ABSTRACT
Exercise training results in adaptations to numerous organ systems and offers protection against metabolic disorders including obesity and type 2 diabetes, and recent reports suggest that adipose tissue may play a role in these beneficial effects of exercise on overall health. Multiple studies have investigated the effects of exercise training on both white adipose tissue (WAT) and brown adipose tissue (BAT), as well as the induction of beige adipocytes. Studies from both rodents and humans show that there are exercise training-induced changes in WAT including decreased cell size and lipid content, and increased mitochondrial activity. In rodents, exercise training causes an increased beiging of WAT. Whether exercise training causes a beiging of human scWAT, as well as which factors contribute to the exercise-induced beiging of WAT are areas of current investigation. Studies investigating the effects of exercise training on BAT mass and function have yielded conflicting data, and hence, is another area of intensive investigation. This review will focus on studies aimed at elucidating the mechanisms regulating exercise training induced-adaptations to adipose tissue.
KEYWORDS: beiging, brown adipose tissue, exercise, myokines, white adipose tissue
Introduction
Regular physical exercise has beneficial effects on overall metabolic health including improvements in glucose tolerance, insulin sensitivity, and the lowering of circulating lipid concentrations.1-3 While improvements in systemic glucose homeostasis have largely been attributed to adaptations in skeletal muscle and liver, exercise training likely results in alterations to almost all tissues in the body. Investigations aimed at understanding the effects of exercise training on many of these non-muscle tissues have been much more limited; however, in recent years there has been increased interest in investigating the effects of exercise training on adipose tissue.
Adipose tissue
Adipose tissue is a critical modulator of energy metabolism. There are 3 distinct types of adipocytes in humans and rodents; white adipocytes, brown adipocytes, and beige adipocytes. White adipose tissue (WAT) is primarily composed of white adipocytes and the stromal vascular fraction (SVF), which is comprised of multiple cell types including progenitor cells and immune cells. WAT is comprised of unilocular adipocytes that can store large amounts of triglycerides as chemical energy. WAT is also involved in hormone production, immune function, and local tissue architecture,4 and increases in white adipose tissue mass (adiposity) are directly associated with increased rates of metabolic diseases such as type 2 diabetes and obesity.5 Importantly, the location of the adiposity within the body plays a significant role in determining the degree of impaired systemic metabolism. WAT has been classified into 2 major depots: visceral (vWAT) and subcutaneous (scWAT). vWAT refers to the adipose tissue that surrounds the internal organs, while scWAT is the adipose tissue found around the thighs and buttocks. An accumulation of vWAT is associated with insulin resistance, increased risk of type 2 diabetes, and mortality, whereas a predisposition for preferential accumulation of scWAT correlates with improved insulin sensitivity and a reduced risk to develop metabolic disease and type 2 diabetes.5-7
In contrast to WAT, brown adipose tissue (BAT) is made up of multilocular brown adipocytes that contain numerous mitochondria that function to mediate adaptive thermogenesis and protect against hypothermia and obesity.8 BAT is characterized by high levels of expression of uncoupling protein 1 (UCP1), the protein responsible for non-shivering thermogenesis, as well as high levels of expression of many other genes including PRDM16, PGC1α, Type 2 Deiodinase (DIO2), and CIDEA, all of which play a role in energy metabolism and mitochondrial biogenesis. BAT oxidative metabolism has been shown to be a significant contributor to whole body energy expenditure.9,10 The relatively recent “re-discovery” of BAT in adult humans and its known role in adaptive thermogenesis has made activation of this tissue a prominent focus of metabolic research. The amount of BAT in humans has been shown to vary with age, BMI, and gender, compounding the complexity of these studies.11-13
Beige adipocytes are found interspersed within the WAT,14-16 specifically the subcutaneous WAT (scWAT), in response to various stimuli including cold exposure and β-adrenergic stimulation. Beige cells come from a Myf5- lineage distinguishing them from BAT, but can, upon stimulation, express high levels of UCP1 and contribute to non-shivering thermogenesis.17 These cells also possess distinct beige adipocyte cell surface markers such as Tmem26 and CD13716 and are capable of increased fuel oxidation and thermogenesis compared to white adipocytes.16 Ablation of beige adipocytes makes mice more prone to obesity and insulin resistance,18 suggesting that these adipocytes are important in the regulation of systemic energy metabolism.
Effects of exercise on WAT
It is well established that aerobic or endurance exercise training, defined as repeated bouts of exercise over a period of days, weeks, or years, increases white adipose tissue lipolysis and free fatty acid mobilization,19,20 decreases adiposity,19,20 and increases the expression of several metabolic proteins including GLUT4 and PGC1α.19-24 Exercise training-induced decreases in adipocyte size and lipid content, and increases in GLUT4 and PGC1α expression have been observed in both scWAT20,23 and vWAT.19,20,22-24 Importantly, many of these metabolic adaptations to adipose tissue can occur independently of significant weight loss 20 demonstrating that adipose tissue can be an important contributor to metabolic health, independent of changes in body weight. In addition, we have recently reported that transplantation of scWAT from exercise-trained mice into the visceral cavity of sedentary recipient mice results in improved glucose homeostasis in the recipient mice,21 also demonstrating that training-induced changes in adipose tissue can have important metabolic effects on organism health.
