Abstract
Background
Strongyloidiasis is a gut infection with Strongyloides stercoralis which is common world wide. Chronic infection usually causes a skin rash, vomiting, diarrhoea or constipation, and respiratory problems, and it can be fatal in people with immune deficiency. It may be treated with ivermectin or albendazole or thiabendazole.
Objectives
To assess the effects of ivermectin versus benzimidazoles (albendazole and thiabendazole) for treating chronic strongyloides infection.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (24 August 2015); the Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE (January 1966 to August 2015); EMBASE (January 1980 to August 2015); LILACS (August 2015); and reference lists of articles. We also searched the metaRegister of Controlled Trials (mRCT) using 'strongyloid*' as a search term, reference lists, and conference abstracts.
Selection criteria
Randomized controlled trials of ivermectin versus albendazole or thiabendazole for treating chronic strongyloides infection.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias in the included trials. We used risk ratios (RRs) with 95% confidence intervals (CIs) and fixed‐ or random‐effects models. We pooled adverse event data if the trials were sufficiently similar in their adverse event definitions.
Main results
We included seven trials, enrolling 1147 participants, conducted between 1994 and 2011 in different locations (Africa, Southeast Asia, America and Europe).
In trials comparing ivermectin with albendazole, parasitological cure was higher with ivermectin (RR 1.79, 95% CI 1.55 to 2.08; 478 participants, four trials, moderate quality evidence). There were no statistically significant differences in adverse events (RR 0.80, 95% CI 0.59 to 1.09; 518 participants, four trials, low quality evidence).
In trials comparing ivermectin with thiabendazole, there was little or no difference in parasitological cure (RR 1.07, 95% CI 0.96 to 1.20; 467 participants, three trials, low quality evidence). However, adverse events were less common with ivermectin (RR 0.31, 95% CI 0.20 to 0.50; 507 participants; three trials, moderate quality evidence).
In trials comparing different dosages of ivermectin, taking a second dose of 200 μg/kg of ivermectin was not associated with higher cure in a small subgroup of participants (RR 1.02, 95% CI 0.94 to 1.11; 94 participants, two trials).
Dizziness, nausea, and disorientation were commonly reported in all drug groups. There were no reports of serious adverse events or death.
Authors' conclusions
Ivermectin results in more people cured than albendazole, and is at least as well tolerated. In trials of ivermectin with thiabendazole, parasitological cure is similar but there are more adverse events with thiabendazole.
16 April 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted in April 2019 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review.
Plain language summary
Ivermectin versus benzimidazoles for treating Strongyloides stercoralis infection
What is strongyloides infection and how might ivermectin work
Strongyloides stercoralis is a parasite that lives in the gut of infected people. The infection is not serious for most people, but it can be fatal in people with immune deficiency. People become infected when they come in contact with soil or water contaminated with infectious worms. The chronic infection usually causes skin rash, vomiting, diarrhoea, and constipation, and respiratory problems, such as asthma‐like illness. This disease may be treated with ivermectin or albendazole or thiabendazole. We wanted to know if ivermectin was better or worse than the other alternative therapies.
What the research says
We reviewed the evidence about the effect of ivermectin compared with albendazole and thiabendazole. After searching for relevant trials up to August 2015, we included seven randomized controlled trials, enrolling 1147 adults with chronic strongyloides infection, conducted between 1994 and 2011 in different locations (Africa, Southeast Asia, America, and Europe). Four trials assessed the effectiveness of ivermectin compared with albendazole and three trials assessed the effectiveness of ivermectin compared with thiabendazole.
Comparison ivermectin versus albendazole
Treatment with ivermectin probably cures more people than albendazole (moderate quality evidence), and may be equally or better tolerated (low quality evidence). The included trials did not report serious adverse events or death.
Comparison ivermectin versus thiabendazole
Treatment with ivermectin and thiabendazole may cure similar numbers of people with strongyloides infection (low quality evidence), but ivermectin is probably better tolerated (moderate quality evidence). The included trials did not report serious adverse events or death.
Summary of findings
Background
Description of the condition
Healthcare problem
Strongyloidiasis is an infection caused by the intestinal parasitic worm Strongyloides stercoralis. This parasite is tropical and subtropical regions (Olsen 2009). Most infected people are asymptomatic, allowing the infection to remain undiagnosed and untreated for years (Bisoffi 2013). However, the infection can cause a serious and sometimes fatal illness in immunosuppressed people (Keiser 2004; Olsen 2009).
Geographic distribution
S. stercoralis is a common intestinal nematode that is more prevalent over 70 subtropical and tropical countries distributed across sub‐Saharan Africa, South‐East Asia, and Central and South America (Olsen 2009). The global prevalence was estimated at 39 million cases in 1947 and 100 million cases in 1996 (Bethony 2006). The highest prevalence in the world is in rural and remote aboriginal communities, and is a public health problem due to delayed presentation and reduced access to clinical and tertiary care. Strongyloidiasis can be found in non‐endemic areas owing to increases in travel and migration from endemic to non‐endemic countries (Montes 2010).
