Abstract
Purpose of Review
To describe methodological challenges, gaps, and opportunities in U.S. transgender health research.
Recent Findings
Lack of large prospective observational studies and intervention trials, limited data on risks and benefits of gender affirmation (e.g., hormones and surgical interventions), and inconsistent use of definitions across studies hinder evidence-based care for transgender people. Systematic high-quality observational and intervention-testing studies may be carried out using several approaches, including general population-based, health systems-based, clinic-based, venue-based, and hybrid designs. Each of these approaches has its strength and limitations; however, harmonization of research efforts is needed. Ongoing development of evidence-based clinical recommendations will benefit from a series of observational and intervention studies aimed at identification, recruitment, and follow-up of transgender people of different ages, from different racial, ethnic, and socioeconomic backgrounds and with diverse gender identities.
Summary
Transgender health research faces challenges that include standardization of lexicon, agreed-upon population definitions, study design, sampling, measurement, outcome ascertainment, and sample size. Application of existing and new methods is needed to fill existing gaps, increase the scientific rigor and reach of transgender health research, and inform evidence-based prevention and care for this underserved population.
Keywords: transgender, research methods, health disparity
Introduction
Expansion of the evidence-base to inform transgender clinical care requires rich systematically collected data that at present are scarce or lacking in the U.S. [1]. The available data pertaining to transgender health are often based on convenience samples and the majority of published studies in the U.S. are cross-sectional [2–7] or retrospective [8–10]. The few published prospective follow up studies are small and have examined a limited number of outcomes [11,12]. Most U.S. studies evaluating transgender health focus on substance use and abuse, sexual health, and mental health issues (Figure 1). Relatively little emphasis has been placed on other relevant issues such as healthcare access and utilization patterns over time, determinants of hormonal and surgical treatment complications, and rates of chronic age-related conditions thought to be affected by hormone exposures [13,14].
Figure 1.
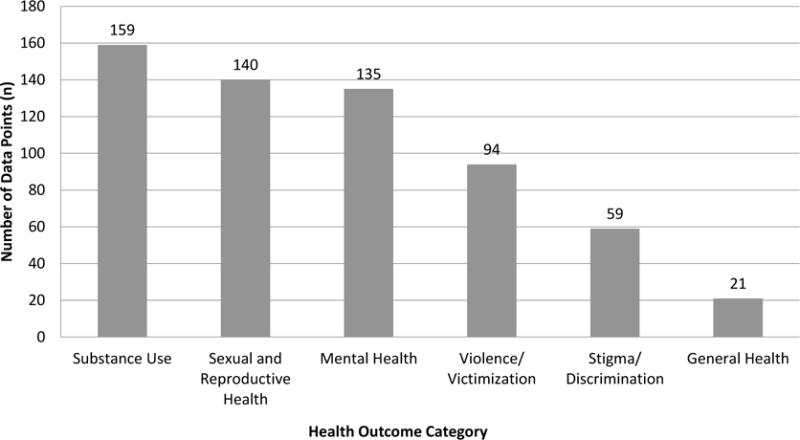
Distribution of health outcomes in U.S. Transgender Research, 2008–2014.
An important methodological issue specific to transgender health research is identification, measurement, and operationalization of “transgender” [15]. Additional challenges facing transgender health research include heterogeneity of settings, limited numbers of trained physicians and researchers with a specific focus in transgender medicine, lack of uniform data collection, and potential problems of non-participation, retention, and drop-out.
Important unanswered questions in transgender health research may be placed into two broad categories: 1) those that deal with estimating population parameters, and 2) those concerned with evaluating causal effects of exposures on health outcomes [16]. Both categories of research are needed in transgender health. An essential methodological requirement of studies aimed at answering questions in the first category is a representative sample of the population of interest. By contrast, for the second category, representativeness is of a lesser concern [17]; the main issue is whether a particular sample allows testing a causal hypothesis through comparability of exposed and non-exposed groups [18]. Studies that most often meet this criterion are randomized placebo controlled clinical trials; however, in transgender clinical research individual randomized controlled trials (RCTs) may not always be feasible or ethically acceptable [19,20]. For example, randomizing transgender people to receive or not receive hormone therapy would violate the principle of equipoise, given guidelines recommending hormonal treatment to alleviate gender dysphoria [21,22]. In those circumstances, observational comparative effectiveness research is needed. Other research questions do lend themselves to RCTs; for example, comparing different hormone regimens, or giving some transgender women aspirin and some placebo to see if rates of deep vein thrombosis or coronary artery disease differ.
The acquisition of high-quality study samples to examine transgender health may be carried out via four main systematic approaches: 1) general population-based, 2) health systems-based, 3) clinic-based, and 4) venue-based. Although in some circumstances other approaches such as respondent-driven sampling or internet-based recruitment may offer useful alternatives, those are considered a form of convenience sampling [23] and remain beyond the scope of this review.
In the next sections of this communication, we describe each of the four main sampling approaches, discuss their advantages and disadvantages, and provide illustrative examples of completed or ongoing studies. We then address additional methodological challenges facing transgender health research and propose paths forward toward the goal of identifying and filling current methods gaps through various observational and interventional studies.
General population-based approach
A population-based study is feasible if the members of a given population can be enumerated to enable drawing of random samples or even inclusion of the entire population of interest [24]. Common sources of data for population-based studies include the Department of Motor Vehicles (DMV) records, voter registration lists, and various commercial population directories [25]. For population-based transgender health research, these data sources would only be useable if they systematically collected information on gender beyond binary natal “sex” categories [26,27]. A number of recent publications urge expansion of existing data capture to identify transgender individuals in a systematic fashion, including use of the two-step method [28–30]; however, to-date these efforts have not translated into standard practice in population-level research.
