ABSTRACT
Brown adipose tissue is a promising therapeutic target for opposing obesity, glucose intolerance and insulin resistance. The ability to modulate gene expression in mature brown adipocytes is important to understand brown adipocyte function and delineate novel regulatory mechanisms of non-shivering thermogenesis. The aim of this study was to optimize a lipofection-based small interfering RNA (siRNA) transfection protocol for efficient silencing of gene expression in mature brown adipocytes. We determined that a critical parameter was to deliver the siRNA to mature adipocytes by reverse transfection, i.e. transfection of non-adherent cells. Using this protocol, we effectively knocked down both high- and low-abundance transcripts in a model of mature brown adipocytes (WT-1) as well as in primary mature mouse brown adipocytes. A functional consequence of the knockdown was confirmed by an attenuated increase in uncoupled respiration (thermogenesis) in response to β-adrenergic stimulation of mature WT-1 brown adipocytes transfected with uncoupling protein 1 siRNA. Efficient gene silencing was also obtained in various mouse and human white adipocyte models (3T3-L1, primary mouse white adipocytes, hMADS) with the ability to undergo “browning.” In summary, we report an easy and versatile reverse siRNA transfection protocol to achieve specific silencing of gene expression in various models of mature brown and browning-competent white adipocytes, including primary cells.
KEYWORDS: brown adipocytes, human adipocytes, insulin signaling, knockdown, lipolysis, primary adipocytes, reverse transfection, Seahorse, siRNA, Ucp1
Introduction
Brown adipose tissue (BAT) is specialized in non-shivering thermogenesis, by which energy is used for the generation of heat.1-4 The protein responsible for this process is uncoupling protein 1 (UCP1), located in the inner mitochondrial membrane, where it uncouples the electron transport chain from ATP production. BAT activity is important for rodents to defend their body temperature during prolonged cold exposure and has been shown to counteract many of the harmful effects of a high-fat diet, such as obesity, glucose intolerance and insulin resistance.1-4 Evidence from rodent studies demonstrates that BAT plays a significant role in glucose and lipid metabolism as well as in whole-body energy homeostasis. The observations that adult humans possess metabolically active BAT, that BAT in humans is recruitable and that BAT mass influences whole-body energy homeostasis make brown adipocytes an interesting target in combatting metabolic disease.5,6
Probing the involvement of selected genes in brown adipocyte function can be achieved through loss- and gain-of-function approaches. Generation of transgenic or gene knockout organisms has been of crucial importance for deciphering BAT development and function, but it is both time-consuming and expensive, and moreover, this approach is limited to rodent models. Therefore, an efficient method to silence gene expression in cultured mature brown adipocytes is a relevant supplement to genetically modified mice or rats. RNA interference (RNAi) is a powerful tool to achieve gene silencing; however, using RNAi in mature adipocytes is challenging due to the ineffective delivery of siRNA and short-hairpin RNA (shRNA) by non-viral and viral means, respectively. Using RNAi in pre-adipocytes is straightforward, but is of limited use if the aim is to understand the function of the targeted gene in the mature adipocyte state: silencing of gene expression by RNAi (by means of siRNA delivery or stable viral expression of shRNA) may affect differentiation per se, and even if differentiation is unaffected, gene silencing by siRNA delivery is transient and is not likely to last until the mature adipocyte state is reached. It is possible to efficiently transduce mature adipocytes with adeno- or lentiviral vectors, however, such an approach is relatively labor intensive and requires special safety precautions.7,8 Of non-viral approaches, electroporation has been used for delivery of siRNA into fully differentiated adipocytes;9-12 however, this strategy requires large amounts of siRNA and/or results in a poor transfection and knockdown efficiency. Recently, significant advances have been made by using lipid-based transfection reagents for siRNA transfection of mature white adipocytes.13-15 To our knowledge, no studies have reported efficient siRNA-based knockdown of gene expression in mature brown adipocytes. Therefore, based on the protocol reported by Kilroy et al.,13 we set out to develop a protocol for efficient gene silencing in mature brown adipocytes.
We here report an easy and versatile reverse siRNA transfection protocol to silence specific gene expression in mature brown adipocytes and in mature white adipocytes capable of undergoing “browning.” Importantly, the protocol works well in human adipocytes as well as in primary mouse adipocytes, and it can be used in combination with downstream analyses such as studies of insulin signaling, lipolysis and thermogenesis.
Results
Efficient silencing of gene expression in mature mouse brown adipocytes by reverse siRNA transfection
In order to silence specific genes in mature brown adipocytes without interfering with adipogenesis, we set out to optimize a lipofection-based siRNA transfection protocol. We based our optimization on a protocol develo-ped by Kilroy et al. to obtain effective silencing of gene expression in white adipocytes.13 Using the Lipofectamine RNAiMAX Transfection Reagent, we probed the importance of experimental parameters such as siRNA concentration and incubation time. For various immortalized cell models, we found that the optimal final concentration of siRNA was 50 nM and the optimal incubation time of the siRNA/RNAiMAX mix was 25 min. As described below, optimal conditions for primary mouse adipocytes were slightly different. In 96-well format, we compared siRNA transfection of adherent (forward transfection) and non-adherent (reverse transfection) pre-adipocytes and adipocytes. An overview of the reverse transfection workflow is shown in Figure 1. We used an siRNA targeting pyruvate kinase M (Pkm) and a non-silencing (negative) siRNA as control. Forward transfection of immortalized WT-1 brown pre-adipocytes and mature adipocytes with siRNA resulted in a similar Pkm knockdown efficiency of ∼55% as determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) (Fig. 2A and B). Reverse transfection with Pkm siRNA yielded a 74% and 93% knockdown in pre-adipocytes and mature adipocytes, respectively (Fig. 2A and B). Thus, the reverse transfection protocol proved overall to be most efficient, particularly in the mature WT-1 brown adipocytes.
