Abstract
BACKGROUND & AIMS
Intestinal thiamin uptake process is vital for maintaining normal body homeostasis of the vitamin; in vitro studies suggest that both thiamin transporter-1 (THTR-1) and -2 (THTR-2) are involved. Mutations in THTR-1 cause thiamin-responsive megaloblastic anemia (TRMA), a tissue specific disease associated with diabetes mellitus, megaloblastic anemia, and sensorineural deafness. However in patients with TRMA, plasma thiamin levels are within normal range, indicating that THTR2 (or another carrier) could provide sufficient intestinal thiamin absorption. We tested this possibility and examined the role of THTR-2 in uptake of thiamin in the intestine of mice.
METHODS
THTR-2 deficient mice were generated by SLC19A3 gene knockout and used to examine intestinal uptake of thiamin in vitro (isolated cells) and in vivo (intact intestinal loops). We also examined intestinal thiamin uptake in THTR-1 deficient mice.
RESULTS
Intestine of THTR-2 deficient mice had reduced uptake of thiamin compared to those of wild –type littermate mice (p<0.01); this reduction was associated with a decrease (p<0.01) in blood thiamin levels in THTR-2 deficient mice. However, intestinal uptake of thiamin in THTR-1 deficient mice was not significantly different from that of wild-type littermate animals. Level of expression of THTR-1 was not altered in the intestine of THTR-2 deficient mice, but level of expression of THTR-2 was up-regulated in the intestine of THTR-1 deficient mice.
CONCLUSION
THTR-2 is required for normal uptake of thiamin in the intestine and can fulfill normal levels of uptake in conditions associated with THTR-1 dysfunction.
Keywords: Vitamin B1, THTR-1, THTR-2, gene-knockout
Introduction
Vitamin B1 (thiamin) is essential for survival of humans and other mammals. Thiamin plays a fundamental role in metabolism and energy production as the coenzyme thiamin pyrophosphate; a metabolite required for normal cellular functions, growth and development (1). Further, thiamin plays a role in reducing cellular oxidative stress via its role in bridging the glycolytic and the pentose phosphate metabolic pathways (2, 3). Insufficient amounts of dietary thiamin can lead to a variety of clinical abnormalities that include neurological and cardiovascular disorders (1, 4, 5). An inability to synthesize thiamin has generated, in mammals, a reliance on the intestinal epithelium to maintain normal thiamin body homeostasis. Using a variety of human and animal intestinal preparations, the process of intestinal thiamin uptake was shown to occur via a specialized carrier-mediated mechanism (reviewed in 6). Other studies have shown that both thiamin transporters-1 and -2 (THTR-1 and THTR-2) are expressed in human and mouse intestine at the protein and mRNA levels (6 – 8). Further, recent in vitro investigations utilizing a gene-silencing approach (i.e., gene-specific siRNA) in cultured human-derived intestinal epithelial Caco-2 cells have shown that both THTR-1 and THTR-2 are involved in thiamin uptake across the apical membrane domain and that together they account for total carrier-mediated uptake of the vitamin (7). However, confirmation of these findings in a more physiologically relevant setting is currently lacking.
The autosomal recessive disorder thiamin-responsive megaloblastic anemia (TRMA) is caused by mutations in THTR-1 (9–11). TRMA is associated with megaloblastic anemia, diabetes mellitus, and auditory deafness that develop as a result of the localized thiamin deficiency that occurs in affected tissues (9–11). However, plasma level of thiamin in TRMA patients remains within the normal range (12), suggesting that THTR-2 (or another as yet unidentified carrier) could provide sufficient intestinal thiamin uptake in THTR-1 deficiency. These observations were reproduced experimentally in THTR-1 deficient mice (13, 14). Our aims in this study are to examine the role of THTR-2 in intestinal thiamin uptake in a physiologically relevant setting and to test the possibility that this transporter can provide sufficient intestinal thiamin uptake in the absence of functional THTR-1. To do this, we generated a THTR-2-deficient mouse model and examined the effect of knocking out the SLC19A3 gene (the gene that encodes THTR-2) on intestinal thiamin uptake. We also obtained THTR-1 deficient mice (13) and investigated the impact of knocking out the SLC19A2 gene on intestinal thiamin uptake. Our results showed that intestinal thiamin uptake in the THTR-2 (but not THTR-1) deficient mice to be significantly lower than that of wild-type littermate mice. This impairment in intestinal thiamin uptake was associated with a significant decrease in blood thiamin level in the THTR-2 deficient mice. Further, while knocking out THTR-2 is not associated with changes in the level of expression of THTR-1 in the intestine, knocking out THTR-1 is associated with a marked induction in the level of intestinal THTR-2.
Materials and Methods
Materials
[3H]-Thiamin and [3H]-biotin (sp act > 30Ci/mmol; radiochemical purity>98%) were purchased from ARC (St Louis. MO). All chemicals and reagents used in this study were of analytical/molecular biology grade and were purchased from commercial sources. Cellulose nitrate filters (0.45 µm pore size) used in the uptake studies were purchased from Sartorius filters (Hayward, CA).
Generation of THTR-2-deficient mice
A conventional targeting vector was constructed using a 13.9kb region of the THTR-2 gene sub cloned from a positively identified BAC clone (inGenious Targeting Laboratory, Inc., Stony Brook, NY). The vector was designed to allow homologous recombination to occur with a neo cassette replacing 4.2kb of the gene including exons 1 and 2 (contains ATG start codon). ES cells were transfected, selected and screened, then positive clones were microinjected into foster mice to produce chimera pups. Subsequent breeding with wild-type C57Bl6 mice produced F1 pups. Six heterozygous knockout mice (Slc19a3+/−) were confirmed by PCR and Southern blot and shipped to our facility. Inbreeding was used to produce homozygous knockout (Slc19a3−/−) mice and maintain the colony. Genotyping of pups was performed using PCR screening with oligonucleotides that confirm insertion of the neo cassette (F-5’-TGCGAGGCCAGAGGCCACTTGTGTAGC-3’ and R-5’-TGACCTGATTTCCTTGAGGG-3’) as well as those that allow detection of the wild-type gene (F-5’-AGTCCTATGGGAACACAAGGCACCCTC-3’ and R-5’-TCGTGATCTTCCTGCTACAG-3’). The primer sets amplify fragments of approximately 2 and 2.1 kb in length respectively. All mouse studies performed were reviewed and approved prior to animal use by the Long Beach VA Subcommittee on Animal Studies and the UCI Institutional Animal Care and Use Committee, both AAALAC accredited institutions.
