Abstract
Purpose
Robotic assisted radical prostatectomy has largely replaced open radical prostatectomy for the surgical management of prostate cancer despite conflicting evidence of superiority with respect to disease control or functional sequelae. Using population cohort data, in this study we examined sexual and urinary function in men undergoing open radical prostatectomy vs those undergoing robotic assisted radical prostatectomy.
Materials and Methods
Subjects surgically treated for prostate cancer were selected from 2 large population based prospective cohort studies, the Prostate Cancer Outcomes Study (enrolled 1994 to 1995) and the Comparative Effectiveness Analysis of Surgery and Radiation (enrolled 2011 to 2012). Subjects completed baseline, 6-month and 12-month standardized patient reported outcome measures. Main outcomes were between-group differences in functional outcome scores at 6 and 12 months using linear regression, and adjusting for baseline function, sociodemographic and clinical characteristics. Sensitivity analyses were used to evaluate outcomes between patients undergoing open radical prostatectomy and robotic assisted radical prostatectomy within and across CEASAR and PCOS.
Results
The combined cohort consisted of 2,438 men, 1,505 of whom underwent open radical prostatectomy and 933 of whom underwent robotic assisted radical prostatectomy. Men treated with robotic assisted radical prostatectomy reported better urinary function at 6 months (mean difference 3.77 points, 95% CI 1.09–6.44) but not at 12 months (1.19, −1.32–3.71). Subjects treated with robotic assisted radical prostatectomy also reported superior sexual function at 6 months (8.31, 6.02–10.56) and at 12 months (7.64, 5.25–10.03). Sensitivity analyses largely supported the sexual function findings with inconsistent support for urinary function results.
Conclusions
This population based study reveals that men undergoing robotic assisted radical prostatectomy likely experience less decline in early urinary continence and sexual function than those undergoing open radical prostatectomy. The clinical meaning of these differences is uncertain and longer followup will be required to establish whether these benefits are durable.
Keywords: prostatectomy, robotics, patient outcome assessment
Robotic assisted radical prostatectomy for the treatment of prostate cancer has largely supplanted open radical prostatectomy despite a lack of evidence demonstrating superior oncologic or functional outcomes.1 Various studies of RALP have reported benefits over RRP, including less blood loss and shorter length of hospital stay, with inconsistent findings of fewer bladder neck contractures, positive surgical margins, and quicker recovery of erectile function and urinary control.2–8
Many of these reports are based on data from single surgeon/institution reports, lack controls for patient comorbidities and evaluate short-term outcomes. In some studies the functional outcomes have been excluded altogether, are assessed too early postoperatively or are measured using nonstandard instruments. Community based analyses are a more representative method to assess the real-world use of these techniques rather than idealized comparisons in tertiary referral centers. To date, such studies have consistently demonstrated shorter hospital stay and less blood loss with RALP, but with variable rates of perioperative complications and positive surgical margins.9–15 Additionally, assessments of PROM using validated and reliable instruments are often lacking, and investigators have relied on administrative data sources to extrapolate disease specific function. However, it remains unknown whether this adequately reflects the patient survivorship experience.9
Despite more than a decade of experience, considerable uncertainty remains surrounding the comparative effectiveness and harms of RALP and RRP in the context of the questionable cost-effectiveness of RALP.15,16 Unfortunately, a prospective randomized trial (NCT01365143) designed to address many of these shortcomings was closed due to lack of accrual.17 A single-institution randomized trial is ongoing in Australia but may be limited by methodological concerns.18
The goal of this study was to compare sexual and urinary function between men with prostate cancer selected in a population based manner undergoing RRP or RALP, using established measurement strategies while controlling for a large number of potential confounders. We used data from the PCOS and CEASAR, both of which are population based cohorts of men treated for prostate cancer, and contain data using validated and reliable PROM.
METHODS
Patients
Data were obtained from 2 large, population based, prospective cohort studies. The PCOS enrolled patients with incident prostate cancer from 6 participating SEER sites between October 1, 1994 and October 31, 1995. Details of PCOS methods have been previously reported.19
CEASAR recruited men from 5 SEER registries from January 2011 to February 2012 with a small proportion of subjects from CaPSURE™, an observational prostate cancer registry.20 Details and objectives for the CEASAR study have also been previously reported.21 Institutional review boards at all participating sites including the Vanderbilt University coordinating site approved the studies.
In total, PCOS initially enrolled 5,672 subjects while CEASAR enrolled 3,691 subjects. We selected men in either parent study who underwent radical prostatectomy and completed baseline PROM.