Exercise and the beiging of white adipose tissue
Numerous stimuli including cold exposure,14 β3-selective adrenergic agonists,15 and exposure to an enriched environment25 have been shown to induce the beiging of adipocytes. In the last several years there have also been a number of reports showing that exercise training in the mouse results in an increased presence of beige adipocytes in scWAT.21,23,25-27 In rodent studies, 3–4 weeks of exposure to an enriched environment, which included the presence of a running wheel, resulted in the emergence of beige cells in scWAT.25-27 These beige cells were identified by an increase in multilocular adipocytes and an increase in Ucp1, Prdm16 and other markers of BAT or beiging.25-27 In another study, exercise training by swimming mice for 90 min per day for 30 d resulted in a significant beiging of scWAT as evidenced by increases in Ucp1 and Prdm16.26 Our work demonstrated that exercise training by voluntary running in a cage containing a wheel for only 11 d resulted in a marked upregulation of numerous beige adipocyte marker genes including Ucp1, Prdm16, Cidea, Elovl3, Pgc1α, Pparγ, Cox8b, Dio2, otopetrin, and Tbx1.21 The wheel cage-trained mice had dramatically increased UCP1 immunofluorescence and the presence of multilocular cells in the scWAT, all of which are consistent with the beiging of scWAT.21 Thus, exercise training in rodents increases the presence of beige adipocytes in the subcutaneous adipose tissue depots.
Although mice clearly respond to exercise training with an increase in beiging of scWAT, from a physiological perspective, there are several reasons that exercise-induced beiging seems counterintuitive.8,28-30 First, it is surprising that exercise training would lead to the induction of a thermogenic, heat-producing cell type such as beige cells, since the contracting muscles generate significant amounts of heat during exercise.8,30 Second, exercise is an energy-consuming process, so it is perplexing that exercise would induce the expression of cells that inherently increase energy expenditure, resulting in even greater fuel requirements for the organism. Finally, during exercise, skeletal muscle is supplied with energy from other tissues, specifically free fatty acids released from WAT; thus it is puzzling that exercise would result in adaptations to the scWAT that result in the burning of residual fat stores, potentially making less substrate available for the working skeletal muscle.29 One hypothesis to explain the exercise-induced beiging of scWAT is that exercise training-induced decreases in cell size and lipid content in scWAT decreases insulation of the body, necessitating increased heat production and resulting in the beiging of scWAT.30,31 While this is an intriguing hypothesis, research aimed at directly addressing this question and other hypotheses will be necessary to determine the primary physiological function of exercise-induced beiging.
Does exercise cause beiging of WAT in humans?
While it is well established that exercise training causes a pronounced beiging of scWAT in rodents, the effects of exercise training on beiging in human adipose tissue is much less clear. One study compared scWAT from lean, sedentary young men (VO2max = 40.5 ± 2.2 ml/kg/min) with age and weight-matched endurance-trained men (VO2max = 59.2 ± 3.6 ml/kg/min) and reported no differences in UCP1, PGC1A, TMEM26, CIDEA, or CD137.32 In another study, male subjects with normoglycemia or hyperglycemia (pre-diabetes) participated in a 12 week exercise training program incorporating both endurance exercise and strength training, and abdominal subcutaneous adipose tissue biopsies were obtained before and after training. The authors report that each training group had a tendency for an increase in UCP1 mRNA in response to training, but that the increase was statistically significant only when the authors combined data from both groups (1.82-fold increase, P < 0.05, n = 24). Training did not increase the beiging markers PRDM16 and CD137, but did increase the beige marker TMEM26 when data from the 2 groups were combined.33 Our own preliminary studies show that 12 weeks of aerobic exercise training in young healthy male subjects significantly increased UCP1 and VEFGA mRNA expression by approximately 2-fold, whereas other beiging markers such as PGC1α, ADRB3, and DIO2 were not increased in the scWAT of these subjects.76 It is possible that the type of exercise, the ambient temperature in which the exercise occurs, the pre-training BMI, and the initial cardiorespiratory fitness levels of the subjects may all be important factors in determining if exercise training in humans induces a beiging of WAT. Another potential explanation is that similar to the variable effects of cold on BAT activity in humans,11,13 some individuals may have a greater genetic propensity to increase beiging of scWAT in response to exercise training. Additional studies are needed to fully understand if beiging of scWAT is an important physiological response to exercise in human subjects, and in subjects that demonstrate beiging of scWAT, if there are metabolic consequences to this adaptation.
Mechanisms regulating increases in beiging
Most non-exercise stimuli are believed to cause beiging of scWAT through increased heat loss and compensatory adrenergic stimulation.30,34-36 In the case of cold exposure, heat loss causes increased thermogenic demand resulting in augmented sympathetic tone and expression of UCP1 to produce more heat.30 Exercise increases sympathetic innervation in scWAT,37 making it possible that the increased sympathetic innervation could contribute to the beiging of scWAT.30,37 Interestingly, several additional or alternative mechanisms have been proposed to be the underlying molecular mechanisms that cause the beiging of scWAT with exercise in rodents.
A potential mechanism that has generated much interest in this field is that exercise results in the release of myokines from the contracting skeletal muscles that signal the scWAT to induce expression of beige adipocytes (Fig. 1).27,38 Lactate is a well-established myokine that could potentially function in this regard, and most types of exercise increase circulating lactate concentrations. Although not directly measured as a response to exercise, increasing circulating lactate concentrations, by either cold exposure or exogenous administration, was shown to increase the beiging of scWAT as determined by increased Ucp1 and Cidea expression.39 In future studies, it will be interesting to determine if lactate plays a fundamental role in the beiging of WAT. In addition, to lactate several other putative myokines that have been implicated in beiging include irisin,27 meteorin-like 1,40 myostatin,41 and β-aminoisobutyric acid.42 It has also been proposed that exercise training results in secretion of hypothalamic brain-derived neurotrophic factor (BDNF) that signals the beiging phenotype.25
Figure 1.