TransPOP is an example of an ongoing federally-funded population-based study [31]. Conducted at the Williams Institute in collaboration with Gallup, Inc., the study’s primary goal is to collect a national probability-based sample to examine sociodemographic characteristics and health status of the U.S. transgender population.
A well-designed and executed population-based study maximizes external validity and offers accurate estimation of frequency and distribution of various health-related measures, such as incidence of disease or prevalence of risks [32]. On the other hand, this approach may be inefficient, if the goal is to assemble a cohort of sufficient power to examine relatively rare events, or to assess clinically relevant exposure-outcome associations.
Health systems-based approach
Electronic medical records (EMR) offer a number of research opportunities, especially in integrated healthcare systems. The most notable examples of integrated systems are the Veterans Health Administration (VHA) and health maintenance organizations (HMOs).
Several studies examined transgender health within the VHA using International Classification of Diseases Ninth edition (ICD-9) codes. Kauth and colleagues examined all VHA encounters 2006–2013 for the following three ICD-9 codes: 302.85 (gender identity disorder in adolescent or adult); 302.6 (gender identity disorder not otherwise specified) and 302.5 (transsexualism) [33]. Additional VHA-based studies examined incidence of breast cancer [10] and lifetime prevalence of various health conditions [9,33] among veterans with and without transgender-related diagnoses.
Another possible source of integrated health systems data are HMOs, which often collaborate via the Health Care Systems Research Network [34]. HMO data make it possible to construct historical and prospective multicenter cohorts, and track the enrollment and healthcare utilization histories over extended periods of time [35].
The research infrastructure available through HMOs is used in the ongoing federally-funded study “Comparative risk and benefits of gender confirmation therapies” [35]. The study is designed as a mixed historic/prospective cohort of transgender people enrolled in Kaiser Permanente plans at three sites (Atlanta Georgia, Northern California, and the Greater Los Angeles area) (Figure 2). Cohort candidates are identified based on relevant ICD-9 codes or presence of free-text keywords in the medical notes [36].
Figure 2.
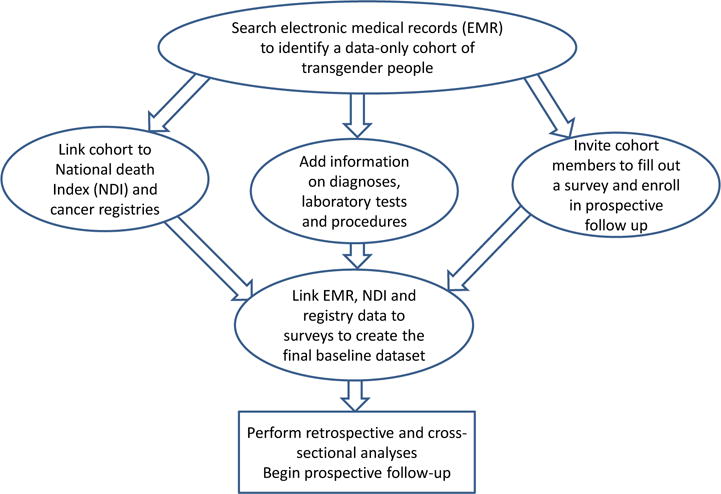
A flow diagram depicting data collection and design of a mixed retrospective/prospective health systems-based study.
The advantages of a health systems-based approach include its relative efficiency in identifying eligible subjects, availability of cisgender (non-transgender) controls who can be matched to transgender participants, and ability to involve multiple sites. On the other hand, this approach may miss key hard-to-reach (e.g., uninsured or not engaged in healthcare) transgender population subgroups. Additionally, if data collection procedures are limited to EMR, the resulting information may lack detail.
Clinic-based approach
A clinic-based approach uses institutions that provide care to the patient population of interest. An example of successful implementation of this approach is the European Network for the Investigation of Gender Incongruence (ENIGI). ENIGI is a collaboration of several Western European gender identity clinics [37–40]. The project recruits and prospectively follows people that apply at these gender identity clinic ages 17 years or older from clinical entry (before initiation of hormonal therapy or surgical gender affirmation) onwards. Data collection procedures include periodic administration of survey instruments, systematic abstraction of medical records, and acquisition and storage of biospecimens [39].
Clinic-based studies of U.S. transgender patients will also benefit from the experience of other U.S. clinic-based multicenter projects such as the Centers for AIDS Research [41]. One approach would be to establish a multisite cohort study of transgender patients by joining federally qualified health centers (FQHCs), also known as community health centers [42]. For example, Fenway Health in Boston, Massachusetts is a FQHC that served more than 1,700 unique transgender patients in 2014 [43]. Other FQHCs with large transgender patient populations are located in most major cities, including Callen-Lorde Community Health Center in New York and similar centers in Philadelphia, Washington, Chicago, Los Angeles, and San Francisco.
A clinic-based approach offers excellent opportunities for in-depth data collection, biospecimen acquisition, and both patient- and provider-reported information. Many clinics, particularly community health centers, also serve underserved transgender subgroups such as uninsured or underinsured individuals, racial/ethnic minorities, patients with non-binary gender identities, and HIV-infected patients. Putting together a large clinic-based cohort requires multiple study sites, and per participant cost and effort is relatively high; however, scientific knowledge to be gained is high.
