Abstract
Symptoms guide disease management, and patients frequently report HIV-related symptoms, but HIV symptom patterns reported by patients have not been described in the era of improved antiretroviral treatment. The objectives of our study were to investigate the prevalence and burden of symptoms in people living with HIV and attending an outpatient clinic. The prevalence, burden, and bothersomeness of symptoms reported by patients in routine clinic visits during 2011 were assessed using the 20-item HIV Symptom Index. Principal component analysis was used to identify symptom clusters and relationships between groups using appropriate statistic techniques. Two main clusters were identified. The most prevalent and bothersome symptoms were muscle aches/joint pain, fatigue, and poor sleep. A third of patients had seven or more symptoms, including the most burdensome symptoms. Even with improved antiretroviral drug side-effect profiles, symptom prevalence and burden, independent of HIV viral load and CD4+ T cell count, are high.
Keywords: fatigue, HIV, pain, symptom burden, symptom prevalence, symptoms
HIV disease has always been associated with a high symptom burden, yet clusters of symptoms have not been defined in the current literature encompassing the contemporary era of improved combination antiretroviral therapy (cART). Available evidence has indicated poor agreement in provider and patient assessments of the symptom experience, and patient reports of symptoms are often overlooked or under-recognized by health care providers (Justice et al., 2001; Justice, Rabeneck, Hays, Wu, & Bozzette, 1999). Patient-reported symptoms may drive treatment interruption or discontinuation, leading to poor health outcomes and decreased quality of life and/or function (Deeks, 2011; Erlandson et al., 2013; Kempf et al., 2009). Therefore, acknowledging symptoms and incorporating symptom management into clinical visits is an important strategy to improve patient–provider relations and health outcomes.
Many factors are associated with the development of symptoms in the context of HIV disease, including comorbidities, treatment side effects, and inflammatory processes (Dodd et al., 2001; Lenz, Pugh, Milligan, Gift, & Suppe, 1997). Within the symptom experience, symptoms interact with each other by moderating and mediating one another (Lenz et al., 1997). For example, pain symptoms may lead to sleep dysfunction and fatigue, which in turn may increase the experience of pain. Depressive symptoms are also associated with increased experience of pain (Merlin et al., 2012), and the combined effect of sleep and unresolved pain may further influence depression or sadness. This dynamic relationship between symptoms makes research targeting the symptom experience challenging (Cheung, Le, & Zimmermann, 2009; Lenz et al., 1997). Situational factors arising from the social and physical environment can also influence the development, experience, and interpretation of symptoms (Dodd et al., 2001; Lenz et al., 1997).
Other disease models use symptom patterns to guide management and diagnosis. For instance, polyphagia, polydipsia, polyuria, and weight loss represent a symptom cluster associated with hyperglycemia and a diagnosis of diabetes (American Diabetes Association, 2014). The symptom cluster of bloating, abdominal pain, chronic diarrhea, and/or constipation may indicate exacerbation of irritable bowel syndrome or inflammatory bowel disease (Tontini, Vecchi, Pastorelli, Neurath, & Neumann, 2015). The objectives of our study were to investigate the prevalence and burden of self-reported symptoms in people living with HIV disease (PLWH) attending routine care at an outpatient clinic in the southern United States. This knowledge could help clinicians understand symptom patterns and inform them of underlying disease processes as seen in other diseases such as diabetes or inflammatory bowel disease.
Methods
Study Design and Subjects
The University of Alabama at Birmingham (UAB) 1917 Clinic is a Ryan White-funded ambulatory HIV clinic providing comprehensive medical care and social services including primary and specialty HIV care, mental health, and dental care, serving 3,000 adults living with HIV. Patients are able to access the site’s Liver Clinic for hepatitis treatment and clinical trials. The UAB 1917 Clinic is the largest HIV health care facility in the state of Alabama. Providers are infectious disease board-certified physicians and HIV specialty nurse practitioners. Practitioners provide specialty care clinics in dermatology, endocrinology, neurology, palliative care, psychiatry, and women’s health. Prior to scheduled clinic appointments, all patients privately report symptoms at an electronic kiosk using the HIV Symptom Index, a 20-item survey routinely used for clinical care and research with PLWH to capture prevalence and magnitude of HIV-related symptoms. The index was developed to identify and describe symptoms for the purpose of developing targeted interventions. It is a useful tool to consider patterns of symptoms and the impact on patient quality of life (Justice et al., 2001). Patients identify symptoms experienced and then rate each reported symptom as to the level of bothersomeness on a 5-point Likert-type scale ranging from symptom not present (0) to bothers me a lot (4). The HIV Symptom Index has demonstrated construct validity with high test-retest reliability (intra-class correlation coefficient = 0.92), and internal consistency (α = 0.79; Justice et al., 2001; Whalen, Antani, Carey, & Landefeld, 1994).
Data collected from patients using the HIV Symptom Index are automatically entered into the Center for AIDS Research Network of Integrated Clinical Systems database as part of an ongoing longitudinal study following HIV disease outcomes (Kitahata et al., 2008). We chose a 12-month timeframe to conduct a retrospective analysis of patients seen in 2011 to capture all seasons and investigate the patterns of symptoms reported by patients with HIV disease. The Center for AIDS Research Network of Integrated Clinical Systems database includes patient demographic and clinical information, and was used to identify all eligible subjects between 19 and 79 years of age seen in the 1917 Clinic for routine office visits between January 1 and December 31, 2011. For the purpose of this article, patients were considered to have a diagnosis of HIV regardless of being symptomatic or asymptomatic. The UAB Institutional Review Board approved the study.
