ABSTRACT
Brown and beige (or brite) fat cells are capable of evoking non-shivering thermogenesis in response to cold and β-adrenergic stimulation. By metabolizing lipids and carbohydrate via uncoupled respiration these cells directly convert energy to heat. The discovery of brown and brown-like adipocytes in adult humans has reinvigorated interest in stimulating brown and beige fat development to combat the obesity epidemic. This review focuses on the role that cytoskeleton dynamics play in the regulation of adipocyte biology, specifically beige and brown fat development and how newly discovered adipogenic morphogens affect these processes.
KEYWORDS: brown fat, beige fat, morphogens, cytoskeleton dynamics, cell shape
Introduction
White adipose tissue (WAT) functions to maintain energy homeostasis by storing excess calories in the form of triglycerides, while releasing energy as free fatty acids in response to caloric demand. However, excess white fat leads to metabolic dysfunction, including type 2 diabetes, dyslipidemia, non-alcoholic fatty liver and cardiovascular disease. On the other hand, brown adipose tissue (BAT), which has abundant mitochondria, stores little fat and instead disposes of excess energy via uncoupled respiration and adaptive thermogenesis. BAT expresses a unique mitochondrial protein, uncoupling protein 1 (UCP1), that dissipates the proton gradient and uncouples respiration from ATP synthesis to generate heat. This process of uncoupled respiration is the primary mechanism maintaining body temperature in small rodents and newborn humans in hypothermic conditions. This non-shivering thermogenic property of brown fat is inherently anti-obesogenic and has profound pharmacological potential.
Numerous recent studies have demonstrated the ability to impart brown-like function within WAT depots by β-adrenergic stimulation or cold exposure, a process referred to as browning. These brown-like adipocytes referred to as brite (brown-in-white) or beige cells, are primarily present in the subcutaneous fat depot.26 The origin of beige cells is still a matter of debate, whether they arise from transdifferentiation of white preadipocytes or preexisting mature white adipocytes2,9 or via de novo differentiation of resident progenitors.28 Although the source of beige adipocytes is currently debated, the origins of classical brown and white adipocytes are clearer. Lineage tracing studies have established that classical brown adipocytes arise from a Myf5+ skeletal muscle lineage,18 while adult white adipocytes arise from SMA+ mural cells present in the adipose vasculature.8 Over the past several years, the field of brown and beige fat biology has seen an increase in the number of reports implicating various growth factors and cytoskeleton dynamics in the regulation of brown and beige fat development. More recently, we and others have demonstrated that beige adipocytes arise from a smooth-muscle origin,10,12 suggesting that morphogenetic signals may regulate a cell fate switch between smooth muscle cells and beige adipocytes. In this review, we will focus on the effects of morphogens on cell shape and cytoskeleton dynamics. Finally, we will examine the mechanisms by which actin cytoskeleton regulates brown, beige and white fat development.
The basic control of adipose development and function
Brown, beige and white adipocytes express a common set of core transcription factors that regulate general aspects of differentiation. The central component regulating this general adipogenic differentiation program is peroxisome proliferator-activated receptor (PPAR)-γ, which is indispensable for both white and brown adipocyte development. CCAAT/enhancer binding protein (C/EBP) family members, C/EBPβ and C/EBPδ are present early in adipocyte differentiation and induce the expression of PPARγ. C/EBPα is expressed later in adipogenesis and serves to maintain PPARγ expression. Finally, PPARγ and C/EBPα cooperatively induce the expression of adipocyte specific genes such as lipid metabolism and storage and secretion of cytokines. Brown and beige adipocyte differentiation is built upon this fundamental program of adipogenesis, with the inclusion of additional transcription factors that regulate mitochondrial biogenesis and fatty acid oxidation, notably PGC1α and PRDM16. A distinct genetic profile of white, brown and beige adipocytes in mice and humans has been identified, however, few selective regulators of beige versus brown fat development have been reported.
