ABSTRACT
Chromosome ends are complex structures, which require a panel of factors for their elongation, replication, and protection. We describe here the mechanics of mammalian telomeres, dynamics and maintainance in relation to lamins. Multiple biochemical connections, including association of telomeres to the nuclear envelope and matrix, of telomeric proteins to lamins, and of lamin-associated proteins to chromosome ends, underline the interplay between lamins and telomeres. Paths toward senescence, such as defective telomere replication, altered heterochromatin organization, and impaired DNA repair, are common to lamins' and telomeres' dysfunction. The convergence of phenotypes can be interpreted through a model of dynamic, lamin-controlled functional platforms dedicated to the function of telomeres as fragile sites. The features of telomeropathies and laminopathies, and of animal models underline further overlapping aspects, including the alteration of stem cell compartments. We expect that future studies of basic biology and on aging will benefit from the analysis of this telomere-lamina interplay.
KEYWORDS: aging, lamin, nuclear lamina, Progeria, telomeres
Introduction
Telomeres and lamins are directly connected with 2 important and expanding areas of research, the biology of aging and the studies relating nuclear architecture to genome function. However, research areas on telomeres and lamins do hardly talk to each other and there is a clear need for more integrated pictures. In this perspective, it is relevant to compare and combine not only telomeres and lamins researches, but also analyses performed in different molecular, cellular or organismal systems. In an attempt to contribute to an integrated view, we report here data going from the basics of telomere mechanics to the description and points of convergence of lamins- and telomeres-related phenotypes, in cells, mouse models and in human diseases.
Telomeres as challenging structures
Mammalian telomeres are complex structures protecting chromosome ends, capped with a multiprotein complex, containing arrays of TTAGGG repeats which in humans can extend up to 10–20 kb. They end in a single G-rich overhang at 3′, bending into a t-loop, in which the telomeric double strand portion folds back allowing the G-rich single strand to invade the double-stranded region, creating a displacement loop (D-loop). To secure their correct function and homeostasis, telomeres have to face several challenges. A main one is the end replication problem, a consequence of the inability of conventional DNA polymerases to fully replicate the DNA lagging strand, generating a 5′ gap after removal of the 5′-most RNA primer. A second problem comes up when the semiconservative canonical DNA replication machinery has to confront with the complexity of telomeric structure. A third problem is the protection from the DNA damage sensing system that has to distinguish chromosome ends from DNA double-strand breaks.
Telomerase activity circumvents the end replication problem
The end replication problem is solved by telomerase, a catalytic complex including an RNA-dependent DNA polymerase (TERT) that adds telomeric DNA to chromosome ends by copying a template sequence within the RNA component of the enzyme (TERC).1,2 The telomerase holoenzyme works in association with 2 dyskerin–NHP2–NOP10–GAR1 complexes and with the protein TCAB1 (reviewed in 2). The complex works in the S phase of the cycle3 and is active only in specific cell types, including embryonic stem cells and, at low levels, adult stem cells.4 In cancer cells, to bypass cell crisis generated by excessive division cycles and checkpoint dysfunction, constitutive expression of telomerase represents a mechanism that robustly contributes to tumor cells' indefinite proliferation.5
In wild-type telomerase-positive cells, the average telomere length is the result of the dynamic process including loss due incomplete replication and telomerase-mediated elongation. This equilibrium is not only controlled by telomerase holoenzyme levels,6 but also by the associated proteins such as TCAB1.7,8
The activity of telomerase is also linked to the shelterin, a telomere-capping structure that includes 6 different proteins (Fig. 1a). Of these, the shelterins TRF1 and TRF2 homodimerize, and bind the TTAGGG double strand array of repeats. TRFs are interconnected with the other shelterin members POT1, RAP1, TIN2 and TPP1. POT1 binds the single stranded TTAGGG overhang stretch protruding at 3′ of telomeric DNA and/or to internal regions of single stranded TTAGGG repeats displaced from the complementary strand in telomere t-loop structures. TIN2 and TPP1 bridge POT1 to the TRF proteins.9,10 RAP1 directly interacts with TRF2. The basic model of functional interaction of telomerase with shelterins postulates a TRFs' control at TTAGGG repeats, which would regulate POT1 binding to the G-tail, and, in turn, POT1 would restrict telomerase access and telomere elongation. Recently, a more complex interplay has been suggested, including not only TRFs and POT1, but also TPP1, as a regulator of telomerase recruitment at telomeres, while the implication of TIN2 is still under investigation.2 In addition to these associations, telomerase interacts with another enzyme, tankyrase. Tankyrase, is an ankyrin-related protein whose activity is regulated by Polo-like-kinase-1, and works by releasing TRF1 from telomeres thereby allowing access to telomerase.11,12
Figure 1.
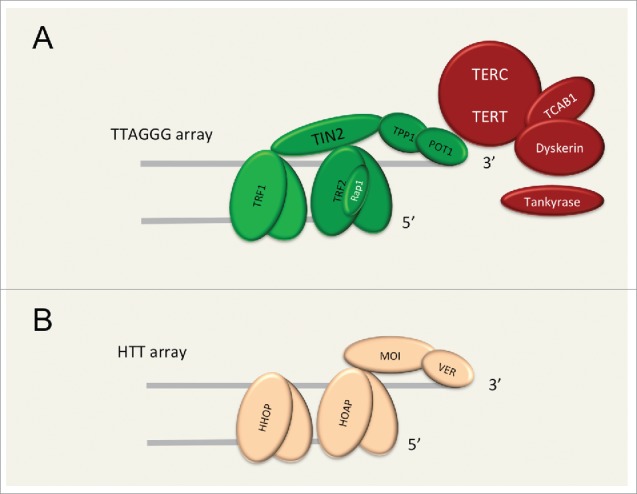
Human and Drosophila telomeres. Schematic representation of the principal capping and replicative proteins in mammals (a) and in flies (b). In flies, telomeres are not composed of TTAGGG repeats typically present in mammalian telomeres, but are characterized by an array of retrotransposons called HeT-A, TART, and TAHRE (collectively abbreviated as HTT). In mammals, the protecting capping complex contains the 6 shelterin components TRF1, TRF2, POT1, TPP1, TIN2 and RAP1. In flies, the capping complex is named terminin and includes HOAP, HHOAP, MOI and VER. In mammals, telomere elongation is accomplished by the telomerase complex, including the TERC and TERT subunits, and with the contribution of dyskerins, TCAB1 and of tankyrase. In flies, telomeres are elongated by retrotransposition. Mammalian and Drosophila telomeres are both functionally constrained into a complex heterochromatic and capped structure, which represents a challenge to the replication machinery.
Semiconservative telomere replication in somatic cells
Beyond the end replication problem, telomeres represent a challenge for the replication machinery due to the repetitive nature of the DNA sequence, the presence of the capping complex and their tridimensional organization including t-loop folding and G quadruplex pairing in the G-rich telomeric repeat strand. Indeed, telomeres have been related to fragile sites, which are specific chromosomal regions that challenge replication, especially under stress conditions such as in the presence of limiting nucleotide pools or of DNA polymerase inhibitors.13 For example, in the presence of low levels of aphidicolin, which induces breaks and gaps at common fragile sites in metaphase chromosomes, an increase in the frequency of telomere aberrations was observed as compared to control cells.13 Moreover, the shelterin TRF1 and active helicases (i.e. BLM and RTEL1) are required to remove the G quadruplex DNA structure at chromosome ends13-15 and to repress the fragile telomere phenotype. TRF1, BLM and RTEL1 therefore represent one block of a telomere-operating platform, to which other factors are expected to add. One natural further member of this operational platform is the proliferating cell nuclear antigen (PCNA), a canonical component of the replisome, which was proven to biochemically and functionally interact with RTEL1 contributing to replication fork dynamics and origin usage during telomere replication.15 Beyond shelterins, PCNA and helicases, telomere replication requires many other factors. Among these, the CTC1–STN1–TEN1 (CST) complex has been implicated in overhang control, and the TRF2-recruited 5′-3′ exonuclease Apollo has been implicated in C-strand resection, a step happening after leading-strand synthesis and preceding t-loop refolding.2
Most higher eukaryotes chromosome ends are organized as in mammals, however there are exceptions. An intriguing case is that of Drosophila and other dipterans. In flies telomeres do not contain the TTAGGG sequence but an array of specialized retrotransposons that contribute, in place of mammalian telomerase, to telomere homeostasis.16 Mammalian and Drosophila telomeres thus do not share telomerase activity and sequence properties, but are however both functionally constrained into a complex heterochromatic and capped (by terminins in flies,17 Fig. 1b) structure, which represents a challenge to the replication machinery. Therefore it was proposed that the study of mammalian orthologues of fly telomeric proteins can lead to the identification of proteins implicated in the complexity of telomere function in mammals. Accordingly we have recently identified a mammalian telomeric protein linked to telomere replication on the basis of its homology to the Drosophila counterpart. This protein, named AKTIP in humans, binds to telomeres, and interacts directly with TRF1 and TRF2. In addition AKTIP binds to the replication machinery component PCNA and is important for its association to replicative chromatin.18,19
Shelterins and DNA damage protection
The third challenge for chromosome ends is dealing with the DNA damage response system. End protection through shelterins has been proven to be instrumental to face this challenge.13,20-22 The shelterin TRF2 controls the ATM arm of the DNA damage response pathway, protecting telomeres from classical non homologous end joining (c-NHEJ) (22,23 and reviewed in 24). Mutant TRF2 induces telomere fusions, whose formation depends on DNA-protein kinases, on ligase IV and on the Ku70–Ku80 heterodimer. The ATR-controlled branch of the DNA damage response would also be activated by telomere ends if there wasn't the protection of the shelterin members TPP1, POT1 and TIN2 (25 and reviewed in 24). These proteins act by creating steric hindrance for binding of the replication protein A (RPA) to chromosome ends at the single-stranded overhang and at stalled replication forks. In addition to biochemical and genetic analyses, work on chromatin structure has shown that shelterin induces physical compaction of telomeric chromatin, which contributes to the protection of chromosome ends against the DNA damage response machinery.26
The analysis of the DNA damage response in relation to telomeres has revealed a further level of complexity. On the one side, as described above, DNA damage related factors are negatively controlled by shelterins, on the other hand the activity of specific DNA damage players is implicated in the maintenance of telomeres. For example, the activity of BRCA2 and Rad51 facilitates telomere replication and capping.27
One final aspect related to telomere function is the role played by the long noncoding RNA telomeric repeat–containing RNA (TERRA) (reviewed in 28). TERRA is transcribed from subtelomeric and telomeric repeat sequences and is involved in telomere maintenance in a telomerase-dependent and a telomerase-independent manner. TERRA activities include the control of telomeric chromatin structure, depending on the interaction with SUV39H1, which induces an increase in H3K9me3, an epigenetic mark of heterochromatin. Alterations of the epigenetic status of telomeres along with variations in TERRA lead to telomere aberrations and to the activation of the DNA damage response pathway.