Upon enrollment, PCOS subjects completed a baseline survey including items regarding clinical and sociodemographic variables, comorbidities and disease specific outcomes using the UCLA Prostate Cancer Index, a reliable and validated PROM of sexual function, urinary incontinence and bowel function related to prostate cancer and its treatment.22 CEASAR participants completed a similar baseline assessment using the EPIC, valid and reliable PROM developed from and containing many similar/identical items as the UCLA Prostate Cancer Index. Previous work has reported similar psychometric performance for these instruments.23 To minimize bias from differences in the 2 PROM, we included 4 common measures of urinary incontinence and 3 common measures of sexual function, and derived modified domain summary scores scaled 0 to 100, with 100 representing optimal function.24
All subjects in PCOS who had surgery underwent RRP because RALP was first introduced more than 5 years after enrollment ended.25 The majority of CEASAR participants who had surgery underwent RALP (78%).
Statistical Analysis
We compared differences between baseline characteristics including inflation adjusted income for PCOS participants using nonparametric tests. In addition to standard socio-demographic and clinical characteristics, we evaluated differences in number of comorbidities, baseline general health and whether nerve sparing (any degree vs none) was performed.
Primary Outcomes
To assess differences in function between patients undergoing RRP vs RALP, we fit 4 multivariable linear regression models, one for each domain summary score (sexual and urinary function) and time point (6 and 12-month surveys). Covariate adjustment was used to control for age (continuous), race, income, education, marital and health insurance status, study site, days since treatment, PSA, Gleason score, margin and nerve sparing status, pathological stage (pT2c or lower vs pT3 or higher), use of androgen deprivation, self-reported overall health and baseline function. Sexual function models also adjusted for use of erectile dysfunction treatments such as injections, vacuum pump, penile prosthesis or medications. Missing values for baseline characteristics included in these models were multiply imputed through predictive mean matching.
To account for documented differences in baseline function between PCOS and CEASAR subjects,24 mean function was modeled with an interaction term between baseline function and surgical procedure (RRP vs RALP). Primary comparisons were assessed using these models, estimating the mean difference in function between patients undergoing the 2 procedures. Model based contrast tests using t-statistics were used at varying levels of baseline function to identify a threshold, above which between-group differences were significant.
Secondary Analyses
A second set of 4 multivariable linear regression models was developed by adding study cohort (CEASAR and PCOS) to the models used for the primary analysis. Sensitivity analyses were performed to evaluate differences attributable to the lack of availability of PD5 inhibitors during PCOS, and to mitigate possible confounding associated with differences in baseline reporting of baseline function.24 This enabled the exploration of between-group differences in outcomes for 3 comparisons of 1) RRP in PCOS vs RRP in CEASAR, 2) RRP in CEASAR vs RALP and 3) RRP in PCOS vs RALP. If observed differences in primary outcomes were explained by differences in surgical technique, one would expect within-group differences between study cohorts not to be significant in comparison 1, but between-group differences to remain significant in comparisons 2 and 3.
To better characterize the impact of surgical approach on treatment in a fashion more meaningful to patients, we fit logistic regression models using each of the 7 individual items aggregated for the domain specific scores. These items were dichotomously recoded from the original Likert-type scales using clinically relevant cut points.
All p values were 2-sided and p <0.05 was considered statistically significant. Confidence intervals are all 95 percent. The analysis was completed using R software v.3.1.2 (R Foundation, Vienna, Austria).
RESULTS
The study cohort comprised 2,438 men, of whom 933 underwent RALP while 1,505 underwent RRP (82.6% from PCOS and 17.4% from CEASAR). All sociodemographic characteristics between the groups were different except for marital status. Participants who underwent RALP were younger, and more likely to be white, employed full-time, and have higher income and education, and private insurance (table 1). Differences were also noted for baseline clinical characteristics. Unadjusted negative margin rate and nerve sparing rate were highest in RALP. Baseline scores for sexual and urinary function were highest in the PCOS group, consistent with previous work (table 2).24
Table 1.