Route of infection
The parasite has a complex life cycle including a direct, an autoinfective and a non‐parasitic free‐living cycle. Infected people pass first stage larvae in the faeces; these develop on the soil to infective larvae which penetrate the skin of the next host. After a blood‐lung migration, females larvae moult and develop into adult female worms embedded in the submucosa of the duodenum and parthenogenetically produce dozens of embryonated eggs a day. Eggs hatch and produce first stage larvae in the intestinal lumen. Most of these pass out in the faeces and either develop into infective third‐stage larvae or into free‐living adult males and females. Alternatively, larvae may develop to the third stage within the intestinal lumen and penetrate the intestinal mucosa or perianal skin, restarting a new infection cycle without ever leaving their host. The occurrence of the autoinfective larvae is the main reason strongyloidiasis is such a serious disease (Streit 2008; Olsen 2009).
Population at risk
The following populations are considered to be at risk of strongyloidiasis (Walzer 1982; Berk 1987; Buonfrate 2012):
People living in endemic regions.
People with chronic malnutrition.
Alcoholics.
Travellers.
Immigrants.
People with malignancies, organ transplantation.
People affected by diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic renal failure.
Breast milk from an infected mother.
Occupation involving soil.
People who use corticosteroids or other immunosuppressant drugs, have immune deficiency disorders (HTLV‐I or HIV) or who are malnourished are at increased risk of hyperinfection syndrome (Nucci 1995; Courouble 2004; Schär 2013). Interestingly, although strongyloidiasis is common among acquired immunodeficiency syndrome (AIDS) patients in endemic areas, hyperinfection syndrome is rarely noted (Montes 2010).
Clinical effects
Three clinical presentations of strongyloidiasis are acute infection, chronic intestinal infection and hyperinfection with dissemination.
Acute infection is rarely reported. It may cause local inflammation at the area of larval penetration, appearing as pruritic skin reaction (acute urticaria and itching) of the buttocks, groin and trunk. Pulmonary migration causes respiratory symptoms as the worms travel through the lungs, specifically cough, shortness of breath, and transient wheezing. Diffuse nodular interstitial infiltrates may be seen on chest radiograph or computed tomography (Loeffler´s syndrome). Gastrointestinal symptoms (diarrhoea, constipation, anorexia, and abdominal pain) begin about two weeks after infection and are common in patients with severe strongyloidiasis (Freedman 1991). Symptomatic or occult gastrointestinal bleeding is a frequent sign at presentation (Fardet 2007). Skin reaction and persistent diarrhoea has been described in international travellers (Nuesch 2005; Angheben 2011).
In chronic infection, the worms maintain a low level of reproduction. Most often it is asymptomatic, but gastrointestinal symptoms such as vomiting, diarrhoea, constipation and borborygmus have been reported. Chronic infection is commonly seen in endemic regions and occasionally seen in international travellers and refugees (Keiser 2004).
Hyperinfection/disseminated syndrome describes an accelerated autoinfection (Miller 2008), and the diagnosis implies the presence of signs and symptoms attributable to increased larval migration to organs beyond the range of the pulmonary autoinfective cycle (dissemination). The invasion of helminths into the mucosa is often associated with Gram‐negative bacterial infections. Mortality, even with treatment, is estimated at 83% to 87% (Maguire 2005; Mejia 2012). Diseminated infection is seen in patients with steroid therapy (Fardet 2007), in HTLV‐1 carriers (Hirata 2006), alcoholics (Zago‐Gomes 2002), diabetics (Coovadia 1993), people with hematologic malignancies and organ transplant recipients (Patel 2008).
Diagnosis
Conventional diagnostic methods, such as the direct smear, formalin ether concentration and filter paper culture methods, cannot produce sufficient sensitivity. Several specimens should be collected on different days to improve detection rate. However, the sensitivity of microscopic‐based techniques might not be good enough, especially in chronic infections where larval output is very low (Requena‐Méndez 2013). However the most sensitive techniques, the Baermann and agar plate methods, are too labour‐intensive to be used in an extensive population (Zaha 2000; Yori 2006). Enzyme‐Linked Immunosorbent Assay (ELISA), Immunofluorescence Antibody Test or Indirect Immune Fluorescent Antibody Technique (IFAT), and Western blot have good negative predictive value but cross‐reactivity is observed with filaria (van Doorn 2007; Mejia 2012; Bisoffi 2014). Strongyloides DNA detection in human stool samples by real‐time polymerase chain reaction (PCR) is highly specific with improved sensitivity compared to microscopy (Ten Hove 2009). Luciferase immunoprecipitation system (LIPS) assays are newer immunologic techniques with high sensitivity (Ramanathan 2008).
Description of the intervention
The benzimidazoles (albendazole and thiabendazole) and ivermectin are the drugs most commonly used to treat strongyloidiasis. The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) recommend ivermectin as the drug of choice. Thiabendazole or albendazole are considered as alternative therapies (CDC 2013; The Medical Letter 2013). A combination therapy with albendazole and ivermectin is recommended in some endemic areas with presence of soil‐transmitted helminthiasis, onchocerciasis and lymphatic filariasis (WHO 2006).
Benzimidazoles
The benzimidazole drugs available for the treatment of strongyloidiasis in humans include thiabendazole and albendazole. Mebendazole is not used for strongyloidiasis for lack of activity. For each of these drugs, the pregnancy risk factor is C, that is, human trials are lacking and animal trials are either positive for foetal risk or lacking as well; however, potential benefits may justify the potential risk (Cook 1992).