Isolation of mouse intestinal epithelial cells, in vivo intestinal loop and uptake studies
Intestinal epithelial cells were isolated from adult mice (2 to 3 months) as described by us before (15) using a well-established fractionation procedure (16). We collected five consecutive fractions that represent mostly villus tip cells (15). Uptake of [3H]-thiamin or [3H]-biotin by cells was measured as described by us previously (15) using an established rapid-filtration technique (17) at 37°C in Krebs-Ringer (KR) buffer at pH 7.4. Labeled and unlabeled vitamin was added to the incubation medium at the onset of incubation, and uptake was examined during the initial linear period of uptake (data not shown). Protein concentrations were measured using a Bio-Rad (Hercules, CA) protein determination kit. Uptake data were expressed in fmol/mg protein/10 min. Intact intestinal loops (5 cm) in vivo were prepared in the jejunal area [as described previously (18)] and filled with 250 µl of KR buffer containing labeled alone or labeled plus unlabeled thiamin or biotin. Uptake was measured after 10 min (linear phase of uptake, data not shown). Uptake data were expressed in fmol/mg tissue wet weight/10 min. All in vitro and in vivo uptake experiments with knockout mice were run simultaneously with sex-matched wild type littermate mice.
Establishment of THTR-1-deficient mice colony
Founders of the THTR-1-deficient mouse colony were kindly provided by Dr. Bruce D. Gelb (Mount Sinai School of Medicine, New York, NY) (13). Genotyping of pups was performed as described before (13) using PCR screening with oligonucleotides that confirm insertion of the neo cassette (F-5’-CTCGTCCTGCAGTTCATTCA-3’ and R-5’-AGACAATCGGCTGCTCTGAT-3’) as well as those that allow detection of the wild-type gene (F-5’-TTACCTGCTGCTGCTGTTTC-3’ and R-5’-GATGGTTAGCTGCTGGGGTA-3’). The primer sets amplify fragments of approximately 120 bp and 500 bp in length, respectively.
Quantitative real-time PCR (qPCR)
qPCR was performed using the Bio-Rad iCycler (Hercules, CA) and a Qiagen Quantitect SYBR green PCR kit (Valencia, CA). Semi-quantitative PCR was performed using the Clontech Advantage Polymerase 2 Kit (Mountain View, CA). RNA from adult (2 to 3 months) mouse tissue was isolated using Trizol (Invitrogen, Carlsbad, CA) and the manufacturers procedure. The RNA was DNase treated and first strand cDNA was made from 5 µg of the isolated total RNA primed with oligo dT using an Invitrogen Superscript synthesis system. A dilution series of the RT products (1, 1/10, and 1/100) was then used in the subsequent qPCR. A 1/25 dilution of RT products was used for semi-quantitative PCR. Primers used in the qPCR were specific for the mouse THTR-1 (F-5’-GTTCCTCACGCCCTACCTTC-3’, R-5’-GCATGAACCACGTCACAATC-3’), the mouse THTR-2 (F-5’-TCATGCAAACAGCTGAGTTCT-3’, R-5’-ACTCCGACAGTAGCTGCTCA-3’) and the mouse β-actin (F-5’-AGCCAGACCGTCTCCTTGTA-3’, R-5’-TAGAGAGGGCCCACCACAC-3’). Quantitative PCR consisted of a 15 second 95°C melt followed by 40 cycles of 95°C melt for 30 sec, 58°C annealing for 30 sec, and 72°C extension and data collection for 1 min. Negative control reactions without RT product were used with every experiment. To compare the relative relationship between THTR-1 and THTR-2 mRNA in tissues of different mice we utilized a calculation method provided by the iCycler manufacturer (Biorad, Hercules, CA) described previously (19). The semi-quantitative PCR consisted of a 3 minute 95°C melt followed by 30 cycles of 95°C melt for 30 sec, 55°C annealing for 30 sec, 72°C extension for 30 sec, and final 72°C extension for 7 minutes. PCR products were loaded on a 3% agarose gel and images were captured using UVP BIODOC-It™ System (Bio-Imaging Systems, Upland, CA). The semi-quantitative PCR yielded only one detectable band and band intensity of THTR-1 or THTR-2 specific products were normalized to the β-actin.
Membrane protein isolation and Western blot analysis
Isolation of membrane proteins and Western blot analysis was performed as previously described (19). Homogenates from freshly isolated intestinal epithelial cells were centrifuged at 1,800 g for 10 min to remove nuclei and non-broken cells, followed by 9,000 g spin in a Beckman ultracentrifuge for 30 min. The pellet was re-suspended to a final concentration of 10 mg/ml, and 30 µg of membrane protein per lane was loaded for SDS-PAGE. Western blot analysis was performed using specific and previously characterized polyclonal antibodies against mTHTR-1 and mTHTR-2 (Sigma-Genosys, The Woodlands, TX).
Phenotype assessment
The assessment included animal weight, ability to produce progeny, overall gross anatomy at autopsy, and microscopic histopathology of tissues including intestine, liver, lung, kidney, heart and brain.
Histopathology analysis
Brain, heart, liver and kidneys from THTR-2 deficient mice were removed immediately after euthanasia, sectioned, 10% formalin fixed over night and submitted for paraffin embedding and H&E stained microscopic slide preparation using standard histological techniques (LBVA MC Histology Laboratory). Gross and microscopic evaluation and reporting was carried out by a board certified anatomic pathologist.
Statistical analysis
Transport data presented in this paper are the result of at least three separate experiments from at least three different mice and are expressed as mean ± S.E.M. in fmol/mg protein (or tissue wet weight)/10 min. Appropriate controls using sex-matched littermate mice were run simultaneously. Data were analyzed using the Students t-test. Carrier-mediated thiamin (or biotin) uptake was determined by subtracting uptake of an identical amount of labeled thiamin in the presence of a high pharmacological concentration of unlabeled thiamin (1 mM) from uptake in its absence. Western blot analysis and qPCR were all performed on at least three separate biological repeats.