Interactions between telomeres and lamins
Although the mechanics of telomere maintenance has been thoroughly explored, a lot has still to be determined about its intranuclear logistics and dynamics. In this respect, the potential involvement of intracellular substructures is giving new insights in telomere function.
Lamins as nuclear envelope and matrix components
The inner membrane of mammalian nucleus contains an organized web of proteins known as the lamina, assembled from intermediate filaments that extend throughout the nuclear matrix. The fundamental building blocks of the lamina are the A- and B-type lamins. In humans, there are 3 lamin genes: LMNA, LMNB1 and LMNB2. Lamins A and C are alternatively spliced isoforms of the LMNA gene. Lamins B1 and B2 are the products of the LMNB1 and LMNB2 genes. The tridimensional structure of single lamins includes a central rod of α-helical heptad repeats flanked by head and tail domains, the C-terminal tail domain contains a motif similar to an immunoglobulin fold (Ig-fold).29,30 Lamins A, B1, and B2 are initially synthesized as precursors, known as prelamins A, B1, and B2. The processing of these precursors into mature lamins involves a sequential series of post-translational modifications, including farnesylation.31 B-type lamins are expressed in most cell types and are critical for survival. Indeed, B-type lamin RNAi in HeLa cells leads to cell death; B1- and B2 lamin-deficient mice dye after birth; however, lamin B1 KO fibroblasts can grow in culture.32,33 Moreover, lamin B1 levels decline in senescent cells.34 Lamin A/C expression is restricted to differentiated cells and is not essential.29
The nuclear lamina is disassembled for mitosis with both A and B-type lamins becoming distributed throughout the cytoplasm and ER membrane, respectively. This process is regulated by the phosphorylation of lamins operated by kinases.35 During nuclear reformation, which requires phosphatase activity, A and B-type lamins follow different pathways of assembly. In the anaphase-telophase stage, lamin B1 is found in association with chromosomes and with the spindle, subsequently assembling into a polymer. Lamin A organization starts later in time after the pore complexes and the nuclear membrane have been formed and is then transported into the nucleoplasm and progressively incorporated into the lamina. In later stages both A- and B-type lamins form complex polymers throughout interphase nuclei.36,37
Lamins play an overall scaffolding role and have been implicated in diverse processes including the maintenance of proper nuclear architecture, DNA replication, DNA repair, and regulation of transcription.29,31,38 Mammalian chromatin interacts with the nuclear lamina through the lamina-associated domains (LADs) and this interactions contributes to stable gene repression.39 In embryonic stem cells lamin A regulates chromatin dynamics and epigenetic pathways that, in turn, determine their differentiation potential.40
Physical connections of telomeres with lamins
Telomeres in budding yeast Saccharomyces cerevisiae typically cluster and tether at the nuclear periphery.41,42 Although yeast do not express lamins, these findings initiated the search for a potential connection of mammalian telomeres with the nuclear peripheral structures and particularly with lamins. Actually, different organisms, from plants to mammals, display a typical organization termed ‘bouquet’, in first meiotic prophase, in which chromosome ends are identified at the nuclear periphery (reviewed in 43). Mammalian meiotic chromosomes contain the telomere-associated proteins TRF1, TRF2, RAP1 and tankyrase, and, in first meiotic prophase they locate to the nuclear envelope, outside of regions of nucelar pore complexes, eventually forming the bouquet, in close proximity to the centrosome.43 Lamin C2, the A-type lamin isoform expressed in mammalian meiotic cells, is required for telomere-positioning, and is linked to cytoplasmic structures through the members of the Linker of Nucleoskeleton and Cytoskeleton (LINC) complex SUN1 and SUN2.44 In meiotic cells, this subnuclear localization of telomeres is critical for chromosome pairing and homologous recombination.45
Connections between nuclear structures and telomeres in mammalian somatic cells are less well studied. More than 15 y ago the pioneering leading researcher in the field of mammalian telomeres, Titia de Lange, reported biochemical data based on nuclear fractionation showing that telomeres are attached to the nuclear matrix via their TTAGGG repeats.46 De Lange and colleagues reported in a later paper that an interaction occurs among telomeric DNA, TRFs and the nuclear matrix.47 In a more recent work, lamin B1 was reported to interact with the shelterin TRF1 in samples enriched for postmitotic cells, although telomers are not preferentially localized at the peripheral nuclear lamina in somatic cells.38 We observed that telomeric chromatin is immunoprecipitated by anti-lamin A/C antibodies in human primary fibroblasts and that TRF1 interacts with lamin B1 (I. Saggio, unpublished data).
In addition to these data concerning the telomeric complex sensu stricto, other connections link telomeres to lamins, at the periphery and through the nucleoplasm, including, for example, the properties of the 2 proteins LAP2α and AKTIP. LAP2alpha, which is a binding partner of lamins A/C, interacts with telomeres and lamin C in telophase, and with nucleoplasmic lamin A/C foci and with the lamina in interphase.48,49 AKTIP, which is functional to telomere replication, in interphase is typically enriched at the nuclear lamina.18
Beyond these data, other associations with the nuclear periphery and/or matrix have been described, such as that of the telomere function-related protein tankyrase, which, in mitosis, co-localizes with TRF1 at chromosomes ends and is found in association with centrosomes, and, in interphase nuclei, is detected at nuclear pore complexes.50 Another example is that of a TIN2 isoform (TIN2L) - that contains additional 97 amino acids as compared to canonical shelterin component TIN2-, and associates with the nuclear matrix.51
A link has also been pointed out between lamins and telomere-related DNA sequences, different from the canonical TTAGGGs at chromosome ends. Wood and collaborators describe a functional interconnection between TRF2, lamin A/C and interstitial telomeric sequences. The paper proposes that A-lamins and TRF2 are critical for the formation of chromosome loops between telomeres and interstitial telomeric sequences, which would work as an additional mechanism of telomere protection.52 Furthermore human subtelomeric sequences, notably the D4Z4 repeat, identified as a CTCF and A-type lamins-dependent insulator, have been shown to contribute telomere positioning at the nuclear periphery in somatic cells.53
Altogether, these data support a biochemical connection between telomere complexes (TTAGGGs, TRF1, TRF2), telomere associated proteins (tankyrase, TIN2L, AKTIP), lamins (A- and B-type) or lamin-associated proteins (AKTIP, LAP2α), and specific DNA sequences (subtelomeric regions and interstitial TTAGGGs) (Fig. 2).
Figure 2.