Venue-based approach
Developed in the mid-1990s, venue-based sampling combines outreach activities with standard methods of sampling to study hard-to-reach groups [44]. Following success of this approach in several cities [45], venue-based sampling was adapted for the National HIV Behavioral Surveillance studies of men who have sex with men (MSM) [46].
Venue-based methods are currently applied in the ongoing federally-funded multisite study “Identity Development, Risk, and Resilience among Gender Diverse Populations.” The study uses purposive, venue-based sampling to recruit transgender people in three U.S. cities (New York, San Francisco, Atlanta). Venues include commercial establishments (e.g., bars, coffee shops, beauty salons) and outdoor spaces (e.g., streets, parks); groups (e.g., community organizations and groups organized around culture, sports, seniors); and events (e.g., LGBT Pride). Sampling is stratified by age and gender identity, while maximizing ethnic and racial diversity. The goal of the study is to test an adaptation of the minority stress model to investigate vulnerability, risk, and resilience in the context of transgender identity development across the lifespan [47].
Access to hard-to-reach participants identified via venue-based recruitment can improve generalizability and allow correcting selection biases [48]. This approach enables a single study team to cover an entire city or geographic area. Limitations include difficulty of maintaining high response and retention rates, and problems with recruiting large numbers of participants.
Other methodological issues in transgender health research
In addition to selecting an appropriate sampling strategy, additional methodological issues that need to be considered in transgender health studies include defining the target population, using optimal study design for the research question, routinely collecting gender identity data, identifying and recruiting transgender people for participation in research, developing accurate measures and efficient data collection protocols, and using consistent terminology. A summary of these issues is presented in Table 1.
Table 1.
Data needs and research priorities.
Data Needs in Transgender Health Research:
|
|
|
Methodological Needs in Transgender Health Research:
|
Routine collection of gender identity data is critical for transgender health clinical practice and research. The two-step method is recommended [15,49], including by the World Professional Association for Transgender Health (WPATH) [50]. The two-step method asks questions about current gender identity and assigned sex at birth (see Table 2) [50]. Cross-tabulating these items allows for different sex and gender combinations to identify transgender patients. Such an approach has been found to identify twice as many transgender people as a single-question method [51].
Table 2.
Routine data collection using the two-step method.
|
About the Two-Step Method Standardization of data collection is critical for transgender health clinical practice and research. The two-step method is recommended, including by the World Professional Association for Transgender Health (WPATH). The two-step method asks questions about both assigned sex at birth and current gender identity. Cross-tabulating these questions allows for different sex and gender combinations to assess transgender health. Recommended Two-Step Method [50] Question 1: How do you describe your gender identity? (check one)
Question 2: What sex were you assigned at birth, on your original birth certificate?
Cross-tabulating these questions gives a two by four (2×6) contingency table with 12ccells demonstrating different sex and gender combinations. | ||
|
| ||
| Assigned Sex at Birth | ||
| Current Gender Identity | Male | Female |
| Male | Cisgender | Transgender* |
| Female | Transgender* | Cisgender |
| Transmale/Trans Man/Female-to-male (FTM) | – | Transgender* |
| Transfemale/Trans Woman/Male-to-female (MTF) | Transgender* | – |
| Genderqueer/Gender Nonconforming | Transgender* | Transgender* |
| Different Identity: Please State: ________________ | Transgender* | Transgender* |
Cisgender = Non-Transgender.
Adding these cells results in overall prevalence of Transgender.
Enrollment of a large multisite cohort of U.S. transgender patients has not yet been executed. Such a cohort could act as a vehicle and infrastructure for multiple analyses and ancillary studies. A series of protocols for identification, recruitment, and follow-up of diverse transgender people in age, racial, ethnic, and socioeconomic backgrounds, and with diverse gender identities could be developed. It is conceivable that such a multisite research project could enroll a cohort study with as many as 10,000–25,000 participants (Figure 3). Sample size considerations for such a multisite cohort study are summarized in Table 3 [52–54].
Figure 3.
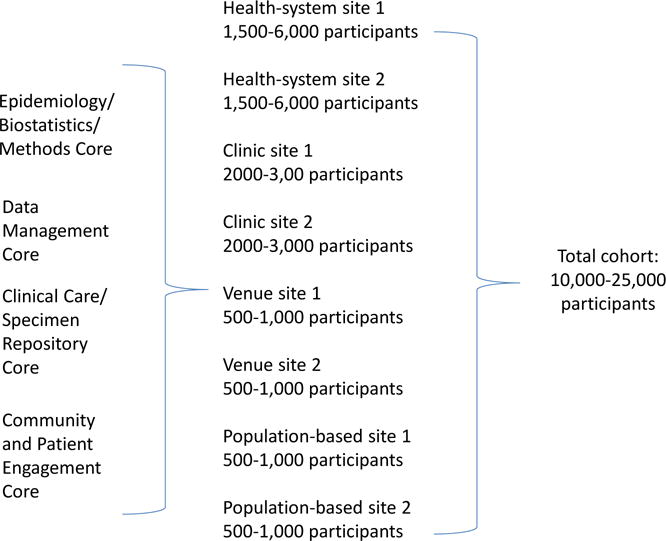
A concept of a multisite U.S. national transgender cohort study.
Table 3.
