy, AMNART corrected | 0.98 (0.94 to to 1.02), 0.253 | * |
| Cognitive activities, days per week^ | * | 0.92 (0.86 to −0.98), 0.006 |
| Age | 0.97 (0.91 to 1.04), 0.364 | 0.95 (0.88 to −1.02), 0.130 |
| Sex | 1.16 (0.46 to −2.96), 0.750 | 1.01 (0.38 to −2.71), 0.978 |
| Charlson Comorbidity Index | 0.96 (0.39 to −2.38), 0.937 | 1.04 (0.40 to −2.69), 0.937 |
| Mini-Mental State Examination | 0.67 (0.52 to 0.86), 0.002 | 0.67 (0.52 to 0.85), 0.001 |
Abbreviations: OR, odds ratio; CI confidence interval; AMNART, American National Adult Reading Test
Leisure activities include reading newspapers, reading books, knitting, playing cards, playing board games, playing computer games, doing crossword puzzles, playing bingo, electronic mail, singing, writing, and participating in group meetings. Scale ranged from 0 to 84.
Variable not included in model. Either literacy or cognitive activities were included in each model. Results including both variables in one model are presented in the Results section.
Figure 1.
Post-surgical delirium incidence (%) by tertile of cognitive leisure activity (1a) and tertile of literacy (1b).
Reading books (OR 0.85, 95% CI 0.72 to 0.99), email (OR 0.81, 95% CI 0.68 to 0.96), and computer games (OR 0.60, 95% CI 0.39 to 0.95) out of the 12 activities were associated with lower risk of delirium incidence in the full models.
Delirium severity
Overall delirium severity on the Memorial Delirium Assessment Scale was 5.4 ± 2.8 points. The Cognitive Activity Scale but not literacy predicted delirium severity when examined individually (Table 3). Cognitive activities (B = −0.06, 95% CI −0.11 to −0.01) but not literacy (−0.01, 95% CI −0.06 to 0.04), predicted delirium severity when examined together.
Table 3.
Linear regression model of delirium severity with cognitive reserve measures. Literacy and cognitive activities were examined individually (see Methods).
| Variables | Beta Coefficient (95% CI), P value |
Beta Coefficient (95% CI), P value |
|---|---|---|
| Literacy, AMNART corrected | −0.02 (−0.07 to 0.03), 0.390 | * |
| Cognitive leisure activities, days per week | * | −0.06 (−0.11 to −0.01), 0.019 |
| Age | −0.02 (−0.08 to 0.05), 0.596 | −0.03 (−0.09 to 0.04), 0.374 |
| Sex | 0.24 (−0.75 to 1.22), 0.634 | 0.02 (−0.97 to 1.01), 0.966 |
| Charlson Comorbidity Index | 0.14 (−0.81 to 1.09), 0.776 | 0.23 (−0.71 to 1.17), 0.625 |
| Mini-Mental State Examination | −0.39 (−0.66 to −0.13), 0.003 | −0.34 (−0.57 to −0.10), 0.005 |
Abbreviations: AMNART, American National Adult Reading Test
Variable not included in model. Either literacy or cognitive activities were included in each model. Results including both variables in one model are presented in the Results section.
Only computer games (B 0.20, 95% CI −0.39 to −0.02) and singing (B −0.43, 95% CI − 0.80 to −0.07) predicted lower delirium severity.
Sensitivity analyses
There were 93 (65.5%) participants with MMSE scores of 28-30 (cognitively normal) and 49 (34.5%) with scores of 24-27 (minimal cognitively impaired). Cognitive activities predicted delirium incidence in cognitively normal participants (OR adjusted for age, sex and CCI 0.90, 95% CI 0.820 to 0.98) and in participants with minimal cognitive impairments (OR 0.89, 95% CI 0.79 to 0.99). Literacy did not predict delirium incidence in minimally cognitively impaired participants (OR 0.99, 95% CI 0.93 to 1.05), but was borderline significant in cognitively normal participants (OR 0.94, 95% CI 0.88 to 1.00).
Sensitivity analyses comparing association of the cognitive reserve measures with delirium incidence by sex (men vs. women), surgery type (hip versus knee replacement surgery) or anesthesia received (general versus spinal versus epidural) were not different, and not presented.