Results
Studies with THTR-2 deficient mice
Generation of THTR-2 knockout animals and establishment of the colony
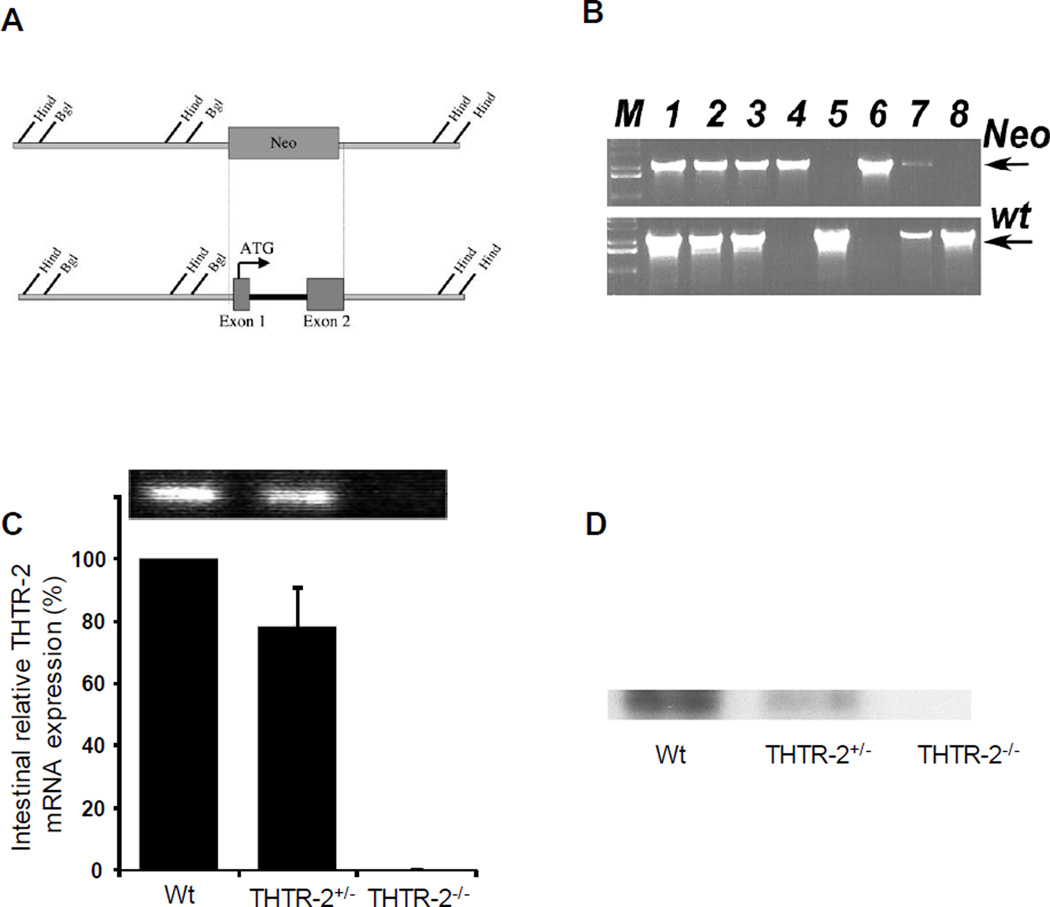
A conventional targeting vector was constructed to insert a neomycin resistance cassette into 4.2kb of the THTR-2 (Slc19a3) gene including exons 1 and 2 that contains the ATG start codon (Fig. 1A). ES cells were transfected, selected and screened. Positive clones were microinjected into foster mice to produce chimeric pups. Subsequent breeding with wild-type (THTR-2+/+) C57Bl6 mice produced F1 pups. Inbreeding was used to produce homozygous knockout (THTR-2−/−) mice, confirmed by PCR (Fig 1B). Quantitative real-time PCR of reverse transcribed intestinal mRNA demonstrated a reduction in the levels of the THTR-2 message in THTR-2+/− mice and no detectable message in the THTR-2−/− mice (Fig 1C) compared to sex-matched wild-type littermates. Western blots of isolated intestinal membrane proteins with THTR-2 specific antibodies showed reduction of the THTR-2 protein level in THTR-2+/− mice compared to sex-matched wild-type littermates and no detectable THTR-2 protein in THTR-2−/− mice (Fig 1D).
Figure 1. Generation of THTR-2−/− mice, validation of THTR-2 loss at the gene, mRNA and protein level.
A) Schematic representation of homologous recombination event between neomycin cassette and exons 1 and 2 of Slc19a3. Representative restriction sites and the initiator ATG are shown. B) Genomic PCR of F1 pubs showing mice positive for neomycin cassette replacement of exons 1 and 2 of Slc19a3 and/or mice carrying the wild type gene. Lanes 1, 2, 3, and 7 show mice found to be heterozygous knockout (THTR-2+/−), lanes 4 and 6 show homozygous knockout (THTR-2−/−) mice, and lanes 5 and 8 are wild type (THTR-2wt) mice. (C) Representative quantitative RT-PCR and (D) Western blot analysis using a THTR-2 specific antibody showing relative expression of THTR-2 mRNA and protein level in the intestine of THTR-2+/+, THTR-2+/− and THTR-2−/− mice.