Anticipated sample sizes required for risk ratios (RR) of various magnitude assuming binary outcomes, statistical power of 80% and a range of outcome occurrence estimates among the non-exposed (P0)*
| Two-sided α-error=0.05, exposed-to-unexposed ratio=1:10 | |||
|
| |||
| P0** | RR=1.5 | RR=1.75 | RR=2.0 |
|
| |||
| 0.005 | 7290 | 3688 | 1874 |
| 0.01 | 3541 | 1639 | 933 |
| 0.05 | 686 | 312 | 179 |
|
| |||
| Two-sided α-error=0.01, exposed-to-unexposed ratio=1:10 | |||
|
| |||
| P0** | RR=1.5 | RR=1.75 | RR=2.0 |
|
| |||
| 0.005 | 10847 | 4812 | 2789 |
| 0.01 | 5268 | 2435 | 1388 |
| 0.05 | 1021 | 464 | 266 |
|
| |||
| Two-sided α-error=0.05, exposed-to-unexposed ratio=1:1 | |||
|
| |||
| P0** | RR=1.5 | RR=1.75 | RR=2.0 |
|
| |||
| 0.005 | 15813 | 7396 | 4678 |
| 0.01 | 7686 | 3814 | 2321 |
| 0.05 | 1475 | 718 | 437 |
|
| |||
| Two-sided α-error=0.01, exposed-to-unexposed ratio=1:1 | |||
|
| |||
| P0** | RR=1.5 | RR=1.75 | RR=2.0 |
|
| |||
| 0.005 | 23528 | 11004 | 6956 |
| 0.01 | 11437 | 5674 | 3454 |
| 0.05 | 2194 | 1068 | 650 |
Each cell shows number of exposed subjects. A P0 of 0.5% corresponds to an approximate annual incidence of prostate cancer among natal males over 50 years of age, or a 15-year risk of ovarian cancer in natal females over 40 years of age [52]. A P0 of just under 1% is an estimate of all-cause mortality in the US [53], and a P0 of about 10% is prevalence of diabetes mellitus in U.S. adults [54].
In addition to supporting within-cohort analyses, the study could also identify comparable reference groups of cisgender males and females to conduct comparative studies, including nested case-cohort designs.
In a longitudinal cohort study of transgender patients, additional data can be obtained through a variety of methods during follow-up. Linkages with external data sources such as the National Death Index, cancer registries, and Medicare and Medicaid may offer an efficient way of assessing morbidity and mortality. In addition, new diagnoses and changes in medications, procedures, and test results can be obtained from periodic reviews of medical records, using electronic data searches and abstraction methods [35,55]. A number of important outcomes, such as quality of life and satisfaction with care, can only be obtained by self-report (in-person, or via mailed or online surveys) as commonly done in other areas of comparative effectiveness research [56–58]. Rich data can also be obtained through collection of biological specimens, a sine qua non for contemporary cohort studies [59–61]. Availability of a biorepository in a multisite cohort study would offer a variety of opportunities for case-cohort and nested case-control studies.
A priority in transgender health research is evaluation of risk and benefits of gender affirmation therapies, such as hormones and surgical interventions. Although randomized clinical trials of gender affirmation are not always possible or ethical, multiple areas of equipoise remain. For example, one could design and implement clinical trials comparing different delivery modes and schedules for hormonal therapy. Another area that lends itself to interventional studies is patient management. Practice-level randomized studies could test various patient navigation methods or physician-decision algorithms aimed at standardizing and optimizing gender affirmation including referrals, hormonal treatment, and post-surgery management and follow-up.
In the absence of randomized studies, some of the questions in transgender health can be answered through observational comparative effectiveness research or implementation science methods [62]. To achieve this goal, data capture should include a full history of hormonal exposures and surgical procedures, therapy received both within and outside of professional care settings, and gender-affirming treatments both in the U.S. and abroad. Validated tools are needed to standardize collection of these patient data elements.
Regardless of study design or research question, there is a need for standardized terminology to describe transgender identity and history. Demographic groupings by gender identity should be grounded in current best practices to ensure appropriate comparisons across genotypic, phenotypic, and identity categories. Table 4 contains specific identity terminology recommendations, taking into consideration the increasing proportion of transgender people who have an identity and sexual orientation outside of historical binaries. Terminology for gender affirming treatments should also be consistent and descriptive.
Table 4.
Recommended terminology for various descriptors, based on identified or affirmed gender. An increasing proportion of transgender people have an identity and sexual orientation identity outside of historical binaries. The term “queer” describes a range of fluid and non-binary gender and sexual identities and may be used in combination with the terms listed to describe particular subpopulations.