Data Analysis
Descriptive statistics were used to summarize demographics, symptoms, and disease characteristics. For the purpose of calculating symptom prevalence, we selected the first visit of 2011 for each patient. A symptom was present and counted if the score was greater than 0 (I do not have the symptom) and reported as 1 to 4 on the HIV Symptom Index, with 1 = I have the symptom, does not bother me, 2 = bothers me a little, 3 = bothers me some, 4 = bothers me a lot (Justice et al., 2001). Symptom distress was defined by a score of 2-4 on the HIV Symptom Index. Symptom burden was defined as the count of symptoms reported.
To examine if there were differences in symptom prevalence in earlier versus later linkage to HIV infection care, we reported symptoms as bothersome for patients diagnosed less than 12 months (n = 147) and patients diagnosed 12 months or more (n = 1,738). Only the first clinic visit of the year per patient was used for this comparison using chi-squared analysis. Note that the recorded date of diagnosis was not representative of the date of infection, nor did it allow for calculating the true time since infection, but rather provided an estimate. Kendall’s tau was used to estimate and test the association between HIV-1 viral load, CD4+ T cell count, and symptom burden count.
A principal component analysis with oblimin rotation (Abdi & Williams, 2010) was conducted on within-subject mean item scores of all 20 items on the HIV Symptom Index across visits and stratified by HIV-1 viremia to determine clusters of symptoms that co-varied or clustered together independent of other subsets of symptoms at varying levels of viremia.
Missing data were defined as data that were not entered by the patient, where the patient left the question blank, and the data were deleted list-wise for analysis. Only 804 patients responded to all 20 symptoms on the HIV Symptom Index for the first visit of the year. Statistical analyses were performed using IBM SPSS Version 22 (IBM Corporation, Armonk, NY).
Results
Symptom Description
In total, 5,738 clinic visits were documented for the 1,945 patients seen for routine HIV care, with 1,885 patients reporting at least one symptom during the year. The mean clinic visit count during the 12-month study period was three encounters per patient. Mean age of study subjects was 44 years, with 96% of patients on HIV antiretroviral therapy, 76% of patients having a viral load of <500 copies/mL, and 71% having a CD4+ T cell count of >500 cells/mm3 (Table 1). Almost a third of patients (31%) had a high symptom burden, reporting seven or more symptoms with 8% of all patients having 10 or more symptoms, including those ranking as most bothersome. There was no statistically significant correlation between symptom burden and viral load or CD4+ T cell count. Two main clusters were identified, encompassing both physical and psychological symptoms in the clinic population studied. No statistically significant difference between presence of symptoms based on HIV-1 RNA viremia >500 copies/mL and viral load suppression (<500 copies/mL of HIV-1 RNA) was found. The most prevalent symptoms reported were poor sleep, muscle aches/joint pain, fatigue, nervous/anxious, and sadness (Table 2; Figure 1). Twelve of the 20 symptoms assessed were reported as bothersome in more than 30% of the sample (Table 2). Of all symptoms reported, the symptoms rated as most bothersome were muscle aches/joint pain, fatigue, bloating/abdominal pain, numbness/pain in feet, poor sleep, nervous/anxious, and sex problems. Of symptoms reported as bothersome, those bothering patients a lot (scoring 4 on the scale) were muscle aches/joint pain, numbness/pain in feet, sex problems, poor sleep, fatigue, and bloating/abdominal pain. Muscle aches/joint pain, fatigue, and poor sleep were present in the top five most prevalent and most bothersome symptoms reported (Table 2; Figure 1).
Table 1.
Demographic and Clinical Characteristics of Patients with HIV Included in the Study (N = 1,945)
| Age (years) | |
| Mean (range) | 44 (19–78) |
| Gender | |
| Male | 1,511 (78%) |
| Female | 434 (22%) |
| Race/ethnicity | |
| African American | 1,024 (53%) |
| Caucasian | 883 (45%) |
| Hispanic | 22 (1%) |
| Other | 16 (1%) |
| Plasma HIV RNA (copies/mL) | |
| <50–200 | 1,403 (73%) |
| 201–499 | 64 (3%) |
| 500–1,000 | 40 (2%) |
| 1,001–10,000 | 125 (7%) |
| ≥10,001 | 294 (15%) |
| Transmission risk factors | |
| Heterosexual | 694 (26%) |
| MSM | 1,029 (53%) |
| IVDU | 162 (8%) |
| Other | 60 (3%) |
| Insurance | |
| Private | 609 (31%) |
| Public | 717 (37%) |
| Uninsured | 619 (32%) |
| CD4 count in cells/mm3 | |
| <50 | 62 (4%) |
| 51–200 | 142 (9%) |
| 201–350 | 255 (16%) |
| 351–500 | 317 (20%) |
| >500 | 828 (51%) |
| Other characteristics | |
| Currently on cART | 1,873 (96%) |
| Depression diagnosis | 276 (11%) |
| Anxiety diagnosis | 249 (10%) |
| Current smokers | 889 (35%) |
| Hepatitis C co-infection | 221 (11%) |
Note. IVDU = intravenous drug use, MSM = men who have sex with men, cART = combination antiretroviral therapy.
Table 2.