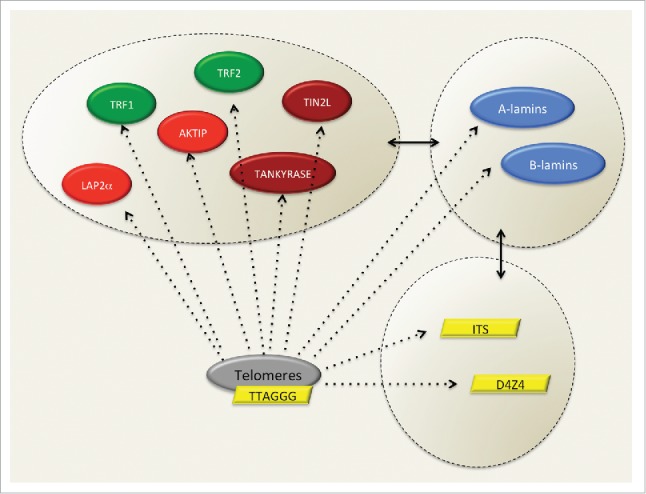
Factors biochemically and/or topologically linked with telomeres and lamins. Several factors contribute to an interplay between lamins and telomeres. These include lamins, shelterins, telomere- and lamin-associated proteins, along with specific DNA sequences. In green, shelterins, in red telomeres- and lamins-associated proteins, in brown telomeres-associated proteins, in blue lamins, in yellow DNA sequences associated with telomeres and lamins.
Functional connections of telomeres with lamins
In accordance with the biochemical data described above, lamins affect several properties of mammalian telomeres. One aspect influenced by lamins is the intranuclear topology of telomeres. In interphase cells intranuclear telomere diffusion could be either a Brownian motion or, if subject to constraining external forces, an anomalous subdiffusion. Interphase telomeres display slow anomalous diffusion, which lamin A depletion changes into a fast and normal diffusion, suggesting for crosslinking events occurring across the nuclear matrix between lamin A and telomeric chromatin controlling telomere dynamics.54 However, in early postmitotic cells, during nuclear assembly, telomere locate preferentially (> 40%) at the nuclear periphery.38 Crabbe and coworkers suggest that the attachment of chromosome ends to the nuclear envelope during nuclear assembly is functional to reorganizing the status of chromatin in the daughter cell.38
The second set of functional connections is related to the influence of lamins on telomere homeostasis and epigenetics. Loss of lamin A causes telomere shortening,55 while loss of the lamina associated telomeric protein AKTIP generates multiple telomeric signals (also called fragile telomeres) which are in principle related to telomere replication defects.18 In addition, lamins and LAP2α affect chromatin epigenetics, conditioning, for example, the level of the heterochromatic mark H3K27me3.49,55 Intriguingly, the fly homolog of AKTIP causes telomere aberrations preferentially involving the Y chromosome that, in Drosophila, is fully heterochromatic19
The third functional aspect of lamins in telomere regulation is their role at dysfunctional telomeres. Specifically, A-type lamins contribute to the NHEJ processing of dysfunctional telomeres by stabilizing 53BP1 protein levels.56 A recent work further established, by advanced time-lapse imaging applied to TRF2-mutant MEFs, the connection between NHEJ occurring at uncapped telomeres, and the proteins 53BP1 and SUN1/2, along with microtubules.57 These data, together with those on the SUN2-dependent nuclear tethering of telomeres reported by Crabbe and co-workers, highlight an interesting direction of genome studies investigates the role of the cytoskeleton for determining intranuclear processes. It is to note that SUN2 is implicated as well in meiotic telomeres attachment at the nuclear envelope as described above.44
Altogether these observations imply an interplay between lamins and telomeres in different cellular processes, including epigenetics, telomere dynamics, telomere homeostasis and DNA repair at dysfunctional telomeres, which, actually, in turn, are among them interconnected (Fig. 3).
Figure 4.
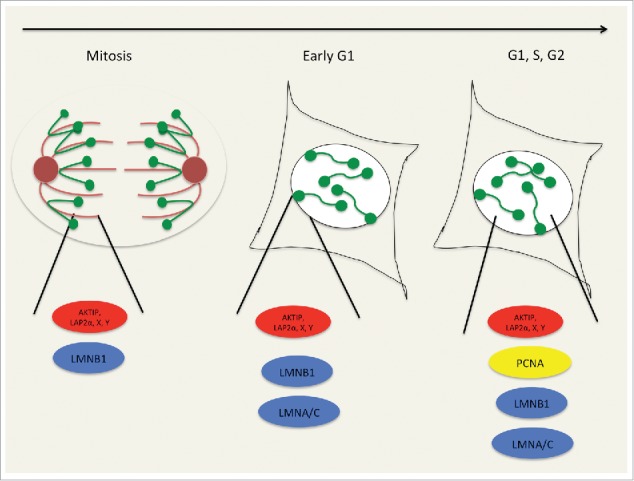
Hub model: a dynamic platform for telomere. Dynamics of the lamin-telomere interaction is described with a hypothetic model, according to which in mid and late mitosis the nuclear division organizational process would involve telomeres (green circles) and specific proteins (in red), which would be architecturally framed with B-lamins (in blue) at mitotic structures (i.e. at the spindle, at the spindle matrix or at centrosomes). Upon lamina reorganization, post-mitotically, complexes would be tethered at the nuclear periphery together with A- and B-lamins. Subsequently, these telomere-protein hubs would move into the matrix. In interphase, hubs' components dynamics and intranuclear subdiffusion would be functional to telomere and chromatin function, also though the recruitment of other factors, i.e., the replicative factor PCNA (in yellow).
Figure 3.
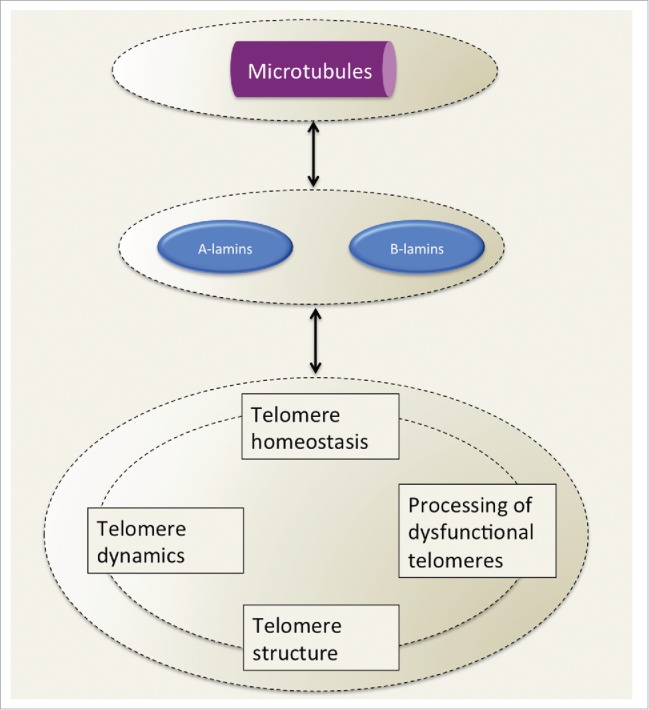
Telomere processes affected by lamins. Lamins, also in connection with microtubules through the LINC complex, regulate multiple aspects of telomere function, including intranuclear diffusion, replication, elongation, heterochromatic organization and processing by NHEJ upon dysfunction, processes that, in turn, are intertwined among them.
Disease phenotypes associated with lamins and telomeres
Defects in pathways controlled by lamins and telomeres have been linked to multifaceted phenotypes, but sharing overlapping aspects, and recalling physiological aging traits. The studies that have been functional to the establishment of these links have used different model systems including transgenic cells, patient-derived samples and organismal disease models.
Telomeres and lamins implication in cell senescence
In 1961, Hayflick and Moorhead observed that normal human fibroblasts did not grow indefinitely in culture.58 Indeed, after 60 to 80 population doublings somatic cells stop dividing and enter a state named cell senescence. These cells are morphologically flat, typically express a senescence-associated β-galactosidase (SAβgal),59 and they do not proceed into synthesising DNA. They are not quiescent or terminally differentiated, and growth arrest is not reversed physiologically.60 Senescence is induced by diverse stimuli, including persistent DNA damage and oxidative stress, but telomere erosion is a main and direct cause for senescence in somatic cells. Indeed, progressive telomere shortening induces a p53- and p16/Rb-dependent replicative senescence.5,60 By analyzing TRF2, Karlseder and coworkers have established that senescence is induced by critically short telomeres, which do not bind sufficient TRF2 to achieve a protective conformation.61 Moreover, a proof of the importance of telomeres in relation to cell senescence comes from the fact that the expression of TERT prevents senescence.61
Senescence is observed also in laminopathic cells including samples derived from patients with mutations in the LMNA gene.62,63 Along and/or upstream to senescence, LMNA mutations induce telomere aberrations, DNA repair impairment, altered heterochromatin organization and dysmorphic nuclei.55,64,65 As in the case of telomere-induced senescence, lamin-induced senescence is prevented by TERT.66 However it was also demonstrated that if TERT counteracts telomere loss and prevents the telomeric DNA damage upon lamin dysfunction, it is rather the expression of LAP2α than that of TERT, which is important for rescuing heterochromatin alterations.49 These results suggest that the crosstalk between lamins and telomeres and the consequences on senescence are intertwined but also multifaceted.