Effect of loss of THTR-2 on intestinal uptake of thiamin
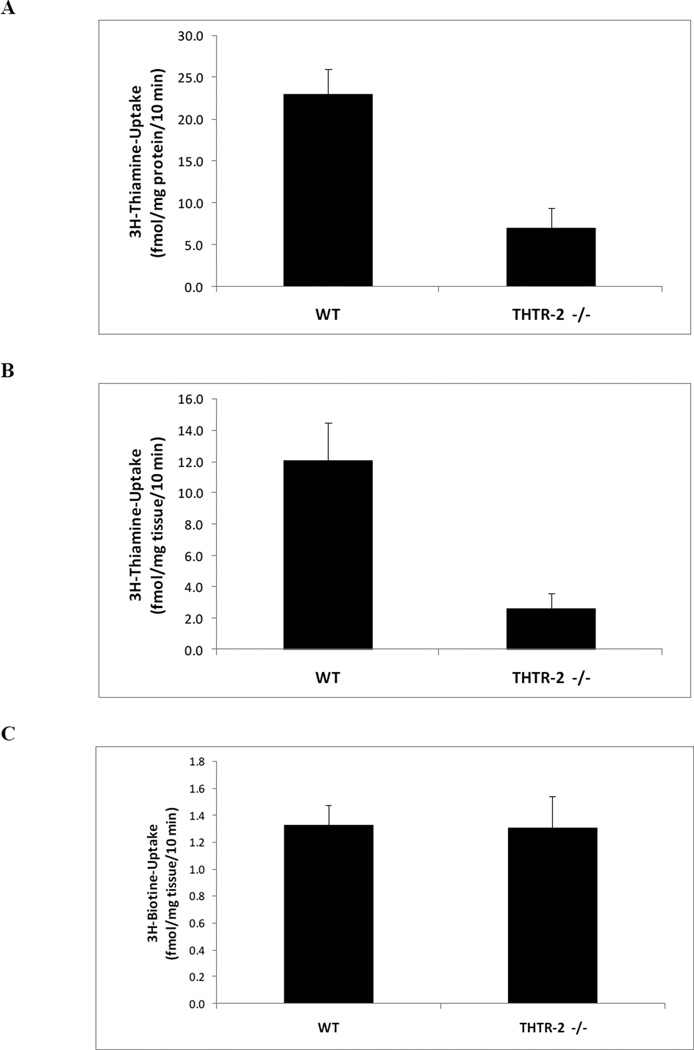
We utilized an in vitro system of freshly isolated intestinal epithelial cells and complemented the findings in vivo with intact intestinal loop studies. Our results showed that THTR-2 deficiency causes a significant decrease in thiamin uptake compared to sex-matched wild-type littermates in freshly isolated intestinal epithelial cells in vitro (Fig 2A, p < 0.002) and in intestinal loops in vivo (Fig 2B, p < 0.01). To show the specificity of the defect, no change in the in vivo intestinal loop uptake of the unrelated labeled vitamin [3H]-biotin was observed in the mouse model (Fig 2C).
Figure 2. Determination of effect of loss of THTR-2 on thiamin uptake.
A) Initial rate of carrier-mediated thiamin uptake in vitro by freshly isolated intestinal epithelial cells from sex matched wild type litter mates (THTR-2+/+) compared to homozygous THTR-2 knockout mice (THTR-2−/−) mice after 10 min incubation (*p < 0.002). B) Initial rate of carrier-mediated thiamin uptake in vivo by intact intestinal loops from THTR-2+/+ and THTR-2−/− mice after 10 min incubation (*p < 0.01). C) Initial rate of carrier-mediated biotin uptake in vivo by intact intestinal loops from THTR-2+/+ and THTR-2−/− mice. Data are mean ± SEM of at least 3 to 5 separate uptake determinations performed on at least three distinct sets of mice (*p < 0.05; **p < 0.01).
Thiamin blood levels
Thiamin blood levels, determined using a commercial service (Vitamin Diagnostics, Cliffwood Beach, NJ), were significantly decreased (p < 0.01) in THTR-2−/− mice compared to sex-matched wild type littermates (53 ± 2 vs. 74 ± 4 µg/L, respectively). However, there was no statistical difference in blood biotin, glucose and insulin levels in THTR-2−/− mice compared to sex-matched wild type litter mates (data not shown).
mRNA and protein analysis
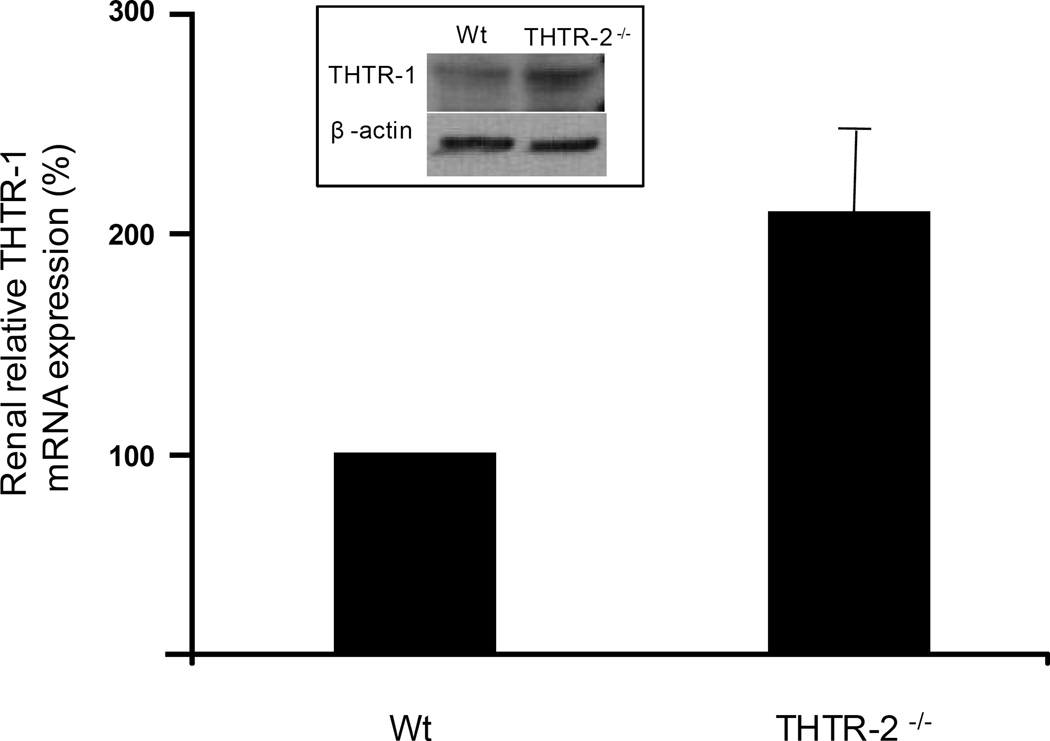
Real time quantitative PCR demonstrated that loss of THTR-2 does not affect the level of THTR-1 mRNA in the intestine (data not shown). However in the kidney, a marked increase in THTR-1 mRNA with concurrent increase in protein level was observed in THTR-2 deficient mice (Fig 3) compared to sex-matched wild type littermates.