Morphogens
Transforming growth factor-β (TGFβ)
TGFβ signaling plays an important role in growth, development and cell fate determination in many tissues, including fat, bone and cartilage. TGFβ levels in adipose tissue and plasma correlates with obesity in humans and mice,1,6 suggesting a role for TGFβ signaling in the regulation of energy homeostasis and the pathology of metabolic disease. TGFβ signals through serine/threonine kinase receptors and induces the expression of smooth muscle contractile genes through the transcription factor SMAD3. TGFβ promotes smooth muscle differentiation, while inhibiting adipocyte differentiation, via the association of SMAD3 with C/EBPβ, preventing the expression of adipogenic genes. SMAD3 null mice, for which TGFβ signaling is attenuated, show decreased white fat mass and are protected from diet-induced obesity.30 Furthermore, SMAD3 null mice have increased brown-like adipocytes in white fat depots and increased mitochondria, indicating SMAD3 is a negative regulator of beige fat development.
Bone morphogenetic proteins
Bone morphogenetic proteins (BMPs) are members of the TGFβ superfamily that promote the differentiation of white and brown adipocytes. BMP2 and BMP4 enhance the commitment of mesenchymal stem cells (MSCs) to an adipogenic lineage and drive preadipocytes to differentiate into mature adipocytes.3,27 Transgenic mice expressing BMP4 in WAT exhibit brown-like adipocytes in white fat, while BMP4-deficient mice have larger white adipocytes.13 BMP7 drives mesenchymal progenitor cells to a brown cell fate, via p38 MAPK, and BMP7 null mice show a reduction in brown fat. BMP7 treatment via adenovirus expression results in increased brown fat mass, enhanced energy expenditure and protection from diet-induced obesity in mice.25
BMP7 has also been shown to activate the thermogenic program in Sca1+ progenitors isolated from white and brown fat.16 In mature BAT, BMP8B is highly expressed and BMP8B null mice exhibit decreased energy expenditure and increased body weight, while still exhibiting normal BAT morphology. BMP8B also potentiates the thermogenic response of β-adrenergic stimulation, suggesting that BMP8B is a regulator of thermogenic activity in mature BAT, as opposed to other BMPs, which tend to drive differentiation and cell fate determination.29 Furthermore, ablation of BMP receptors (BMP receptor type 1A, BMPR1A and activin A receptor type 1, ACVR1) in Myf5+ brown adipocytes results in a marked reduction in BAT mass and compensatory recruitment of brite/beige cells in white fat depots.17 It remains to be elucidated whether cross talk between TGFβ and BMP signaling exists. Nevertheless, BMP7 and TGFβ treated cells exhibit marked morphological changes, implying that topology of microenvironment and cell shape may play a principal role in the determination of cell fate toward a white or beige adipocyte.
Morphology and morphogenesis
Extracellular matrix
The extracellular matrix (ECM) is pivotal in the lineage commitment and differentiation of MSCs, which are recruited from the vascular niche to the ECM network and whose fate is influenced by their microenvironment. Pluripotent MSC progenitors are capable of differentiating into various cell types including fat, bone and cartilage and this decision appears to be based on mechanical signals in the microenvironment, such as matrix rigidity and topology. In vitro studies have suggested a regulatory role of the ECM in cell fate determination by demonstrating that cells grown on a rigid surface exhibit cell spreading, decreased adipogenic differentiation and increased osteogenesis.5,20 Transient receptor potential vanilloid 4 (TRPV4) has been shown to be a mechanical stress sensor.14 TRPV4 null mice have an increased number of brown-like cells in subcutaneous WAT31 and are protected from diet induced obesity and insulin resistance and exhibit elevated energy expenditure. Thus, these data suggest a relationship between mechanical sensing by TRPV4 and cell fate determination of MSCs. The ECM can transmit cellular signals via direct interaction with cell surface receptors and also by regulating growth factor bioavailability. For example, fibrillin-1 controls TGFβ activation by binding and sequestering TGFβ in the ECM. Limb-specific fibrillin-1 deficient mice exhibit reduced adipogenic potential and have less marrow fat.19 In addition to the ECM regulating lineage commitment of MSCs, MCSs have the ability to remodel the ECM to influence its own cell-fate. Expression of membrane-type 1 matrix metalloproteinase (MT1-MMP), a dominant pericellular collagenase enzyme in MSCs, regulates the commitment between osteoblasts, preadipocytes and chondrocytes. Ablation of MT1-MMP expression in MSCs results in enhanced marrow fat and cartilage formation, while impairing bone formation.24 MT1-MMP-dependent ECM remodeling triggers MSC commitment and highlights the importance of MSC-ECM interactions in lineage commitment during early development. In obese patients, the expression and secretion of ECM components are highly upregulated, suggesting that the altered microenvironment may play an important role in obesity and metabolic disease.15,22 Thus, it's becoming clear that the ECM, which orchestrates mechanical signals, cell morphology and cytoskeleton dynamics, can affect the fate of white and beige adipocytes.