The link between organismal aging and cellular senescence is based on the presence of senescent cells in aging organisms. Indeed, in mouse liver, SAβgal-positive cells represent ∼8% and ∼17%, in young and old animals, respectively.67 Results in the same order of magnitude were obtained in other organs/tissues, including skin, lung, and spleen.67 Thus, although aging is a more complex matter than just senescence, and, in turn, senescence is more complex than just telomere and/or lamin dysfunction (reviewed in68), cell senescence establishes at least 1 pathway of a close connection of telomeres and lamins with aging.
Telomeropathies and laminopathies
Telomeropathies (also named impaired telomere syndromes or telomere maintenance spectrum disorders (reviewed in 69) further highlight the importance of telomere homeostasis in organismal physiology and in aging. Their clinical onset displays anticipation with each generation70 and manifests as alterations of different tissues and organs, principally being the marrow, the lung, and the liver, along with overall accelerated aging, with several degrees of penetrance and severity, which are influenced by species-specific responses and are sensitive to the pressure of diverse environmental contexts (reviewed in 69,71). The presence of defects in tissues with high turnover suggests that dysfunctional telomeres affect the homeostasis and proliferative capacity of the stem cell compartments. Indeed, for example, recent work has assessed that mesenchymal stem cells derived from the bone marrow stroma from patients affected by telomeropathies display reduced clonogenicity, altered differentiation properties, and increased senescence, along with failure to form hematopoiesis-competent ossicles upon in vivo transplantation into immunodeficient mice.72 A well-studied accelerated aging telomeric syndrome involves diskerin mutations, which cause a disease named Dyskeratosis Congenita.73 Dyskerin dysfunction destabilizes the telomerase complex inducing a clinical phenotype typically including dystrophic nails, leukoplakia, and hypopigmented skin, and characterized by early mortality due to bone marrow failure.71 Other forms of Dyskeratosis involve other telomeric genes including TERC, TERT, NOP10, NHP2, TIN2 or TCAB1.74 Biallelic mutations in RTEL1, one of the helicases required to remove the G quadruplex DNA structure at chromosome ends,15 are associated with the Diskeratosis Congenita severe variant Hoyeraal-Hreidarsson Syndrome.75
Altogether the complex properties of telomeropathies render the dissection of the pathophysiological mechanisms a challenging area of investigation and genetic models obtained by altering telomeric proteins in mice have contributed to their interpretation (reviewed in 76). A paradigmatic work in mice comes from the analysis of telomerase deficient animals that highlight the importance of telomerase activity for homeostasis of the reproductive and haematopoietic systems and for determining an aging phenotype.77,78 In these mice the phenotype is rescued by TERT reactivation.78 The connection of telomeres with aging in mice is further strengthened by data showing that physiological aging is counterbalanced in adult wild type mice by systemic AAV-mediated transduction of TERT.79 Further animal models of telomere dysfunction are those generated by shelterin impairment, characterized by rapid decline of the regenerative capacity of tissues and accelerated aging. TRF1-depleted mice, for example, display cellular senescence and premature tissue degeneration.80 Further mouse models have highlighted the implication of the interaction between the helicase RTEL1 and the replication machinery component PCNA in protecting from cell senescence, fragile telomeres and genome instability.15 Moreover, mice genetically engineered to have short telomeres display phenotypic traits of human Dyskeratosis Congenita and of physiological aging, including defects in bone marrow production and haematopoietic progenitor niche, and combined immunodeficiency.70
Telomeropathies and respective animal models share overlapping traits with human syndromes linked to lamins, grouped into the so-called laminopathies (reviewed in 81,82), a large family of diseases for which the full understanding of the pathophysiological mechanisms has yet to be fully understood. Among laminopathies, the best characterized example is the Hutchinson Gilford Progeria Syndrome (HGPS) linked to a mutation in exon 11 of the LMNA gene, which induces the expression of a cryptic splice site, in turn determining the production of the truncated form of lamin A named progerin. A major toxic feature of progerin is its permanent farnesylation as the farnesyl group is not removed as in the processing of wild type pre-lamin A.83,84 HGPS cells have shorter telomeres than age matched controls 85 and TERT prevents progerin-dependent cell proliferation defects.49,66,86 HGPS patients appear normal at birth, but develop severe multi-organ abnormalities within 2 years, including skeletal defects and absence of subcutaneous fat. Death occurs in the teenage years and is prevalently caused by heart attacks and strokes. TERT reduces progerin-induced DNA-damage signaling and rescues cell proliferation correlating the disease to telomere dysfunction.86 Sporadic endogenous cryptic splice site exposure and consequent progerin expression has been highlighted also in wild type aged human cells, suggesting a further link between lamins and aging.65 Progeroid clinical cases other than HGPS have also been described at the genetic level and have reinforced the idea that lamins play a pivotal role in determining the symptoms of premature aging. Indeed, for example, restrictive dermopathy patients have recessive mutations in ZMPSTE24, the gene encoding the proteolytic enzyme involved in lamin A maturation.87 In the same line, Cabanillas and coworkers have described a novel premature aging condition (the Nestor-Guillermo Progeria Syndrome), characterized by chronic onset, caused by mutations in BANF1, a gene whose product is implicated in nuclear assembly and interacts with lamin A.88 Many different animal models have been produced containing mutations in lamins or in lamin-related genes to study the etiopathology of laminopathies. Mice lacking ZMPSTE24, for example, recapitulate most progeroid traits and are considered a candidate model system of HGPS. They are characterized by skeletal defects and reduced subcutaneous adipose tissue.89,90 Partial recovery of the ZMPSTE24−/− phenotype is obtained by co-depletion of p53, suggesting a key role of DNA damage in the pathophysiology of these mice.90 More recently, it has been shown that the depletion of SUV39H191 also partially rescues the ZMPSTE24−/− phenotype pointing to an intertwining of lamin function with epigenetic control of senescence. As for telomeropathies, a road toward the interpretation of laminopathies is coming from experiments stem cells. It has been shown, for example, that progerin expression impairs the differentiation potential of mesenchymal stem cells, which could explain the defects in fat and bone tissues observed in HGPS patients.92 Epidermal stem cells have also been implicated in HGPS both in vitro and in animal models.93,94
Overall these data show that phenotypes of laminopathies and telomeropathies, along with those of organismal models, share links with aging, with the DNA replication (e.g. RTEL1) and damage pathways (e.g., 53BP1), with heterochromatin organization (e.g. SUV39H1), and with telomere homeostasis, specifically supported by notion that TERT rescues senescence. In addition to this, defects of stem cells compartments observed upon both lamin and telomere dysfunction further tie the roads for lamin- and telomere-dependent disease phenotype interpretations.
Conclusion
Numerous aspects at the biochemical, cellular and organismal level suggest points of convergence between lamins and telomeres (Table 1). Namely, telomeres interact directly and indirectly with lamins and with lamin interacting proteins, via strictly telomeric proteins and telomere-associated factors. Along with this, the topology and dynamics of telomeres is connected with the nuclear lamina and with the nuclear matrix. Moreover, the study of senescent cells, and of laminopathies and telomeropathies, along with that of organismal models highlights functional convergences. The most striking link is the ability of TERT to rescue senescence induced by both lamins and telomeres alterations in cellular and organismal systems. In addition to this, although less defined at the mechanistic level, the exhaustion of the stem cells compartments is observed upon both lamin and telomere dysfunction. Moreover, cellular and disease models and human diseases have highlighted a functional connection of phenotypes with defects in the DNA replication, DNA damage response, and heterochromatin organization, which can possibly be ascribed to common operational platforms in the nucleus, controlled by the nuclear lamina and matrix. We propose for these platforms a hypothetic model, according to which in mid and late mitosis the nuclear division organizational process would be architecturally linked with B-lamins, at mitotic structures (i.e., at the spindle, at the spindle matrix or at centrosomes), telomeres and specific proteins. Post-mitotically, telomeres and related factors would be first tethered at the nuclear periphery together with A- and B-lamins, which would contribute to global organization of the chromatin. Subsequently, these telomere-protein hubs would move from the nuclear envelope into the matrix. In interphase, dynamics of the components of these hubs and their intranuclear subdiffusion would function in telomere and chromatin function, securing the topological and biochemical status of platforms needed for telomere replication and repair. We would further speculate that the proposed lamins-related hub organization could be valid also for other complex chromatin structures. Upon telomere and/or lamin dysfunction the activity of these platforms would be impaired, which would affect highly proliferative organs and stem cell compartments, eventually causing organ dysfunction and accelerated aging.