Symptoms Ranked by Prevalence and Distress for Symptoms Identified in Main Symptom Clusters
| Most Prevalent Symptoms |
Total N Reporting on HIV Symptom Index* |
A. Prevalence of Persons Reporting Symptom as Present on HIV Symptom Index |
B. Reporting Symptom Bothersome on HIV Symptom Index |
C. Reporting Symptom as Bothers A Lota from those reporting symptom as Bothersome |
|||
|---|---|---|---|---|---|---|---|
| N | n | % | n | % | n | % | |
| Poor sleep | 1,784 | 908 | 51 | 747 | 82 | 264 | 35 |
| Muscle aches/Joint pain | 1,815 | 865 | 48 | 732 | 85 | 310 | 42 |
| Fatigue | 1,774 | 834 | 47 | 676 | 84 | 208 | 31 |
| Nervous/anxious | 1,782 | 763 | 43 | 618 | 81 | 198 | 32 |
| Sadness | 1,784 | 714 | 40 | 547 | 77 | 135 | 25 |
| Numbness/pain in feet | 1,808 | 658 | 36 | 540 | 82 | 214 | 40 |
| Headache | 1,796 | 645 | 36 | 485 | 75 | 134 | 28 |
| Memory loss | 1,797 | 626 | 35 | 456 | 73 | 93 | 20 |
| Sex problems | 1,797 | 503 | 28 | 397 | 80 | 152 | 38 |
| Cough/shortness of breath | 1,792 | 508 | 28 | 374 | 74 | 102 | 27 |
| Fever/chills/sweats | 1,797 | 489 | 27 | 387 | 79 | 93 | 24 |
| Dizzy | 1,760 | 444 | 25 | 336 | 76 | 63 | 19 |
| Bloating/abdominal pain | 1,785 | 442 | 25 | 366 | 83 | 105 | 29 |
| Poor appetite | 1,785 | 428 | 24 | 316 | 74 | 97 | 31 |
| Diarrhea | 1,784 | 423 | 24 | 300 | 71 | 76 | 25 |
| Nausea/vomiting | 1,792 | 376 | 21 | 270 | 72 | 62 | 28 |
Note. N size is equal to those reporting the symptom on the HIV Symptom Index. Total N differed by symptom due to missing data for particular symptom. Prevalence is the number (n) of people reporting the symptom as 1-4: 0 = I do not have the symptom; 1 = have, but does not bother; 2 = bothers a little; 3 = bothers some; 4 = bothers a lot. Column A reports the % of persons reporting having the symptom. Column B reports the % bothersome for the proportion of people reporting the symptom between 2 and 4; this excludes persons reporting that they do not have the symptoms and those that reported the symptom did not bother them. Column C reports the number of persons who reported the symptom as bothers a lot of those reporting symptom as bothersome in Column B.
Figure 1.
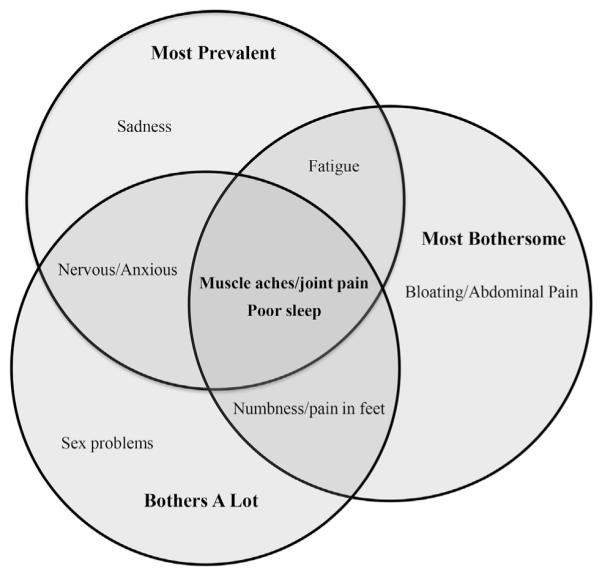
Venn diagram of the top 5 ranked symptoms with prevalence, most bothersome, and bothers a lot reported on the HIV Symptom Index.
We examined differences in symptoms based on race and gender using chi-squared analysis. No statistically significant differences in reported symptoms based on gender were found. However, for race, specifically among African American, White, and Hispanic groups, there were differences in fatigue, sadness, poor sleep, nervous/anxious, and memory loss, with White patients reporting a higher prevalence for these symptoms than non-White groups (p < .01). Hispanics reported higher prevalence of sex problems than Whites and African Americans (p < .01). Whites and Hispanics reported higher muscle aches/joint pain and diarrhea than African Americans (p < .01). African Americans and Hispanics reported a higher prevalence of headaches than Whites (p = .03). There were no statistically significant differences in bloating/abdominal pain, numbness/pain in feet, cough/shortness of breath, poor appetite, nausea/vomiting, fever/chills/sweats, or feeling dizzy.
When examining differences in whether persons reported symptoms as bothersome, Whites reported that symptoms of fatigue, memory, sadness, anxiety, poor sleep, and sex (p < .01), muscle aches/joint pain, and nausea (p = .02) bothered them more than the rates of bothersomeness reported by African Americans for these symptoms. Diarrhea (p < .01) and hair loss (p = .03) bothered Whites, but African Americans were more likely to report these symptoms as bothers a lot than Whites. African Americans reported rash as being more bothersome than Whites (p < .01).
Symptom Clusters
Across all patient routine clinic visits, four symptom clusters were identified. The two main clusters identified attributed 42% and 7% of total variability in the 20 symptoms reported, having Eigen values greater than 1.0 (Table 3). Eleven of the 20 symptoms loaded on Factor 1 and explained 41% of the total variation in symptoms reported. The symptom cluster represented in Factor 2 accounted for 7% of the total variation. The remaining two clusters accounted for 5% of the variability each and had Eigen values of 1.0. The loadings (correlations between symptom and factor) of symptoms on each factor or cluster are displayed in Table 3, and Figure 2 displays a Venn diagram illustrating the breakdown of symptoms of the major clusters, including their overlap. In this sample, the 20-item index had an internal consistency of α = .92.