Cytoskeleton and cell morphology
The round shape of fat-laden adipocytes suits the cells function and maximizes lipid storage. The hallmark of adipocyte differentiation is a pronounced change in cell shape, which is the result of actin cytoskeleton dynamics. Several studies have shown that changes in cell shape and cytoskeleton tension can influence the fate of MSCs. Cell spreading induced by fibronectin-coated plates inhibits adipogenic differentiation and the inhibitory effect of cell spreading in 3T3-F442A cells on adipogenesis can be reversed with actin disruption.21 Increase cytoskeleton formation, by overexpression of SMA, induces cell spreading and inhibits adipocyte differentiation.23 There is a complex cross talk between cell shape and RhoA activity, as demonstrated by McBeath et. al. that cell shape can impact RhoA activity. Conversely, it has also been shown that RhoA can affect cell shape by inducing cytoskeleton tension. Ultimately, however, it seems clear that both cell shape and RhoA activity dictate lineage commitment. The role of cytoskeletal tension in brown and beige adipocyte differentiation has yet to be investigated, although our data suggest that actin dynamics drives progenitors toward smooth muscle cells as opposed to beige adipocytes.
Effects of cytoskeleton dynamics
Myocardian-related transcription factor A
Our lab has shown TGFβ and BMP7 affect cell morphology and, respectively elongate or broaden cell shape via actin dynamics. Rho-associated protein kinase (ROCK), a major effector of the RhoA pathway, controls the monomeric G-actin and filamentous actin levels. Dominant negative RhoA commit MSCs to an adipocyte cell fate, while constitutively active RhoA commit cells to an osteogenic cell fate.11 Myocardin related transcription factor (MRTFA) senses cytoplasmic actin polymerization by reversibly binding to G-actin. The incorporation of G-actin into F-actin releases MRTFA, which translocates into the nucleus to induce serum response factor target (SRF) genes, such as smooth muscle actin (SMA). We have demonstrated that overexpression of MRTFA in MCSs inhibits adipogenesis and MRTFA null mice are protected from diet-induced obesity and exhibit UCP1+ multilocular cells, establishing MRTFA as a regulator of beige adipocyte development.12 Stromal vascular cells from MTRFA null mice exhibit enhanced beige adipogenesis and reduced expression of smooth muscle proteins, suggesting that MRTFA regulates the switch between smooth-muscle cells and beige adipocytes in progenitors. Our recent data indicate that MRTFA also regulates the balance between adipogenesis and osteogenesis in bone-marrow derived MSCs, and MRTFA null mice exhibit decreased bone mass and increased marrow fat. Furthermore, nuclear translocation of MRTFA and actin polymerization have been shown to be downstream of the actions of the TRPV4 mechanosenor.14 Thus, the process by which MRTFA controls cell fate commitment is likely not due to alteration of a single gene but due to the expression of an entire program of genes coding for cell shape, cytoskeleton and extracellular matrix.