Table 1.
Telomeres- and lamins-associated phenotypes. Phenotype traits of telomere-related animal models (5-6th generation TERC knock out, G5/G6-mTerc KO; skin-targeted TRF1 KO, K5-TRF1 KO), of telomere-related disease Dyskeratosis Congenital (DKC1 Xq28), of lamin-related animal models (L530P and LMNA knock in models, respectively LmnaL503P/L5, LmnaG609G/G609G, and Zmpste24 KO), and of lamin-related disease Hutchinson Gilford Progeria Syndrome (HGPS). HSC - haematopoietic stem cell; BMSC - bone marrow stem cell; VSMC - Vascular smooth muscle cell; MSC - mesenchymal stem cell; nd - not determined; nc - not changed.
| G5/G6-mTerc K.O. | K5-TRF1 K.O. | Dyskeratosis Congenita | LmnaL503P/L503P | LmnaG609G/G609G | Zmpste24 K.O. | HGPS | ||
|---|---|---|---|---|---|---|---|---|
| Organismal | Lifespan | 18 months 95 | 3 d 96 | 30 y 97 | 4-5 weeks 98 | 14 weeks 99 | 20 weeks 89,100 | 12.6 y101 |
| Body weight | Reduced 95 | Reduced 96 | Short stature 74 | Reduced 98 | Reduced 99 | Reduced 89,100 | Reduced102 | |
| Tissue/Organ | Skin | Hair follicles defects 95 | Hair follicles defects 96 | Skin defects72,74 | Hair follicles and skin defects 98 | Hair follicles defects 99 | Alopecia 89,100 | Alopecia, skin defects102 |
| Subcutaneous fat | Loss 95 | nd | nd | Loss 98 | Loss 99 | Loss 89,100 | Atrophy102 | |
| Skeletal system | Bone defects 103 | nd | Bone defects 74 | Bone defects 98,104 | Bone defects 99 | Bone defects 89,100 | Bone defects102 | |
| Reproductive system | Infertile 77 | nd | nd | nd | Infertile99 | nd | nd | |
| Haematopoietic system | HSC defects 77 | nd | nd | nd | nd | nd | nd | |
| Bone marrow | BMSC defects 70 | nd | Failure 74 MSC defects72 | nd | nd | nd | VSMC/MSC defects 105 | |
| Cellular/Molecular | Cellular senescence | Increased 106 | Increased 96 | Increased 107 | Increased 108 | Increased 99 | Increased 90 | Increased 109 |
| Nuclear aberrations | nd | nd | nd | Increased 98 | Increased 99 | Increased 100 | Increased 83 | |
| Chromatin changes | nd | nd | nd | nd | nd | Aggregates 100 | Detachment from periphery 110 | |
| Telomeric Aberrations | Fusions 106 | Increased 96 | Fusions 111 | nd | nd | nd | nd | |
| Telomeres Length | Shortening 106 | nc 96 | Shortening 112 | nc 98 | nd | nc 100 | Shortening 85 | |
| Genomic instability | Fusions 113 | Breaks 96 | nc 114 | nc 108 | nd | Breaks115 | nd | |
| DNA damage response | Active 67 | Active 96 | Active 116 | nc 108 | Active 99 | Active 115 | Active115 | |
| DNA repair | Defective NHEJ 117 | nd | nd | nd | nd | Defective HR 115 | Defective HR115 |
In conclusion, we expect that looking at this complex interplay in more detail will contribute to a better understanding and interpretation of data obtained in lamin and telomere studies.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Paolo Bianco for exceptionally interesting scientific discussion.
Funding
I.S. is receiving grants from the EU FP7 #286071 Brainvectors, and Telethon GEP15033. R.B is financed by AIRC IG 16020. M.L.T. is financed by Pasteur Cenci Bolognetti.
References
- [1].Vega LR, Mateyak MK, Zakian VA. Getting to the end: telomerase access in yeast and humans. Nat Rev Mol Cell Biol 2003; 4:948-59; PMID:14685173; http://dx.doi.org/ 10.1038/nrm1256 [DOI] [PubMed] [Google Scholar]
- [2].Hockemeyer D, Collins K. Control of telomerase action at human telomeres. Nat Struct Mol Biol 2015; 22:848-52; PMID:26581518; http://dx.doi.org/ 10.1038/nsmb.3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tomlinson RL, Ziegler TD, Supakorndej T, Terns RM, Terns MP. Cell cycle-regulated trafficking of human telomerase to telomeres. Mol Biol Cell 2006; 17:955-65; PMID:16339074; http://dx.doi.org/ 10.1091/mbc.E05-09-0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer 2007; 96:1020-4; PMID:17353922; http://dx.doi.org/ 10.1038/sj.bjc.6603671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Novo CL, Londono-Vallejo JA. Telomeres and the nucleus. Semin Cancer Biol 2013; 23:116-24; PMID:22330096; http://dx.doi.org/ 10.1016/j.semcancer.2012.02.001 [DOI] [PubMed] [Google Scholar]
- [6].Cristofari G, Lingner J. Telomere length homeostasis requires that telomerase levels are limiting. EMBO J 2006; 25:565-74; PMID:16424902; http://dx.doi.org/ 10.1038/sj.emboj.7600952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Venteicher AS, Abreu EB, Meng Z, McCann KE, Terns RM, Veenstra TD, Terns MP, Artandi SE. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science 2009; 323:644-8; PMID:19179534; http://dx.doi.org/ 10.1126/science.1165357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, Chen R, Alter BP, Artandi SE. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev 2011; 25:11-6; PMID:21205863; http://dx.doi.org/ 10.1101/gad.2006411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005; 19:2100-10; PMID:16166375; http://dx.doi.org/ 10.1101/gad.1346005 [DOI] [PubMed] [Google Scholar]
- [10].Palm W, de Lange T. How shelterin protects mammalian telomeres. Ann Rev Genet 2008; 42:301-34; PMID:18680434; http://dx.doi.org/ 10.1146/annurev.genet.41.110306.130350 [DOI] [PubMed] [Google Scholar]
- [11].Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 1998; 282:1484-7; PMID:9822378; http://dx.doi.org/ 10.1126/science.282.5393.1484 [DOI] [PubMed] [Google Scholar]
- [12].Ha GH, Kim HS, Go H, Lee H, Seimiya H, Chung DH, Lee CW. Tankyrase-1 function at telomeres and during mitosis is regulated by Polo-like kinase-1-mediated phosphorylation. Cell Death Differ 2012; 19:321-32; PMID:21818122; http://dx.doi.org/ 10.1038/cdd.2011.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, de Lange T. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 2009; 138:90-103; PMID:19596237; http://dx.doi.org/ 10.1016/j.cell.2009.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Drosopoulos WC, Kosiyatrakul ST, Schildkraut CL. BLM helicase facilitates telomere replication during leading strand synthesis of telomeres. J Cell Biol 2015; 210:191-208; PMID:26195664; http://dx.doi.org/ 10.1083/jcb.201410061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vannier JB, Sandhu S, Petalcorin MI, Wu X, Nabi Z, Ding H, Boulton SJ. RTEL1 is a replisome-associated helicase that promotes telomere and genome-wide replication. Science 2013; 342:239-42; PMID:24115439; http://dx.doi.org/ 10.1126/science.1241779 [DOI] [PubMed] [Google Scholar]
- [16].Mason JM, Frydrychova RC, Biessmann H. Drosophila telomeres: an exception providing new insights. Bioessays 2008; 30:25-37; PMID:18081009; http://dx.doi.org/ 10.1002/bies.20688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Raffa GD, Cenci G, Ciapponi L, Gatti M. Organization and evolution of drosophila terminin: Similarities and differences between drosophila and human telomeres. Front Oncol 2013; 3:112; PMID:23675571; http://dx.doi.org/ 10.3389/fonc.2013.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Burla R, Carcuro M, Raffa GD, Galati A, Raimondo D, Rizzo A, La Torre M, Micheli E, Ciapponi L, Cenci G, et al.. AKTIP/Ft1, a new shelterin-interacting factor required for telomere maintenance. PLoS genetics 2015; 11:e1005167; PMID:26110528; http://dx.doi.org/ 10.1371/journal.pgen.1005167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cenci G, Ciapponi L, Marzullo M, Raffa GD, Morciano P, Raimondo D, Burla R, Saggio I, Gatti M. The analysis of pendolino (peo) mutants reveals differences in the fusigenic potential among drosophila telomeres. PLoS genetics 2015; 11:e1005260; PMID:26110638; http://dx.doi.org/ 10.1371/journal.pgen.1005260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell 1998; 6:401-13; PMID:9476899; http://dx.doi.org/ 10.1016/S0092-8674(00)80932-0 [DOI] [PubMed] [Google Scholar]
- [21].Takai H, Smogorzewska A, de Lange T. DNA damage foci at dysfunctional telomeres. Curr Biol 2003; 13:6533-41; http://dx.doi.org/ 10.1016/S0960-9822(03)00542-6 [DOI] [PubMed] [Google Scholar]