Table 3.
Principal Components Analysis Determining Patterns of Symptoms Reported
|
N = 1,885 | ||||
|---|---|---|---|---|
| HIV Symptom Index (N = 20) |
Factor 1 | Factor 2 | Factor 3 | Factor 4 |
| Fatigue | .45 | .46 | −.02 | −.14 |
| Fever/chills/sweats | .76 | −.02 | .05 | .03 |
| Dizziness | .61 | .22 | .01 | −.06 |
| Numbness/pain in feet | .34 | .26 | .18 | −.29 |
| Memory loss | .21 | .58 | .05 | −.07 |
| Nausea/vomiting | .73 | .04 | −.04 | .17 |
| Diarrhea | .56 | .13 | −.03 | .15 |
| Sadness | .18 | .77 | −.11 | .05 |
| Nervous/anxious | .22 | .75 | −.11 | .04 |
| Poor sleep | .25 | .63 | −.05 | .02 |
| Rash | .18 | −.16 | .66 | .06 |
| Cough/shortness of breath | .64 | −.11 | .19 | −.03 |
| Headache | .74 | .04 | −.01 | −.04 |
| Loss of appetite | .37 | .27 | .18 | .55 |
| Bloating/abdominal pain | .54 | .10 | .22 | −.22 |
| Muscle aches/joint pain | .36 | .37 | .15 | −.20 |
| Sex problems | −.25 | .72 | .20 | .01 |
| Fat deposit/weight gain | .05 | .30 | .22 | −.60 |
| Weight loss/wasting | .07 | .30 | .40 | .62 |
| Hair loss changes | −.05 | .10 | .73 | −.04 |
| Eigen values | 8.14 | 1.41 | 1.04 | 1.02 |
| Proportion | 0.41 | .07 | .05 | .05 |
Note. Factor 1 (Symptom Cluster 1) contributed to 41% of the variance in symptoms while Factor 2 (Symptom Cluster 2) contributed 7%. Factors 1 and 2 had a strong inter-factor correlation of .53 (p value < .001). Factors 3 and 4 were excluded from further analysis due to low variability.
Figure 2.

Venn diagram of the two main symptom clusters.
Symptom cluster 1
Of the symptoms reported in cluster 1, the most prevalent symptoms occurring in at least a third of the sample population were muscle aches/joint pain (48%), fatigue (47%), numbness/pain in feet (36%), and headache (36%). Cough/shortness of breath (28%), fever/chills/sweats (28%), and bloating/abdominal pain (25%) were prevalent in at least a quarter of those reporting symptoms. Muscle aches/joint pain (85%), fatigue (84%), bloating/abdominal pain (83%), and numbness/pain in feet (82%) were reported as the most bothersome, with at least 40% of those reporting muscle aches/joint pain (42%) and numbness/pain in feet (40%) being reported as bothers a lot on the HIV Symptom Index.
Symptom cluster 2
Of the symptoms reported in cluster 2, the most prevalent, occurring in at least a third of the sample population, were poor sleep (51%), muscle aches/joint pain (48%), fatigue (47%), nervous/anxious (43%), sadness (40%), and memory loss (35%). Sex problems occurred in 28% of patients reporting symptoms. Furthermore, of those reporting symptoms, muscle aches/joint pain (85%), fatigue (84%), poor sleep (82%), and nervous/anxious (81%) were reported as the most bothersome. Muscle aches/joint pain (42%) and numbness/pain in feet (40%) were reported as bothers a lot in at least 40% of those reporting symptoms on the HIV Symptom Index. Nearly a third reported poor sleep (35%) and sex problems (38%) as bothers a lot.
Associations Between Symptom Clusters
There was a strong correlation between Symptom Cluster 1 and Symptom Cluster 2 (r = .53, p < .01). Fatigue and muscle pain/joint aches loaded on both factors and were reported among the most prevalent and most bothersome symptoms. The high prevalence of fatigue and muscle pain/joint aches persisted in subjects with both clusters with HIV-1 viremia (≥500 copies/mL) or aviremia (<500 copies/mL). There were no statistical differences in clusters based on gender or race.
In symptom clusters in patients with HIV-1 viral loads >500 copies/mL, symptom clusters did not have any overlap. The first cluster accounted for 42% of the variability in reported symptoms, with rash added to the symptoms listed in comparison to the full population model. The second cluster loaded symptoms with an inverse correlation between weight variables and appetite, and accounted for 8% of the variability of symptoms reported (Table 4).
Table 4.