Baseline sociodemographic characteristics by surgery type and cohort
| RALP | RRP (total) | RRP (PCOS) | RRP (CEASAR) | p Value*
|
||
|---|---|---|---|---|---|---|
| Test 1† | Test 2‡ | |||||
| No. pts | 933 | 1,505 | 1,243 | 262 | ||
| No. pt age (%): | 0.004 | 0.002 | ||||
| Less than 55 | 173 (18.5) | 239 (15.9) | 208 (16.7) | 31 (11.8) | ||
| 55–59 | 234 (25.1) | 328 (21.8) | 272 (21.9) | 56 (21.4) | ||
| 60–64 | 244 (26.2) | 352 (23.4) | 278 (22.4) | 74 (28.2) | ||
| 65–69 | 181 (19.4) | 376 (25.0) | 312 (25.1) | 64 (24.4) | ||
| 70–74 | 90 (9.6) | 183 (12.2) | 150 (12.1) | 33 (12.6) | ||
| 75 or Greater | 11 (1.2) | 27 (1.8) | 23 (1.9) | 4 (1.5) | ||
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| No. race (%): | <0.001 | <0.001 | ||||
| Black | 80 (8.6) | 243 (16.1) | 207 (16.7) | 36 (13.7) | ||
| Hispanic | 61 (6.5) | 191 (12.7) | 173 (13.9) | 18 (6.9) | ||
| Other | 38 (4.1) | 11 (0.7) | 0 (0.0) | 11 (4.2) | ||
| White | 739 (79.2) | 1,057 (70.2) | 863 (69.4) | 194 (74.0) | ||
| Missing | 15 (1.6) | 3 (0.2) | 0 (0.0) | 3 (1.1) | ||
| No. income (%): | <0.001 | <0.001 | ||||
| Less than $30,000 | 102 (10.9) | 287 (19.1) | 228 (18.3) | 59 (22.5) | ||
| $30,000–$100,000 | 446 (47.8) | 794 (52.8) | 673 (54.1) | 121 (46.2) | ||
| More than $100,000 | 319 (34.2) | 322 (21.4) | 261 (21.0) | 61 (23.3) | ||
| Missing | 66 (7.1) | 102 (6.8) | 81 (6.5) | 21 (8.0) | ||
| No. education (%): | <0.001 | <0.001 | ||||
| High school or less | 229 (24.5) | 567 (37.7) | 478 (38.5) | 89 (34.0) | ||
| Some college | 200 (21.4) | 365 (24.3) | 307 (24.7) | 58 (22.1) | ||
| College graduate | 224 (24.0) | 219 (14.6) | 166 (13.4) | 53 (20.2) | ||
| Advanced degree | 254 (27.2) | 334 (22.2) | 282 (22.7) | 52 (19.8) | ||
| Missing | 26 (2.8) | 20 (1.3) | 10 (0.8) | 10 (3.8) | ||
| No. employment (%): | <0.001 | <0.001 | ||||
| Other | 30 (3.2) | 74 (4.9) | 54 (4.3) | 20 (7.6) | ||
| Part-time | 63 (6.8) | 141 (9.4) | 114 (9.2) | 27 (10.3) | ||
| Full-time | 557 (59.7) | 570 (37.9) | 461 (37.1) | 109 (41.6) | ||
| Retired | 271 (29.0) | 710 (47.2) | 606 (48.8) | 104 (39.7) | ||
| Missing | 12 (1.3) | 10 (0.7) | 8 (0.6) | 2 (0.8) | ||
| No. not married (%): | 137 (14.7) | 230 (15.3) | 185 (14.9) | 45 (17.2) | 0.494 | 0.844 |
| Missing | 28 (3.0) | 15 (1.0) | 5 (0.4) | 10 (3.8) | ||
| No. privately insured (%): | 620 (66.5) | 842 (55.9) | 706 (56.8) | 136 (51.9) | <0.001 | <0.001 |
| Missing | 9 (1.0) | 100 (6.6) | 98 (7.9) | 2 (0.8) | ||
Pearson chi-square test.
Comparisons are between RALP, RRP (PCOS) and RRP (CEASAR).
Comparisons are between RALP (total) and RRP (total).
Table 2.
Additional characteristics by surgery type and cohort
| RALP | RRP (total) | RRP (PCOS) | RRP (CEASAR) | p Value*
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test 1* | Test 2† | |||||||||
| No. pts | 933 | 1,505 | 1,243 | 262 | ||||||
| Clinicopathological characteristics | ||||||||||
| No. pathological staging (%): | ||||||||||
| pT2 | 735 | (78.8) | 1,015 | (67.4) | 810 | (65.2) | 205 | (78.2) | <0.001‡ | <0.001‡ |
| pT3 (not otherwise specified) | 4 | (0.4) | 105 | (7.0) | 100 | (8.0) | 5 | (1.9) | ||
| pT3a | 114 | (12.2) | 202 | (13.4) | 179 | (14.4) | 23 | (8.8) | ||
| pT3b | 32 | (3.4) | 108 | (7.2) | 92 | (7.4) | 16 | (6.1) | ||
| pT4 | 0 | (0.0) | 42 | (2.8) | 42 | (3.4) | 0 | (0.0) | ||
| Other | 3 | (0.3) | 9 | (0.6) | 6 | (0.5) | 3 | (1.1) | ||
| Missing | 45 | (4.8) | 24 | (1.6) | 14 | (1.1) | 10 | (3.8) | ||
| Median ng/ml PSA (IQR) | 5.0 | (4.1–6.7) | 6.6 | (4.8–9.9) | 6.9 | (5.0–10.5) | 5.2 | (4.2–7.6) | <0.001§ | <0.001§ |
| No. PSA missing (%) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | ||
| No. Gleason score (%): | <0.001‡ | <0.001‡ | ||||||||
| 6 or Less | 294 | (31.5) | 762 | (50.6) | 664 | (53.4) | 98 | (37.4) | ||
| 7 | 557 | (59.7) | 462 | (30.7) | 333 | (26.8) | 129 | (49.2) | ||
| 8–10 | 59 | (6.3) | 126 | (8.4) | 96 | (7.7) | 30 | (11.5) | ||