YAP/TAZ
YAP (Yes associated protein) and TAZ (transcriptional coactivator containing PDZ binding motif) have emerged as important regulators of MSC responsiveness to mechanical stress. Gene expression analysis of mammary epithelial cells grown on high and low ECM stiffness has identified the activation of YAP/TAZ signaling.4 These recent studies have also found that ECM stiffness and cell spreading activates Rho-GTPase and cytoskeletal tension, resulting in nuclear localization of YAP/TAZ and mirroring aspects of MRTFA-SRF regulation. Expression of SMA in MSCs induces stress fibers, YAP/TAZ nuclear accumulation and suppresses adipocyte differentiation, which was reversed with YAP siRNA and YAP nuclear activity inhibitor.23 YAP/TAZ nuclear translocation is also induced by ECM remodeling mediated through MT1-MMP, which activates β-integrin signaling and Rho-GTPase.24 These studies link the mechanotransduction of physical cues in the microenvironment by YAP/TAZ to the differentiation of MSCs into several distinct cell types, including adipocytes and osteoblasts. TAZ has been shown to modulate the balance between osteoblast and adipocyte differentiation by activating RUNX2 while repressing PPARγ transcription.7 Surprisingly, BMP2 was shown to induce TAZ translocation, RUNX2-dependent expression of osteocalcin and osteoblast differentiation. Thus, obvious parallels can be drawn between these important mechanotransducers of matrix stiffness, MRTFA and YAP/TAZ, and, given our discovery of MTRFA as a regulator of beige adipocyte differentiation, it leads us to question the role of YAP/TAZ in brown and beige adipocyte differentiation and formation.
Human health relevance
The unabating obesity epidemic is contributing to the prevalence of type 2 diabetes, hypertension, and cardiovascular disease and highlights the need for new anti-obesity therapeutics. Brown fat has received special attention for its ability to protect against obesity and metabolic disease via UCP1 mediated non-shivering adaptive thermogenesis. In humans, the correlation between leanness and resistance to obesity with the presence of brown and beige fat suggests that brown fat activity may contribute to the regulation of body weight. While activating brown fat has obvious pharmacological potential for obesity and diabetes therapeutics, there have been few studies on the role of brown and beige fat in humans and this research is still in its infancy. The development of a diet pill, which robustly activates brown fat and elicits substantial weight loss will be a difficult challenge.
Future perspectives and therapeutic potential
In recent years, substantial investigation has gone into understanding the recruitment and activation of beige and brown fat, however, many questions still remain. In particular, it's not yet clear whether transcription factors that influence the ECM are involved in cell fate determination. Lineage tracing studies have indicated that a population of beige adipocytes arise from vascular smooth muscle-like cells, suggesting that progenitor cells must make a lineage commitment decision between smooth muscle cells and beige adipocytes and we have shown the transcription coactivator MRTFA to be a regulator of such lineage commitment. Additionally, YAP/TAZ has also emerged as a major regulator of both cell-fate determination and a sensor of mechanical stress. Thus, we hypothesize that transcription factors involved in ECM function, such as MRTFA and YAP/TAZ, may be able to influence whether progenitors become smooth-muscle cells or beige adipocytes by altering actin cytoskeleton dynamics (Figure 1). We also question the role of the microenvironment. Can it be altered to influence the development and function of adipose tissue and to promote beige adipocyte differentiation. If so, then a better understanding of the relation between YAP/TAZ, MRTFA signaling and the actin cytoskeleton could reveal strategies to promote beige fat development. While we believe that recent research points in this direction, further study will be needed to clarify whether modulation of factors involved in ECM function may have value in combating metabolic disease.
Figure 1.