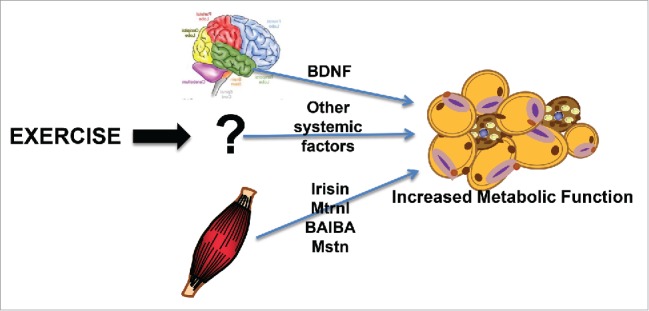
Exercise and the beiging of scWAT. Exercise causes alterations in several factors from both muscle and brain that result in a beiging of scWAT.
Irisin
One search for a secreted protein that could cause beiging in scWAT stemmed from studies in transgenic mice with increased skeletal muscle PGC1α expression.27 These mice were shown to have beige adipocytes within their scWAT, an effect that was further amplified when mice were exercise trained.27 Irisin was identified as a polypeptide cleaved from the membrane protein Fibronectin type III domain-containing protein 5 (Fndc5a), and 8 weeks of voluntary wheel-cage running of mice increased circulating irisin concentrations. Inhibition of irisin by injection of a neutralizing FNDC5 antibody reduced the training-induced beiging. This study also demonstrated that a 10 wk endurance training program in young healthy male subjects increased plasma irisin by 2-fold. These data indicated that chronic exercise in both mice and humans significantly increase blood irisin concentrations, and in mice, this increase significantly contributes to the beiging of scWAT.27
While these intriguing data suggested a role for irisin in response to exercise training in both mice and humans, results from several follow up studies came to varying conclusions.27,33,43-48 These studies have all addressed the questions of whether exercise training increases circulating irisin concentrations and some of the investigations determined if exercise-induced increases in irisin cause a beiging of scWAT in humans. A combined endurance and strength-training program in male subjects had no effect on circulating irisin concentrations,33 although interestingly, and as discussed above, these training programs only resulted in modest, if any increase in beiging of scWAT. Another study examined the effects of 8 weeks of either endurance or strength training exercise on circulating irisin in young men who were overweight or obese. Endurance aerobic exercise training did not increase circulating irisin concentrations, whereas resistance exercise resulted in a significant increase in irisin concentrations.45 In contrast, strength training for 12 wks in women ages 20–32 y that resulted in increased lean body mass and improved strength did not result in changes in circulating irisin concentration.43 In another study, young, healthy women preformed vibration exercise, a moderate intensity exercise that resembles shivering. While a single bout of vibration exercise resulted in a statistically significant ~2-fold increase in circulating irisin, there was no effect of 6 wks of vibration exercise on serum irisin concentrations.44
One possible reason for the discrepancies in the effects of exercise training on circulating irisin concentrations is the technique used to measure irisin. Studies have indicated that human irisin antibodies used in commercial ELISA kits may not accurately detect irisin47,49 because the antibody used was measuring the transmembrane domain of FNDC5 and not the secreted irisin peptide. This may have resulted in an inaccurate measurement of circulating irisin concentration in response to exercise. More recently, studies have used mass spectrometry to quantify the amount of circulating irisin post-exercise in humans.46,50 This technique allows for quantification of circulating human irisin in an antibody-independent manner. Using this technique circulating irisin was increased by both acute exercise (35% increase; P < 0.001) 50 and chronic exercise (1.2-fold in human subjects after 12 wks of aerobic interval training; P < 0.05).46,50 Thus, studies have shown that both resistance45 and endurance exercise 27,46,50 can increase circulating irisin in human subjects. The function of the exercise-induced increase in circulating irisin has been difficult to determine in human subjects. While cell culture experiments have indicated that incubation of mouse or rat pre-adipocytes with irisin increases markers of beiging including Ucp1,27,51,52 irisin did not increase beiging of isolated human preadipocytes.53 Thus, more studies are needed to determine if irisin functions as an exercise-induced myokine responsible for the beiging of scWAT in human subjects. As will be discussed below, this interesting molecule has been implicated as a mediator of other putative myokines and their metabolic functions.
Myostatin
Myostatin, also known as growth and differentiation factor 8 (GDF8), is a member of the TGF-β super family. In skeletal muscle, myostatin inhibits muscle cell growth and differentiation,54,55 and myostatin knockout mice have an increased muscle mass and decreased fat mass.56 Interestingly, these knockout mice have a dramatic increase in beiging of scWAT.51 Incubation of cells cultured from the stromal vascular fraction of scWAT and incubated with media conditioned with skeletal muscle from myostatin knockout mice resulted in a marked beiging of the SVF cells. These data suggest a unique role for myostatin as a muscle myokine that inhibits the beiging of scWAT.51,57
Meteorin-like (Metrnl)
Metrnl was identified from primary myotubes overexpressing PGC1α440, the PGC1α splice isoform that had been shown to regulates muscle hypertrophy and energy expenditure.58 Increasing circulating Metrnl by 5-6-fold in mice by injection of adenoviral vectors increased whole-body energy expenditure, an effect associated with an increase in beiging of scWAT.40 In addition, administration of an anti-Metrnl antibody partially prevented cold-induced beiging of scWAT, suggesting that Metrnl could be an important hormone mediating the beiging phenotype. A single bout of downhill running of mice, a form of exercise that has a large component of eccentric contractions that causes significant muscle damage, increased Metrnl mRNA expression by 3-fold in triceps, but not quadriceps muscles, 6 hours post-exercise. These animals also had a significant increase in circulating Metrnl 24 hours post-exercise, and similarly, young healthy male human subjects who underwent a single bout of combined resistance and aerobic exercise had a significant increase in circulating Metrnl concentrations both 1 and 4 hours post-exercise. Interestingly, wheel-cage exercise training in mice, which is well established to cause beiging of scWAT, did not increase Metrnl expression. Taken together, these intriguing data suggest that Metrnl functions in the beiging of scWAT and responds to resistance forms of exercise in mice and humans. In future work, it will be important to determine if Metrnl mediates the effects of beiging that occurs with exercise training.