Principal Components Analysis Determining Patterns of Symptoms Reported Stratified by HIV-1 Viremia <500 copies/mL and ≥500 copies/mL
|
N = 1,884 | ||||
|---|---|---|---|---|
| HIV Symptom Index (N = 20) |
Aviremia <500 Copies/mL (n = 1,437) |
Viremia ≥500 Copies/mL (n = 447) |
||
| Factor 1 | Factor 2 | Factor 1 | Factor 2 | |
| Fatigue | .44 | −.15 | .28 | .18 |
| Fever/chills/sweats | .75 | .02 | .73 | .04 |
| Dizziness | .61 | −.08 | .58 | .09 |
| Numbness/pain in feet | .26 | −.30 | .30 | .30 |
| Memory loss | .22 | −.08 | .32 | .21 |
| Nausea/vomiting | .76 | .14 | .71 | −.19 |
| Diarrhea | .61 | .13 | .54 | −.12 |
| Sadness | .21 | .05 | −.06 | −.05 |
| Nervous/anxious | .25 | .03 | −.01 | −.15 |
| Poor sleep | .29 | .04 | −.01 | .03 |
| Rash | .14 | .06 | .52 | .17 |
| Cough/shortness of breath | .59 | −.01 | .64 | .16 |
| Headache | .77 | −.06 | .43 | .00 |
| Loss of appetite | .41 | .56 | .65 | −.39 |
| Bloating/abdominal pain | .54 | −.25 | .49 | .28 |
| Muscle aches/joint pain | .28 | −.19 | .26 | .27 |
| Sex problems | −.27 | .02 | .03 | .20 |
| Fat deposit/weight gain | .04 | −.59 | −.08 | .75 |
| Weight loss/wasting | .06 | .63 | .73 | −.30 |
| Hair loss changes | −.07 | −.02 | .43 | .44 |
| Eigen values | 8.1 | 1.4 | 8.4 | 1.5 |
| Proportion | .40 | .07 | .42 | .07 |
In patients with HIV-1 viral loads <500 copies/mL, muscle aches/joint pain dropped from the first cluster model and fatigue did not load on the second cluster. The first cluster accounted for 40% of the variability of symptoms reported (Table 4). Loss of appetite loaded on both clusters. The second cluster, accounting for 7% of the variability, did not differ from the full population model.
Symptom Burden
Symptoms reported by each patient at the first visit of the year ranged from 0 to 16 (median 3 symptoms). Of all patients, 29% reported no symptoms, while 28% reported 1–3 symptoms, 14% reported 4–6 symptoms, 10% reported 7–9 symptoms, 14% reported 10–15 symptoms, and 5% reported 16 symptoms in the symptom clusters. In patients who reported symptoms, the association between symptom burden and viral load and between symptom burden and CD4+ T cell count was evaluated for a subset of patients without missing data for viral load (n = 804) and for a subset of patients without missing data for CD4+ T cell count (n = 655), using Kendall’s tau. No association emerged between viral load and symptom burden (τ = .032, p = .20) or CD4+ T cell count and symptom burden (τ = −.015, p = .622). There was no statistically significant correlation between Patient Health Questionnaire-9 scores and symptom burden. However, there was a weak association with Patient Health Questionnaire-9A scores (measuring anxiety) and symptom burden (r[752] = .07, p = .035).
Symptom Distress and Recent HIV Diagnosis
We compared the proportion of symptoms reported as bothersome by patients diagnosed for less than 12 months versus more than 12 months. For patients diagnosed more than 12 months, a higher proportion of patients reported fatigue (p = .002), numbness/pain in feet (p = .041), memory loss, and fat deposit/weight gain (p = .007) as bothersome; this same group experienced a lower proportion of patients who reported sadness (p = .006), rash (p = .002), and loss of appetite (p = .010) as bothersome. These results are displayed in Table 5. No other symptom in the symptom index had statistically significant differences between time of recorded diagnosis and distress of symptom report.
Table 5.
Proportion of Symptoms Reported as Bothersome at < 12 Months of Diagnosis Versus ≥12 Months of Diagnosis of HIV Infection
| Symptom Reported as Bothersome |
<12 Months n = 147 % |
≥12 Months n = 1,738 % |
p Value* |
|---|---|---|---|
| Fatigue | 32 | 39 | .002 |
| Fever/chills/sweats | 21 | 22 | .905 |
| Dizzy | 19 | 19 | .635 |
| Numbness/pain in feet | 21 | 31 | .041 |
| Memory loss | 19 | 26 | .007 |
| Nausea/vomiting | 18 | 15 | .089 |
| Diarrhea | 18 | 17 | .744 |
| Sad | 41 | 30 | .006 |
| Anxious/nervous | 40 | 34 | .288 |
| Poor sleep | 39 | 42 | .430 |
| Rash | 22 | 12 | .002 |
| Cough/shortness of breath | 21 | 21 | .739 |
| Headache | 30 | 27 | .588 |
| Loss of appetite | 26 | 17 | .010 |
| Bloating/abdominal pain | 20 | 21 | .208 |
| Muscle aches/joint pain | 35 | 41 | .201 |
| Sex problems | 17 | 22 | .130 |
| Fat deposit/weight gain | 11 | 20 | .008 |
| Wasting/weight loss | 17 | 14 | .314 |
| Hair loss | 9 | 11 | .351 |
Note. Pearson chi-squared used to determine difference between groups.
Discussion
High symptom prevalence, distress, and burden were identified in this sample of patients with HIV infection, despite high levels of HIV-1 suppression and immunologic stability. Physical and mental health symptoms were both common. The most prevalent symptoms were fatigue, muscle pain/joint pain, sadness, numbness/pain in feet, and poor sleep, which have been reported in inflammatory-related conditions. This was consistent with recent work conducted by McGowan and colleagues (2014) in which fatigue, poor sleep, and muscle ache/joint pain were among the most prevalent and distressing symptoms. The majority of the most prevalent symptoms were represented in Symptom Cluster 1 regardless of HIV-1 viremia. Poor sleep and muscle aches/joint pain were among the most prevalent symptoms.
Of people reporting any symptom, more than 80% reported their symptoms as bothersome, with a significant proportion of patients reporting some symptoms as bothering them a lot. Symptoms perceived as bothersome can affect quality of life and even health outcomes. Symptoms such as pain, poor sleep, and sexual issues should be proactively addressed in clinical visits. Pain has been associated with mental health disorders and has frequently been reported with other symptoms (Merlin et al., 2012). However, the report of perceived adverse effects or symptoms indicate possible underlying inflammation, which has been implicated as a key predictor in HIV disease progression, early aging, and non-HIV-related morbidity and mortality (El-Sadr et al., 2006; Sandler et al., 2011). Inflammatory markers have not been investigated in the context of symptom development in HIV disease but should be given consideration to further understand the underlying physiologic processes of symptom development in HIV disease.