| Missing | 23 | (2.5) | 155 | (10.3) | 150 | (12.1) | 5 | (1.9) | ||
| No. neg margins (%): | 710 | (76.1) | 936 | (62.2) | 756 | (60.8) | 180 | (68.7) | <0.001‡ | <0.001‡ |
| Missing | 28 | (3.0) | 100 | (6.6) | 92 | (7.4) | 8 | (3.1) | ||
| No. nerve sparing (%): | 728 | (78.0) | 702 | (46.6) | 517 | (41.6) | 185 | (70.6) | <0.001‡ | <0.001‡ |
| Missing | 145 | (15.5) | 302 | (20.1) | 242 | (19.5) | 60 | (22.9) | ||
| No. comorbidities (%): | <0.001‡ | 0.001‡ | ||||||||
| 0 | 355 | (38.0) | 794 | (52.8) | 717 | (57.7) | 77 | (29.4) | ||
| 1 | 362 | (38.8) | 452 | (30.0) | 344 | (27.7) | 108 | (41.2) | ||
| 2 | 114 | (12.2) | 163 | (10.8) | 131 | (10.5) | 32 | (12.2) | ||
| 3 or Greater | 26 | (2.8) | 60 | (4.0) | 51 | (4.1) | 9 | (3.4) | ||
| Missing | 76 | (8.1) | 36 | (2.4) | 0 | (0.0) | 36 | (13.7) | ||
| Baseline pt reported outcomes | ||||||||||
| No. self-reported overall health (%): | <0.001‡ | <0.001‡ | ||||||||
| Poor | 6 | (0.6) | 21 | (1.4) | 17 | (1.4) | 4 | (1.5) | ||
| Fair | 35 | (3.8) | 108 | (7.2) | 90 | (7.2) | 18 | (6.9) | ||
| Good | 221 | (23.7) | 438 | (29.1) | 351 | (28.2) | 87 | (33.2) | ||
| Very good | 429 | (46.0) | 592 | (39.3) | 485 | (39.0) | 107 | (40.8) | ||
| Excellent | 240 | (25.7) | 342 | (22.7) | 296 | (23.8) | 46 | (17.6) | ||
| Missing | 2 | (0.2) | 4 | (0.3) | 4 | (0.3) | 0 | (0.0) | ||
| Median urinary function summary score (IQR) | 100.0 | (85.0–100.0) | 100 | (100.0–100.0) | 100.0 | (100.0–100.0) | 100.0 | (88.8–100.0) | <0.001§ | <0.001§ |
| Median sexual function summary score (IQR) | 86.7 | (58.3–100.0) | 88.9 | (66.7–100.0) | 93.3 | (73.3–100.0) | 79.2 | (51.7–93.3) | <0.001§ | <0.001§ |
Comparisons are among RALP, RRP (PCOS) and RRP (CEASAR).
Comparisons are between RALP (total) and RRP (total).
Pearson chi-square test.
Kruskal-Wallis test.
Primary Outcomes
Multivariable models revealed that patients undergoing RALP reported higher urinary function scores at 6 months and sexual function at 6 and 12 months (table 3). However, patients undergoing RALP did not report superior urinary function at 12 months. The relationship between the interaction of surgical approach with baseline function and postoperative functional outcome was also significant for the same periods. Age, baseline function and general health were also associated with postoperative functional outcomes, while income and race were not.
Table 3.
Multivariable linear regression models for domain summary scores by time of survey
| Urinary Function Summary Scores
|
Sexual Function Summary Scores*
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 6 Mos
|
12 Mos
|
6 Mos
|
12 Mos
|
|||||
| Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | |
| Surgery: RALP vs RRP | 3.77 | 0.005 | 1.20 | 0.35 | 8.31 | <0.001 | 7.64 | <0.001 |
| Baseline function (10 pts)† | 0.61 | 0.21 | 1.87 | <0.001 | 1.20 | <0.001 | 1.54 | <0.001 |
| Interactions | ||||||||
| Surgery-baseline function (10 pts) | 1.96 | 0.003 | 0.55 | 0.39 | 1.43 | <0.001 | 2.01 | <0.001 |
| Age-10 yrs, continuous | −2.78 | 0.001 | −2.63 | 0.001 | −1.43 | 0.06 | −3.22 | <0.001 |
| Race: white vs other | 0.75 | 0.56 | −0.06 | 0.96 | −0.33 | 0.76 | −0.65 | 0.58 |
| Income vs less than $30,000: | ||||||||
| $30,000–$100,000 | −0.80 | 0.59 | −0.26 | 0.86 | −1.13 | 0.39 | −0.12 | 0.93 |
| Greater than $100,000 | 3.17 | 0.09 | 3.12 | 0.08 | 1.75 | 0.28 | 1.34 | 0.45 |
| General health vs excellent: | ||||||||
| Poor | −23.59 | <0.001 | −24.22 | <0.001 | −12.15 | 0.002 | −15.94 | <0.001 |
| Fair | −6.04 | 0.008 | −6.95 | 0.002 | −5.83 | 0.003 | −10.83 | <0.001 |
| Good | −9.38 | <0.001 | −9.91 | <0.001 | −4.57 | <0.001 | −4.15 | 0.001 |
| Very good | −2.67 | 0.03 | −4.17 | <0.001 | −1.75 | 0.09 | −3.16 | 0.004 |
Adjusted for education, marital and health insurance status, study site, days since treatment, PSA, Gleason score, margin and nerve sparing status, pathological stage, and use of androgen deprivation.