β-Aminoisobutyric acid
Another molecule that has recently been suggested to play a role in the beiging of scWAT in response to exercise is β-Aminoisobutyric Acid (BAIBA).42 BAIBA was identified as a potential myokine by LC-MS metabolic profiling of human myocytes that overexpressed PGC1α. Incubation of primary SVF cells isolated from mouse WAT with BAIBA, and addition of BAIBA to the drinking water of mice significantly increased expression of the beiging genes Ucp1 and Cidea. Three weeks of exercise training in mice by voluntary wheel-cage running and 20 wks of supervised submaximal aerobic exercise training (HERITAGE Family Study) in 80 human subjects resulted in a significant increase in circulating BAIBA.42 Human pluripotent stem cells that were exposed to BAIBA while undergoing differentiation to mature white adipocytes had an increased expression of the beiging markers UCP1, CIDEA, and PRDM16. These data suggest that exercise in both mice and human subjects results in a significant increase in circulating BAIBA, and in rodents and isolated human cells, this increase may play a role in increased beiging of scWAT.
Brain-Derived neurotrophic factor (BDNF)
BDNF is a secreted factor released from the hypothalamus and is a key element in energy homeostasis.59 BDNF has also been identified as a molecule that can increase beiging of scWAT in mice.25 Mice that were exposed to 4 weeks of voluntary wheel-cage exercise, an enriched environment (which included the presence of mazes and toys), or both exercise and an enriched environment had an increased beiging of scWAT.25 The mice that were exposed to both a wheel-cage and an enriched environment had a significant upregulation of hypothalamic BDNF and a greater beiging of scWAT. Overexpression of BDNF using adenovirus also led to increased beiging of scWAT, while inhibition of BDNF using miR-Bdnf during exercise training significantly inhibited the training-induced beiging of scWAT. These data indicate an important role for exercise training-induced hypothalamic BDNF in the beiging of scWAT.25
In another study, endurance exercise by 30 d of voluntary wheel-cage running significantly increased Fndc5 expression in the brain, corresponding with in increased hippocampal BDNF.60 This increase was impaired in Pgc1α-/- mice,60 indicating that the increase in BDNF was mediated through the PGC1α/FNDC5 pathway. It had been previously shown that increased FNDC527 leads to increased irisin, which increases the beiging of scWAT. To determine if increased peripheral FNDC5 resulted in an increase in hippocampal BDNF, an adenoviral vector containing FNDC5 was overexpressed in the liver. This resulted in an increase in circulating irisin, increased hippocampal BDNF, and an increased beiging of scWAT. While it is clear that increased activation of this pathway (PGC1α/FNDC5/BDNF) results in an increase in beiging of scWAT, it is not clear whether the increase is a result of the increased circulating irisin, or of the resultant increase in BDNF.60
Taken together, these studies indicate that there are several secreted factors that have been reported to contribute to the exercise-induced beiging of scWAT. How these molecules interact, as well as how they may be regulated in response to exercise in humans, is still a subject of much interest and investigation.
Exercise effects on brown adipose tissue activity
Several investigations have examined the effects of exercise training on BAT, with conflicting results (Fig. 2). Some studies have demonstrated increased BAT activity with exercise,61-66 others showed no change in BAT activity,67-70 while a third set of studies reported a decrease in BAT activity with exercise.32,71-74
Figure 2.
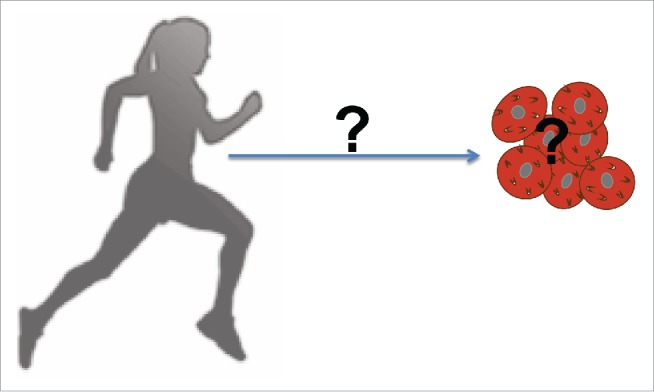
Exercise and BAT. The effects of exercise on BAT function and activity, as well as potential factors that may influence these effects, remain elusive.