| Gender Identity | Sex Assigned at Birth | Term | Androphilic+ Orientation Identity | Gynephilic Orientation Identity | Mixed Sexual Orientation Identity |
|---|---|---|---|---|---|
| Female | Female | Cisgender Woman | Heterosexual/Straight | Homosexual/Lesbian | Bisexual/Pansexual/Queer |
| Male | Male | Cisgender Man | Homosexual/Gay | Heterosexual/Straight | Bisexual/Pansexual/Queer |
| Female | Male | Trans(gender) Woman | Heterosexual/Straight | Homosexual/Lesbian | Bisexual/Pansexual/Queer |
| Transgender female/transfemale/male-to-female (MTF) | Male | Trans(gender) Woman | Heterosexual/Straight | Homosexual/Lesbian | Bisexual/Pansexual/Queer |
| Male | Female | Trans(gender) Man | Homosexual/Gay | Heterosexual/Straight | Bisexual/Pansexual/Queer |
| Transgender male/transmale/female-to-male (FTM) | Female | Trans(gender) Man | Homosexual/Gay | Heterosexual/Straight | Bisexual/Pansexual/Queer |
| Non-binary/fluid/genderqueer | Male | Trans Feminine spectrum individual | Varies by individual | Varies by individual | Varies by individual |
| Non-binary/fluid/genderqueer | Female | Trans Masculine spectrum individual | Varies by individual | Varies by individual | Varies by individual |
| Gender identity not listed here (avoid using the word “other”) | Either | Varies by individual | Varies by individual | Varies by individual | Varies by individual |
Note: Transgender men and women may identify only as “male/female” or as “transgender male/female.” Androphilic and gynephilic are terms used to describe sexual orientation identity. Androphilic describes sexual attraction to men or masculinity. Gynephilic describes sexual attraction to women or femininity. The terms are used to identify a person’s attraction without attributing gender identity or sex assignment to the individual. Cisgender refers to being non-transgender. This table refers to sexual orientation identity – additional questions regarding sexual behaviors and sexual organs involved in the sexual practices are recommended in clinical settings.
A critical feature of research with transgender people is patient-centeredness [63]. Engaging local transgender community members from the start will ensure that study design and data collection methods are feasible and acceptable to participants [35]. Moreover, in longitudinal studies, for example in a prospective cohort, long-term follow up and ability to re-contact participants are keys to success given attrition is the primary threat to validity [64]. Ways of maximizing retention and protocol adherence should be explored though formative qualitative and pilot studies, as in other areas of research with hard-to-reach populations [65,66]. Transgender health studies will likely benefit from applying community-based research principles to work “with” not “on” transgender people [67] in the design, implementation, and dissemination of research and to foster trust and synergy between researchers and local communities [68,69].
Conclusion
Transgender health research would benefit from methodological advances that ensure adequately powered statistical analyses, representation of hard-to-reach subgroups, a consistent agreed-upon shared lexicon, unified protocols across studies for collecting data on treatment and health outcomes over time, ability to pool data across sites, and ample opportunities for ancillary and nested studies, including clinical trials. Based on the strengths and limitations of different approaches discussed in the previous sections, some transgender health studies should be health systems-based, some clinic-based, some should conduct venue-based sampling, and some should deploy hybrid designs. It is expected that studies will have different methodological strengths and address diverse aspects of the transgender health research agenda. Broadening methodologies used in transgender health research represents a key next step to further scientific and clinical knowledge and advance health equity for transgender people.
Key Points.
Challenges in transgender health research include standardization of lexicon, agreed-upon population definitions, study design, sampling, measurement, outcome ascertainment, and sample size.
Ongoing development and refinement of clinical recommendations will benefit from a series of evidence-based observational and intervention studies aimed at identifying, recruiting, and following diverse cohorts of transgender people.
Applying existing and new methodologies will increase the scientific rigor and reach of transgender health research, and inform evidence-based prevention and care for this underserved patient population.
Acknowledgments
None
Financial support and sponsorship
This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R13HD084267), the Endocrine Society, the Tawani Foundation, the World Professional Association for Transgender Health (WPATH), and the Program in Human Sexuality at the University of Minnesota Medical School. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health, the Endocrine Society, WPATH, or the Department of Veterans Affairs
Footnotes
Conflicts of Interest: None.
References
- 1.Institute of Medicine. The health of lesbian, gay, bisexual, and transgender people: Building a foundation for better understanding. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 2.Bockting WO, Miner MH, Swinburne Romine RE, Hamilton A, Coleman E. Stigma, mental health, and resilience in an online sample of the us transgender population. Am J Public Health. 2013;103(5):943–951. doi: 10.2105/AJPH.2013.301241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budge SL, Adelson JL, Howard KA. Anxiety and depression in transgender individuals: The roles of transition status, loss, social support, and coping. J Consult Clin Psychol. 2013;81(3):545–557. doi: 10.1037/a0031774. [DOI] [PubMed] [Google Scholar]
- 4.Habarta N, Wang G, Mulatu MS, Larish N. Hiv testing by transgender status at centers for disease control and prevention-funded sites in the united states, puerto rico, and us virgin islands, 2009–2011. Am J Public Health. 2015;105(9):1917–1925. doi: 10.2105/AJPH.2015.302659. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Special interest: Article describing trends in HIV testing data from the Centers for Disease Control and Prevention by transgender status.
- 5.Coulter RW, Blosnich JR, Bukowski LA, Herrick AL, Siconolfi DE, Stall RD. Differences in alcohol use and alcohol-related problems between transgender- and nontransgender-identified young adults. Drug Alcohol Depend. 2015;33(3):287–295. doi: 10.1016/j.drugalcdep.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman J, Romine RS, Bockting WO. Hiv risk behaviors in the u.S. Transgender population: Prevalence and predictors in a large internet sample. J Homosex. 2014;61(11):1558–1588. doi: 10.1080/00918369.2014.944048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reisner SL, Bailey Z, Sevelius J. Racial/ethnic disparities in history of incarceration, experiences of victimization, and associated health indicators among transgender women in the u.S. Women & health. 2014;54(8):750–767. doi: 10.1080/03630242.2014.932891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blosnich JR, Brown GR, Shipherd Phd JC, Kauth M, Piegari RI, Bossarte RM. Prevalence of gender identity disorder and suicide risk among transgender veterans utilizing veterans health administration care. Am J Public Health. 2013;103(10):e27–32. doi: 10.2105/AJPH.2013.301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown GR, Jones KT. Health correlates of criminal justice involvement in 4,793 transgender veterans. LGBT Health. 2015 doi: 10.1089/lgbt.2015.0052. e-publicaton ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191–198. doi: 10.1007/s10549-014-3213-2. [DOI] [PubMed] [Google Scholar]; *Special interest: Article presents breast cancer incidence and risk data from a large cohort of transgender veterans.