- [22].Smogorzewska A, Karlseder J, Holtgreve-Grez H, Jauch A, de Lange T. DNA ligase IV-dependent NHEJ of deprotected mammalian telomeres in G1 and G2. Curr Biol 2002; 12:1635-44; PMID:12361565; http://dx.doi.org/ 10.1016/S0960-9822(02)01179-X [DOI] [PubMed] [Google Scholar]
- [23].Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 1999; 283:1321-5; PMID:10037601; http://dx.doi.org/ 10.1126/science.283.5406.1321 [DOI] [PubMed] [Google Scholar]
- [24].Arnoult N, Karlseder J. Complex interactions between the DNA-damage response and mammalian telomeres. Nat Struct Mol Biol 2015; 22:859-66; PMID:26581520; http://dx.doi.org/ 10.1038/nsmb.3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Denchi E, de Lange T. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 2007; 448:1068-71; PMID:17687332; http://dx.doi.org/ 10.1038/nature06065 [DOI] [PubMed] [Google Scholar]
- [26].Bandaria JN, Qin P, Berk V, Chu S, Yildiz A. Shelterin protects chromosome ends by compacting telomeric chromatin. Cell 2016; 164:735-46; PMID:26871633; http://dx.doi.org/ 10.1016/j.cell.2016.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Badie S, Escandell JM, Bouwman P, Carlos AR, Thanasoula M, Gallardo MM, Suram A, Jaco I, Benitez J, Herbig U, et al.. BRCA2 acts as a RAD51 loader to facilitate telomere replication and capping. Nat Struct Mol Biol 2010; 17:1461-9; PMID:21076401; http://dx.doi.org/ 10.1038/nsmb.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rippe K, Luke B. TERRA and the state of the telomere. Nat Struct Mol Biol 2015; 22:853-8; PMID:26581519; http://dx.doi.org/ 10.1038/nsmb.3078 [DOI] [PubMed] [Google Scholar]
- [29].Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker D, Solimando L, Goldman R. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 2008; 22:832-53; PMID:18381888; http://dx.doi.org/ 10.1101/gad.1652708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shumaker D, Solimando L, Sengupta K, Shimi T, Adam S, Grunwald A, Strelkov S, Aebi U, Cardoso M, Goldman R. The highly conserved nuclear lamin Ig-fold binds to PCNA: its role in DNA replication. J Cell Biol 2008; 181:269-80; PMID:18426975; http://dx.doi.org/ 10.1083/jcb.200708155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet 2012; 28:464-71; PMID:22795640; http://dx.doi.org/ 10.1016/j.tig.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vergnes L, Peterfy M, Bergo MO, Young SG, Reue K. Lamin B1 is required for mouse development and nuclear integrity. Proc Natl Acad Sci U S A 2004; 101:10428-33; PMID:15232008; http://dx.doi.org/ 10.1073/pnas.0401424101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang SH, Jung HJ, Coffinier C, Fong LG, Young SG. Are B-type lamins essential in all mammalian cells? Nucleus 2011; 2:562-9; PMID:22127257; http://dx.doi.org/ 10.4161/nucl.2.6.18085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shimi T, Butin-Israeli V, Adam SA, Hamanaka RB, Goldman AE, Lucas CA, Shumaker DK, Kosak ST, Chandel NS, Goldman RD. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev 2011; 25:2579-93; PMID:22155925; http://dx.doi.org/ 10.1101/gad.179515.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Eriksson JE, Dechat T, Grin B, Helfand B, Mendez M, Pallari HM, Goldman RD. Introducing intermediate filaments: from discovery to disease. J Clin Invest 2009; 119:1763-71; PMID:19587451; http://dx.doi.org/ 10.1172/JCI38339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Moir RD, Yoon M, Khuon S, Goldman RD. Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J Cell Biol 2000; 151:1155-68; PMID:11121432; http://dx.doi.org/ 10.1083/jcb.151.6.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schellhaus AK, De Magistris P, Antonin W. Nuclear Reformation at the End of Mitosis. J Mol Biol 2015; PMID:26423234 [DOI] [PubMed] [Google Scholar]
- [38].Crabbe L, Cesare AJ, Kasuboski JM, Fitzpatrick JA, Karlseder J. Human telomeres are tethered to the nuclear envelope during postmitotic nuclear assembly. Cell Rep 2012; 2:1521-9; PMID:23260663; http://dx.doi.org/ 10.1016/j.celrep.2012.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kind J, Pagie L, de Vries SS, Nahidiazar L, Dey SS, Bienko M, Zhan Y, Lajoie B, de Graaf CA, Amendola M, et al.. Genome-wide Maps of Nuclear Lamina Interactions in Single Human Cells. Cell 2015; 163:134-47; PMID:26365489; http://dx.doi.org/ 10.1016/j.cell.2015.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Melcer S, Hezroni H, Rand E, Nissim-Rafinia M, Skoultchi A, Stewart CL, Bustin M, Meshorer E. Histone modifications and lamin A regulate chromatin protein dynamics in early embryonic stem cell differentiation. Nat Commun 2012; 3:910; PMID:22713752; http://dx.doi.org/ 10.1038/ncomms1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gilson E, Laroche T, Gasser SM. Telomeres and the functional architecture of the nucleus. Trends Cell Biol 1993; 3:128-34; PMID:14731767; http://dx.doi.org/ 10.1016/0962-8924(93)90175-Z [DOI] [PubMed] [Google Scholar]
- [42].Taddei A, Hediger F, Neumann FR, Gasser SM. The function of nuclear architecture: a genetic approach. Annu Rev Genet 2004; 38:305-45; PMID:15568979; http://dx.doi.org/ 10.1146/annurev.genet.37.110801.142705 [DOI] [PubMed] [Google Scholar]
- [43].Scherthan H, Jerratsch M, Li B, Smith S, Hulten M, Lock T, de Lange T. Mammalian meiotic telomeres: protein composition and redistribution in relation to nuclear pores. Mol Biol Cell 2000; 11:4189-203; PMID:11102517; http://dx.doi.org/ 10.1091/mbc.11.12.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schmitt J, Benavente R, Hodzic D, Hoog C, Stewart CL, Alsheimer M. Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc Natl Acad Sci U S A 2007; 104:7426-31; PMID:17452644; http://dx.doi.org/ 10.1073/pnas.0609198104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Link J, Jahn D, Schmitt J, Gob E, Baar J, Ortega S, Benavente R, Alsheimer M. The meiotic nuclear lamina regulates chromosome dynamics and promotes efficient homologous recombination in the mouse. PLoS genetics 2013; 9:e1003261; PMID:23382700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].de Lange T. Human telomeres are attached to the nuclear matrix. EMBO J 1992; 11:717-24; PMID:1537344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ludérus M, van Steensel B, Chong L, Sibon O, Cremers F, de Lange T. Structure, subnuclear distribution, and nuclear matrix association of the mammalian telomeric complex. J Cell Biol 1996; 135:867-81; PMID:Can't; http://dx.doi.org/ 10.1083/jcb.135.4.867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dechat T, Gajewski A, Korbei B, Gerlich D, Daigle N, Haraguchi T, Furukawa K, Ellenberg J, Foisner R. LAP2alpha and BAF transiently localize to telomeres and specific regions on chromatin during nuclear assembly. J Cell Sci 2004; 117:6117-28; PMID:15546916; http://dx.doi.org/ 10.1242/jcs.01529 [DOI] [PubMed] [Google Scholar]
- [49].Chojnowski A, Ong PF, Wong ES, Lim JS, Mutalif RA, Navasankari R, Dutta B, Yang H, Liow YY, Sze SK, et al.. Progerin reduces LAP2alpha-telomere association in Hutchinson-Gilford progeria. eLife 2015; 4; PMID:26312502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Smith S, de Lange T. Cell cycle dependent localization of the telomeric PARP, tankyrase, to nuclear pore complexes and centrosomes. J Cell Sci 1999; 112 (Pt 21):3649-56; PMID:10523501 [DOI] [PubMed] [Google Scholar]
- [51].Kaminker PG, Kim SH, Desprez PY, Campisi J. A novel form of the telomere-associated protein TIN2 localizes to the nuclear matrix. Cell Cycle 2009; 8:931-9; PMID:19229133; http://dx.doi.org/ 10.4161/cc.8.6.7941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wood AM, Laster K, Rice EL, Kosak ST. A beginning of the end: new insights into the functional organization of telomeres. Nucleus 2015; 6:172-8; PMID:25961132; http://dx.doi.org/ 10.1080/19491034.2015.1048407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ottaviani A, Schluth-Bolard C, Rival-Gervier S, Boussouar A, Rondier D, Foerster AM, Morere J, Bauwens S, Gazzo S, Callet-Bauchu E, et al.. Identification of a perinuclear positioning element in human subtelomeres that requires A-type lamins and CTCF. EMBO J 2009; 28:2428-36; PMID:19644448; http://dx.doi.org/ 10.1038/emboj.2009.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bronshtein I, Kepten E, Kanter I, Berezin S, Lindner M, Redwood AB, Mai S, Gonzalo S, Foisner R, Shav-Tal Y, et al.. Loss of lamin A function increases chromatin dynamics in the nuclear interior. Nat Commun 2015; 6:8044; PMID:26299252; http://dx.doi.org/ 10.1038/ncomms9044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gonzalez-Suarez I, Redwood A, Gonzalo S. Loss of A-type lamins and genomic instability. Cell Cycle 2009; 8:3860-5; PMID:19901537; http://dx.doi.org/ 10.4161/cc.8.23.10092 [DOI] [PubMed] [Google Scholar]