Figure 1.
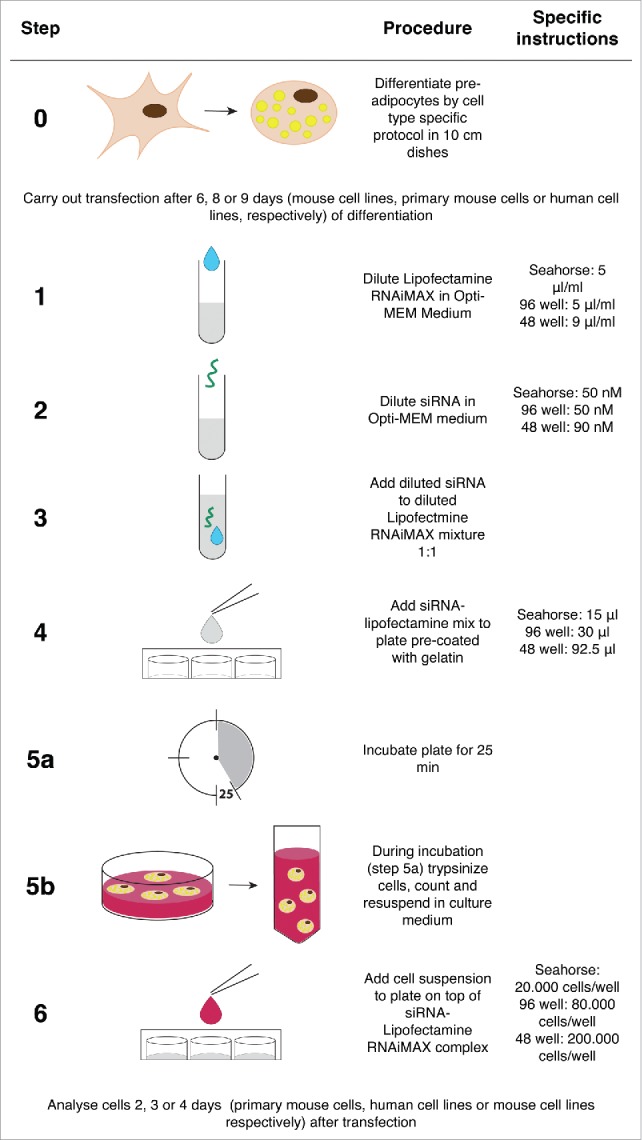
Schematic overview of the reverse siRNA transfection protocol.
Figure 2.
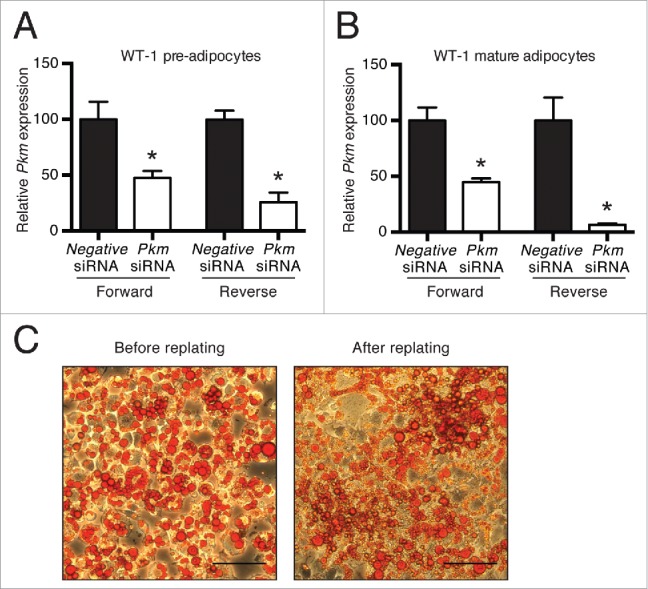
Comparison of Pkm knockdown efficiency in pre-adipocytes and adipocytes using forward and reverse siRNA transfection. Forward and reverse siRNA transfections of WT-1 pre-adipocytes and mature adipocytes with an siRNA against Pkm or a universal negative siRNA as control. The transfections were performed when the cells were ˜70% confluent for the pre-adipocytes or at day 6 of differentiation for the mature adipocytes. The pre-adipocytes were harvested 2 d after transfection, and the mature adipocytes were harvested 4 d after transfection. Relative mRNA expression (measured by RT-qPCR) of Pkm was determined by normalization to expression levels of TATA-binding protein (Tbp). (A) Forward and reverse siRNA transfection of WT-1 pre-adipocytes. (B) Forward and reverse siRNA transfection of mature WT-1 adipocytes. Data represent mean of means +SEM (n = 3). *, p < 0.05 versus universal negative siRNA. (C) Oil red O staining of mature WT-1 adipocytes before and 4 d after replating. The scale bar equals 100 μm.
To render probable that the reverse transfected cells were primarily fully differentiated adipocytes, we confirmed that mature WT-1 adipocytes were able to attach after replating. Micrographs of an oil red O-stained 10-cm dish before replating and 48-well after replating showed that most of the adherent cells stained positive, demonstrating that mature adipocytes were able to re-attach after replating (Fig. 2C).