- 11.Nuttbrock L, Bockting W, Rosenblum A, Hwahng S, Mason M, Macri M, Becker J. Gender abuse and major depression among transgender women: A prospective study of vulnerability and resilience. Am J Public Health. 2014;104(11):2191–2198. doi: 10.2105/AJPH.2013.301545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keo-Meier CL, Herman LI, Reisner SL, Pardo ST, Sharp C, Babcock JC. Testosterone treatment and mmpi-2 improvement in transgender men: A prospective controlled study. J Consult Clin Psychol. 2015;83(1):143–156. doi: 10.1037/a0037599. [DOI] [PubMed] [Google Scholar]
- 13.MacCarthy S, Reisner SL, Nunn A, Perez-Brumer A, Operario D. The time is now: Attention increases to transgender health in the united states but scientific knowledge gaps remain. LGBT Health. 2015 doi: 10.1089/lgbt.2014.0073. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisner SL, Poteat T, Keatley J, Cabral M, Mothopeng T, Dunham E, Holland CE, Max R, Baral SD. Global health burden and needs of transgender populations: A systematic review. Lancet. 2015 doi: 10.1016/S0140-6736(16)00684-X. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Special interest: Epidemiologic review of global transgender health research.
- 15.Reisner S, Conron K, Scout N, Baker K, Herman J, Lombardi E, Greytak E, Gill A, Matthews A. “Counting” transgender and gender nonconforming adults in health research: Recommendations from the gender identity in u.S. Surveillance group. Transgender Studies Quarterly. 2015;2(1):34–57. [Google Scholar]
- 16.Keyes KM, Galea S. Epidemiology matters. Oxford University Press; New York, NY: 2014. [Google Scholar]
- 17.Rothman KJ, Gallacher JE, Hatch EE. Why representativeness should be avoided. Int J Epidemiol. 2013;42(4):1012–1014. doi: 10.1093/ije/dys223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernan MA. A definition of causal effect for epidemiological research. J Epidemiol Community Health. 2004;58(4):265–271. doi: 10.1136/jech.2002.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283(20):2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 20.Lilford RJ, Jackson J. Equipoise and the ethics of randomization. J R Soc Med. 1995;88(10):552–559. [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman E, Bockting WO, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, Fraser L, Green J, Knudson G, Meyer W. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgenderism. 2012;13(4):165–232. [Google Scholar]
- 22.Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, 3rd, Spack NP, Tangpricha V, Montori VM. Endocrine treatment of transsexual persons: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94(9):3132–3154. doi: 10.1210/jc.2009-0345. [DOI] [PubMed] [Google Scholar]
- 23.McCreesh N, Frost SD, Seeley J, Katongole J, Tarsh MN, Ndunguse R, Jichi F, Lunel NL, Maher D, Johnston LG, Sonnenberg P, et al. Evaluation of respondent-driven sampling. Epidemiology. 2012;23(1):138–147. doi: 10.1097/EDE.0b013e31823ac17c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman KJ. Epidemiology; an introduction. Oxford University Press; New York, NY: 2002. [Google Scholar]
- 25.Chintapalli S, Goodman M, Allen M, Ward K, Liff J, Young J, Terry P. Assessment of a commercial searchable population directory as a means of selecting controls for case-control studies. Public health reports. 2009;124(3):378–383. doi: 10.1177/003335490912400306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conron KJ, Landers SJ, Reisner SL, Sell RL. Sex and gender in the us health surveillance system: A call to action. Am J Public Health. 2014;104(6):970–976. doi: 10.2105/AJPH.2013.301831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conron KJ, Scott G, Stowell GS, Landers SJ. Transgender health in massachusetts: Results from a household probability sample of adults. Am J Public Health. 2012;102(1):118–122. doi: 10.2105/AJPH.2011.300315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deutsch MB, Buchholz D. Electronic health records and transgender patients–practical recommendations for the collection of gender identity data. Journal of general internal medicine. 2015;30(6):843–847. doi: 10.1007/s11606-014-3148-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Special interest: Practical recommendations for working with electronic health records for transgender patient care.