DISCUSSION
In this prospective cohort study, higher participation in cognitive activities but not higher literacy was associated with decreased delirium incidence and severity in older surgical patients. Each additional day of participation in cognitive activities was associated with an eight percent reduced risk of postoperative delirium even after accounting for potential confounders. The sensitivity analysis suggests that this finding applies for both cognitively normal and minimal cognitively impaired individuals. Delirium severity was mild reflecting the relatively healthy nature of our population that was undergoing elective and not emergency surgeries. Since delirium severity is linked with negative outcomes,4 reducing severity is a worthwhile endpoint even if delirium incidence cannot be prevented.
Literacy was not a significant predictor of delirium incidence or severity. Literacy serves as a proxy for early life cognitive reserve, while Cognitive Activity Scale reflects reserve built up and maintained in later life.17 A caveat is that literacy scores might also reflect later life engagement in vocabulary enhancing activities such as reading books. Our findings suggest that while early life learning is important, it may be even more important to maintain later life cognitive activities to reduce delirium risk.
A previous study29 found that participation in leisure activities was associated with decreased delirium incidence in hospitalized patients, and mediated the relationship between education and delirium. Unlike our prospective study, this retrospective study 29 found that physical activity had stronger effect on delirium incidence than cognitive or social activities. Higher literacy was associated with lower risk of delirium in surgical patients in the ‘Successful Aging after Elective Surgery’ (SAGES) study.16 Physical and cognitive activities in SAGES did not predict delirium incidence. The investigators suggested that cognitive and physical activities might be risk factors for dementia and not delirium as dementia cases were excluded based on interviews or modified MMSE cut score.16 We also excluded patients with dementia based on physician diagnosis and MMSE cut score. Unlike SAGES, cognitive activities were significant in our normal group. We assessed current level of cognitive activity participation, whereas the SAGES study assessed lifetime cognitive activity of participants (mean age 76.7 years) at four points starting at age 12, which may be prone to recall bias. The SAGES study assessed six cognitive activities (visiting library, reading newspapers, reading magazines, reading books, writing letters, and playing board or card games).16 We did not assess visiting library. Of the other five common activities only reading books was significant in our analysis. On the other hand, three (computer games, e-mail, and singing) out of four cognitive activities associated with delirium incidence or severity in our study were not assessed in SAGES. The association of specific cognitive activities with delirium should be further examined.
Engaging in computer games protected against both incidence and severity of delirium. Computer based cognitive remediation approaches have been reported to improve cognitive function and demonstrate neuroplasticity.30 The 2013 American Community Survey reported that 71.0% of Americans aged 65 or older reported computer or internet usage.31 Given the ubiquity of computers in American homes (65.1% of homes with a householder aged 65 or older31), computer games should be further tested as a preoperative delirium preventive strategy.
Study strengths include its prospective nature, blinding of assessors, and use of validated and reliable measures to assess cognitive status.
Limitations
Though clinical, neuroimaging and pathological correlates of cognitive reserve have been described; a direct cognitive reserve measure has not been established. Hence, cognitive reserve proxies were used as done in prior studies.5, 8, 18, 32 Further research on the biology of cognitive reserve and delirium is needed to establish etiological factors. While we did not include exclude participants based on education, future studies should include more educationally diverse groups. Only two patients had surgeries cancelled after their baseline assessment. It was logistically challenging to conduct the assessments on the day of surgery. We might have missed patients who developed and resolved delirium in the first post-operative day. Lowered participation in cognitive activities might occur during early stages of dementia. However, the association of cognitive activities with delirium risk remained in the cognitively normal subgroup, suggesting that reverse causation may not account for our findings. The observational nature of the study does not establish causality, which needs to be tested in future clinical trials. While we did not have formal MCI or dementia diagnostic procedures, our cognitively normal participants are unlikely to meet objective cognitive impairment criterion for either MCI or dementia. Hence, the prevalence of MCI in this sample is expected to be very low, and will not be a major influence on findings. The cut score of 28 on MMSE is more stringent than cut scores used to define cognitive normalcy in many aging studies.