- [56].Gonzalez-Suarez I, Redwood A, Perkins S, Vermolen B, Lichtensztejin D, Grotsky D, Morgado-Palacin L, Gapud E, Sleckman B, Sullivan T, et al.. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J 2009; 28:2414-27; PMID:19629036; http://dx.doi.org/ 10.1038/emboj.2009.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lottersberger F, Karssemeijer RA, Dimitrova N, de Lange T. 53BP1 and the LINC Complex Promote Microtubule-Dependent DSB Mobility and DNA Repair. Cell 2015; 163:880-93; PMID:26544937; http://dx.doi.org/ 10.1016/j.cell.2015.09.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science 1998; 279:349-52; PMID:9454332; http://dx.doi.org/ 10.1126/science.279.5349.349 [DOI] [PubMed] [Google Scholar]
- [59].Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, et al.. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995; 92:9363-7; PMID:7568133; http://dx.doi.org/ 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011; 192:547-56; PMID:21321098; http://dx.doi.org/ 10.1083/jcb.201009094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Karlseder J, Smogorzewska A, de Lange T. Senescence induced by altered telomere state, not telomere loss. Science 2002; 295:2446-9; PMID:11923537; http://dx.doi.org/ 10.1126/science.1069523 [DOI] [PubMed] [Google Scholar]
- [62].McClintock D, Gordon LB, Djabali K. Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc Natl Acad Sci U S A 2006; 103:2154-9; PMID:16461887; http://dx.doi.org/ 10.1073/pnas.0511133103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Taimen P, Pfleghaar K, Shimi T, Moller D, Ben-Harush K, Erdos MR, Adam SA, Herrmann H, Medalia O, Collins FS, et al.. A progeria mutation reveals functions for lamin A in nuclear assembly, architecture, and chromosome organization. Proc Natl Acad Sci U S A 2009; 106:20788-93; PMID:19926845; http://dx.doi.org/ 10.1073/pnas.0911895106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Das A, Grotsky DA, Neumann MA, Kreienkamp R, Gonzalez-Suarez I, Redwood AB, Kennedy BK, Stewart CL, Gonzalo S. Lamin A Deltaexon9 mutation leads to telomere and chromatin defects but not genomic instability. Nucleus 2013; 4:410-9; PMID:24153156; http://dx.doi.org/ 10.4161/nucl.26873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science 2006; 312:1059-63; PMID:16645051; http://dx.doi.org/ 10.1126/science.1127168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kudlow BA, Stanfel MN, Burtner CR, Johnston ED, Kennedy BK. Suppression of proliferative defects associated with processing-defective lamin A mutants by hTERT or inactivation of p53. Mol Biol Cell 2008; 19:5238-48; PMID:18843043; http://dx.doi.org/ 10.1091/mbc.E08-05-0492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging cell 2009; 8:311-23; PMID:19627270; http://dx.doi.org/ 10.1111/j.1474-9726.2009.00481.x [DOI] [PubMed] [Google Scholar]
- [68].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153:1194-217; PMID:23746838; http://dx.doi.org/ 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Holohan B, Wright WE, Shay JW. Cell biology of disease: Telomeropathies: an emerging spectrum disorder. J Cell Biol 2014; 205:289-99; PMID:24821837; http://dx.doi.org/ 10.1083/jcb.201401012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet 2009; 85:823-32; PMID:19944403; http://dx.doi.org/ 10.1016/j.ajhg.2009.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood 2014; 124:2775-83; PMID:25237198; http://dx.doi.org/ 10.1182/blood-2014-05-526285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Balakumaran A, Mishra PJ, Pawelczyk E, Yoshizawa S, Sworder BJ, Cherman N, Kuznetsov SA, Bianco P, Giri N, Savage SA, et al.. Bone marrow skeletal stem/progenitor cell defects in dyskeratosis congenita and telomere biology disorders. Blood 2015; 125:793-802; PMID:25499762; http://dx.doi.org/ 10.1182/blood-2014-06-566810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet 1998; 19:32-8; PMID:9590285; http://dx.doi.org/ 10.1038/ng0598-32 [DOI] [PubMed] [Google Scholar]
- [74].Dokal I. Dyskeratosis congenita. Hematol Am Soc Hematol Educ Program 2011; 2011:480-6; PMID:22160078; http://dx.doi.org/ 10.1182/asheducation-2011.1.480 [DOI] [PubMed] [Google Scholar]
- [75].Walne AJ, Vulliamy T, Kirwan M, Plagnol V, Dokal I. Constitutional mutations in RTEL1 cause severe dyskeratosis congenita. Am J Hum Genet 2013; 92:448-53; PMID:23453664; http://dx.doi.org/ 10.1016/j.ajhg.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Liao CY, Kennedy BK. Mouse models and aging: longevity and progeria. Curr Top Dev Biol 2014; 109:249-85; PMID:24947239; http://dx.doi.org/ 10.1016/B978-0-12-397920-9.00003-2 [DOI] [PubMed] [Google Scholar]
- [77].Lee HW, Blasco MA, Gottlieb GJ, Horner JW 2nd, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature 1998; 392:569-74; PMID:9560153; http://dx.doi.org/ 10.1038/33345 [DOI] [PubMed] [Google Scholar]
- [78].Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, et al.. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 2011; 469:102-6; PMID:21113150; http://dx.doi.org/ 10.1038/nature09603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med 2012; 4:691-704; PMID:22585399; http://dx.doi.org/ 10.1002/emmm.201200245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Martínez P, Thanasoula M, Muñoz P, Liao C, Tejera A, McNees C, Flores J, Fernández-Capetillo O, Tarsounas M, Blasco M. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev 2009; 23:2060-75; PMID:Can't; http://dx.doi.org/ 10.1101/gad.543509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Worman H, Fong L, Muchir A, Young S. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest 2009; 119:1825-36; PMID:19587457; http://dx.doi.org/ 10.1172/JCI37679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Camozzi D, Capanni C, Cenni V, Mattioli E, Columbaro M, Squarzoni S, Lattanzi G. Diverse lamin-dependent mechanisms interact to control chromatin dynamics. Focus on laminopathies. Nucleus 2014; 5:427-40; PMID:25482195; http://dx.doi.org/ 10.4161/nucl.36289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, Lyonnet S, Stewart CL, Munnich A, Le Merrer M, et al.. Lamin a truncation in Hutchinson-Gilford progeria. Science 2003; 300:2055; PMID:12702809; http://dx.doi.org/ 10.1126/science.1084125 [DOI] [PubMed] [Google Scholar]
- [84].Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, Berglund P, et al.. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 2003; 423:293-8; PMID:12714972; http://dx.doi.org/ 10.1038/nature01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Decker ML, Chavez E, Vulto I, Lansdorp PM. Telomere length in Hutchinson-Gilford progeria syndrome. Mech Ageing Dev 2009; 130:377-83; PMID:19428457; http://dx.doi.org/ 10.1016/j.mad.2009.03.001 [DOI] [PubMed] [Google Scholar]
- [86].Benson E, Lee S, Aaronson S. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J Cell Sci 2010; 123:2605-12; PMID:20605919; http://dx.doi.org/ 10.1242/jcs.067306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Barrowman J, Wiley PA, Hudon-Miller SE, Hrycyna CA, Michaelis S. Human ZMPSTE24 disease mutations: residual proteolytic activity correlates with disease severity. Hum Mol Genet 2012; 21:4084-93; PMID:22718200; http://dx.doi.org/ 10.1093/hmg/dds233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cabanillas R, Cadinanos J, Villameytide JA, Perez M, Longo J, Richard JM, Alvarez R, Duran NS, Illan R, Gonzalez DJ, et al.. Nestor-Guillermo progeria syndrome: a novel premature aging condition with early onset and chronic development caused by BANF1 mutations. Am J Med Genet A 2011; 155A:2617-25; PMID:21932319; http://dx.doi.org/ 10.1002/ajmg.a.34249 [DOI] [PubMed] [Google Scholar]