- 29.Deutsch MB, Feldman JL. Updated recommendations from the world professional association for transgender health standards of care. American family physician. 2013;87(2):89–93. [PubMed] [Google Scholar]
- 30.Reisner SL, Conron KJ, Tardiff LA, Jarvi S, Gordon AR, Austin SB. Monitoring the health of transgender and other gender minority populations: Validity of natal sex and gender identity survey items in a u.S. National cohort of young adults. BMC public health. 2014;14(1224) doi: 10.1186/1471-2458-14-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams institute launches first-of-its-kind study of u.S. Transgender population. The Williams Institute UCLA School of Law; Los Angeles: 2015. http://williamsinstitute.law.ucla.edu/press/press-releases/transpop-announcement-march-2015/#sthash.TgQmKHHC.dpuf. [Google Scholar]
- 32.Szklo M. Population-based cohort studies. Epidemiologic reviews. 1998;20(1):81–90. doi: 10.1093/oxfordjournals.epirev.a017974. [DOI] [PubMed] [Google Scholar]
- 33.Kauth MR, Shipherd JC, Lindsay J, Blosnich JR, Brown GR, Jones KT. Access to care for transgender veterans in the veterans health administration: 2006–2013. Am J Public Health. 2014;104(S4):S532–S534. doi: 10.2105/AJPH.2014.302086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieu TA, Hinrichsen VL, Moreira A, Platt R. Collaborations in population-based health research: The 17th annual hmo research network conference, march 23–25, 2011, boston, massachusetts, USA. Clinical medicine & research. 2011;9(3–4):137–140. doi: 10.3121/cmr.2011.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodman M, Fletcher RH, Doria-Rose VP, Jensen CD, Zebrowski AM, Becerra TA, Quinn VP, Zauber AG, Corley DA, Doubeni CA. Observational methods to assess the effectiveness of screening colonoscopy in reducing right colon cancer mortality risk: Scolar. Journal of comparative effectiveness research. 2015:1–11. doi: 10.2217/cer.15.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quinn V, Becerra T, Gillespie T, Hunkeler E, Baird T, Baisch N, Owen-Smith A, Roblin D, Stephenson R, Tangpricha V, Valentine C, et al. Embedding patients, providers, and community stakeholders in research to improve transgender health. J Patient-Centered Res Rev. 2015;2(2):114–115. [Google Scholar]
- 37.Van Caenegem E, Wierckx K, Taes Y, Schreiner T, Vandewalle S, Toye K, Lapauw B, Kaufman JM, T’Sjoen G. Body composition, bone turnover, and bone mass in trans men during testosterone treatment: 1-year follow-up data from a prospective case-controlled study (enigi) Eur J Endocrinol. 2015;172(2):163–171. doi: 10.1530/EJE-14-0586. [DOI] [PubMed] [Google Scholar]
- 38.Wierckx K, Van Caenegem E, Schreiner T, Haraldsen I, Fisher A, Toye K, Kaufman JM, T’Sjoen G. Cross-sex hormone therapy in trans persons is safe and effective at short-time follow-up: Results from the european network for the investigation of gender incongruence. J Sex Med. 2014;11(8):1999–2011. doi: 10.1111/jsm.12571. [DOI] [PubMed] [Google Scholar]; *Special interest: Evidence from a European cohort of safety and effectiveness of cross-sex hormone therapy for transgender people.
- 39.Kreukels BP, Haraldsen IR, De Cuypere G, Richter-Appelt H, Gijs L, Cohen-Kettenis PT. A european network for the investigation of gender incongruence: The enigi initiative. Eur Psychiatry. 2012;27(6):445–450. doi: 10.1016/j.eurpsy.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 40.Fuss J, Hellweg R, Van Caenegem E, Briken P, Stalla GK, T’Sjoen G, Auer MK. Cross-sex hormone treatment in male-to-female transsexual persons reduces serum brain-derived neurotrophic factor (bdnf) Eur Neuropsychopharmacol. 2015;25(1):95–99. doi: 10.1016/j.euroneuro.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, Lober WB, Van Rompaey SE, Crane HM, Moore RD, Bertram M, et al. Cohort profile: The centers for aids research network of integrated clinical systems. Int J Epidemiol. 2008;37(5):948–955. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.What are federally qualified health centers (fqhcs)? 2015 Http://www.Hrsa.Gov/healthit/toolbox/ruralhealthittoolbox/introduction/qualified.Html: http://www.hrsa.gov/healthit/toolbox/RuralHealthITtoolbox/Introduction/qualified.html.
- 43.Reisner SL, Bradford J, Hopwood R, Gonzalez A, Makadon H, Todisco D, Cavanaugh T, VanDerwarker R, Grasso C, Zaslow S, Boswell SL, et al. Comprehensive transgender healthcare: The gender affirming clinical and public health model of fenway health. Journal of urban health: bulletin of the New York Academy of Medicine. 2015;92(3):584–592. doi: 10.1007/s11524-015-9947-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacKellar D, Valleroy L, Karon J, Lemp G, Janssen R. The young men’s survey: Methods for estimating hiv seroprevalence and risk factors among young men who have sex with men. Public health reports. 1996;111(Suppl 1):138–144. [PMC free article] [PubMed] [Google Scholar]
- 45.Muhib FB, Lin LS, Stueve A, Miller RL, Ford WL, Johnson WD, Smith PJ, Community Intervention Trial for Youth Study T A venue-based method for sampling hard-to-reach populations. Public health reports. 2001;116(Suppl 1):216–222. doi: 10.1093/phr/116.S1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacKellar DA, Gallagher KM, Finlayson T, Sanchez T, Lansky A, Sullivan PS. Surveillance of hiv risk and prevention behaviors of men who have sex with men–a national application of venue-based, time-space sampling. Public health reports. 2007;122(Suppl 1):39–47. doi: 10.1177/00333549071220S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Identity development, risk, and resilience among gender diverse populations. 2014 Https://www.Collectiveip.Com/grants/nih:8815604. https://www.collectiveip.com/grants/NIH:8815604.
- 48.Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for hiv surveillance. AIDS. 2005;19(Suppl 2):S67–72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- 49.Cahill S, Singal R, Grasso C, King D, Baker K, Makadon H. Do ask, do tell: High levels of acceptability by patients of routine collection of sexual orientation and gender identity data in four diverse american community health centers. PLoS ONE. 2014;9(9):e107104. doi: 10.1371/journal.pone.0107104. [DOI] [PMC free article] [PubMed] [Google Scholar]; *Special interest: Study of community health centers showing high levels of acceptability in collecting patient dat a on g ender identity and sexual orienation.