In accordance with the decrease in Pkm mRNA levels, transfection with Pkm siRNA resulted in a substantial decrease in PKM protein levels (Fig. 3A and B). To verify that the knockdown did not affect basic adipocyte function or affected the maturity of the adipocytes, we evaluated the effect of Pkm siRNA transfection on selected parameters. Knockdown of Pkm did not affect expression of typical adipocyte-enriched genes, such as fatty acid-binding protein 4 (Fabp4) and glucose transporter 4 (Glut4) (Fig. 3C and 3D), suggesting that the ratio of mature adipocytes to undifferentiated cells that re-attached after reverse transfection was similar irrespective of the siRNA used. Stimulation with insulin demonstrated that insulin signaling, measured by Ser473 phosphorylation of AKT, was not influenced by Pkm siRNA compared to negative control siRNA (Fig. 3B). Similarly, Pkm knockdown had no effect on adrenergically induced lipolysis, since a 6–7-fold increase in glycerol release after stimulation with the β-adrenergic agonist isoproterenol (ISO) was observed both in control and Pkm siRNA-transfected cells (Fig. 3E).
Figure 3.
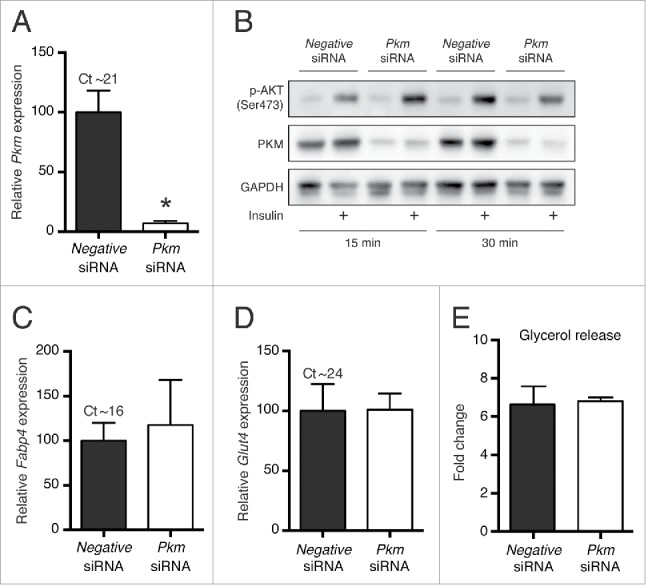
Effect of reverse siRNA transfection on brown adipocyte function. Mature WT-1 adipocytes reverse transfected with Pkm siRNA or universal negative siRNA at day 6 of adipocyte differentiation and harvested 4 d later. Relative mRNA expression levels (measured by RT-qPCR) of (A) Pkm, (C) Fabp4, and (D) Glut4 were determined by normalization to expression levels of Tbp. Data represent mean of means +SEM (n = 3). *, p < 0.05 vs. universal negative siRNA. (B) Immunoblotting analyses of Ser473-phosphorylated AKT (p-AKT) and PKM following stimulation with 5 μg/ml insulin for 15 or 30 min. GAPDH was used as a loading control (n = 2). (E) Cell culture medium glycerol content from reverse transfected cells stimulated with 1 μM isoproterenol for 6 h, depicted as fold increase compared to non-stimulated cells. Data represent mean of means +SEM (n = 3).
In summary, WT-1 brown adipocytes reverse transfected with siRNA displayed a normal response to insulin and β-adrenergic stimulation.
Efficient knockdown irrespective of target mRNA abundance
Pkm is expressed at relatively high levels in mature brown adipocytes (Ct value ∼20). To compare the knockdown efficiency of high- and low-abundance mRNAs using reverse transfection of mature WT-1 adipocytes, we applied siRNA targeting Pkm or calcium/calmodulin-dependent protein kinase IIα (Camk2a), the latter being expressed at relatively low levels as determined by RT-qPCR (Ct value ∼27). Pkm mRNA was knocked down by 89% upon transfection with Pkm siRNA (Fig. 4A), whereas Camk2a mRNA was reduced by 82% in response to Camk2a siRNA (Fig. 4B). Hence, both high- and low-abundance mRNAs were efficiently silenced in mature brown adipocytes by reverse siRNA transfection. Expression of Fabp4 and Glut4 mRNAs was not differentially influenced by the siRNAs (Fig. 4C and 4D).
Figure 4.
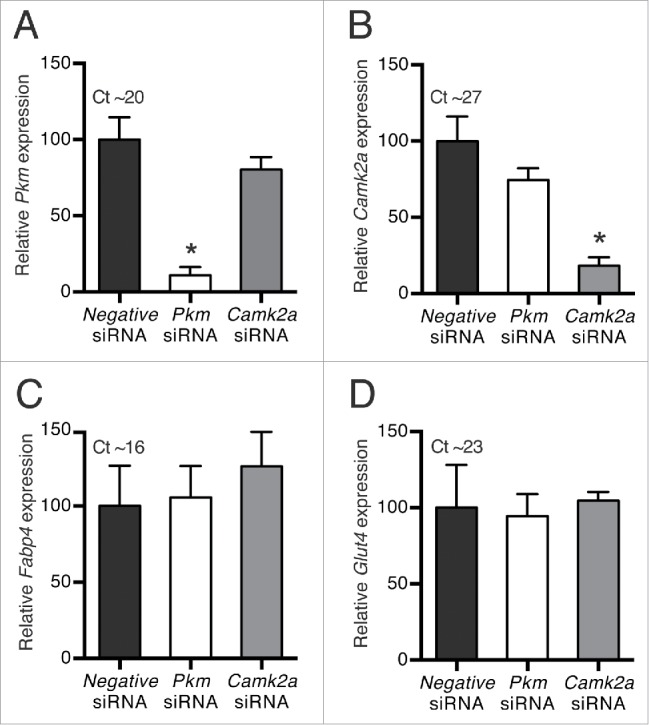
Knockdown efficiency of high- and low-abundance targets. Reverse siRNA transfections of mature WT-1 adipocytes with siRNA against Pkm, Camk2a or a universal negative siRNA as control. The transfections were performed at day 6 of differentiation and adipocytes were harvested 4 d later. Relative mRNA expression levels (RT-qPCR) of (A) Pkm, (B) Camk2a, (C) Fabp4, and (D) Glut4 were determined by normalization to expression levels of Tbp. Data represent mean of means +SEM (n = 4). *, p < 0.05 versus universal negative siRNA.