Few publications have reported symptom prevalence using the HIV Symptom Index. However, in validating the HIV Symptom Index, Justice and colleagues (2001) reported symptom prevalence in 115 PLWH in 1998-1999. Overall symptom prevalence has decreased from a median of 15 symptoms reported to a median of 3, as reported in our study. The most common symptoms in 1999 versus 2011 were fatigue (81% vs. 47%), diarrhea (77% vs. 24%), anxiety (77% vs. 43%), sadness (76% vs. 40%), and difficulty sleeping (76% vs. 51%). In our study, the most common symptoms were poor sleep (difficulty sleeping; 51%), muscle aches/joint pain (48%), fatigue (47%), nervous/anxious (anxiety; 43%), and sadness (40%). Although there has been an overall decrease in reported symptom prevalence, fatigue, sleeping difficulties, anxiety, and sadness have remained as the most common symptoms reported. Diarrhea has improved, decreasing from 77% to 24%, which is to be expected given improvements in protease inhibitors. However, muscle aches/joint pain has become one of the more prevalent symptoms, although decreasing in overall frequency from 72% to 48%. Symptom burden has changed and decreased with improvements in cART, but persists despite viral suppression. Earlier HIV regimens had high side-effect profiles and, unbeknownst at the time, were the cause of high levels of inflammation. Now that side-effect profiles have improved, patients may be dealing with the effects of underlying pathophysiological processes of inflammation manifesting symptoms and the symptomatic effects from developed or developing comorbidities such as cardiovascular, bone, and liver disease.
The most bothersome symptoms in 1999 versus 2011 were fatigue (77% vs. 84%), sadness (67% vs. 77%), anxiety (66% vs. 81%), sleep difficulties (65% vs. 82%), and diarrhea (61% vs. 71%). The most bothersome symptoms reported also changed with the highest reported symptoms being muscle aches/joint pain (85%), fatigue (84%), bloating/abdominal pain (83%), and numbness/pain in feet and poor sleep (82%). While fatigue remained as a bothersome and prevalent symptom, pain (muscle, joint, abdominal, and neuropathic) became more prevalent and psychological symptoms (e.g., sadness and anxiety) became less bothersome. Of note, the 2001 study (Justice et al., 2001) reflected different geographic and ethnographic populations and a smaller sample than in our study, but were used to compare reported symptoms using the same instrument. While symptoms have diminished overall, symptoms still persist, and in those experiencing symptoms, they contribute to a greater patient burden. Racial differences in symptom experiences reported are crucial because they indicate potential barriers to adherence in already vulnerable populations. If side effects are likely to develop for particular medications, discussing these symptoms with patients in advance may address perceived threats, and provide steps and interventions that may support a higher likelihood of continuing therapy or seeking immediate health care in the event of symptom development (Janz & Becker, 1984).
Symptom burden impacts adherence to cART and health outcomes along the HIV disease care continuum (Baran et al., 2014; Gay et al., 2011; Kempf et al., 2009; Mugavero, Amico, Horn, & Thompson, 2013). During clinic visits and while addressing adherence to cART, providers should be particularly sensitive to identifying and managing symptoms that are causing patient distress, regardless of the source of the symptoms. While individual symptoms of the identified clusters are a part of the larger complete review of systems in a clinical assessment, asking patients specifically if they have symptoms of muscle or joint pain, poor sleep, numbness/pain in feet, fatigue, bloating/abdominal pain, and sex problems will help clinicians manage strategies targeted to patient concerns. Given the apparent symptom clusters observed in our study, the review of systems may need to be reorganized to recognize symptom cluster patterns in clinics focused on HIV disease management, proactively focusing on symptom clusters identified in persons with suppressed viral load versus viremia. With a better understanding of possible underlying physiological factors, clinicians may develop targeted strategies to address and resolve symptom clusters experienced by patients. Furthermore, clinicians may want to focus on managing symptoms most distressing to their patient populations.
Patients who had been diagnosed with HIV for 12 or more months were bothered by fatigue, neuropathy, fat deposit/weight gain, and memory loss. These symptoms were likely related to long-term cART; however, newer therapies have lower side-effect profiles. Other explanations included long-term effects of inflammation (Wilson et al., 2014). Fatigue (Klimas, Broderick, & Fletcher, 2012), neuropathy (Harezlak et al., 2011; Zheng et al., 2011), obesity (Koethe et al., 2013), and cognitive decline including memory loss (Ancuta et al., 2008; Deeks, 2011; Kamat et al., 2012) have been associated with inflammation. Understanding the etiology of symptoms, especially those identified in chronic HIV disease, has been an ongoing consideration in clinical HIV research. Are symptoms a result of side effects or the ongoing effects of inflammation and residual viral replication? Further studies validating these symptoms and the variables contributing to their presence are warranted.
Other symptoms, including poor appetite, sadness, and rash, tended to become less bothersome after 12 months in clinical care. It is possible that a decrease in reported sadness was related to adjustment to diagnosis after the first 12 months; this emphasizes the importance of supporting patients through the first year of HIV diagnosis. According to the HIV treatment cascade in the United States, roughly 40% of persons diagnosed with HIV infection and linked into care are actually retained in care (Mugavero et al., 2013). Therefore, identifying symptoms that are particularly distressing to patients should be anticipated and addressed to improve retention in care, especially during the first 12 months of diagnosis and linkage to care.