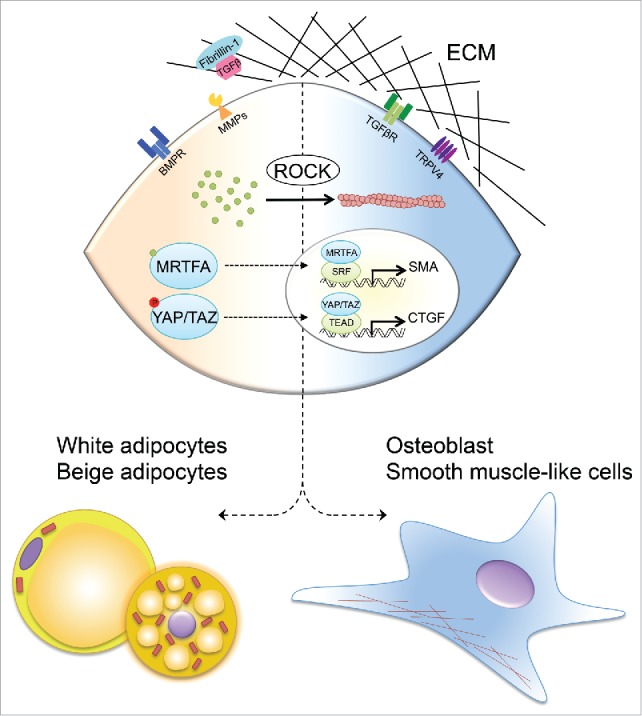
Model of the ECM mediating cell fate of MSCs via interactions with mechanosensors and by modulating the bioavailability of hormones, such as TGFβ. Matrix stiffness and cell shape activates ROCK signaling cascade causing actin polymerization and formation of stress fibers (right). Stress fiber formation drives nuclear translocation of MRTFA and YAP/TAZ and transcriptional expression of SMA and osteogenic genes, which drives commitment to osteoblast and smooth muscle-like cell lineages. BMPs and MMPs decrease ROCK activity, causing actin disruption and preventing nuclear translocation of actin-bound MRTFA and phosphorylated YAP/TAZ, which facilitate white and beige adipogenic lineage commitment (left).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Alessi MC, Bastelica D, Morange P, Berthet B, Leduc I, Verdier M, Geel O, Juhan-Vague I. Plasminogen activator inhibitor 1, transforming growth factor-beta1, and BMI are closely associated in human adipose tissue during morbid obesity. Diabetes 2000; 49:1374-80; PMID:10923640; http://dx.doi.org/ 10.2337/diabetes.49.8.1374 [DOI] [PubMed] [Google Scholar]
- 2.Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010; 298:E1244-53; PMID:20354155; http://dx.doi.org/ 10.1152/ajpendo.00600.2009 [DOI] [PubMed] [Google Scholar]
- 3.Bowers RR, Lane MD. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle Georget Tex 2007; 6:385-9. [DOI] [PubMed] [Google Scholar]
- 4.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al.. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474:179-83; PMID:21654799; http://dx.doi.org/ 10.1038/nature10137 [DOI] [PubMed] [Google Scholar]
- 5.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126:677-89; PMID:16923388 [DOI] [PubMed] [Google Scholar]
- 6.Herder C, Zierer A, Koenig W, Roden M, Meisinger C, Thorand B. Transforming growth factor-beta1 and incident type 2 diabetes: results from the MONICA/KORA case-cohort study, 1984-2002. Diabetes Care 2009; 32:1921-3; PMID:19592635; http://dx.doi.org/ 10.2337/dc09-0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, Mueller E, Benjamin T, Spiegelman BM, Sharp PA, et al.. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 2005; 309:1074-78; PMID:16099986; http://dx.doi.org/ 10.1126/science.1110955 [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 2014; 9:1007-22; PMID:25437556; http://dx.doi.org/ 10.1016/j.celrep.2014.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YH, Petkova AP, Konkar AA, Granneman JG. Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J Off Publ Fed Am Soc Exp Biol 2015; 29:286-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, et al.. A smooth muscle-like origin for beige adipocytes. Cell Metab 2014; 19:810-20; PMID:24709624; http://dx.doi.org/ 10.1016/j.cmet.2014.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004; 6:483-95. [DOI] [PubMed] [Google Scholar]
- 12.McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell 2015; 160:105-18; PMID:25579684; http://dx.doi.org/ 10.1016/j.cell.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY, Xue RD, Yu HY, Guo L, Gao HD, et al.. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci USA 2013; 110:E798-807; PMID:23388637; http://dx.doi.org/ 10.1073/pnas.1215236110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahaman SO, Grove LM, Paruchuri S, Southern BD, Abraham S, Niese KA, Scheraga RG, Ghosh S, Thodeti CK, Zhang DX, et al.. TRPV4 mediates myofibroblast differentiation and pulmonary fibrosis in mice. J Clin Invest 2014; 124:5225-38; PMID:25365224; http://dx.doi.org/ 10.1172/JCI75331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roca-Rivada A, Belen Bravo S, Pérez-Sotelo D, Alonso J, Isabel Castro A, Baamonde I, Baltar J, Casanueva FF, Pardo M. CILAIR-Based secretome analysis of obese visceral and subcutaneous adipose tissues reveals distinctive ECM remodeling and inflammation mediators. Sci Rep 2015; 5:12214; PMID:26198096; http://dx.doi.org/ 10.1038/srep12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al.. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A 2011; 108:143-8; PMID:21173238; http://dx.doi.org/ 10.1073/pnas.1010929108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng YH. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 2013; 495:379-83; PMID:23485971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al.. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454:961-7; PMID:18719582; http://dx.doi.org/ 10.1038/nature07182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smaldone S, Clayton NP, Del Solar M, Pascual-Gonzales G, Cheng SH, Wentworth BM, Schaffler MB, Ramirez F. Fibrillin-1 Regulates Skeletal Stem Cell Differentiation by Modulating Tgfβ Activity Within the Marrow Niche. J Bone Miner Res Off J Am Soc Bone Miner Res 2015; 31(1):86-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegelman BM, Farmer SR. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell 1982; 29:53-60; PMID:7105184; http://dx.doi.org/ 10.1016/0092-8674(82)90089-7 [DOI] [PubMed] [Google Scholar]
- 21.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expsression in 3T3-adipocytes. Cell 1983; 35:657-66; PMID:6686086; http://dx.doi.org/ 10.1016/0092-8674(83)90098-3 [DOI] [PubMed] [Google Scholar]
- 22.Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab 2013; 18:470-7; PMID:23954640; http://dx.doi.org/ 10.1016/j.cmet.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talele NP, Fradette J, Davies JE, Kapus A, Hinz B. Expression of α-smooth muscle actin determines the fate of mesenchymal stromal cells. Stem Cell Rep 2015; 4:1016-30; http://dx.doi.org/ 10.1016/j.stemcr.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, Rowe RG, Botvinick EL, Kurup A, Putnam AJ, Seiki M, Weaver VM, Keller ET, Goldstein S, Dai J, et al.. MT1-MMP-dependent control of skeletal stem cell commitment via a β1-integrin/YAP/TAZ signaling axis. Dev Cell 2013; 25:402-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, et al.. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454:1000-4; PMID:18719589; http://dx.doi.org/ 10.1038/nature07221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 2012; 53:619-29; PMID:22271685; http://dx.doi.org/ 10.1194/jlr.M018846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors Chur Switz 1993; 9:57-71; http://dx.doi.org/ 10.3109/08977199308991582 [DOI] [PubMed] [Google Scholar]
- 28.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19:1338-44; PMID:23995282; http://dx.doi.org/ 10.1038/nm.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez MJ, Morgan D, Csikasz RI, Gallego R, Rodriguez-Cuenca S, et al.. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012; 149:871-85; PMID:22579288; http://dx.doi.org/ 10.1016/j.cell.2012.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, et al.. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab 2011; 14:67-79; PMID:21723505; http://dx.doi.org/ 10.1016/j.cmet.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, Cohen P, Khandekar MJ, Boström P, Mepani RJ, et al.. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 2012; 151:96-110; PMID:23021218; http://dx.doi.org/ 10.1016/j.cell.2012.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]