Silencing of gene expression in human multipotent adipose-derived stem cell (hMADS), in the 3T3-L1 cell line and in primary mouse adipocytes
We further analyzed the efficiency and applicability of the reverse transfection protocol in 4 additional adipocyte cell models of human and mouse origin: hMADS, 3T3-L1 and primary mouse adipocytes from BAT and the inguinal white adipose tissue (iWAT) (Fig. 5). hMADS, 3T3-L1 and primary iWAT adipocytes are considered models of white adipogenesis, but all have the ability under specific conditions to convert into UCP1-positive, thermogenic adipocytes, a process known as “browning.”16-18 Reverse transfection of hMADS and 3T3-L1 adipocytes was carried out as for WT-1 adipocytes (Fig. 1). In order to obtain sufficient material for downstream analyses, primary mouse adipocytes were reverse transfected in 48-well plates, using 2.5-fold more cells per well and 90 nM siRNA (Fig. 1). We used siRNA against glyceraldehyde-3-phosphate (GAPDH) in the human hMADS adipocytes and Pkm siRNA in the 3 mouse adipocyte models. GAPDH mRNA was knocked down by 90% in hMADS adipocytes, and knockdown efficiencies of Pkm mRNA in 3T3-L1 and primary BAT and iWAT adipocytes were 90%, 73% and 80%, respectively (Fig. 5A-D). Hence, the optimized protocol was also applicable to human adipocytes (hMADS), mouse white adipocytes (3T3-L1 and primary iWAT) and primary mouse brown adipocytes.
Figure 5.
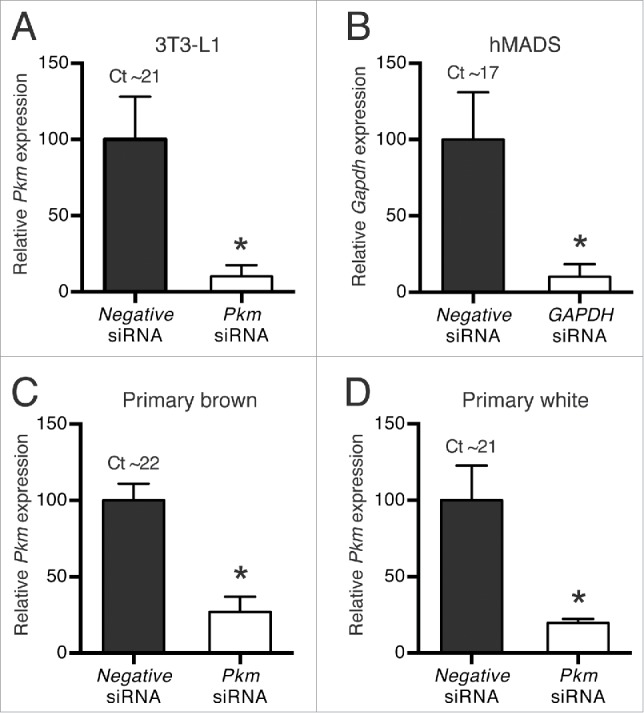
Knockdown efficiency in different adipocyte cell models. Reverse siRNA transfections of mature adipocytes with siRNA against Pkm for mouse cell models, GAPDH for human hMADS cells or a universal negative siRNA as control. The transfections were performed in (A) 3T3-L1, (B) hMADS, and (C) primary brown and (D) primary white adipocytes. The reverse siRNA transfections were performed at day 6 (3T3-L1), day 9 (hMADS) or day 8 (primary cells) of differentiation and were harvested 4 d, 3 d and 2 d later, respectively. Relative mRNA expression levels (measured by RT-qPCR) of Pkm or GAPDH were determined by normalization to expression levels of Tbp. Data represent mean of means +SEM (n = 3 for panels A and B, n = 4 for panels C and D). *, p < 0.05 vs. universal negative siRNA.
Attenuation of β-adrenergically stimulated oxygen consumption in mouse brown adipocytes by Ucp1 knockdown
To demonstrate a functional consequence of gene silencing in brown adipocytes, we reverse transfected mature WT-1 adipocytes with an siRNA against Ucp1, which resulted in a 78% decrease in Ucp1 mRNA levels, or with a control siRNA (Fig. 6A). Since Ucp1 is barely expressed in pre-adipocytes, the Ucp1 knockdown demonstrated that mature adipocytes had been transfected. To investigate the efficiency of the UCP1 knockdown on adrenergically induced thermogenesis, we performed the reverse transfection with Ucp1 siRNA directly in gelatin-coated XF96 Cell Culture Microplates, to be used for analysis on the Seahorse XF analyzer. Four days after transfection, we determined oxygen consumption rate (OCR) during sequential addition of ISO, carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP) and rotenone/antimycin A (Fig. 6B). This was carried out under experimental conditions where it has been demonstrated that adrenergically induced OCR is UCP1-dependent.19 Brown adipocytes transfected with the control siRNA increased their OCR by 70% in response to acute stimulation with ISO, whereas Ucp1 siRNA-transfected cells increased their OCR by only 30% (Fig. 6B and C), demonstrating both the functional efficiency of the knockdown and the significance of UCP1 for the induced thermogenesis. Ucp1 knockdown cells pretreated with oligomycin failed to increase oxygen consumption in response to ISO, whereas OCR increased with 30% in oligomycin-pretreated control cells (data not shown). Similar to the situation in freshly isolated mouse brown adipocytes,20 this demonstrates that UCP1 is essential for adrenergically induced thermogenesis in mature WT-1 cells.