Thiabendazole (International Nonproprietary Name: tiabendazole) was the first benzimidazole developed and licensed for human use in 1962 (Horton 2000). Thiabendazole was approved in the USA in 1967 but has subsequently been withdrawn because better tolerated antihelmintic agents are available, such as ivermectin or albendazole. However, thiabendazole is still available in many countries and it is used in veterinary medicine in the USA. Although thiabendazole is active against a variety of intestinal parasites it produces frequent adverse events (nausea, malaise or dizziness (Grove 1982; Gann 1994). Recommended schedule of thiabendazole for parasitic infection is: 50 mg/kg/day divided every 12 hours (maximum 3 g/day) for two days. Many other schedules are used (longer time of treatment or other route of administration than oral). Rectal administration has been reported as successful for treating a patient with hyperinfection and bowel obstruction (Boken 1993).
Albendazole has been used widely since 1982 to treat intestinal parasites. The recommended schedule is an oral dose of 400 mg every 12 hours for seven days. The adverse events have been reported as minor (Nahmias 1994); severe adverse events are uncommon, although caution is indicated (Liu 1996).
Ivermectin
Ivermectin is an extremely potent, broad‐spectrum, anthelmintic drug that was first introduced for animal use around 1981 and approved for human use in 1988 (Campbell 1991). It is a semi‐synthetic macrocyclic lactone (molecular name) derived from avermectin (lactones) of the soil mould, Streptomyces avermitilis, causing paralysis in many intestinal parasites through its effect on ion‐channels in cell membranes (Campbell 1991). The recommended schedule is 200 µg/kg/day for two days. Many other schedules are used (single dose or a second dose one week later than first one). Ivermectin has been given per rectum as an enema with some success (Tarr 2003). Subcutaneous doses of 200 µg/kg every 48 hours has been used with success (Marty 2005; Pacanowski 2005; Salluh 2005). Many adverse reactions have been reported, but they usually do not require discontinuation of the drug (Ottesen 1994). The pregnancy risk factor is C, that is, human trials are lacking and animal trials are either positive for fetal risk or lacking as well (Merck 2007); however, potential benefits may justify the potential risk (Merck 2007).
Ivermectin is being increasingly used worldwide to combat human tropical diseases, such as onchocerciasis (18 million people infected), strongyloidiasis (100 million people infected), scabies (300 million cases annually), pediculosis, gnathostomiasis and myiasis (dos Santos 2009). Safety trials have shown no serious adverse events in patients treated with ivermectin (Crump 2011). Ivermectin, as well as albendazole and diethylcarbamazine, is also massively used to eliminate lymphatic filariasis through the Global Programme to Eliminate Lymphatic Filariasis (Ottesen 2008).
How the intervention might work
By binding to free β‐tubulin, benzimidazoles inhibit the polymerization of tubulin and the uptake of glucose causing disruption of microtubule formation in the parasite (Lacey 1990).
Ivermectin has potent activity at Gaba‐amino‐butyric‐acid (GABA)‐gated Cl and K channels and glutamate‐gated Cl and K channels, interfering with neural transmission causing paralysis in invertebrates (Campbell 1991; Geary 2005).
Why it is important to do this review
The control of strongyloidiasis as a public health problem is not a priority for governments (Olsen 2009). Moreover, the treatment is not universally available, although drugs are listed in the essential medicines of the WHO (WHO 2015). The introduction of treatment with ivermectin as annually mass treatment in endemic communities of onchocerciasis has shown a reduction in transmission in endemic communities and reduce the expected number of new infections (Traore 2012). Ivermectin is currently employed by the African Programme for Onchocerciasis Control (APOC) and the Onchocerciasis Elimination Programme for the Americas (OEPA) for mass treatment in endemic communities. Trials of long‐term treatment with ivermectin to control lymphatic filariasis have shown that use of the drug is additionally associated with significant reduction in the prevalence of infection with any soil‐transmitted helminth parasites, most or all of which are deemed to be major causes of the morbidity arising from poor childhood nutrition and growth (Moncayo 2008). Mass treatment with ivermectin have been effective to eliminate both infections and seems to be the ideal drug for such interventions (Heukelbach 2004).
This Cochrane Review aimed to summarise systematically all the evidence from randomized controlled trials (RCTs) relating to the effectiveness of ivermectin in chronic strongyloidiasis in order to provide current best evidence on which to base decisions for practice and further research.
Objectives
To assess the effects of ivermectin versus benzimidazoles (albendazole and thiabendazole) for treating chronic strongyloides infection.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Participants were people (all ages) who were immunocompetent or immunocompromised, and with chronic infection by S. stercoralis confirmed by parasitological examination (at least one positive specimen) or serology tests (IFAT).
We defined immunocompromised people as those affected by haematological malignancies, bone marrow and kidney transplants, hypogammaglobulinaemia (low gamma globulin in blood), malnutrition, HTLV‐1/HIV infection or co‐infection, or who are using corticosteroids.
Types of interventions
Ivermectin versus albendazole or thiabendazole.
Types of outcome measures
Primary outcomes
Elimination of infection or parasitological cure: defined as any parasitological exam negative during follow‐up period (more than two stool samples negative).
Secondary outcomes
Death;
-
Adverse events as reported in trials:
Serious adverse events (requires inpatient hospitalization or prolongation of existing hospitalization; persistent or significant disability/incapacity; or is life threatening).
Adverse events leading to discontinuation of treatment.
Other adverse events.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press and in progress).