- [89].Bergo MO, Gavino B, Ross J, Schmidt WK, Hong C, Kendall LV, Mohr A, Meta M, Genant H, Jiang Y, et al.. Zmpste24 deficiency in mice causes spontaneous bone fractures, muscle weakness, and a prelamin A processing defect. Proc Natl Acad Sci U S A 2002; 99:13049-54; PMID:12235369; http://dx.doi.org/ 10.1073/pnas.192460799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, et al.. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature 2005; 437:564-8; PMID:16079796; http://dx.doi.org/ 10.1038/nature04019 [DOI] [PubMed] [Google Scholar]
- [91].Liu B, Wang Z, Zhang L, Ghosh S, Zheng H, Zhou Z. Depleting the methyltransferase Suv39h1 improves DNA repair and extends lifespan in a progeria mouse model. Nat Commun 2013; 4:1868; PMID:23695662; http://dx.doi.org/ 10.1038/ncomms2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Scaffidi P, Misteli T. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat Cell Biol 2008; 10:452-9; PMID:18311132; http://dx.doi.org/ 10.1038/ncb1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Espada J, Varela I, Flores I, Ugalde AP, Cadinanos J, Pendas AM, Stewart CL, Tryggvason K, Blasco MA, Freije JM, et al.. Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. J Cell Biol 2008; 181:27-35; PMID:18378773; http://dx.doi.org/ 10.1083/jcb.200801096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rosengardten Y, McKenna T, Grochova D, Eriksson M. Stem cell depletion in Hutchinson-Gilford progeria syndrome. Aging Cell 2011; 10:1011-20; PMID:21902803; http://dx.doi.org/ 10.1111/j.1474-9726.2011.00743.x [DOI] [PubMed] [Google Scholar]
- [95].Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 1999; 96:701-12; PMID:10089885; http://dx.doi.org/ 10.1016/S0092-8674(00)80580-2 [DOI] [PubMed] [Google Scholar]
- [96].Martinez P, Thanasoula M, Munoz P, Liao C, Tejera A, McNees C, Flores JM, Fernandez-Capetillo O, Tarsounas M, Blasco MA. Increased telomere fragility and fusions resulting from TRF1 deficiency lead to degenerative pathologies and increased cancer in mice. Genes Dev 2009; 23:2060-75; PMID:19679647; http://dx.doi.org/ 10.1101/gad.543509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Mason PJ, Bessler M. The genetics of dyskeratosis congenita. Cancer Genet 2011; 204:635-45; PMID:22285015; http://dx.doi.org/ 10.1016/j.cancergen.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Mounkes LC, Kozlov S, Hernandez L, Sullivan T, Stewart CL. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 2003; 423:298-301; PMID:12748643; http://dx.doi.org/ 10.1038/nature01631 [DOI] [PubMed] [Google Scholar]
- [99].Osorio FG, Navarro CL, Cadinanos J, Lopez-Mejia IC, Quiros PM, Bartoli C, Rivera J, Tazi J, Guzman G, Varela I, et al.. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med 2011; 3:106ra7; PMID:NOT_FOUND [DOI] [PubMed] [Google Scholar]
- [100].Pendas AM, Zhou Z, Cadinanos J, Freije JM, Wang J, Hultenby K, Astudillo A, Wernerson A, Rodriguez F, Tryggvason K, et al.. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat Genet 2002; 31:94-9; PMID:11923874 [DOI] [PubMed] [Google Scholar]
- [101].Hennekam RC. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am J Med Genet A 2006; 140:2603-24; PMID:16838330; http://dx.doi.org/ 10.1002/ajmg.a.31346 [DOI] [PubMed] [Google Scholar]
- [102].Pereira S, Bourgeois P, Navarro C, Esteves-Vieira V, Cau P, De Sandre-Giovannoli A, Levy N. HGPS and related premature aging disorders: from genomic identification to the first therapeutic approaches. Mech Ageing Dev 2008; 129:449-59; PMID:18513784; http://dx.doi.org/ 10.1016/j.mad.2008.04.003 [DOI] [PubMed] [Google Scholar]
- [103].Saeed H, Abdallah BM, Ditzel N, Catala-Lehnen P, Qiu W, Amling M, Kassem M. Telomerase-deficient mice exhibit bone loss owing to defects in osteoblasts and increased osteoclastogenesis by inflammatory microenvironment. J Bone Miner Res 2011; 26:1494-505; PMID:21308778; http://dx.doi.org/ 10.1002/jbmr.349 [DOI] [PubMed] [Google Scholar]
- [104].Chen CY, Chi YH, Mutalif RA, Starost MF, Myers TG, Anderson SA, Stewart CL, Jeang KT. Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies. Cell 2012; 149:565-77; PMID:22541428; http://dx.doi.org/ 10.1016/j.cell.2012.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Zhang J, Lian Q, Zhu G, Zhou F, Sui L, Tan C, Mutalif RA, Navasankari R, Zhang Y, Tse HF, et al.. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 2011; 8:31-45; PMID:21185252; http://dx.doi.org/ 10.1016/j.stem.2010.12.002 [DOI] [PubMed] [Google Scholar]
- [106].Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 1997; 91:25-34; PMID:9335332; http://dx.doi.org/ 10.1016/S0092-8674(01)80006-4 [DOI] [PubMed] [Google Scholar]
- [107].Agarwal S, Loh YH, McLoughlin EM, Huang J, Park IH, Miller JD, Huo H, Okuka M, Dos Reis RM, Loewer S, et al.. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature 2010; 464:292-6; PMID:20164838; http://dx.doi.org/ 10.1038/nature08792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hernandez L, Roux KJ, Wong ES, Mounkes LC, Mutalif R, Navasankari R, Rai B, Cool S, Jeong JW, Wang H, et al.. Functional coupling between the extracellular matrix and nuclear lamina by Wnt signaling in progeria. Dev Cell 2010; 19:413-25; PMID:20833363; http://dx.doi.org/ 10.1016/j.devcel.2010.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, Nabel EG, Collins FS. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest 2011; 121:2833-44; PMID:21670498; http://dx.doi.org/ 10.1172/JCI43578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Oberdoerffer P, Sinclair DA. The role of nuclear architecture in genomic instability and ageing. Nat Rev Mol Cell Biol 2007; 8:692-702; PMID:17700626; http://dx.doi.org/ 10.1038/nrm2238 [DOI] [PubMed] [Google Scholar]
- [111].Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, Dokal I. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature 2001; 413:432-5; PMID:11574891; http://dx.doi.org/ 10.1038/35096585 [DOI] [PubMed] [Google Scholar]
- [112].Vulliamy TJ, Knight SW, Mason PJ, Dokal I. Very short telomeres in the peripheral blood of patients with X-linked and autosomal dyskeratosis congenita. Blood Cells Mol Dis 2001; 27:353-7; PMID:11259155; http://dx.doi.org/ 10.1006/bcmd.2001.0389 [DOI] [PubMed] [Google Scholar]
- [113].Hande MP, Samper E, Lansdorp P, Blasco MA. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J Cell Biol 1999; 144:589-601; PMID:10037783; http://dx.doi.org/ 10.1083/jcb.144.4.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Burgdorf W, Kurvink K, Cervenka J. Sister chromatid exchange in dyskeratosis congenita lymphocytes. J Med Genet 1977; 14:256-7; PMID:926136; http://dx.doi.org/ 10.1136/jmg.14.4.256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Liu B, Wang J, Chan KM, Tjia WM, Deng W, Guan X, Huang JD, Li KM, Chau PY, Chen DJ, et al.. Genomic instability in laminopathy-based premature aging. Nat Med 2005; 11:780-5; PMID:15980864; http://dx.doi.org/ 10.1038/nm1266 [DOI] [PubMed] [Google Scholar]
- [116].Kirwan M, Beswick R, Walne AJ, Hossain U, Casimir C, Vulliamy T, Dokal I. Dyskeratosis congenita and the DNA damage response. Br J Haematol 2011; 153:634-43; PMID:21477209; http://dx.doi.org/ 10.1111/j.1365-2141.2011.08679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Espejel S, Klatt P, Menissier-de Murcia J, Martin-Caballero J, Flores JM, Taccioli G, de Murcia G, Blasco MA. Impact of telomerase ablation on organismal viability, aging, and tumorigenesis in mice lacking the DNA repair proteins PARP-1, Ku86, or DNA-PKcs. J Cell Biol 2004; 167:627-38; PMID:15545322; http://dx.doi.org/ 10.1083/jcb.200407178 [DOI] [PMC free article] [PubMed] [Google Scholar]