Figure 3. Relative expression of THTR-1 mRNA in THTR-2−/− mouse kidney.
Quantitative RT-PCR analysis with THTR-1 specific primers on reverse transcribed mouse renal total RNA samples. Data were normalized to β-actin expression in five independently isolated total renal RNA samples of sex-matched wild type (wt) litter mates and THTR-2−/− pairs of mice. The sex-matched wild type (wt) litter mate THTR-1 levels were set at 100% expression. Inset: Corresponding change in THTR-1 protein level in the kidney of THTR-2−/− mice.
Phenotype assessment
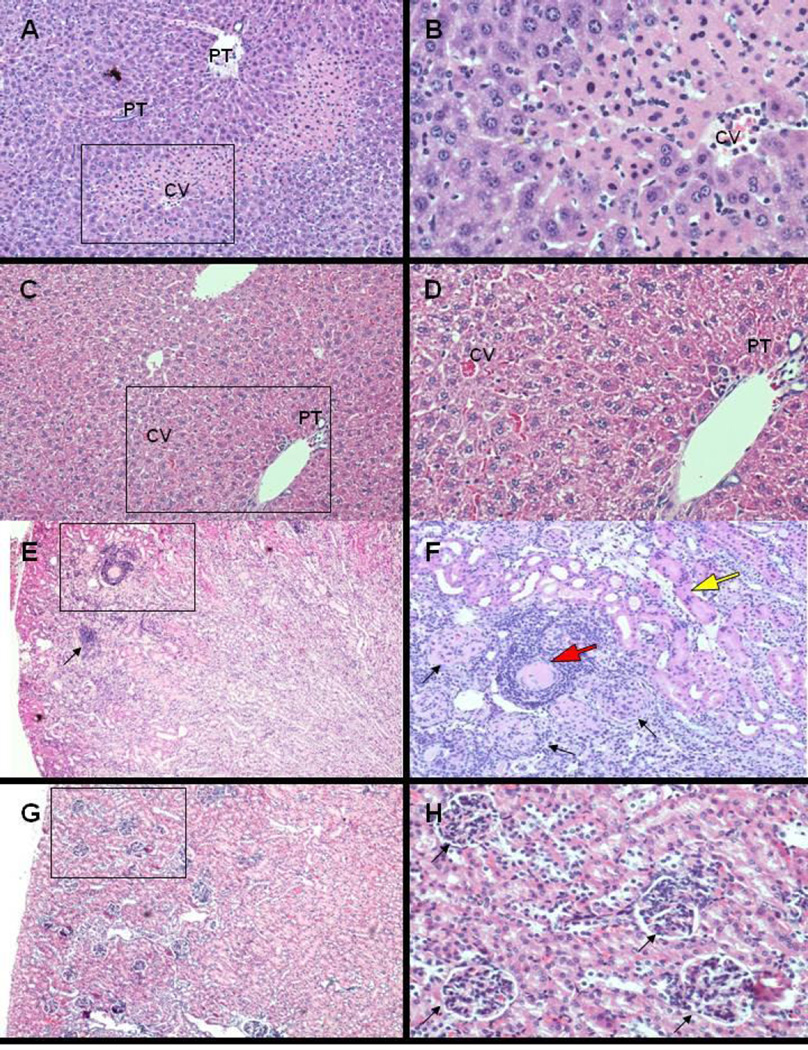
The phenotype of the THTR-2−/− mice showed no initial significant differences when compared with age- and gender-matched wild-type control mice. However, THTR-2−/− mice died prematurely at one year of age and displayed a noticeable change in phenotype that included lethargic behavior and progressive wasting that started at around 2–3 months prior to death. We performed gross anatomical and histo-pathological examination of five THTR-2-deficient mice at 9 month of age and did not observe differences in gross pathological assessment of all organs. Histological analysis of tissue from heart and brain (cortex and cerebellum) did not show any abnormalities. However, histological analysis of liver tissue in four of five THTR-2-deficient mice demonstrated significant injury of the hepatic parenchyma with necrosis and chronic inflammation in zone 2 (mid-zonal) and focally extending periportally into zone 1 (Fig 4 A–D). In addition, histological analysis of renal tissue demonstrated interstitial chronic inflammation of the renal cortex with degeneration of the proximal convoluted tubules, as well as arteriolosclerosis (renal nephrosclerosis) (Fig 4 E–H). Histological analysis of the liver and renal tissues of five sex-matched wild type litter mates did not show any histopathological changes. Whether these changes contribute to the premature death of the THTR-2 deficient mice at about one year of age and whether other (as yet unidentified) abnormalities are involved require further investigations.
Figure 4. Histopathological examination of THTR-2−/− mouse kidneys and livers.
Histological evaluation of renal and hepatic parenchyma (H&E stain of paraffin embedded sections, A,C,E,G, 4× objective, with higher magnification of boxed area in B,D,F,H, 10× objective). Significant injury of the hepatic parenchyma (A,B) in four of five THTR-2−/− mice with hepatocellular necrosis and chronic inflammation primarily in zone 2 (mid-zonal) focally extending periportally into zone 1 (CV: central vein; PT: portal tract). Injury of renal cortex (E,F) in two of five THTR-2−/− mice with acute tubular necrosis (F, yellow arrow, acute change), interstitial chronic nephritis (E, black arrow, subacute change) and nephrosclerosis with arteriolar (F, red arrow) and glomerular (F, closed head arrows) sclerosis (chronic change). Hepatic (C,D) and renal (G,H) histology of age-matched THTR-2wt mice without significant changes.