Electronic searches
We searched the following databases using the search terms detailed in Appendix 1: the Cochrane Infectious Diseases Group (CIDG) Specialized Register (24 August 2015); the Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE (January 1966 to August 2015); EMBASE (January 1980 to August 2015); and LILACS (August 2015). We also searched the metaRegister of Controlled Trials (mRCT) using 'strongyloid*' as a search term.
Searching other resources
We searched the reference lists of identified trials to find additional trials. We searched the following conference proceedings for relevant abstracts: the Annual Congress of the American Society for Tropical Medicine and Hygiene (2005 to 2015); and the European Congress on Tropical Medicine and International Health (2009 to 2015). To help identify unpublished and ongoing trials, we contacted relevant organizations including tropical medicine and infectious disease institutes in Japan, and Peru, and pharmaceutical companies including Merck & Co., Inc. However, our attempts to contact trial authors were unsuccessful.
Data collection and analysis
Selection of studies
Cesar Henriquez‐Camacho (CHC) with assistance from Vittoria Lutje, the CIDG Information Retrieval Specialist, searched the literature and retrieved trials. Juan Echevarria (JE) and Frine Samalvides (FS) retrieved the full reports of potentially relevant trials and then applied the inclusion criteria to the full reports using an eligibility form. If eligibility was unclear, we tried to contact the trial authors for clarification. Eduardo Gotuzzo (EG) resolved any disagreements. We scrutinized the eligible trials to ensure that each trial was included only once. We listed the trials that were not eligible for inclusion and explain the reasons for exclusion.
Data extraction and management
One review author (CHC) extracted the data, and JE and Maria N Plana (MNP) crossed‐check the data with the original paper for accuracy. We used a data extraction form, which was piloted previously. CHC entered the data into Review Manager (RevMan). We resolved discrepancies by discussion.
We extracted data for dichotomous variables as the number of events and the number of participants in each group for all outcomes. We calculated the percentage lost to follow‐up in each group. Also, we extracted and recorded data on the following: characteristics of participants, characteristics of interventions, characteristics of outcome measures, date of trial, trial authors, location of trial, sponsor of trial (specified, known or unknown), design (described as randomized or not), participants (strongyloidiasis confirmed), interventions (treatment, days, doses), outcomes (treatment failure, parasite clearance, adverse events) and data known to have been collected by trialists but not included in the report (where possible).
Assessment of risk of bias in included studies
Two review authors (CHC and MNP) independently assessed the risk of bias of each included trial using the criteria outlined in the Cochrane 'Risk of bias' tool (Higgins 2011). A third review author (EG) resolved any disagreements. We considered the following domains: random sequence generation (selection bias), allocation concealment (selection bias), blinding for participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and other sources of bias. We classified each domain as being at 'low', 'high' or 'unclear' risk of bias. We included a 'Risk of bias' graph (Figure 1) and a 'Risk of bias' summary (Figure 2).
1.
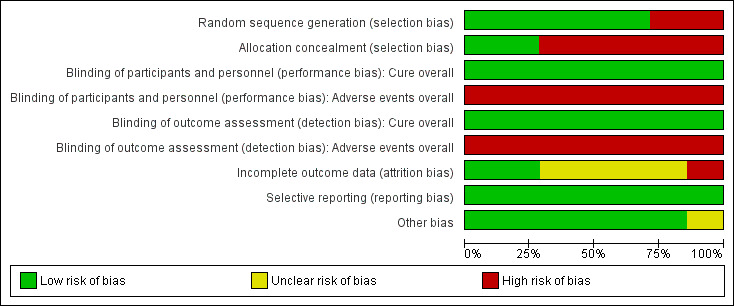
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.
2.
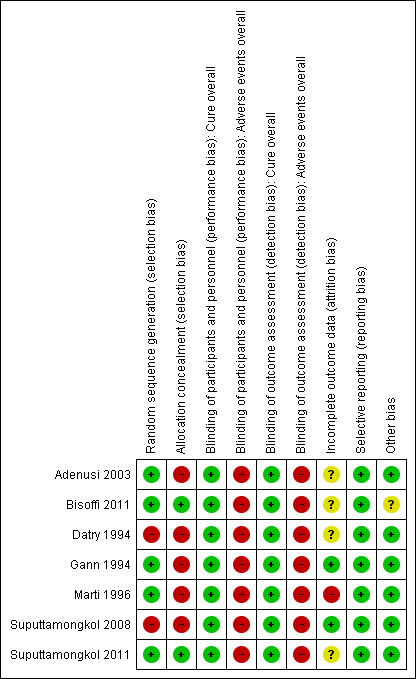
Methodological quality summary: review authors’ judgements about each methodological quality item for each included trial.
Measures of treatment effect
We used risk ratios (RR) with 95% confidence intervals (CIs) and fixed‐effect models to analyse the efficacy data.
Assessment of heterogeneity
We assessed statistical heterogeneity by examining the forest plots and using the I² statistic and Chi² test values. We regarded heterogeneity as substantial if the I² statistic was > 50% or there was a low P value (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We planned to construct funnel plots to look for evidence of publication bias, provided there were a sufficient number of trials included to make this analysis informative.
Data synthesis
We computed pooled estimates of effect separately for each comparison we had data for (ivermectin versus albendazole; and ivermectin versus thiabendazole).
We used Review Manager (RevMan) for data analysis.
For the analysis of adverse events, we needed to ascertain the number of participants who experienced the adverse events. We used the rate ratio to pool adverse event data if the trials were sufficiently similar in their adverse event definitions. We excluded data from trials that only reported the number of adverse events as it is possible that an individual could have more than one adverse event reported. If these adverse events were reported by randomized groups, we included the data in the analysis.