Also adjusted for use of erectile dysfunction treatments.
Refers to baseline summary scores for respective functional domains.
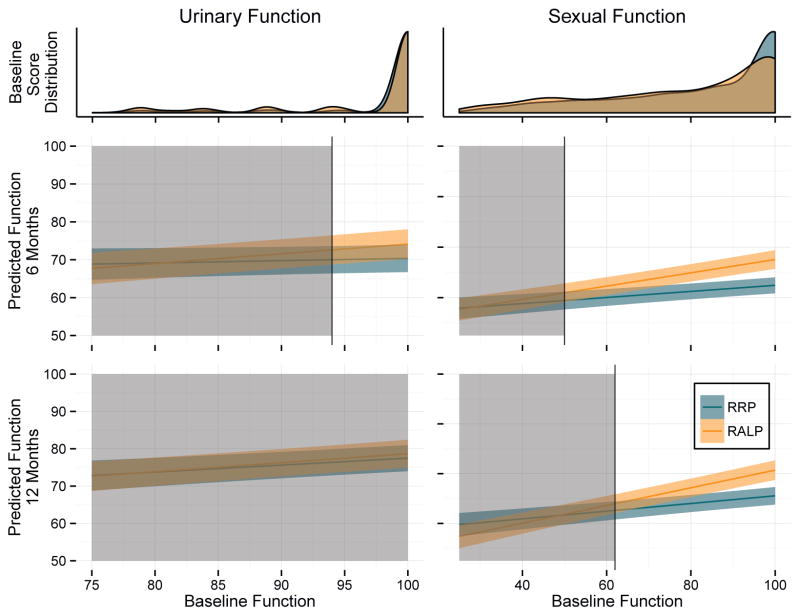
We then identified thresholds for baseline function above which there were statistically significant between-group differences in predicted scores (fig. 1). For urinary function (6 months) significant differences were noted for patients whose baseline function score was 94 or greater, above which the majority of men reported baseline function. Above this threshold the magnitude of the difference in favor of RALP over RRP ranged from 2.59 points (95% CI 0.30–5.08) to 3.77 points (1.11–6.44). At 12 months there were no differences in predicted scores at any threshold.
Figure 1.
Model based predicted postoperative function by baseline function and distribution of baseline function scores. Shaded areas indicate that between-group differences in predicted function are not statistically different at significance level of 0.05. Unshaded areas represent statistically significant differences at p <0.05. Colored bands represent 95% CIs for predicted scores. Overlap of intervals does not indicate that differences are nonsignificant.
Differences in sexual function were noted for those with a baseline function score of at least 50 at 6 months and 62 at 12 months, values also reported by the majority of men in both studies. The magnitude of this difference ranged from 3.06 points (0.14–5.99) to 10.21 points (7.51–12.91) at 6 months and 2.70 points (0.08–5.31) to 10.31 points (7.51–13.13) at 12 months.
Individual Items
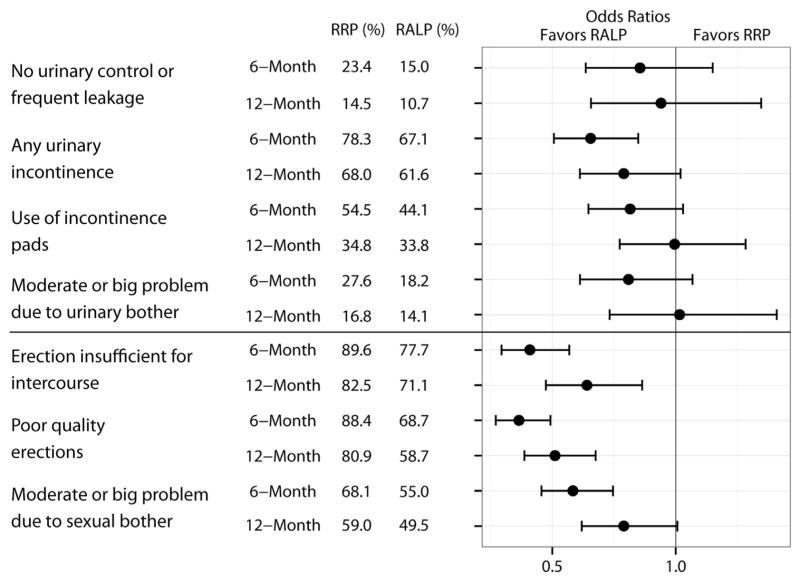
Unadjusted responses to individual PROM items at 6 and 12 months and odds ratios from logistic regression items are presented in figure 2. Several sexual function items at 6 and 12 months were better for RALP and none suggested benefit for RRP. While several urinary function items favored RALP, with the exception of “any urinary incontinence” at 6 months no statistically significant differences were found.