Studies with THTR-1−/− mice
Effect of loss of THTR-1 on intestinal thiamin uptake
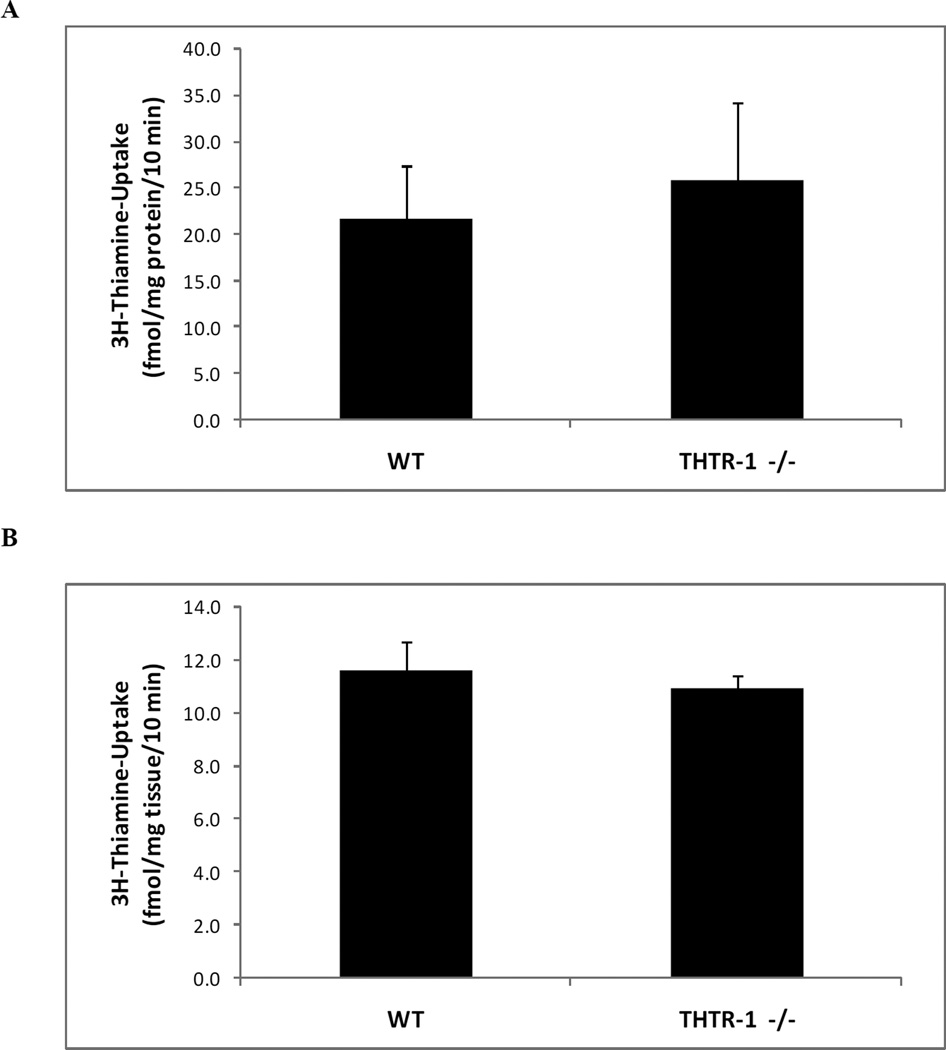
The THTR-1−/− mouse line has been previously generated, phenotyped and used to study different aspects of thiamin nutrition and metabolic roles, but no study was performed on the effect of THTR-1 deficiency on intestinal thiamin uptake (13). We obtained founders from Dr. Bruce D. Gelb (Mount Sinai School of Medicine, New York, NY) (13) and established a colony of these animals at our accredited animal facility. Genotyping of pups was performed as described before (13) and animals were used for transport investigations between 2 to 3 months of age. Using these animals and sex-matched wild-type littermates, we examined thiamin uptake in vitro with freshly isolated intestinal epithelial cell preparations. Our results showed no significant difference in thiamin uptake between THTR-1−/− and sex-matched wild type litter mates (Fig. 5A). Similar observations were obtained when thiamin uptake was examined using intestinal loops in vivo (Fig. 5B).
Figure 5. Determination of effect of loss of THTR-1 on functionality of thiamin uptake in isolated intestinal epithelial cells and in vivo using intact intestinal loops.
A) Initial rate of carrier-mediated thiamin uptake in vitro by freshly isolated intestinal epithelial cells from sex matched wild type litter mates (THTR-1+/+) compared to homozygous THTR-2 knockout mice (THTR-1−/−) mice after 10 min incubation. B) Initial rate of carrier-mediated thiamin uptake in vivo by intact intestinal loops from THTR-1+/+ and THTR-1−/− mice after 10 min. Data are mean ± SEM of at least 3 to 5 separate uptake determinations performed on at least three distinct sets of mice.
mRNA and protein analysis
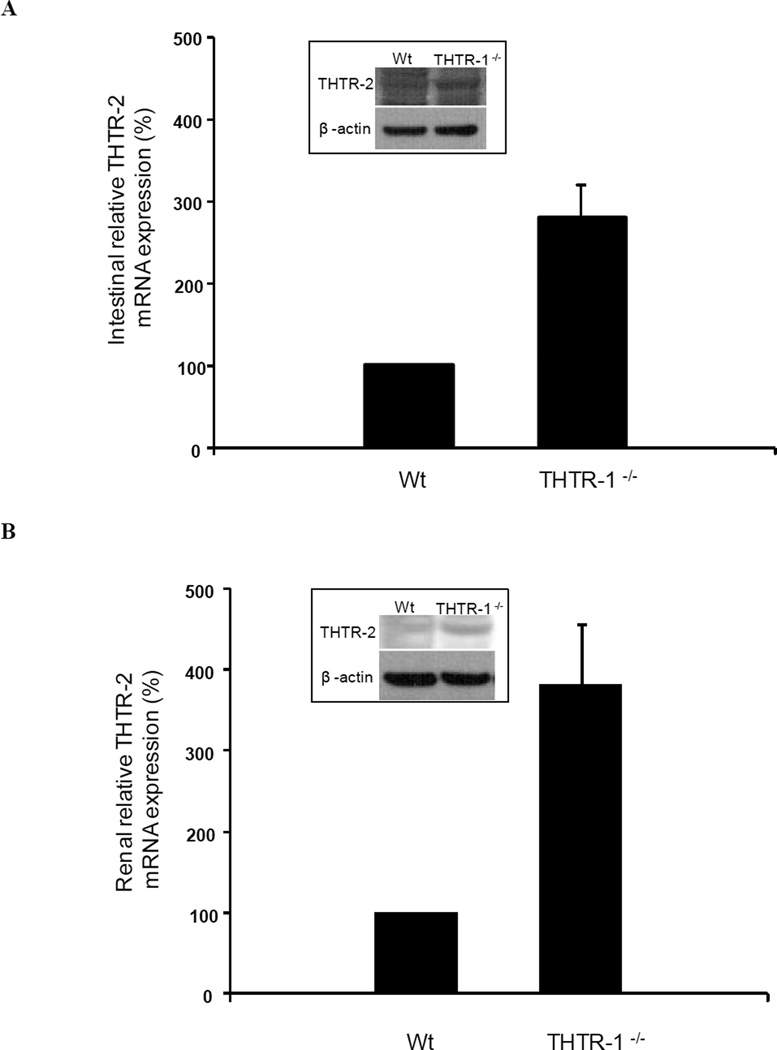
Real time quantitative PCR demonstrated that loss of THTR-1 leads to a marked induction of THTR-2 mRNA with an increase in protein level in the intestine (Fig. 6A) and kidneys of THTR-1 deficient mice compared to sex-matched wild type litter mates (Fig 6B).