We used a fixed‐effect model for combining data where it was reasonable to assume that trials were estimating the same treatment effect. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if we detected substantial statistical heterogeneity, we used a random‐effects model. If we used random‐effects analysis, we presented the results as the average treatment effect with its 95% CIs, and the estimates of the I² statistic.
Subgroup analysis and investigation of heterogeneity
We attempted to explain any heterogeneity through subgroup analyses. We planned to conduct the following subgroup analyses of primary outcome in both comparisons (ivermectin versus albendazole & ivermectin versus thiabendazole): type of population (endemic and non‐endemic areas) and doses of ivermectin (single versus double doses).
Sensitivity analysis
We planned sensitivity analyses to explore whether trials at high risk of bias overestimated the effect of treatment.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies sections.
Results of the search
The electronic search generated 50 citations and abstracts, and three conference reports. We screened these articles and only seven trials including 1147 participants met the inclusion criteria (Figure 3). None were cluster‐randomized. Communication with Merck & Co., Inc, the manufacturers of Mectizan and with experts in the field did not yield information on any further trials. Only two trial authors provided further information about included trials (Marti 1996; Bisoffi 2011).
3.
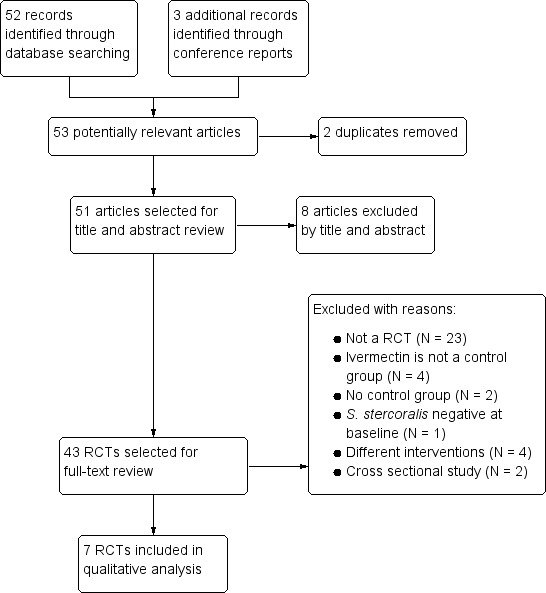
Trial flow diagram.
Included studies
See Characteristics of included studies.
Setting and participants
Four trials took place in endemic communities in Zanzibar (Marti 1996), Nigeria (Adenusi 2003) and Thailand (Suputtamongkol 2008; Suputtamongkol 2011). Three trials recruited participants from endemic areas living in non‐endemic countries in the USA (Gann 1994), France (Datry 1994), and travellers or immigrants residing in Italy (Bisoffi 2011). Two trials included only adults (Suputtamongkol 2008; Suputtamongkol 2011), and five trials included adults and children (Datry 1994; Gann 1994; Marti 1996; Adenusi 2003; Bisoffi 2011). Only two trials included immunocompromised participants (Suputtamongkol 2008; Suputtamongkol 2011), although the number of immunocompromised and immunocompetent participants was unclear. The authors of two trials were contacted and responded (Marti 1996; Bisoffi 2011).
Interventions
In all included trials ivermectin was compared with a benzimidazole (two trials specified MECTIZAN®, one specified IVOMEC® from Merck Sharp Dome, and one VERMECTIN® from Atlantic Laboratories Co. Ltd.). Three trials compared ivermectin versus thiabendazole (one specified MINTEZOL® from Merck Sharp Dome) (Gann 1994; Adenusi 2003; Bisoffi 2011) and four trials compared ivermectin versus albendazole (one specified ALBATEL® from TO Chemicals and one specified ZENTEL® from SmithKline Beecham) (Datry 1994; Marti 1996; Suputtamongkol 2008; Suputtamongkol 2011). The usual dose of ivermectin was 200 μg/kg body weight; however, Gann 1994, and Suputtamongkol 2011 had two treatment groups of one single dose and two doses. The dose of albendazole was 400 mg twice daily for seven days in two trials (Suputtamongkol 2008; Suputtamongkol 2011) and 400 mg twice daily for three days in two trials (Datry 1994; Marti 1996).
Outcome measures
Assessment of outcome measures was by parasitological examination. This included direct stool examination, Kato‐Katz technique, Baermann test, Agar plate culture, formol‐ether concentration and IFAT. The included trials did not define who undertook the outcome assessments. Trials assessed and reported outcome measures differently, depending on the technique used.
Two trials used the Baermann technique as the only diagnostic method (Gann 1994; Adenusi 2003). The rest of the included trials used two or more diagnostic methods. Four trials used stool examination, according to Baermann, as the assessment method. Only one trial, Bisoffi 2011, used a serological test (IFAT) with agar plate.
The number of stool samples varied between two to nine, but the results of each sample were not always reported. There was lack of uniformity in follow‐up (mean of follow‐up: 7.5 weeks (range: two to 24 weeks)).
Only one trial evaluated clinical improvement through medical interview (Gann 1994). There were several adverse events reported, but there were no deaths after administration of drugs or by the disease itself. For more detailed information on individual trials see Characteristics of included studies.
Excluded studies
We excluded 36 trials from the review (see Characteristics of excluded studies).