Figure 2.
Unadjusted responses and logistic regression models for individual items comparing RRP to RALP. Values less than 1 favor RALP. Error bars represent 95% CIs.
Sensitivity Analyses
Primary urinary function results favoring RALP were inconsistently supported in the sensitivity analyses (table 4). Consistent with primary results, there were no differences for comparison 1, that is comparing urinary function outcomes between RRP groups from each of the 2 parent studies. Significant between-group differences persisted when comparing RRP in PCOS to RALP (comparison 3). However, when examining the contemporary cohort (ie between RRP and RALP in CEASAR, comparison 2), significant within-study differences for urinary function were not identified.
Table 4.
Model based predicted scores and contrast tests comparing surgical approach and study cohort stratified by time since treatment
| Predicted Scores | Difference | 95% CI | p Value (t-statistics) | |
|---|---|---|---|---|
| Comparison of RRP (PCOS) vs RRP (CEASAR) | ||||
| Urinary function: | ||||
| 6-Mo | 69.73 vs 72.76 | 3.03 | −1.19–7.24 | 0.16 |
| 12-Mo | 77.67 vs 76.81 | −0.86 | −4.83–3.10 | 0.67 |
| Sexual function: | ||||
| 6-Mo | 42.17 vs 48.68 | 6.52 | 2.81–10.23 | 0.001 |
| 12-Mo | 48.98 vs 50.40 | 1.42 | −2.47–5.31 | 0.47 |
| Comparison of RRP (CEASAR ) vs RALP | ||||
| Urinary function: | ||||
| 6-Mo | 72.76 vs 74.67 | 1.92 | −1.76–5.59 | 0.31 |
| 12-Mo | 76.81 vs 78.39 | 1.58 | −1.83–4.98 | 0.36 |
| Sexual function: | ||||
| 6-Mo | 48.68 vs 53.14 | 4.46 | 1.30–7.62 | 0.006 |
| 12-Mo | 50.40 vs 56.57 | 6.18 | 2.88–9.48 | <0.001 |
| Comparison of RRP (PCOS) vs RALP | ||||
| Urinary function: | ||||
| 6-Mo | 69.73 vs 74.67 | 4.94 | 1.75–8.13 | 0.002 |
| 12-Mo | 77.67 vs 78.39 | 0.71 | −2.34–3.76 | 0.65 |
| Sexual function: | ||||
| 6-Mo | 42.17 vs 53.14 | 10.98 | 8.16–13.80 | <0.001 |
| 12-Mo | 48.98 vs 56.57 | 7.60 | 4.62–10.57 | <0.001 |
Sexual function primary outcomes were consistently supported by the sensitivity analyses. Predicted scores for men undergoing RRP in the contemporary and previous eras (ie CEASAR and PCOS, comparisons 2 and 3) were significantly lower at 6 and 12 months compared to RALP. While within-group differences for those undergoing RRP (comparison 1) were significant at 6 months, by 12 months the differences were not statistically different from zero.
DISCUSSION
Data from 2 large, prospective, population based cohort studies of prostate cancer survivors demonstrate that patients treated with RALP report more favorable sexual function at 6 and 12 months as well as more favorable urinary function scores at 6 months. Similar findings were observed when the analysis used responses to individual items. Of note, there were no significant differences in urinary function between the surgical approaches at 12 months, and sensitivity analyses, with less statistical power, provided inconsistent support of differences in urinary function at 6 months.
These possible outcome benefits for sexual function attributed to RALP have not been previously shown in a consistent manner. Many prior studies were undertaken at high volume tertiary referral centers and patients who seek treatment at these institutions may be different from the general population. Furthermore, surgeons who operate in these centers tend to have high operative volumes and their outcomes may be different from surgeons operating in the community.26 The current study is population based, and includes surgeons from tertiary referral centers and those in community practice and, accordingly, more accurately reflects the experience of prostate cancer survivors undergoing prostatectomy.
Alternatively, the difference between these findings and those of prior studies may be due to our inclusion of baseline function in the multivariable models. Several cross-sectional analyses comparing RRP and RALP that have not demonstrated functional outcome differences failed to adjust for baseline differences,7,9,12 while a recent study demonstrated sexual function benefit for RALP after doing so.27
Differences between RALP and RRP in this study could be due to factors unrelated to robotic technology but from general patterns of prostate cancer care and/or surgical training. During the adoption of RALP, prostate cancer care became more centralized1 and several reports suggest that better outcomes may be achieved at high volume centers.26 Furthermore, instruction designed to teach the operation relied heavily on the use of video demonstrations that may have standardized the technique more than was achievable for RRP. Therefore, differences may be due to the reduction of variation in technique rather than intrinsic advantages of the robot.
That PCOS only included RRP limits bias from treatment selection. Surgeons continuing to perform RRP in the CEASAR era may select patients with adverse pathology, potentially confounding data surrounding functional outcomes. We largely adjusted for possible confounders in our multivariable models but acknowledge the prospect of remaining unmeasured confounding.