Figure 6. Relative expression of THTR-2 mRNA in THTR-1−/− mouse intestine (A) and kidney (B).
Quantitative PCR analysis with THTR-2 specific primers on reverse transcribed intestinal and kidney RNA. Data were normalized to β-actin expression in five independently isolated total RNA samples of intestinal and renal tissue of sex-matched wild type (wt) litter mates and THTR-1−/− pairs of mice. The sex-matched wild type (wt) litter mate THTR-2 levels were set at 100% expression. Insets: Corresponding changes in THTR-2 protein levels in the intestine (A) and kidney (B) of THTR-1−/− mice
Discussion
As mentioned earlier, humans and other mammals cannot synthesize thiamin and thus must obtain the vitamin from exogenous sources via intestinal absorption. Both THTR-1 and THTR-2 are expressed in the human and mouse intestine but the extent of their involvement in the overall intestinal thiamin uptake process is not fully characterized. Further, humans with defective THTR-1 (i.e., TRMA patients) (12), as well as mice that are THTR-1 deficient (13) show normal plasma thiamin levels, suggesting that intestinal THTR-2 may be sufficient to maintain adequate plasma thiamin levels. To evaluate the role of THTR-2 in the intestinal thiamin uptake process in a physiologically relevant setting, we generated THTR-2-deficient mice and examined intestinal thiamin uptake. We also examined thiamin uptake in the intestine of THTR-1 deficient mice that have been previously generated (13). Intestinal thiamin uptake in these animals was compared to uptake in sex- matched wild-type littermate mice. Our findings showed that loss of THTR-2 leads to a significant decrease in intestinal thiamin uptake, which is associated with a significant decrease in blood thiamin levels. On the other hand, intestinal thiamin uptake in THTR-1 deficient mice was not significantly different from that of sex-matched wild type littermates.
The decrease in intestinal thiamin uptake and blood thiamin level observed in the THTR-2 deficient mice appear to be specific for thiamin as intestinal uptake of the unrelated water-soluble vitamin biotin and its blood level were not affected by the loss of THTR-2. The decrease in blood thiamin level is also significant given that a recent publication suggested that mutations in human THTR-2 lead to biotin-responsive basal ganglia disease (20), a condition that is alleviated by high doses of biotin. The latter has led the authors to suggest that THTR-2 may also act as a biotin transporter (20). The current in vivo findings support our previous in vitro findings in cultured intestinal epithelial cells, which concluded that THTR-2 is not a biotin transporter (21).
THTR-2 deficient mice showed a compensatory THTR-1 mRNA and protein level increase in renal tissue while no detectable change in expression level of THTR-1 was observed in the intestine. In contrast, THTR-1 deficient mice showed a marked compensatory increase of THTR-2 mRNA and protein levels in both, intestinal and renal tissue, a finding that may explain why THTR-1 deficient mice (13), and patients with TRMA with mutations in THTR-1 (12) have normal plasma thiamin levels.
The THTR-2 deficient mice did not display any specific phenotype until they reached the age of 9 to 10 months. At that time, they started to display a noticeable change that included lethargic behavior ending in death. The cause of death is not clear but does not appear to be due to abnormalities that occur in the heart and brain resulting from thiamin deficiency (1, 4, 5) as gross anatomy and histopathology of these tissues in THTR-2 deficient mice were normal. This does not exclude the possibility that structural or biochemical abnormalities occur in the brain and heart of THTR-2 deficient mice; further studies are needed to examine this possibility. We did, however, observe marked injury of the hepatic parenchyma (a pattern commonly seen in yellow fever, as well as in toxic reactions as in nitrofurazone poisoning; ref 22) and renal cortex of some of the THTR-2 deficient animals. Whether these changes contributed to the early death of these animals is not clear and again requires further investigations. The main function of thiamin transporters in the liver is to supply the vitamin to hepatocytes for use in the different metabolic reactions that thiamin is involved in. In the kidney, the role of the thiamin transporters is believed to be mainly the reabsorption of the vitamin to prevent losses in the urine.
In contrast to the above-described phenotype of the THTR-2 deficient mice, no early death was reported in the case of THTR-1 deficient mice. Males of the latter mouse line, however, were sterile (13, 14), a situation that did not occur in the case of THTR-2 deficient animals. These findings suggest that while both THTR-1 and THTR-2 are involved in thiamin transport, a defect in their function leads to different pathologies in different tissues. This is most probably due to differences in the role played by these transporters in regulating thiamin homeostasis of different cells.
In summary, results of the current investigations demonstrate that THTR-2 deficiency in mice leads to an impairment in intestinal thiamin uptake and a decrease in blood thiamin levels. In contrast, THTR-1 deficiency in mice does not impair intestinal thiamin uptake most probably due to induction in the level of expression of THTR-2 to compensate for loss of function of former thiamin carrier.
Acknowledgments
Supported by grants from the Department of Veterans Affairs (HMS) and the National Institutes of Health (DK56061 and AA18071 to HMS; DK73032 to JCR)
We would like to thank Ms. Shuling Wang for her dedicated technical assistance in establishing the THTR-2 knockout mouse colony.