Unlike previous hospital-based delirium studies that combined unrelated surgery types,16 our study was restricted to orthopedic patients with hip and knee surgeries (2 additional patients had spine surgery). For instance, the SAGES study included patient undergoing total hip or knee replacement, cervical or lumbar laminectomy, lower extremity arterial bypass, open abdominal aortic aneurysm repair, and colectomy.16 Nonetheless, perioperative features of knee and hip surgeries are expected to be different as are their postoperative management. But, it is unlikely that type of surgery or anesthesia affected results as delirium incidence rates were similar in these different surgical and anesthesia subgroups. The association of cognitive reserve measures with delirium incidence was not different when analyzed by surgery or anesthesia, though power for this subgroup analysis was lower. Post-surgical mobilization procedures for each surgery were also not different and not based on baseline cognitive reserve status. Furthermore, the same rehabilitation team in the same hospital did the mobilization; reducing post-operative variability. The role of cognitive reserve in preventing delirium in other surgeries should be examined.
Higher cognitive reserve, as measured by literacy and cognitive activity participation, is associated with reduced incidence and severity of delirium in older surgical patients. Later life cognitive reserve seems to be more important in preventing delirium and held up in models adjusting for baseline cognitive status, education, and literacy. Our results will apply to the older surgical population, a highly prevalent and high risk group for delirium. Encouragingly, our findings suggest that both individuals with normal and lower cognitive function can benefit from participation in leisure activities. If our findings are validated by other groups, randomized control studies should be conducted to see if postoperative delirium incidence and severity can be reduced by increasing pre-operative participation in cognitive reserve enhancing activities.
ACKNOWLEDGMENTS
We thank Neil Cobelli, MD and Marcie Cobelli, RNP for referring patients. We acknowledge the help of Jeremy Nathaniel, Tanya Verghese, Somechukwu Onuoha, Varada Nair, MaryAnn Zhang, and Deena Peyser with this study.
Study funding: Supported in part by intramural grant from Resnick Gerontology Center, Albert Einstein College of Medicine.
Joe Verghese, Cuiling Wang, and Roee Holtzer received funding support from National Institute on Aging grants (R01 AG039330, RO1AGO44007, AGO44829 and R01AG036921). Joe Verghese is an editorial member of the Journal of the American Geriatrics Society. Amanda Tow was supported in part by NIGMS training grant T32-GM007288 to the Albert Einstein College of Medicine.
Sponsors role: The sponsor study had no role in the design and the collection, analysis, and interpretation of data and the writing of the article and the decision to submit it for publication.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Authors contribution: Amanda Tow and Joe Verghese was responsible for study concept and design, analysis and interpretation of data, and preparation of manuscript. Amanda Tow and Cuiling Wang were responsible for acquisition of data, analysis and interpretation of data, and preparation of manuscript. Roee Holtzer, Alok Sharan, Sun Jin Kim, Aharon Gladstein, and Yossef Blum were responsible for acquisition of data, interpretation of data, and preparation of manuscript.
REFERENCES
- 1.Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiol. 2011;77:448–456. [PMC free article] [PubMed] [Google Scholar]
- 2.Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50:850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 5.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66:821–827. doi: 10.1212/01.wnl.0000202520.68987.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verghese J, Lipton RB, Katz MJ, et al. Leisure Activities and the Risk of Dementia in the Elderly. New Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 8.Jones RN, Fong TG, Metzger E, et al. Aging, brain disease, and reserve: implications for delirium. Am J Geriatr Psychiatry. 2010;18:117–127. doi: 10.1097/JGP.0b013e3181b972e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolanowski AM, Fick DM, Clare L, et al. An intervention for delirium superimposed on dementia based on cognitive reserve theory. Aging Mental Health. 2010;14:232–242. doi: 10.1080/13607860903167853. [DOI] [PubMed] [Google Scholar]