Exercise increases BAT activity
Studies in rats have demonstrated that 6–8 wks of swim training for 2–3 hours per day resulted in increased blood flow to BAT and increased responsiveness to norepinephrine stimulation.63,64 Another study demonstrated that 8 wks of exercise training by swimming significantly increased BAT mitochondrial activity in rats.61 In this study, rats were subjected to an exercise training protocol where they swam in a thermoneutral (31–33°C), 1 gallon, plastic barrel for up to 75 minutes per day. While training did not alter total body weight or food intake, retroperitoneal adipose tissue mass was significantly decreased in response to exercise. Examination of the BAT revealed a significant increase in type 2 deiodinase (dio2) and mitochondrial respiration in trained rats indicating increased BAT activity. Other studies have shown that exercise training in mice by either treadmill running or wheel-cage exposure for 6 wks results in moderate increases in mitochondrial protein content (1.6-fold), cytochrome oxidase activity (3-fold), and oxygen consumption in BAT (2.6-fold),65 as well as increased expression of Ucp1 and other BAT-specific genes including Dio2, Prdm16, Pgc1α.66,75 Exercise training via treadmill exercise for 8 wks (15 m/min, for 40 min per day, 5 days/wk) also significantly increased the number adipose progenitor cells in BAT (defined as Lin-;CD29+;CD34+;Sca1+;CD24).66,75 The BAT adipose progenitor cells isolated from trained mice differentiated into brown adipocytes that expressed significantly more UCP1 when compared to BAT adipose progenitor cells isolated from sedentary mice.66,75 These data suggest that exercise training may increase BAT adipogenesis, enhance oxidative phosphorylation, and increase energy expenditure in BAT.
Exercise does not alter BAT activity
Several other studies examining the effects of treadmill running exercise in rats have come to a different conclusion, indicating that exercise has no effect on BAT activity. In one study, rats exercised on a treadmill for 45 d at either 24°C or 4°C. There was no change in BAT activity or thermogenesis, measured through mitochondrial guanosine 5'-diphosphate binding (GDP) at either temperature.69 Treadmill exercise training in rats for 33 d also did not affect BAT mitochondrial GDP binding, alter energy expenditure or BAT-mediated thermogenesis.70 An additional treadmill exercise study in rats indicated that 6 wks of training for 90 min/day, resulted in no change in BAT mass or blood flow, and no change in thermogenic activity.68 In contrast to the studies discussed in the previous section, these studies indicate that exercise in rodents has little or no effect on BAT thermogenesis or mitochondrial activity regardless of ambient temperature. These studies used different markers to measure BAT activity and gene expression, which could account for some discrepancies between the studies showing an increase in BAT activity compared to the investigations showing no effect of exercise training on BAT activity.
Exercise decreases BAT activity
In contrast to these studies indicating that exercise increases or has no effect on BAT activity, there have been several studies indicating that endurance exercise results in a decrease in BAT activity.32,71-74 In one study rats swam 30 min/day for 21 d in “fast flowing water," causing the rats to swim vigorously. After 21 days, swim-trained rats had decreased BAT activity compared to sedentary rats.74 In another study treadmill running of rats at 75–85% of VO2max 60 min/day, 5 days/wk for 8 wks resulted in a significant decrease in body weight and fat mass, as well as an increased beiging of the scWAT. In contrast, BAT removed from these rats revealed a “whitening” of BAT and the unilocular adipocyte area of the BAT from trained rats was ~4x greater than from sedentary rats. BAT from the trained rats also had significantly decreased PGC1α and UCP1 protein expression, and a marked reduction in fatty acid oxidation. These data indicate that thermogenesis, determined by increased UCP1 expression, is inversely regulated in response to exercise training by BAT and scWAT.71 To determine the effects of chronic exercise on BAT in humans, BAT activity was measured in 24 lean, healthy men, aged 18–35 who were either endurance-trained or sedentary. To measure BAT activity, subjects were given a mild cold exposure for 2 hrs and BAT activity was measured by [18F]FDG-PET/CT scan. Cold-induced BAT activity was significantly lower in the trained group compared with the untrained group, indicating that regular endurance exercise training is associated with decreased cold-induced activity of BAT.32 Thus, these studies indicate that exercise training in both rodents and humans down-regulates BAT activity.
As can be surmised from the studies described above, how BAT adapts to exercise training has not been clearly established. From a physiological perspective, similar to the increased beiging of scWAT with exercise, increased BAT activity during exercise is counterintuitive. BAT is a thermogenic tissue, and an increased heat production may be redundant and metabolically wasteful during exercise. This is not to say that exercise is not having any effect on BAT; it could be altering a secreted factor or perhaps just ‘turning off’ so as not to take energy from working tissues (i.e. skeletal muscle). There are numerous questions that have still not been addressed; making this is an intriguing line of investigation for future studies.
Summary
It is clear that exercise induces a beiging of scWAT in rodents, and at least part of the underlying mechanism for this effect may be mediated by skeletal muscle-derived factors or myokines. These myokines, including irisin, Metrnl, myostatin, BAIBA, and lactate, all increase in response to exercise and under various conditions can result in a beiging of scWAT. Despite these advances, there are a number of unanswered questions. One important question is whether beiging of scWAT in humans is a physiological response to exercise training. The function of beiging of scWAT in response to exercise training also remains a mystery. The effects of exercise on BAT activity remain elusive, with several studies showing increased BAT activity, several studies demonstrating no change in BAT activity with exercise, and several studies showing decreased BAT activity. More work is needed to fully elucidate exercise-induced metabolic adaptations to adipose tissue, and to use this information to elucidate novel therapeutic targets for obesity, type 2 diabetes, and other metabolic diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Institutes of Health grants R01-DK-099511 (to L.J.G.), K01-DK-105109 (to K.I.S.), and 5P30-DK-36836 (Diabetes Research Center at Joslin Diabetes Center).