Risk of bias in included studies
We have listed summary details in the Characteristics of included studies section. Figure 1 and Figure 2 summarise the 'Risk of bias' assessment in the included trials.
Allocation
Two trials were the only trials that reported adequate methods of allocation concealment (Bisoffi 2011; Suputtamongkol 2011). Five trials reported adequate methods of random sequence generation (Gann 1994; Marti 1996; Adenusi 2003; Bisoffi 2011; Suputtamongkol 2011). Only two trials had low risk of bias both for random sequence generation and allocation concealment (Bisoffi 2011; Suputtamongkol 2011).
Blinding
All the trials were unblinded, but the lack of blinding could not have affected the results because the primary outcome (parasitological cure) was objectively measured.
Incomplete outcome data
One trial was considered at high risk of bias because of the high number of losses to follow‐up (Marti 1996). Four of the seven included trials did not provide enough information to assess attrition bias and were classified as having an unclear risk of bias.
Selective reporting
Only one trial protocol was available and could be assessed for selective reporting bias (Bisoffi 2011). However, all trials have been classified as low risk of reporting bias. The principal outcomes (parasitological cure and adverse events) were communicated in all reports.
Other potential sources of bias
Only one trial stopped recruitment early (Bisoffi 2011). There were not explicitly defined criteria for the early conclusion of the trial (see Characteristics of included studies).
Effects of interventions
Summary of findings for the main comparison. Summary of findings table 1.
| Ivermectin versus albendazole for treating strongyloides infection | |||||
| Patient or population: patients with treating strongyloides infection Settings: worldwide Intervention: ivermectin versus albendazole | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Albendazole | Ivermectin | ||||
| Cure overall negative parasitological test Follow‐up: mean 5 weeks | 48 per 100 | 84 per 100 (72 to 98) | RR 1.79 (1.55 to 2.08) | 478 (4 trials) | ⊕⊕⊕⊝ moderate1 |
| Adverse events report of adverse events Follow‐up: mean 5 weeks | 26 per 100 | 21 per 100 (15 to 29) | RR 0.80 (0.59 to 1.09) | 518 (4 trials) | ⊕⊕⊝⊝ low1,2 |
| *The basis for the assumed risk (eg the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded by 1 for risk of bias: two trials did not conceal allocation, and no method of allocation is described. 2Downgraded by 1 for imprecision: wide range of estimates on 3 trials could include substantive fewer events to a few more.
Summary of findings 2. Summary of findings table 2.
| Ivermectin versus thiabendazole for treating strongyloides infection | |||||
| Patient or population: patients with treating strongyloides infection Settings: worldwide Intervention: ivermectin versus thiabendazole | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Thiabendazole | Ivermectin | ||||
| Cure overall negative parasitological test Follow‐up: mean 11 weeks | 69 per 100 | 74 per 100 (66 to 82) | RR 1.07 (0.96 to 1.2) | 467 (3 trials) | ⊕⊕⊝⊝ low1 |
| Adverse events report of adverse events Follow‐up: mean 11 weeks | 73 per 100 | 23 per 100 (15 to 36) | RR 0.31 (0.2 to 0.5) | 507 (3 trials) | ⊕⊕⊕⊝ moderate1 |
| *The basis for the assumed risk (eg the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded by 1 for risk of bias: 2 trials did not conceal allocation, and no method of allocation is described in one trial.
All seven included trials measured parasitological cure at different follow‐up periods (from two to 24 weeks) (Datry 1994; Gann 1994; Marti 1996; Adenusi 2003; Suputtamongkol 2008; Bisoffi 2011; Suputtamongkol 2011). Four trials compared ivermectin versus albendazole (Datry 1994; Marti 1996; Suputtamongkol 2008; Suputtamongkol 2011) and three trials compared ivermectin versus thiabendazole (Gann 1994; Adenusi 2003; Bisoffi 2011).
Comparison 1: Ivermectin versus albendazole
Parasitological cure
See Table 1.
Parasitological cure was higher with ivermectin (RR 1.79, 95% CI 1.55 to 2.08; 478 participants; four trials; Analysis 1.1; Figure 4). This effect was consistent despite the geographical origin of the population (RR 1.75, 95% CI 1.50 to 2.04; 425 participants; three trials in endemic areas; and RR 2.21, 95% CI 1.28 to 3.80; 53 participants; one trial in non‐endemic areas; Analysis 1.2). The subgroup analysis performed by dosage of ivermectin included four trials assessing single doses (200 μg/kg) (Datry 1994; Marti 1996; Suputtamongkol 2008; Suputtamongkol 2011) and one trial assessing double doses (200 μg/kg for two consecutive days; Suputtamongkol 2011). There were no differences when ivermectin single or double dose was compared to albendazole (P = 0.18), low quality evidence;Analysis 1.3).
1.1. Analysis.
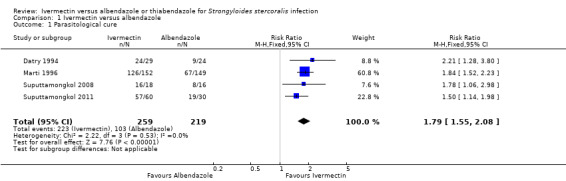
Comparison 1 Ivermectin versus albendazole, Outcome 1 Parasitological cure.
4.