- 50.Deutsch MB, Green J, Keatley J, Mayer G, Hastings J, Hall AM. Electronic medical records and the transgender patient: Recommendations from the world professional association for transgender health emr working group. J Am Med Inform Assoc. 2013;20(4):700–703. doi: 10.1136/amiajnl-2012-001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tate CC, Ledbetter JN, Youssef CP. A two-question method for assessing gender categories in the social and medical sciences. J Sex Res. 2013;50(8):767–776. doi: 10.1080/00224499.2012.690110. [DOI] [PubMed] [Google Scholar]
- 52.Surveillance Epidemiology and End Results Program, National Cancer Institute. Seer*stat database: Incidence - seer 18 regs research data + hurricane katrina impacted louisiana cases, nov 2013 sub (1973–2011 varying) - linked to county attributes - total u.S. 1969–2012 counties, based on the november 2013 submission. 2014 [Google Scholar]
- 53.National Center for Health Statistics. Death in the united states, 2011. Press Release. 2013 [Google Scholar]
- 54.U.S. Department of Health and Human Services. National diabetes statistics report: Estimates of diabetes and its burden in the united states, 2014. Press Release. 2014 [Google Scholar]
- 55.Guy GP, Jr, Lipscomb J, Gillespie TW, Goodman M, Richardson LC, Ward KC. Variations in guideline-concordant breast cancer adjuvant therapy in rural georgia. Health services research. 2015;50(4):1088–1108. doi: 10.1111/1475-6773.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barocas DA, Chen V, Cooperberg M, Goodman M, Graff JJ, Greenfield S, Hamilton A, Hoffman K, Kaplan S, Koyama T, Morgans A, et al. Using a population-based observational cohort study to address difficult comparative effectiveness research questions: The ceasar study. Journal of comparative effectiveness research. 2013;2(4):445–460. doi: 10.2217/cer.13.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grant MD, Marbella A, Wang AT, Pines E, Hoag J, Bonnell C, Ziegler KM, Aronson N. Menopausal symptoms: Comparative effectiveness of therapies. Rockville (MD): 2015. [PubMed] [Google Scholar]
- 58.Boulet LP, Coeytaux RR, McCrory DC, French CT, Chang AB, Birring SS, Smith J, Diekemper RL, Rubin B, Irwin RS, Panel CEC. Tools for assessing outcomes in studies of chronic cough: Chest guideline and expert panel report. Chest. 2015;147(3):804–814. doi: 10.1378/chest.14-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 60.Frank B, Ariza L, Lamparter H, Grossmann V, Prochaska JH, Ullmann A, Kindler F, Weisser G, Walter U, Lackner KJ, Espinola-Klein C, et al. Rationale and design of three observational, prospective cohort studies including biobanking to evaluate and improve diagnostics, management strategies and risk stratification in venous thromboembolism: The vteval project. BMJ open. 2015;5(7):e008157. doi: 10.1136/bmjopen-2015-008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riboli E, Kaaks R. The epic project: Rationale and study design. European prospective investigation into cancer and nutrition. International journal of epidemiology. 1997;26(Suppl 1):S6–14. doi: 10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 62.Madon T, Hofman KJ, Kupfer L, Glass RI. Public health. Implementation science. Science. 2007;318(5857):1728–1729. doi: 10.1126/science.1150009. [DOI] [PubMed] [Google Scholar]
- 63.Gabriel SE, Normand SL. Getting the methods right–the foundation of patient-centered outcomes research. The New England journal of medicine. 2012;367(9):787–790. doi: 10.1056/NEJMp1207437. [DOI] [PubMed] [Google Scholar]
- 64.Rothman KJ, Greenland S. Cohort studies. In: Rothman KJ, Greenland S, Lash TL, editors. Modern epidemiology. Lippincott Williams and Wilkins; Philadelphia, PA: 2008. [Google Scholar]
- 65.Ofstedal MB, Weir DR. Recruitment and retention of minority participants in the health and retirement study. The Gerontologist. 2011;51(Suppl 1):S8–20. doi: 10.1093/geront/gnq100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wallace DC, Bartlett R. Recruitment and retention of african american and hispanic girls and women in research. Public health nursing. 2013;30(2):159–166. doi: 10.1111/phn.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leung MW, Yen IH, Minkler M. Community based participatory research: A promising approach for increasing epidemiology’s relevance in the 21st century. Int J Epidemiol. 2004;33(3):499–506. doi: 10.1093/ije/dyh010. [DOI] [PubMed] [Google Scholar]
- 68.Radix AE, Lelutiu-Weinberger C, Gamarel KE. Satisfaction and healthcare utilization of transgender and gender nonconforming individuals in nyc: A community-based participatory study. LGBT Health. 2014;1(4):302–308. doi: 10.1089/lgbt.2013.0042. [DOI] [PubMed] [Google Scholar]
- 69.Reisner SL, Gamarel KE, Dunham E, Hopwood R, Hwahng S. Female-to-male transmasculine adult health: A mixed-methods community-based needs assessment. Journal of the American Psychiatric Nurses Association. 2013;19(5):293–303. doi: 10.1177/1078390313500693. [DOI] [PubMed] [Google Scholar]