References
- 1.Bonadonna RC, Del Prato S, Saccomani MP, Bonora E, Gulli G, Ferrannini E, Bier D, Cobelli C, DeFronzo RA. Transmembrane glucose transport in skeletal muscle of patients with non-insulin-dependent diabetes. J Clin Invest 1993; 92:486-494; PMID:8326013; http://dx.doi.org/ 10.1172/JCI116592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 2009; 587:5551-8; PMID:19736305; http://dx.doi.org/ 10.1113/jphysiol.2009.179432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Ann Rev Med 1998; 49:235-61; PMID:9509261; http://dx.doi.org/ 10.1146/annurev.med.49.1.235 [DOI] [PubMed] [Google Scholar]
- 4.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol 2010; 6:195-213; PMID:20195269; http://dx.doi.org/ 10.1038/nrendo.2010.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr 2005; 81:555-63; PMID:15755822 [DOI] [PubMed] [Google Scholar]
- 6.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol 1997; 145:614-9; PMID:9098178; http://dx.doi.org/ 10.1093/oxfordjournals.aje.a009158 [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation 2008; 117:1658-67; PMID:18362231; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.107.739714 [DOI] [PubMed] [Google Scholar]
- 8.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84:277-359; PMID:14715917; http://dx.doi.org/ 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- 9.Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012; 122:545-52; PMID:22269323; http://dx.doi.org/ 10.1172/JCI60433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature 2000; 404:652-60; PMID:10766252; http://dx.doi.org/ 10.1038/35007527 [DOI] [PubMed] [Google Scholar]
- 11.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al.. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58:1526-31; PMID:19401428; http://dx.doi.org/ 10.2337/db09-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360:1500-8; PMID:19357405; http://dx.doi.org/ 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- 13.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al.. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360:1509-17; PMID:19357406; http://dx.doi.org/ 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010; 285:7153-64; PMID:20028987; http://dx.doi.org/ 10.1074/jbc.M109.053942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishibashi J, Seale P. Medicine. Beige can be slimming. Science (New York, N.Y.) 2010; 328:1113-4; doi: 10.1126/science.1190816; PMID:20448151; http://dx.doi.org/ 10.1126/science.1190816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al.. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366-76; PMID:22796012; http://dx.doi.org/ 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, et al.. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454:961-7; PMID:18719582; http://dx.doi.org/ 10.1038/nature07182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, et al.. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014; 156:304-16; PMID:24439384; http://dx.doi.org/ 10.1016/j.cell.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig BW, Hammons GT, Garthwaite SM, Jarett L, Holloszy JO. Adaptation of fat cells to exercise: response of glucose uptake and oxidation to insulin. J Appl Physiol Respir Environ Exerc Physiol 1981; 51:1500-6; PMID:7033193 [DOI] [PubMed] [Google Scholar]
- 20.Gollisch KS, Brandauer J, Jessen N, Toyoda T, Nayer A, Hirshman MF, Goodyear LJ. Effects of exercise training on subcutaneous and visceral adipose tissue in normal- and high-fat diet-fed rats. Am J Physiol Endocrinol Metab 2009; 297:E495-504; PMID:19491293; http://dx.doi.org/ 10.1152/ajpendo.90424.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanford KI, Middelbeek RJ, Townsend KL, Lee MY, Takahashi H, So K, Hitchcox KM, Markan KR, Hellbach K, Hirshman MF, et al.. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015; 64:2002-14; PMID:25605808; http://dx.doi.org/ 10.2337/db14-0704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stallknecht B, Vinten J, Ploug T, Galbo H. Increased activities of mitochondrial enzymes in white adipose tissue in trained rats. Am J Physiol 1991; 261:E410-414; PMID:1653528 [DOI] [PubMed] [Google Scholar]
- 23.Sutherland LN, Bomhof MR, Capozzi LC, Basaraba SA, Wright DC. Exercise and adrenaline increase PGC-1{alpha} mRNA expression in rat adipose tissue. J Physiol 2009; 587:1607-17; PMID:19221126; http://dx.doi.org/ 10.1113/jphysiol.2008.165464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirshman MF, Wardzala LJ, Goodyear LJ, Fuller SP, Horton ED, Horton ES.. Exercise training increases the number of glucose transporters in rat adipose cells. Am J Physiol 1989; 257:E520-30; PMID:2801935 [DOI] [PubMed] [Google Scholar]
- 25.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011; 14:324-38; PMID:21907139; http://dx.doi.org/ 10.1016/j.cmet.2011.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trevellin E, Scorzeto M, Olivieri M, Granzotto M, Valerio A, Tedesco L, Fabris R, Serra R, Quarta M, Reggiani C, et al.. Exercise training induces mitochondrial biogenesis and glucose uptake in subcutaneous adipose tissue through eNOS-dependent mechanisms. Diabetes 2014; 63:2800-11; PMID:24622799; http://dx.doi.org/ 10.2337/db13-1234 [DOI] [PubMed] [Google Scholar]
- 27.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, et al.. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481:463-8; PMID:22237023; http://dx.doi.org/ 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsen M, Raschke S, Eckel J. Browning of white fat: does irisin play a role in humans? J Endocrinol 2014; 222:R25-38; PMID:24781257; http://dx.doi.org/ 10.1530/JOE-14-0189 [DOI] [PubMed] [Google Scholar]