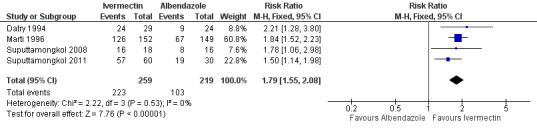
Forest plot of comparison: 1 Ivermectin versus albendazole, outcome: 1.1 Parasitological cure.
1.2. Analysis.
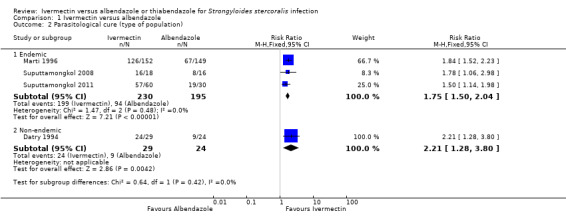
Comparison 1 Ivermectin versus albendazole, Outcome 2 Parasitological cure (type of population).
1.3. Analysis.
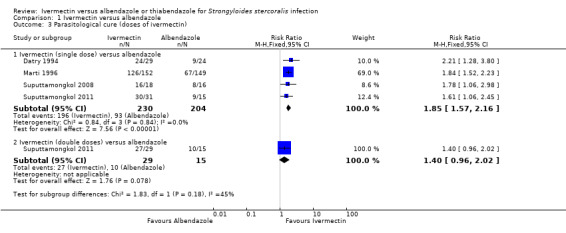
Comparison 1 Ivermectin versus albendazole, Outcome 3 Parasitological cure (doses of ivermectin).
Only two trials included immunocompromised patients, although the number of patients was unclear (Suputtamongkol 2008; Suputtamongkol 2011). These trials showed higher cure with ivermectin (RR 1.78, 95% CI 1.06 to 2.98 and RR 1.50, 95% CI 1.14 to 1.98, respectively). These trials did not provide any subgroup analyses for immunocompromised patients.
Sensitivity analysis excluding trials with unclear number of immunocompromised patients (Suputtamongkol 2008; Suputtamongkol 2011) had no impact on the estimated efficacy of ivermectin (RR 1.89, 95% CI 1.58 to 2.27; 354 participants; two trials; Analysis 1.4).
1.4. Analysis.
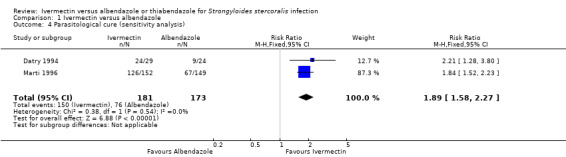
Comparison 1 Ivermectin versus albendazole, Outcome 4 Parasitological cure (sensitivity analysis).
One trial, Bisoffi 2011, excluded participants with immunodeficiencies and the remaining trials reported the exclusion of hematologic abnormalities. However it was unclear whether participants were assessed for immunocompetence.
Death
There was no mortality reported as related to treatment. Suputtamongkol 2011 reported 15 deaths related to underlying diseases as solid tumours, haematological malignancies, diabetes, lupus, myocardial infarction and sepsis.
Adverse events
There were no reports of serious adverse events. Ivermectin was at least as well tolerated as albendazole (RR 0.80, 95% CI 0.59 to 1.09; 518 participants, four trials, very low quality evidence; Analysis 1.5; Figure 5). Table 3 summarises further the information related to adverse events of the primary trials.
1.5. Analysis.
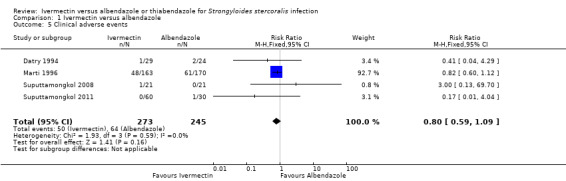
Comparison 1 Ivermectin versus albendazole, Outcome 5 Clinical adverse events.
5.
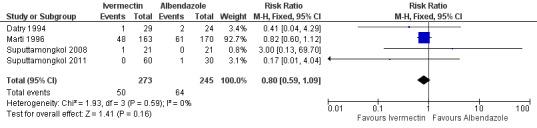
Forest plot of comparison: 1 Ivermectin versus albendazole, outcome: 1.5 Clinical adverse events.
1. Comparison 1: Ivermectin versus albendazole. Adverse events (clinical and laboratory).
| Trial | Assessment method | Timing | Ivermectin | Albendazole |
| Datry 1994 | "Tolerance were evaluated at each visit by a thorough questionnaire and physical examination. Hepatic, renal and haematological functions were investigated before treatment and on day 7" | "Tolerance of drugs were evaluated on days 7, 30 and 90" | Evaluted for AEs 29 patients: fatigue, nausea, tremor: 1 (3%); elevation of transaminases: 1 (3%); anaemia and leukopenia: 1 (3%) |
Evaluted for AEs 24 patients: abdominal pain, nausea and dizziness: 2 (8%); elevation of transaminases: 1 (4%) |
| Marti 1996 | Standardized questionnaire | "The assessment for side effects of the drug regimen was performed three days after start of treatment...and three weeks after the end of treatment" | Evaluated for AEs 163 patients:
|
Evaluated for AEs 170 patients:
|
| Suputtamongkol 2008 | Biochemical and haematological tests | "one week after enrolment" | Evaluated for AEs 18 patients:
|
Evaluated for AEs 16 patients:
|
| Suputtamongkol 2011 | "Adverse events were defined as symptoms or signs that developed after the trial drug administration and had not been reported prior to the administration of the first dose of the antihelmintic" | Not described | Evaluated for AEs 60 patients:
|
Evaluated for AEs 30 patients:
|
Abbreviations: AEs: adverse events.