Some might suggest that the only way to eliminate selection bias is to perform a randomized trial. A large multicenter trial, required to permit generalizability, is unlikely ever to be done given the current pervasiveness of RALP and a previously failed attempt due to accrual.17 The only ongoing randomized trial of which we are aware is a single center design comparing a high volume open surgeon to a high volume robotic surgeon resulting in significant questions about external validity.18
While this work has demonstrated statistically significant differences, especially for sexual function, it is uncertain if the observed differences achieve clinical significance. A third of the standard deviation has been suggested as an acceptable value for a minimally important difference in EPIC.28 Using this standard, minimal important differences are 4.77 for urinary function and 7.64 for sexual function. Confidence intervals contained these values even in those with the highest levels of baseline function, limiting conclusions that observed differences are clinically meaningful.
Differences in baseline function between the 2 parent studies have been reported with PCOS patients reporting better baseline function.24 For this reason, and given that baseline function may be the most important predictor of posttreatment function,29 we adjusted for this in our models. Furthermore, better baseline function in the PCOS group favors the opposite outcome than what was observed, suggesting that the benefit of RALP may be underestimated. Nonetheless, PCOS and CEASAR, initiated more than 15 years apart, had similar methodology, and provide a means for determining the comparative effectiveness of the 2 approaches, which has otherwise been elusive.
This study has several limitations. The primary measures of sexual and urinary function were created from common items of the Prostate Cancer Index and EPIC. Psychometric validation of this approach has not been established but single-item analysis appeared consistent with the main results. In addition, the introduction of PD5 inhibitors occurred in 1998, after first year data collection in the PCOS. We adjusted for differences in the use of erectile aids but could not adjust for this qualitative difference in treatment. Sensitivity analyses consistently supported findings of improved sexual function at 12 months in those undergoing RALP, suggesting that even with PD5 inhibitors, patients undergoing RALP enjoyed a sexual function benefit.
While this study reveals improvements in patient reported urinary and sexual function attributable to RALP, this study does not evaluate the value assigned to these between-group differences. The widespread use and uptake of robotic technology were largely driven by market forces despite associated increased costs and before demonstrable patient benefit.30 As a society we need to evaluate the value of incremental benefits to determine the appropriateness of innovative technologies to ensure patients are provided with high value care.
CONCLUSIONS
This population based study reveals that patients undergoing RALP likely experience less functional decline in early urinary continence and sexual function compared to those undergoing RRP, but it is uncertain whether these differences are clinically meaningful. Differences in urinary function between the 2 procedures resolve by 12 months while differences in sexual function persist up to 12 months after surgery. Longer followup will be required to establish whether this benefit is durable beyond 1 year and to assess oncologic outcomes.
Acknowledgments
Supported by the U.S. Agency for Healthcare Research and Quality (Grants 1R01HS019356 and 1R01HS022640-01); the National Cancer Institute, National Institutes of Health (Grant R01-CA114524), and the following contracts to each of the participating institutions: N01-PC-67007, N01-PC-67009, N01-PC-67010, N01-PC-67006, N01-PC-67005 and N01-PC-67000, and through a contract from the Patient-Centered Outcomes Research Institute.
The men who participated in CEASAR and PCOS, the physicians in the SEER regions who assisted in data collection from their patients and from medical records, the study managers and chart abstractors, and all the staff for each of the 6 cancer registries provided assistance with these studies.
Abbreviations and Acronyms
- CEASAR
Comparative Effectiveness Analysis of Surgery and Radiation
- EPIC
Expanded Prostate Cancer Index Composite-26
- PCOS
Prostate Cancer Outcomes Study
- PD5
phosphodiesterase type 5
- PROM
patient reported outcome measures
- RALP
robotic assisted radical prostatectomy
- RRP
open radical prostatectomy
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
No direct or indirect commercial incentive associated with publishing this article.