- 29.Kelly DP. Medicine. Irisin, light my fire. Science (New York, N.Y.) 2012; 336:42-3; PMID:22491843; http://dx.doi.org/ 10.1126/science.1221688 [DOI] [PubMed] [Google Scholar]
- 30.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab 2014; 20:396-407; PMID:25127354; http://dx.doi.org/ 10.1016/j.cmet.2014.07.005 [DOI] [PubMed] [Google Scholar]
- 31.Hirata M, Suzuki M, Ishii R, Satow R, Uchida T, Kitazumi T, Sasaki T, Kitamura T, Yamaguchi H, Nakamura Y, et al.. Genetic defect in phospholipase Cdelta1 protects mice from obesity by regulating thermogenesis and adipogenesis. Diabetes 2011; 60:1926-37; PMID:21617180; http://dx.doi.org/ 10.2337/db10-1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA, Broeders EP, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD.. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes 2015; 39:1696-702; PMID:26189600; http://dx.doi.org/ 10.1038/ijo.2015.130 [DOI] [PubMed] [Google Scholar]
- 33.Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA.. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 2014; 281:739-49; PMID:24237962; http://dx.doi.org/ 10.1111/febs.12619 [DOI] [PubMed] [Google Scholar]
- 34.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Pénicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992; 103(Pt 4):931-42; PMID:1362571 [DOI] [PubMed] [Google Scholar]
- 35.Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord 1997; 21:465-75; PMID:9192230; http://dx.doi.org/ 10.1038/sj.ijo.0800432 [DOI] [PubMed] [Google Scholar]
- 36.Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol 1997; 54:121-31; PMID:9296358; http://dx.doi.org/ 10.1016/S0006-2952(97)00162-7 [DOI] [PubMed] [Google Scholar]
- 37.Ranallo RF, Rhodes EC. Lipid metabolism during exercise. Sports Med (Auckland, N.Z.) 1998; 26:29-42; PMID:9739539; http://dx.doi.org/ 10.2165/00007256-199826010-00003 [DOI] [PubMed] [Google Scholar]
- 38.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 2012; 8:457-65; PMID:22473333; http://dx.doi.org/ 10.1038/nrendo.2012.49 [DOI] [PubMed] [Google Scholar]
- 39.Carriere A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, Barreau C, Walther R, Galinier A, Wdziekonski B, et al.. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes 2014; 63:3253-65; PMID:24789919; http://dx.doi.org/ 10.2337/db13-1885 [DOI] [PubMed] [Google Scholar]
- 40.Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, et al.. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014; 157:1279-91; PMID:24906147; http://dx.doi.org/ 10.1016/j.cell.2014.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldman BJ, Streeper RS, Farese RV Jr., Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci U S A 2006; 103:15675-80; PMID:17030820; http://dx.doi.org/ 10.1073/pnas.0607501103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts LD, Boström P, O'Sullivan JF, Schinzel RT, Lewis GD, Dejam A, Lee YK, Palma MJ, Calhoun S, Georgiadi A, et al.. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab 2014; 19:96-108; PMID:24411942; http://dx.doi.org/ 10.1016/j.cmet.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellefsen S, Vikmoen O, Slettaløkken G, Whist JE, Nygaard H, Hollan I, Rauk I, Vegge G, Strand TA, Raastad T, et al.. Irisin and FNDC5: effects of 12-week strength training, and relations to muscle phenotype and body mass composition in untrained women. Eur J Appl Physiol 2014; 114:1875-88; PMID:24906447; http://dx.doi.org/ 10.1007/s00421-014-2922-x [DOI] [PubMed] [Google Scholar]
- 44.Huh JY, Mougios V, Skraparlis A, Kabasakalis A, Mantzoros CS. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism: clinical and experimental 2014; 63:918-21; PMID:24814685; http://dx.doi.org/ 10.1016/j.metabol.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Lee HJ, So B, Son JS, Yoon D, Song W. Effect of aerobic training and resistance training on circulating irisin level and their association with change of body composition in overweight/obese adults: a pilot study. Physiol Res 2015; PMID:26447516 [DOI] [PubMed] [Google Scholar]
- 46.Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab 2015; 22:734-40; PMID:26278051; http://dx.doi.org/ 10.1016/j.cmet.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L, Lee S, Brenmoehl J, Thomas S, Drevon CA, et al.. Irisin - a myth rather than an exercise-inducible myokine. Sci Rep 2015; 5:8889; PMID:25749243; http://dx.doi.org/ 10.1038/srep08889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, Jung R, Wisløff U, Tjønna AE, Raastad T, et al.. Evidence against a beneficial effect of irisin in humans. PloS one 2013; 8:e73680; PMID:24040023; http://dx.doi.org/ 10.1371/journal.pone.0073680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson HP. Irisin and FNDC5 in retrospect: An exercise hormone or a transmembrane receptor? Adipocyte 2013; 2:289-93; PMID:24052909; http://dx.doi.org/ 10.4161/adip.26082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daskalopoulou SS, Cooke AB, Gomez YH, Mutter AF, Filippaios A, Mesfum ET, Mantzoros CS.. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur J Endocrinol 2014; 171:343-52; PMID:24920292; http://dx.doi.org/ 10.1530/EJE-14-0204 [DOI] [PubMed] [Google Scholar]
- 51.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J 2013; 27:1981-9; PMID:23362117; http://dx.doi.org/ 10.1096/fj.12-225755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, et al.. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014; 63:514-25; PMID:24150604; http://dx.doi.org/ 10.2337/db13-1106 [DOI] [PubMed] [Google Scholar]
- 53.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, et al.. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A 2011; 108:143-8; PMID:21173238; http://dx.doi.org/ 10.1073/pnas.1010929108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997; 387:83-90; PMID:9139826; http://dx.doi.org/ 10.1038/387083a0 [DOI] [PubMed] [Google Scholar]