Figure 6.
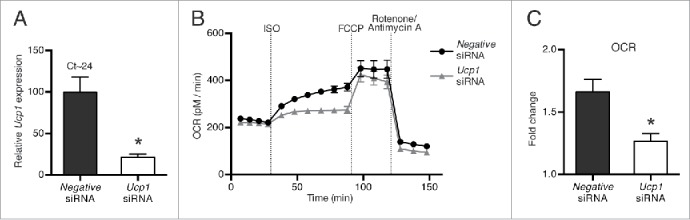
Functional consequences of Ucp1 knockdown. Mature WT-1 brown adipocytes were reverse transfected at day 6 of differentiation with an siRNA against Ucp1 or a universal negative siRNA. The cells were harvested 4 d later. (A) Relative mRNA expression levels (measured by RT-qPCR) of Ucp1 were determined by normalization to expression levels of Tbp. (B) Oxygen consumption rate (OCR) was measured on a Seahorse XF96 Analyzer under basal conditions and during successive addition of 1 μM isoproterenol (ISO), 1 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) and a mixture of 1 μM rotenone and 1 μM antimycin A. The figure shows a representative experiment (out of 4). (C) Fold change in OCR between the basal level and the measurement 1 h after ISO stimulation. Data represents mean of means +SEM (panels A and C) or mean ± SEM (panel B) (n = 4). *, p < 0.05 versus universal negative siRNA.
Discussion
Brown adipocytes are considered promising targets in combatting obesity and diabetes. The ability to tinker with specific genes in mature brown adipocytes is of importance for discovering novel information about brown adipocyte biology. Here we report an easy, rapid and versatile lipofection-based reverse siRNA transfection protocol with which specific genes can be effectively silenced in mature adipocytes of various origins.
Transfecting the mature adipocytes while still in suspension, followed by reattachment, greatly increases knockdown efficiency. This reverse transfection protocol is effective using pre-adipocytes, but surprisingly is more effective using mature adipocytes. The presented protocol, as summarized in Figure 1, has an easy workflow, is time and cost efficient and requires no special equipment. The protocol is easily adaptable to various adipocyte cell models, as demonstrated by its effectiveness in different mouse and human adipogenic cell lines as well as in primary mouse adipocytes. Equally efficient gene silencing was observed in mature brown adipocytes and mature white adipocytes with the ability to undergo browning.
We also demonstrate that the knockdown procedure can be combined with functional assays, as shown by the normal response to insulin and β-adrenergic stimulation after reverse siRNA transfection, and the reduced β-adrenergically induced oxygen consumption following knockdown of Ucp1 expression. Finally, the reverse siRNA transfection protocol has been optimized and standardized for 96-well plates and is therefore adaptable to high-throughput screening of siRNA libraries.
We hope that the reported protocol will be an easily accessible and useful tool for deciphering novel aspects of brown adipocyte biology and browning of white adipocytes in vitro.
Materials and methods
Culture of cell lines
The WT-1 cell line established by immortalization of brown pre-adipocytes from newborn mice with SV40 large T antigen was kindly provided by Dr. C. Ronald Kahn.21 WT-1 cells were propagated in Dulbecco's Modified Eagle's Medium (DMEM) (Life Technologies, 52100) supplemented with 10% fetal bovine serum (FBS) (Life Technologies, 10270), 62.5 μg/ml penicillin and 100 μg/ml streptomycin (Life Technologies, 15140–122). The 3T3-L1 white pre-adipocyte cell line22 was propagated as WT-1 cells, except that 10% calf serum (PPA Laboratories, B15-004) was used instead of FBS. Two days post-confluent WT-1 and 3T3-L1 cells (designated day 0) were induced to differentiate in WT-1 propagation medium supplemented with 1 μM dexamethasone (Sigma-Aldrich, D1756), 0.5 mM isobutylmethylxanthine (Sigma-Aldrich, I5879), 5 μg/ml insulin (Roche, 11376497001) and 1 μM rosiglitazone (Cayman Chemicals, 71740). At day 2, cells were refreshed with medium containing 5 μg/ml insulin and 1 μM rosiglitazone. From day 4, the cells were cultured in WT-1 propagation medium.
hMADS cells23 were obtained from Dr. Christian Dani and propagated in Advanced DMEM/F12 (Life Technologies, 12634) supplemented by 10% FBS, 2 mM L-glutamine (Life Technologies, 25030), 62.5 μg/ml penicillin and 100 μg/ml streptomycin. Two days post-confluent cells (day 0) were induced to differentiate in Advanced DMEM/F12 with 2% FBS and 1 μM dexamethasone, 0.5 mM isobutylmethylxanthine, 5 μg/ml insulin, 1 μM rosiglitazone, 1 μM cortisol (Sigma-Aldrich, H0135) and 1 nM thyroid hormone (T3) (Sigma-Aldrich, T6397). This treatment was repeated at day 3. On day 6, the cells were refed with Advanced DMEM/F12 with 2% FBS containing 1 μM rosiglitazone and 1 nM T3.