- 10.Galanakis P, Bickel H, Gradinger R, et al. Acute confusional state in the elderly following hip surgery: Incidence, risk factors and complications. Int J Geriatr Psychiatry. 2001;16:349–355. doi: 10.1002/gps.327. [DOI] [PubMed] [Google Scholar]
- 11.Pompei P, Foreman M, Rudberg MA, et al. Delirium in hospitalized older persons - outcomes and predictors. J Am Geriatr Soc. 1994;42:809–815. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 12.Jones RN, Yang FM, Zhang Y, et al. Does educational attainment contribute to risk for delirium? A potential role for cognitive reserve. J Gerontol A Biol Sci Med Sci. 2006;61:1307–1311. doi: 10.1093/gerona/61.12.1307. [DOI] [PubMed] [Google Scholar]
- 13.Lerner AJ, Hedera P, Koss E, et al. Delirium in Alzheimer disease. Alzheimer Dis Assoc Disord. 1997;11:16–20. doi: 10.1097/00002093-199703000-00004. [DOI] [PubMed] [Google Scholar]
- 14.McCusker J, Cole M, Dendukuri N, et al. Delirium in older medical inpatients and subsequent cognitive and functional status: A prospective study. CMAJ. 2001;165:575–583. [PMC free article] [PubMed] [Google Scholar]
- 15.Leung JM, Sands LP, Mullen EA, et al. Are preoperative depressive symptoms associated with postoperative delirium in geriatric surgical patients? J Gerontol A Biol Sci Med Sci. 2005;60:1563–1568. doi: 10.1093/gerona/60.12.1563. [DOI] [PubMed] [Google Scholar]
- 16.Saczynski JS, Inouye SK, Kosar C, et al. Cognitive and Brain Reserve and the Risk of Postoperative Delirium in Older Patients. Lancet Psychiatry. 2014;1:437–443. doi: 10.1016/S2215-0366(14)00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall CB, Lipton RB, Sliwinski M, et al. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenzuela MJ, Sachdev P. Brain reserve and dementia: A systematic review. Psychol Med. 2006;36:441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- 19.Verghese J, Cuiling W, Katz MJ, et al. Leisure activities and risk of vascular cognitive impairment in older adults. J Geriatr Psychiatry Neurol. 2009;22:110–118. doi: 10.1177/0891988709332938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pompei P, Foreman M, Rudberg MA, et al. Delirium in hospitalized older persons: outcomes and predictors. J Am Geriatr Soc. 1994;42:809–815. doi: 10.1111/j.1532-5415.1994.tb06551.x. [DOI] [PubMed] [Google Scholar]
- 21.McAvay GJ, Van Ness PH, Bogardus ST, Jr., et al. Depressive symptoms and the risk of incident delirium in older hospitalized adults. J Am Geriatr Soc. 2007;55:684–691. doi: 10.1111/j.1532-5415.2007.01150.x. [DOI] [PubMed] [Google Scholar]
- 22.Verghese J, Holtzer R, Lipton RB, et al. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc. 2012;60:1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Awissi DK, Lebrun G, Fagnan M, et al. Regroupement de Soins Critiques RdSRQ. Alcohol, nicotine, and iatrogenic withdrawals in the ICU. Crit Care Med. 2013;41:S57–68. doi: 10.1097/CCM.0b013e3182a16919. [DOI] [PubMed] [Google Scholar]
- 24.Vaurio LE, Sands LP, Wang Y, et al. Postoperative delirium: The importance of pain and pain management. Anesth Analg. 2006;102:1267–1273. doi: 10.1213/01.ane.0000199156.59226.af. [DOI] [PubMed] [Google Scholar]
- 25.Lynch EP, Lazor MA, Gellis JE, et al. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–785. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Inouye SK, Vandyck CH, Alessi CA, et al. Clarifying Confusion - the Confusion Assessment Method - a New Method for Detection of Delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 27.Breitbart W, Rosenfeld B, Roth A, et al. The memorial delirium assessment scale. J Pain Symptom Manage. 1997;13:128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 28.Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 29.Yang FM, Inouye SK, Fearing MA, et al. Participation in activity and risk for incident delirium. J Am Geriatr Soc. 2008;56:1479–1484. doi: 10.1111/j.1532-5415.2008.01792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anguera JA, Boccanfuso J, Rintoul JL, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.File T, Ryan C. Computer and Internet use in the United States: 2013. In: Bureau USC, editor. American Community Survey Reports. Volume 2015. web: U.S. Census Bureau’s American Community Survey Office; 2014. pp. American Community Survey Reports. [Google Scholar]
- 32.Jones RN, Manly J, Glymour MM, et al. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc. 2011;17:593–601. doi: 10.1017/S1355617710001748. [DOI] [PMC free article] [PubMed] [Google Scholar]