- 55.McNally EM. Powerful genes–myostatin regulation of human muscle mass. N Engl J Med 2004; 350:2642-4; PMID:15215479; http://dx.doi.org/ 10.1056/NEJMp048124 [DOI] [PubMed] [Google Scholar]
- 56.Whittemore LA, Song K, Li X, Aghajanian J, Davies M, Girgenrath S, Hill JJ, Jalenak M, Kelley P, Knight A, et al.. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 2003; 300:965-71; PMID:12559968; http://dx.doi.org/ 10.1016/S0006-291X(02)02953-4 [DOI] [PubMed] [Google Scholar]
- 57.Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int J Obes (2005) 2016; 40:434-42; PMID:26435323; http://dx.doi.org/ 10.1038/ijo.2015.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, et al.. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2012; 151:1319-31; PMID:23217713; http://dx.doi.org/ 10.1016/j.cell.2012.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lapchak PA, Hefti F. BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. Neuroreport 1992; 3:405-8; PMID:1633277; http://dx.doi.org/ 10.1097/00001756-199205000-00007 [DOI] [PubMed] [Google Scholar]
- 60.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM.. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab 2013; 18:649-59; PMID:24120943; http://dx.doi.org/ 10.1016/j.cmet.2013.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ignacio DL, Fortunato RS, Neto RA, da Silva Silvestre DH, Nigro M, Frankenfeld TG, Werneck-de-Castro JP, Carvalho DP. Blunted response of pituitary type 1 and brown adipose tissue type 2 deiodinases to swimming training in ovariectomized rats. Hormone and metabolic research=Hormon- und Stoffwechselforschung=Hormones et metabolisme 2012; 44:797-803; PMID:22815055; http://dx.doi.org/ 10.1055/s-0032-1314875 [DOI] [PubMed] [Google Scholar]
- 62.Xu Z, Xu X, Zhong M, Hotchkiss IP, Lewandowski RP, Wagner JG, Bramble LA, Yang Y, Wang A, Harkema JR, et al.. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol 2011; 8:20; PMID:21745393; http://dx.doi.org/ 10.1186/1743-8977-8-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirata K, Nagasaka T. Enhancement of calorigenic response to cold and to norepinephrine in physically trained rats. Japn J Physiol 1981; 31:657-65; PMID:7328915; http://dx.doi.org/ 10.2170/jjphysiol.31.657 [DOI] [PubMed] [Google Scholar]
- 64.Hirata K. Blood flow to brown adipose tissue and norepinephrine- induced calorigenesis in physically trained rats. Japn J Physiol 1982; 32:279-91; PMID:7109341; http://dx.doi.org/ 10.2170/jjphysiol.32.279 [DOI] [PubMed] [Google Scholar]
- 65.Yoshioka K, Yoshida T, Wakabayashi Y, Nishioka H, Kondo M. Effects of exercise training on brown adipose tissue thermogenesis in ovariectomized obese rats. Endocrinol Japn 1989; 36:403-8; PMID:2555143; http://dx.doi.org/ 10.1507/endocrj1954.36.403 [DOI] [PubMed] [Google Scholar]
- 66.Xu X,Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, et al.. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 2011; 300:R1115-25; PMID:21368268; http://dx.doi.org/ 10.1152/ajpregu.00806.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leblanc J, Dussault J, Lupien D, Richard D. Effect of diet and exercise on norepinephrine-induced thermogenesis in male and female rats. J Appl Physiol Respir Environ Exerc Physiol 1982; 52:556-61; PMID:7068471 [DOI] [PubMed] [Google Scholar]
- 68.Wickler SJ, Stern JS, Glick Z, Horwitz BA. Thermogenic capacity and brown fat in rats exercise-trained by running. Metabolism 1987; 36:76-81; PMID:3796299; http://dx.doi.org/ 10.1016/0026-0495(87)90067-9 [DOI] [PubMed] [Google Scholar]
- 69.Richard D, Arnold J, Leblanc J. Energy balance in exercise-trained rats acclimated at two environmental temperatures. J Appl Physiol 1986; 60:1054-9; PMID:3957820 [DOI] [PubMed] [Google Scholar]
- 70.Richard D, Rochon L, Deshaies Y. Effects of exercise training on energy balance of ovariectomized rats. Am J Physiol 1987; 253:R740-5; PMID:3688275 [DOI] [PubMed] [Google Scholar]
- 71.Wu MV, Bikopoulos G, Hung S, Ceddia RB. Thermogenic capacity is antagonistically regulated in classical brown and white subcutaneous fat depots by high fat diet and endurance training in rats: impact on whole-body energy expenditure. J Biol Chem 2014; 289:34129-40; PMID:25344623; http://dx.doi.org/ 10.1074/jbc.M114.591008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boss O, Samec S, Desplanches D, Mayet MH, Seydoux J, Muzzin P, Giacobino JP. Effect of endurance training on mRNA expression of uncoupling proteins 1, 2, and 3 in the rat. FASEB J 1998; 12:335-9; PMID:9506477 [DOI] [PubMed] [Google Scholar]
- 73.Larue-Achagiotis C, Rieth N, Louis-Sylvestre J. Exercise training modifies nutrient self-selection in rats. Physiol Behav 1994; 56:367-72; PMID:7938251; http://dx.doi.org/ 10.1016/0031-9384(94)90208-9 [DOI] [PubMed] [Google Scholar]
- 74.Sullo A, Brizzi G, Maffulli N. Triiodothyronine deiodinating activity in brown adipose tissue after short cold stimulation test in trained and untrained rats. Physiol Res 2004; 53:69-76; PMID:14984316 [PubMed] [Google Scholar]
- 75.Xu X, Liu C, Xu Z, Tzan K, Wang A, Rajagopalan S, Sun Q. Altered adipocyte progenitor population and adipose-related gene profile in adipose tissue by long-term high-fat diet in mice. Life Sci 2012; 90:1001-9; PMID:22683431; http://dx.doi.org/ 10.1016/j.lfs.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stanford KI et al.. Exercise training alters subcutaneous white adipose tissue (scWAT) from mice and humans [Abstract] In: Proceedings of the 73rd Annual Meeting of the American Diabetes Association; 2013 June 21-25, Chicago, IL, CT-OR14. [Google Scholar]