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
References
- 1.Anderson CB, Penson DF, Ni S, et al. Centralization of radical prostatectomy in the United States. J Urol. 2013;189:500. doi: 10.1016/j.juro.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Farnham SB, Webster TM, Herrell SD, et al. Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology. 2006;67:360. doi: 10.1016/j.urology.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Nelson B, Kaufman M, Broughton G, et al. Comparison of length of hospital stay between radical retropubic prostatectomy and robotic assisted laparoscopic prostatectomy. J Urol. 2007;177:929. doi: 10.1016/j.juro.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 4.Smith JA, Jr, Chan RC, Chang SS, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178:2385. doi: 10.1016/j.juro.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Menon M, Shrivastava A, Kaul S, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007;51:648. doi: 10.1016/j.eururo.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 6.Rocco B, Matei DV, Melegari S, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: a matched-pair analysis. BJU Int. 2009;104:991. doi: 10.1111/j.1464-410X.2009.08532.x. [DOI] [PubMed] [Google Scholar]
- 7.Krambeck AE, DiMarco DS, Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448. doi: 10.1111/j.1464-410X.2008.08012.x. [DOI] [PubMed] [Google Scholar]
- 8.Thompson JE, Egger S, Bohm M, et al. Superior quality of life and improved surgical margins are achievable with robotic radical prostatectomy after a long learning curve: a prospective single-surgeon study of 1552 consecutive cases. Eur Urol. 2014;65:521. doi: 10.1016/j.eururo.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 10.Trinh QD, Sammon J, Sun M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the Nationwide Inpatient Sample. Eur Urol. 2012;61:679. doi: 10.1016/j.eururo.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Kowalczyk KJ, Levy JM, Caplan CF, et al. Temporal national trends of minimally invasive and retropubic radical prostatectomy outcomes from 2003 to 2007: results from the 100% Medicare sample. Eur Urol. 2012;61:803. doi: 10.1016/j.eururo.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Alemozaffar M, Sanda M, Yecies D, et al. Benchmarks for operative outcomes of robotic and open radical prostatectomy: results from the Health Professionals Follow-up Study. Eur Urol. 2014;67:432. doi: 10.1016/j.eururo.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu JC, Gandaglia G, Karakiewicz PI, et al. Comparative effectiveness of robot-assisted versus open radical prostatectomy cancer control. Eur Urol. 2014;66:666. doi: 10.1016/j.eururo.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Wallerstedt A, Tyritzis SI, Thorsteinsdottir T, et al. Short-term results after robot-assisted laparoscopic radical prostatectomy compared to open radical prostatectomy. Eur Urol. 2014;67:660. doi: 10.1016/j.eururo.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Gandaglia G, Sammon JD, Chang SL, et al. Comparative effectiveness of robot-assisted and open radical prostatectomy in the post-dissemination era. J Clin Oncol. 2014;32:1419. doi: 10.1200/JCO.2013.53.5096. [DOI] [PubMed] [Google Scholar]
- 16.Hohwu L, Borre M, Ehlers L, et al. A short-term cost-effectiveness study comparing robot-assisted laparoscopic and open retropubic radical prostatectomy. J Med Econ. 2011;14:403. doi: 10.3111/13696998.2011.586621. [DOI] [PubMed] [Google Scholar]
- 17.Prospective Randomized Trial Comparing Robotic Versus Open Radical Prostatectomy, ClinicalTrials.gov 2015 (NCT01365143).
- 18.Gardiner RA, Yaxley J, Coughlin G, et al. A randomised trial of robotic and open prostatectomy in men with localised prostate cancer. BMC Cancer. 2012;12:189. doi: 10.1186/1471-2407-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potosky AL, Legler J, Albertsen PC, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92:1582. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 20.Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996;48:773. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 21.Barocas DA, Chen V, Cooperberg M, et al. Using a population-based observational cohort study to address difficult comparative effectiveness research questions: the CEASAR study. J Comp Eff Res. 2013;2:445. doi: 10.2217/cer.13.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litwin MS, Hays RD, Fink A, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Namiki S, Takegami M, Kakehi Y, et al. Analysis linking UCLA PCI with Expanded Prostate Cancer Index Composite: an evaluation of health related quality of life in Japanese men with localized prostate cancer. J Urol. 2007;178:473. doi: 10.1016/j.juro.2007.03.113. [DOI] [PubMed] [Google Scholar]
- 24.Resnick MJ, Barocas DA, Morgans AK, et al. The evolution of self-reported urinary and sexual dysfunction over the last two decades: implications for comparative effectiveness research. Eur Urol. 2014;67:1019. doi: 10.1016/j.eururo.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon M, Shrivastava A, Tewari A, et al. Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. 2002;168:945. doi: 10.1016/S0022-5347(05)64548-X. [DOI] [PubMed] [Google Scholar]
- 26.Trinh QD, Bjartell A, Freedland SJ, et al. A systematic review of the volume-outcome relationship for radical prostatectomy. Eur Urol. 2013;64:786. doi: 10.1016/j.eururo.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stolzenburg JU, Graefen M, Kriegel C, et al. Effect of surgical approach on erectile function recovery following bilateral nerve-sparing radical prostatectomy: an evaluation utilizing data from a randomized, double-blind, double-dummy multicenter trial of tadalafil versus placebo. BJU Int. 2015;116:241. doi: 10.1111/bju.13030. [DOI] [PubMed] [Google Scholar]
- 28.Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the expanded prostate cancer index composite short form. Urology. 2015;85:101. doi: 10.1016/j.urology.2014.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol. 2009;27:3916. doi: 10.1200/JCO.2008.18.6486. [DOI] [PubMed] [Google Scholar]
- 30.Barbash GI, Friedman B, Glied SA, et al. Factors associated with adoption of robotic surgical technology in US hospitals and relationship to radical prostatectomy procedure volume. Ann Surg. 2014;259:1. doi: 10.1097/SLA.0b013e3182a5c8b8. [DOI] [PubMed] [Google Scholar]