Cell cultures were kept at 37°C in a humidified atmosphere with 5% CO2. Cells used for reverse siRNA transfections were cultured in 10 cm dishes until transfection.
Isolation and culture of primary adipocytes
Primary brown (from interscapular, cervical and axillary BAT) and inguinal white pre-adipocytes, from 3 to 4 weeks old NMRI mice (males and females), were isolated and cultured essentially as described.24 After the tissue was minced and transferred to a HEPES-buffered solution (pH 7.4) containing 0.2% crude collagenase type II (Sigma-Aldrich, C6885), it was digested at 37°C for 30 min with constant shaking. The cell suspension was filtered through a 250-μm filter and incubated on ice for 15 min to separate the mature adipocytes and the stromal vascular (SV) fraction. The SV fraction was then filtered through a 50-μm filter. After centrifugation (10 min, 700 g), the pellet was resuspended in culture medium (DMEM, 4.5 g D-glucose/liter) (Sigma-Aldrich, D6429), 10% newborn calf serum (Life Technologies, 16010–159), 2.4 nM insulin (Novo Nordisk, 8–0204), 4 mM L-glutamine, 10 mM HEPES (Lonza, BE17-737E), 25 μg/ml sodium ascorbate (Sigma-Aldrich, A4034), 50 IU/ml penicillin and 50 μg/ml streptomycin and centrifuged. The pellet was resuspended in culture medium and plated in 6-well plates. Cultures were incubated in a humidified atmosphere of 8% CO2 at 37°C. The cell culture medium was changed at days 1, 3, 5 and 7 after isolation.
Reverse siRNA transfections
Pre-adipocytes and mature adipocytes were reverse transfected with siRNA as follows: siRNA and Lipofectamine RNAiMAX (Life Technologies, 13778–150) were diluted in Opti-MEM I Reduced Serum Medium (Life Technologies, 31985-062) separately before being mixed by pipetting. The siRNA-RNAiMAX mix was added to gelatin-coated 96- or 48-well cell culture plates or 96-well Seahorse plates, and left to incubate for 25 min at room temperature. The final concentrations of Lipofectamine RNAiMAX and siRNA were 5 μl/ml and 50 nM, respectively, in 96-well cell culture and Seahorse plates, and 9 μl/ml and 90 nM, respectively, in 48-well plates. The final amount of medium per well was 90 μl in a Seahorse plates, 180 μl in 96-well culture plates and 250 μl in 48-well plates.
Pre-adipocytes were reverse transfected when the cells were ˜70% confluent. Pre-adipocytes were detached with 0.05% trypsin (Sigma-Aldrich, T3924) for 5 min, counted and spun down (5 min, 300 g). The cells were resuspended in culture medium and added (20,000 cells per well) to 96-well plates on top of the pre-incubated siRNA-RNAiMAX mix. The cells were harvested 2 d after transfection.
Mature adipocytes were transfected at day 6 of differentiation for mouse cell lines (WT-1 and 3T3-L1), day 8 for primary cultures or day 9 for hMADS cells. Mature adipocytes were trypsinized with 0.25% trypsin (Life Technologies, 25200-072) for 15–20 min, counted and spun down (5 min, 300 g). The cells were resuspended in culture medium and added to Seahorse, 96- or 48-well plates on top of the pre-incubated siRNA-RNAiMAX mix. The final number of cells per well was 20,000, 80,000 and 200,000 in Seahorse, 96- and 48-well culture plates, respectively. Reverse transfection and replating were performed in 96-well plates for mouse and human cell lines and in 48-well plates for primary cells. Figure 1 illustrates the protocol for reverse siRNA transfection.
Mature mouse primary adipocytes and hMADS cells were harvested 2 and 3 d after transfection, respectively. For the mouse cell lines (WT-1 and 3T3-L1 cells) the medium was changed after 2 d and the cells were harvested 4 d after transfection.
The MISSION® siRNAs (Sigma-Aldrich) used were mouse Pkm (SASI_Mm01_00036294), mouse Camk2a (SASI_Mm01_00347598) and mouse Ucp1 (SASI_Mm01_00067600). For hMADS cells, the Silencer Select siRNA against human GAPDH (Life Technologies, 4427038) was used. The MISSION® siRNA Universal Negative Control #1 (Sigma-Aldrich, SIC001) was used as control in all mouse experiments and Negative Control #1 (Life Technologies, 4390843) was used as control in hMADS cells.
Forward siRNA transfections
Pre-adipocytes and mature adipocytes were forward transfected with siRNA when the cells were ˜70% confluent or at day 6 of differentiation, respectively. As with the reverse siRNA transfection, the siRNA and Lipofectamine RNAiMAX were diluted separately and mixed by pipetting. The siRNA-RNAiMAX mix was left to incubate for 25 min at room temperature after which the siRNA-RNAiMAX mix was added on top of the adherent cells. The concentration of Lipofectamine RNAiMAX and siRNA was as described for the reverse siRNA transfection in 96-wells culture plates. The pre-adipocytes were harvested 2 d after transfection. For mature adipocytes the medium was changed after 2 d and the cells were harvested 4 d after transfection.
Oil red O staining
Oil red O staining was performed as previously described.25
Seahorse measurements
Four days after transfection, real-time measurements of OCR were performed using the Seahorse XF96 Extracellular Flux Analyzer (Seahorse Bioscience). One h before the first measurement, the cell culture medium was changed to DMEM (Seahorse Bioscience, 102352) supplemented with 5 mM glucose (Sigma-Aldrich, G7021) and 2% bovine serum albumin (Sigma-Aldrich, A9647) (adjusted to pH 7.4). OCR was measured under basal conditions and during successive addition of 1 μM ISO (Sigma-Aldrich, I5627), 1 μM FCCP and a mixture of 1 μM rotenone and 1 μM antimycin A (Seahorse Bioscience, 101706–100).