Limitations and Future Research
While our study had adequate statistical power to identify relevant symptom clusters and associations, there were a number of limitations, including not categorizing symptom clusters based on immune status or function. The patient sample was recruited at a single clinic at an academic institution with a proportionally well-controlled population in the southeast United States, so the results may not be generalizable to other areas. But the findings of symptom prevalence results were consistent with studies of other HIV-infected populations (Edelman, Gordon, & Justice, 2011; Swan et al., 2014).
Our study did identify symptom clusters and confirmed that symptoms were still common in patients with well-controlled HIV disease. Many of these symptoms could be attributed to cART, but given the newer therapies and lower side-effect profiles, this is unlikely to be the sole explanation. While symptom burden has decreased in comparison to earlier reports in which symptoms were attributed to high viremia and medication side effects, the findings from our study and newer evidence suggests other explanations, such as inflammatory effects due to immune activation, for the consistent prevalence of symptoms. Research in other geographical clinic populations is needed to validate these results with longitudinal investigation of various factors. Predicting symptoms will help clinical scientists identify management strategies to reduce or alleviate symptoms when warranted. Even though this study was meant to describe the prevalence and burden of symptoms experienced and reported by PLWH, a significant limitation of our study was that we did not have a comparison cohort of persons without HIV infection. Comparisons between infected and uninfected populations would support interpretation of the context of the HIV symptom experience. Information on comorbidities, outside of those reported, was not collected. We did not have access to substance abuse information. This limited our study to control for substance use, which can influence symptoms. However, previous studies in the same clinic population have shown substance use reported by 3% of the clinic population (Merlin et al., 2012).
We did not control for or consider medication side effects in our analysis; however, most of the symptoms (i.e., diarrhea, rash, and nausea/vomiting) associated with medication side effects were not ranked among the most prevalent symptoms reported. Variability in literacy, health numeracy, and health literacy of the study sample could have impacted the accuracy of self-reported data and may have impacted the study findings (Gakumo, Vance, Moneyham, Deupree, & Estrada, 2013). As the medical community strives to achieve improved patient-centered health outcomes, additional steps to explore aspects of symptom burden are warranted. The results of our study demonstrated a need to develop and test symptom management strategies that predictably reduce symptom burden and improve clinical management and patient quality of life. Now that people are living longer with HIV and developing comorbidities from inflammation, further research is warranted to examine differences in comorbidities and the effect of inflammation on symptoms. Investigating symptoms in the context of comorbidities and chronic inflammation in HIV populations is also warranted.
Conclusion
Even with improvements to cART side-effect profiles, symptom prevalence and symptom burden continue to be highly independent of HIV viral load and CD4+ T cell count in PLWH. Clinicians should target inquiries to patients regarding highly prevalent symptoms and identify bothersome symptoms for management.
Key Considerations.
Patients living with HIV disease continue to have high symptom burden despite viral suppression and decreased side-effect profiles.
Symptoms experienced in HIV disease may have underlying pathophysiology due to inflammation, even in the context of viral suppression.
Nurses should proactively ask patients about symptoms that contribute to distress.
Nurses should help patients discuss symptoms with their clinicians.
Acknowledgments
This publication resulted from research supported by the University of Alabama at Birmingham Center for AIDS Research (#P30 AI027767), the VA HSR&D Nursing Research Initiative (NRI 13-356-2), and the Veterans Administration National Quality Scholars Program. In addition, this publication was made possible with the support of the Lifespan/Tufts/Brown Center for AIDS Research (R25MH083620).
Footnotes
Disclosures
The authors report no real or perceived vested interests that relate to this article that could be construed as a conflict of interest.
Contributor Information
Natalie L. Wilson, University of Alabama at Birmingham School of Nursing, and a Health Services Researcher, Birmingham Veterans Administration Medical Center, Birmingham, Alabama, USA.
Andres Azuero, University of Alabama at Birmingham School of Nursing, Birmingham, Alabama, USA.
David E. Vance, Office of Research and Scholarship, University of Alabama at Birmingham School of Nursing, Birmingham, Alabama, USA.
Joshua S. Richman, University of Alabama School of Medicine, and Fellow, Birmingham VA Medical Center, Birmingham, Alabama, USA.
Linda D. Moneyham, University of Alabama at Birmingham, Birmingham, Alabama, USA.
James L. Raper, University of Alabama at Birmingham Schools of Nursing and Medicine, and Director/Nurse Practitioner of 1917 HIV/AIDS Outpatient Clinic, Birmingham, Alabama, USA.
Sonya L. Heath, Department of Medicine, Division of Infectious Diseases, University of Alabama at Birmingham School of Medicine, Birmingham, Alabama, USA.
Mirjam-Colette Kempf, University of Alabama at Birmingham School of Nursing and Department of Health Behavior, School of Public Health, Birmingham, Alabama, USA.