Biographies
Hamid Said: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, obtained funding, and study supervision.
Nils Lambrecht: acquisition of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and technical support.
Jack Reidling: acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and technical support.
Mohammad Kassir: technical and material support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Berdanier CD. Water-soluble vitamins. In: Berdanier CD, editor. Advanced Nutrition-Micronutrients. New York: CRC Press; 1998. pp. 73–124. [Google Scholar]
- 2.Oishi K, Barchi M, Au AC, et al. Male infertility due to germ cell apoptosis in mice lacking the thiamin carrier, Tht1. A new insight into the critical role of thiamin in spermatogenesis. Devel Bio. 2004;266:299–309. doi: 10.1016/j.ydbio.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Leevy Cm, Baker H. Vitamins and alcoholism. Am J Clin Nutr. 1968;21:1325–1328. doi: 10.1093/ajcn/21.11.1325. [DOI] [PubMed] [Google Scholar]
- 4.Tanphaichitr V. Vitamin A and Carotenoids. In: Shils ME, Olsen JA, Shike M, editors. Modern Nutrition in Health and Disease. New York: Lea and Febige; 1994. pp. 359–375. r, 359–375. [Google Scholar]
- 5.Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff Syndrome and related neurological disorders due to alcoholism and malnutrition. Philadelphia, F.A.: Davis Company; 1989. [Google Scholar]
- 6.Said HM. Recent advances in carrier-mediated intestinal absorption of water-soluble vitamins. Annu Rev Physiol. 2004;66:419–446. doi: 10.1146/annurev.physiol.66.032102.144611. [DOI] [PubMed] [Google Scholar]
- 7.Said HM, Balamurugan K, Subramanian VS, et al. Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G491–G498. doi: 10.1152/ajpgi.00361.2003. [DOI] [PubMed] [Google Scholar]
- 8.Boulware MJ, Subramanian VS, Said HM, et al. Polarized expression of members of the solute carrier SLC19A gene family of water-soluble multivitamin transporters: implications for physiological function. Biochem J. 2003;376:43–48. doi: 10.1042/BJ20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diaz GA, Banikazemi M, Oishi K, et al. Mutations in a new gene encoding a thiamine transporter cause thiamine-responsive megaloblastic anaemia syndrome. Nature Genetics. 1999;22:309–312. doi: 10.1038/10385. [DOI] [PubMed] [Google Scholar]
- 10.Fleming JC, Tartaglini E, Steinkamp MP, et al. The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nature Genetics. 1999;22:305–308. doi: 10.1038/10379. [DOI] [PubMed] [Google Scholar]
- 11.Labay V, Raz T, Baron D, et al. Mutations in SLC19A2 cause thiamine-responsive megaloblastic anaemia associated with diabetes mellitus and deafness. Nature Genetics. 1999;22:300–304. doi: 10.1038/10372. [DOI] [PubMed] [Google Scholar]
- 12.Neufield EJ, Fleming JC, Tartaglini E, et al. Thiamin-responsive megaloblastic anemia syndrome: a disorder of high-affinity thiamin transport. Blood Cell Mol Dis. 2001;27:135–138. doi: 10.1006/bcmd.2000.0356. [DOI] [PubMed] [Google Scholar]
- 13.Oishi K, Hofmann S, Diaz GA, et al. Targeted disruption of Slc19a2, the gene encoding the high-affinity thiamin transporter Thtr-1, causes diabetes mellitus, sensorineural deafness and megaloblastosis in mice. Hum Mol Genet. 2002;11:2951–2960. doi: 10.1093/hmg/11.23.2951. [DOI] [PubMed] [Google Scholar]
- 14.Fleming JC, Tartaglini E, Kawatsuji R, et al. Male infertility and thiamine-dependent erythroid hypoplasia in mice lacking thiamine transporter Slc19a2. Mol Genet Metab. 2003;80:234–241. doi: 10.1016/s1096-7192(03)00141-0. [DOI] [PubMed] [Google Scholar]
- 15.Nabokina SM, Reidling JC, Said HM. Differentiation-dependent up-regulation of intestinal thiamin uptake: cellular and molecular mechanisms. J Biol Chem. 2005;280:32676–32682. doi: 10.1074/jbc.M505243200. [DOI] [PubMed] [Google Scholar]
- 16.Pinkus LM. Separation and use of enterocytes. Methods Enzymol. 1981;77:154–162. doi: 10.1016/s0076-6879(81)77020-4. [DOI] [PubMed] [Google Scholar]
- 17.Hopfer U, Nelson K, Perrotto J, et al. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973;248:25–32. [PubMed] [Google Scholar]
- 18.Laftah AH, Latunde-Dada GO, Fakih S, et al. Haem and folate transport by proton-coupled folate transporter/haem carrier protein 1 (SLC46A1) Br J Nutr. 2009;101:1150–1156. doi: 10.1017/S0007114508066762. Epub 2008 Sep 10. [DOI] [PubMed] [Google Scholar]
- 19.Reidling JC, Nabokina SM, Balamurugan K, et al. Developmental maturation of intestinal and renal thiamin uptake: studies in wild-type and transgenic mice carrying human THTR-1 and 2 promoters. J Cell Physiol. 2006;206:371–377. doi: 10.1002/jcp.20492. [DOI] [PubMed] [Google Scholar]
- 20.Zeng WQ, Al-Yamani E, Acierno JS, Jr, et al. Biotin-responsive basal ganglia disease maps to 2q36.3 and is due to mutations in SLC19A3. Am J Hum Genet. 2005;77:16–26. doi: 10.1086/431216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian VS, Marchant JS, Said HM. Biotin-responsive basal ganglia disease-linked mutations inhibit thiamine transport via hTHTR2: biotin is not a substrate for hTHTR2. Am J Physiol Cell Physiol. 2006;291:C851–C859. doi: 10.1152/ajpcell.00105.2006. [DOI] [PubMed] [Google Scholar]
- 22.Ito K, Takeuchi A, Nii A, et al. Nitrofurazone at a High Dose Induces Hepatocyte and Adrenal Necrosis in Rats. Journal of Toxicologic Pathology. 2004;17:59–61. [Google Scholar]