RT-qPCR
Total RNA was purified using TRI Reagent (Sigma-Aldrich, T9424) according to the instructions of the manufacturer, and reverse transcription and qPCR were performed as previously described,26 except that the SensiFAST SYBR Lo-ROX Kit was used (Bioline, BIO-94005). Primers used were: mouse Camk2a, fwd-TCTTC TGAGAGCACCAACAC, rev-GGTCGCACATCTTCGT GTA (136 bp); mouse Fabp4, fwd-TGGAAGCTTG TCTCCAGTGA, rev-AATCCCCATTTACGCTGATG (111 bp); mouse Glut4, fwd-ATCATCCGGAACCTGGAGG, rev-CGGTCAGGCGCTTTAGACTC (52 bp); mouse Pkm, fwd-CTGTGGAGATGCTGAAGGAG, rev-CAACAGGACGGTAGAGAATGG (156 bp); mouse Tbp, fwd-ACCCTTCACCAATGACTCCTATG, rev-ATGATGACTGCAGCAAATCGC (190 bp); mouse Ucp1, fwd-AGCCGGCTTAATGACTGGAG, rev-TCTG TAGGCTGCCCAATGAAC (51 bp); human GAPDH, fwd-GCAAGAGCACAAGAGGAAGAG, rev-CTACAT GGCAACTGTGAGGAG (103 bp); human TBP, fwd-CCCGAAACGCCGAATATAA, rev-GAAAATCAGTG CCGTGGTTC (83 bp).
Whole cell extracts and immunoblotting
Cells transfected with Pkm siRNA were harvested 4 d after transfection. Preparation of whole-cell extracts and immunoblotting were done as described.25 Antibodies used were against Ser473 phosphorylated AKT (Cell Signaling Technologies, 4058), GAPDH (Abcam, Ab8245) and PKM (Sigma-Aldrich, SAB4200095).
Glycerol release
Four days after transfection with control or Pkm siRNA, following addition of fresh medium, cells were stimulated with 1 μM ISO. Cell culture medium was collected after 6 h of stimulation and stored at −20°C. Glycerol release was measured using the Adipolysis Assay Kit (Cayman Chemical, 10009381) essentially following the instructions of the manufacturer.
Statistical analyses
For all cell culture studies, 3 samples were harvested for each condition in each experiment, except for primary cultures where only 2 wells were harvested. Data represent mean of means of 3 to 4 independent experiments + standard error of the mean (SEM). Statistical significance in RT-qPCR data and fold change in OCR was determined through 95% confidence intervals as calculated by an unpaired Student's t-test. Bonferroni correction was used when multiple comparisons were applied.
Abbreviations
- BAT
brown adipose tissue
- Camk2a
calcium/calmodulin-dependent protein kinase IIα
- DMEM
Dulbecco's Modified Eagle's Medium
- Fabp4
fatty acid-binding protein 4
- FBS
fetal bovine serum
- FCCP
carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone
- GAPDH
glyceraldehyde-3-phosphate
- Glut4
glucose transporter 4
- hMADS
human multipotent adipose-derived stem cells
- ISO
isoproterenol
- iWAT
inguinal white adipose tissue
- OCR
oxygen consumption rate
- Pkm
pyruvate kinase, muscle
- RNAi
RNA interference
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- SEM
standard error of the mean
- shRNA
short-hairpin RNA
- siRNA
small interfering RNA
- SV
stromal vascular
- T3
thyroid hormone
- Tbp
TATA-binding protein
- Ucp1
uncoupling protein 1
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the EU FP7 project DIABAT (HEALTH-F2-2011-278373). MSI and MCHP were funded in part by The Danish Diabetes Academy supported by The Novo Nordisk Foundation.
References
- 1.Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest 2015; 125:478-86; PMID:25642708; http://dx.doi.org/ 10.1172/JCI78362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell 2014; 156:20-44; PMID:24439368; http://dx.doi.org/ 10.1016/j.cell.2013.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19:1252-63; PMID:24100998; http://dx.doi.org/ 10.1038/nm.3361 [DOI] [PubMed] [Google Scholar]
- 4.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metabol 2010; 11:268-72; PMID:20374959; http://dx.doi.org/ 10.1016/j.cmet.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 5.Schrauwen P, van Marken Lichtenbelt WD, Spiegelman BM. The future of brown adipose tissues in the treatment of type 2 diabetes. Diabetologia 2015; 58:1704-7; PMID:25957230; http://dx.doi.org/ 10.1007/s00125-015-3611-y [DOI] [PubMed] [Google Scholar]
- 6.Betz MJ, Enerback S. Human brown adipose tissue: What we have learned so far. Diabetes 2015; 64:2352-60; PMID:26050667; http://dx.doi.org/ 10.2337/db15-0146 [DOI] [PubMed] [Google Scholar]
- 7.Sharma PM, Egawa K, Gustafson TA, Martin JL, Olefsky JM. Adenovirus-mediated overexpression of IRS-1 interacting domains abolishes insulin-stimulated mitogenesis without affecting glucose transport in 3T3-L1 adipocytes. Mol Cell Biol 1997; 17:7386-97; PMID:9372969; http://dx.doi.org/ 10.1128/MCB.17.12.7386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlotti F, Bazuine M, Kekarainen T, Seppen J, Pognonec P, Maassen JA, Hoeben RC. Lentiviral vectors efficiently transduce quiescent mature 3T3-L1 adipocytes. Mol Ther 2004; 9:209-17; PMID:14759805; http://dx.doi.org/ 10.1016/j.ymthe.2003.11.021 [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Heckmann BL, Liu J. Studying lipolysis in adipocytes by combining siRNA knockdown and adenovirus-mediated overexpression approaches. Methods Cell Biol 2013; 116:83-105; PMID:24099289; http://dx.doi.org/ 10.1016/B978-0-12-408051-5.00006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puri V, Chakladar A, Virbasius JV, Konda S, Powelka AM, Chouinard M, Hagan GN, Perugini R, Czech MP. RNAi-based gene silencing in primary mouse and human adipose tissues. J Lipid Res 2007; 48:465-71; PMID:17093294; http://dx.doi.org/ 10.1194/jlr.D600033-JLR200 [DOI] [PubMed] [Google Scholar]