References
- Abdi H, Williams LJ. Principal component analysis. Wiley Interdisciplinary Reviews: Computational Statistics. 2010;2(4):433–459. [Google Scholar]
- American Diabetes Association Diabetes symptoms. 2014 Retrieved from http://www.diabetes.org/diabetes-basics/symptoms/
- Ancuta P, Kamat A, Kunstman KJ, Kim EY, Autissier P, Wurcel A, Gabuzda D. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran R, Mulcahy F, Krznaric I, Monforte AD, Samarina A, Xi H, Martinez M. Reduced HIV symptoms and improved health-related quality of life correlate with better access to care for HIV-1 infected women: The ELLA study. Journal of the International AIDS Society. 2014;17(4 Suppl 3):19616. doi: 10.7448/IAS.17.4.19616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WY, Le LW, Zimmermann C. Symptom clusters in patients with advanced cancers. Support Care Cancer. 2009;17(9):1223–1230. doi: 10.1007/s00520-009-0577-7. [DOI] [PubMed] [Google Scholar]
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annual Review in Medicine. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd M, Janson S, Facione N, Faucett J, Froelicher ES, Humphreys J, Taylor D. Advancing the science of symptom management. Journal of Nursing Scholarship. 2001;33(5):668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Edelman EJ, Gordon K, Justice AC. Patient and provider-reported symptoms in the post-cART era. AIDS and Behavior. 2011;15(4):853–861. doi: 10.1007/s10461-010-9706-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Rappoport C. CD4+ count-guided interruption of antiretroviral treatment. New England Journal of Medicine. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, Campbell TB. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. Journal of Infectious Diseases. 2013;208(2):249–259. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakumo CA, Vance DE, Moneyham LD, Deupree JP, Estrada CA. Health numeracy and health literacy within the context of management of patients with human immunodeficiency virus. Nursing: Research and Reviews. 2013;3:23–31. [Google Scholar]
- Gay C, Portillo CJ, Kelly R, Coggins T, Davis H, Aouizerat BE, Lee KA. Self-reported medication adherence and symptom experience in adults with HIV. Journal of the Association of Nurses in AIDS Care. 2011;22(4):257–268. doi: 10.1016/j.jana.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harezlak J, Buchthal S, Taylor M, Schifitto G, Zhong J, Daar E, Navia B. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS. 2011;25(5):625–633. doi: 10.1097/QAD.0b013e3283427da7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz NK, Becker MH. The Health Belief Model: A decade later. Health Education Quarterly. 1984;11(1):1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G, Wu AW. Development and validation of a self-completed HIV symptom index. Journal of Clinical Epidemiology. 2001;54(Suppl 1):S77–S90. doi: 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Justice AC, Rabeneck L, Hays RD, Wu AW, Bozzette SA. Sensitivity, specificity, reliability, and clinical validity of provider-reported symptoms: A comparison with self-reported symptoms. Outcomes Committee of the AIDS Clinical Trials Group. Journal of Acquired Immune Deficiency Syndromes. 1999;21(2):126–133. [PubMed] [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2012;60(3):234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf MC, Pisu M, Dumcheva A, Westfall AO, Kilby JM, Saag MS. Gender differences in discontinuation of antiretroviral treatment regimens. Journal of Acquired Immune Deficiency Syndromes. 2009;52(3):336–341. doi: 10.1097/QAI.0b013e3181b628be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, Saag MS. Cohort profile: The Centers for AIDS Research network of integrated clinical systems. International Journal of Epidemiology. 2008;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas NG, Broderick G, Fletcher MA. Biomarkers for chronic fatigue. Brain, Behavior, and Immunity. 2012;26(8):1202–1210. doi: 10.1016/j.bbi.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koethe JR, Dee K, Bian A, Shintani A, Turner M, Bebawy S, Hulgan T. Circulating interleukin-6, soluble CD14, and other inflammation biomarker levels differ between obese and nonobese HIV-infected adults on antiretroviral therapy. AIDS Research and Human Retroviruses. 2013;29(7):1019–1025. doi: 10.1089/aid.2013.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: An update. Advances in Nursing Science. 1997;19(3):14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- McGowan J, Sherr L, Rodger A, Fisher M, Miners A, Johnson M, Lampe F. Effects of age on symptom burden, mental health and quality of life amongst people with HIV in the UK. Journal of the International AIDS Society. 2014;17(4 Suppl 3):19511. doi: 10.7448/IAS.17.4.19511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlin JS, Cen L, Praestgaard A, Turner M, Obando A, Alpert C, Frank I. Pain and physical and psychological symptoms in ambulatory HIV patients in the current treatment era. Journal of Pain and Symptom Management. 2012;43(3):638–645. doi: 10.1016/j.jpainsymman.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: From cascade to continuum to control. Clinical Infectious Diseases. 2013;57(8):1164–1171. doi: 10.1093/cid/cit420. [DOI] [PubMed] [Google Scholar]
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Douec DC. Plasma levels of soluble CD14 independently predict mortality in HIV infection. Journal of Infectious Diseases. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan H, McDannold S, McInnes DK, Glickman M, Reisman J, Gifford AL. Symptom bothersomeness and symptom attribution in adults on HIV medications. Poster presented at the 9th International Conference on HIV Treatment and Prevention Adherence; Miami, FL. Jun, 2014. [Google Scholar]
- Tontini GE, Vecchi M, Pastorelli L, Neurath MF, Neumann H. Differential diagnosis in inflammatory bowel disease colitis: State of the art and future perspectives. World Journal of Gastroenterology. 2015;21(1):21–46. doi: 10.3748/wjg.v21.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen CC, Antani M, Carey J, Landefeld CS. An index of symptoms for infection with human immunodeficiency virus: Reliability and validity. Journal of Clinical Epidemiology. 1994;47(5):537–546. doi: 10.1016/0895-4356(94)90300-x. [DOI] [PubMed] [Google Scholar]
- Wilson NL, Vance DE, Moneyham LD, Raper JL, Mugavero MJ, Heath SL, Kempf MC. Connecting the dots: Could microbial translocation explain commonly reported symptoms in HIV disease? Journal of the Association of Nurses in AIDS Care. 2014;25(6):483–495. doi: 10.1016/j.jana.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Ouyang H, Zheng X, Liu S, Mata M, Fink DJ, Hao S. Glial TNFalpha in the spinal cord regulates neuropathic pain induced by HIV gp120 application in rats. Molecular Pain. 2011;7:40. doi: 10.1186/1744-8069-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]