- 11.Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci U S A 2003; 100:7569-74; PMID:12808134; http://dx.doi.org/ 10.1073/pnas.1332633100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajakumari S, Wu J, Ishibashi J, Lim HW, Giang AH, Won KJ, Reed RR, Seale P. EBF2 determines and maintains brown adipocyte identity. Cell Metabol 2013; 17:562-74; PMID:23499423; http://dx.doi.org/ 10.1016/j.cmet.2013.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kilroy G, Burk DH, Floyd ZE. High efficiency lipid-based siRNA transfection of adipocytes in suspension. PloS One 2009; 4:e6940; PMID:19759827; http://dx.doi.org/ 10.1371/journal.pone.0006940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kajimoto K, Takayanagi S, Sasaki S, Akita H, Harashima H. RNA interference-based silencing reveals the regulatory role of fatty acid-binding protein 4 in the production of IL-6 and vascular endothelial growth factor in 3T3-L1 adipocytes. Endocrinology 2012; 153:5629-36; PMID:23008513; http://dx.doi.org/ 10.1210/en.2012-1456 [DOI] [PubMed] [Google Scholar]
- 15.Lee MJ, Pickering RT, Puri V. Prolonged efficiency of siRNA-mediated gene silencing in primary cultures of human preadipocytes and adipocytes. Obesity (Silver Spring, Md) 2014; 22:1064-9; PMID:24307633; http://dx.doi.org/ 10.1002/oby.20641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O, Petersen R, Penicaud L, Kristiansen K, Bouloumie A, Casteilla L, et al.. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells (Dayton, Ohio) 2009; 27:2753-60; PMID:19697348; http://dx.doi.org/ 10.1002/stem.200 [DOI] [PubMed] [Google Scholar]
- 17.Karamanlidis G, Karamitri A, Docherty K, Hazlerigg DG, Lomax MA. C/EBPbeta reprograms white 3T3-L1 preadipocytes to a Brown adipocyte pattern of gene expression. J Biol Chem 2007; 282:24660-9; PMID:17584738; http://dx.doi.org/ 10.1074/jbc.M703101200 [DOI] [PubMed] [Google Scholar]
- 18.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metabol 2012; 15:395-404; PMID:22405074; http://dx.doi.org/ 10.1016/j.cmet.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Fromme T, Schweizer S, Schottl T, Klingenspor M. Taking control over intracellular fatty acid levels is essential for the analysis of thermogenic function in cultured primary brown and brite/beige adipocytes. EMBO Rep 2014; 15:1069-76; PMID:25135951; http://dx.doi.org/ 10.15252/embr.201438775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J Biol Chem 2000; 275:25073-81; PMID:10825155; http://dx.doi.org/ 10.1074/jbc.M000547200 [DOI] [PubMed] [Google Scholar]
- 21.Fasshauer M, Klein J, Ueki K, Kriauciunas KM, Benito M, White MF, Kahn CR. Essential role of insulin receptor substrate-2 in insulin stimulation of Glut4 translocation and glucose uptake in brown adipocytes. J Biol Chem 2000; 275:25494-501; PMID:10829031; http://dx.doi.org/ 10.1074/jbc.M004046200 [DOI] [PubMed] [Google Scholar]
- 22.Green H. Sublines of mouse 3T3 cells that accumulate lipid. Cell Mol Life Sci 1974; 1:113-6; PMID:NOT_FOUND [Google Scholar]
- 23.Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, Villageois A, Bagnis C, Breittmayer JP, Groux H, et al.. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med 2005; 201:1397-405; PMID:15867092; http://dx.doi.org/ 10.1084/jem.20042224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannon B, Nedergaard J. Cultures of adipose precursor cells from brown adipose tissue and of clonal brown-adipocyte-like cell lines. Methods Mol Biol 2001; 155:213-24; PMID:11293074 [DOI] [PubMed] [Google Scholar]
- 25.Hansen JB, Petersen RK, Larsen BM, Bartkova J, Alsner J, Kristiansen K. Activation of peroxisome proliferator-activated receptor gamma bypasses the function of the retinoblastoma protein in adipocyte differentiation. J Biol Chem 1999; 274:2386-93; PMID:9891007; http://dx.doi.org/ 10.1074/jbc.274.4.2386 [DOI] [PubMed] [Google Scholar]
- 26.Murholm M, Dixen K, Qvortrup K, Hansen LH, Amri EZ, Madsen L, Barbatelli G, Quistorff B, Hansen JB. Dynamic regulation of genes involved in mitochondrial DNA replication and transcription during mouse brown fat cell differentiation and recruitment. PloS One 2009; 4:e8458; PMID:20107496; http://dx.doi.org/ 10.1371/journal.pone.0008458 [DOI] [PMC free article] [PubMed] [Google Scholar]