In ivermectin group, the adverse events most frequently reported were loose stools (10%), cough (7%), headache (9%) and fever (6%) (Marti 1996); fatigue, nausea and tremor (3%) (Datry 1994). In Suputtamongkol 2008, one patient had acute generalized exanthematous pustulosis of moderate severity probably drug‐related.
In albendazole group, the adverse events most frequently reported were headache (11%), loose stools (10%), dizziness (6%) and cough (5%) (Marti 1996); nausea and dizziness (8%) (Datry 1994). None of them caused discontinuation of participants' normal daily activities. Severe nausea and vomiting were reported in one patient in the albendazole group (Suputtamongkol 2011).
Adverse analytical changes
Three trials (Datry 1994; Suputtamongkol 2008; Suputtamongkol 2011) reported a modest elevation of transaminases suggesting hepatotoxicity in both the ivermectin and albendazole treatment arms. Other abnormalities included anaemia and leucopenia in ivermectin group (Datry 1994; see Table 3). Transaminase levels returned to normality within a month (three to four weeks) and the haematological abnormalities disappeared within two months after treatment discontinuation.
Comparison 2: Ivermectin versus thiabendazole
Parasitological cure
See Table 2.
Parasitological cure was not different between ivermectin and thiabendazole (RR 1.07, 95% CI 0.96 to 1.20; 467 participants, three trials; Analysis 2.1; Figure 6). The geographical origin did not modified the effect of either treatments (RR 1.07, 95% CI 0.94 to 1.22; 216 participants, one trial in endemic areas; and RR 1.08, 95% CI 0.90 to 1.29; 251 participants, two trials in non‐endemic areas; Analysis 2.2). The subgroup analysis performed by dosage of ivermectin included two trials assessing single doses (200 μg/kg) (Adenusi 2003; Bisoffi 2011) and one trial assessing double doses (200 μg/kg for two consecutive days) (Gann 1994). There were no differences when ivermectin single or double dose was compared to thiabendazole (P = 0.92),low quality evidence; Analysis 2.3).
2.1. Analysis.
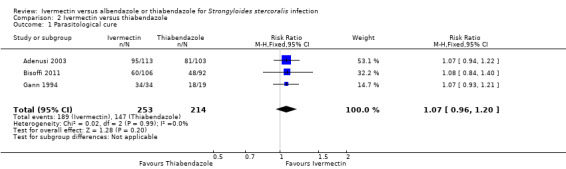
Comparison 2 Ivermectin versus thiabendazole, Outcome 1 Parasitological cure.
6.
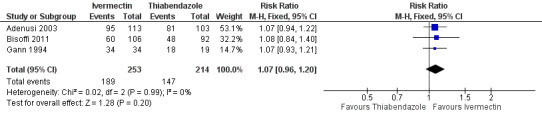
Forest plot of comparison: 2 Ivermectin versus thiabendazole, outcome: 2.1 Parasitological cure.
2.2. Analysis.
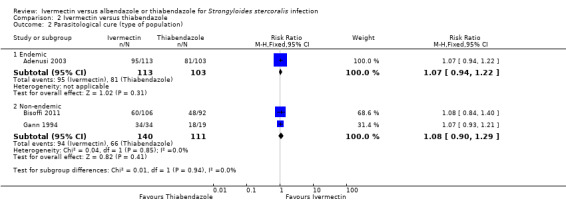
Comparison 2 Ivermectin versus thiabendazole, Outcome 2 Parasitological cure (type of population).
2.3. Analysis.
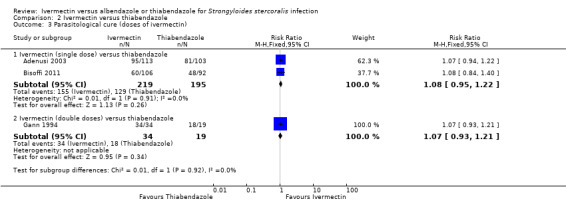
Comparison 2 Ivermectin versus thiabendazole, Outcome 3 Parasitological cure (doses of ivermectin).
Death
There was no mortality reported as related to treatment.
Adverse events
Severe drug reaction was not reported. The incidence of adverse events was higher in the thiabendazole group than in the ivermectin group (RR 0.31, 95% CI 0.20 to 0.50; 507 participants; three trials; Analysis 2.4; Figure 7). Table 4 summarises further the information related to adverse events of the primary trials.
2.4. Analysis.
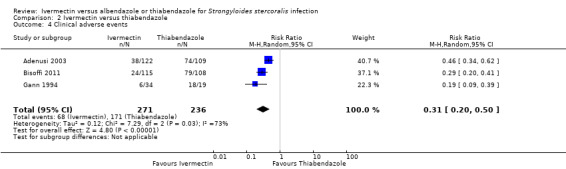
Comparison 2 Ivermectin versus thiabendazole, Outcome 4 Clinical adverse events.
7.
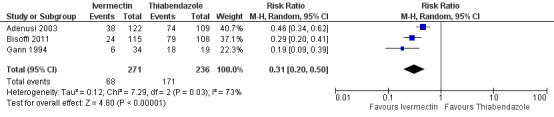
Forest plot of comparison: 2 Ivermectin versus thiabendazole, outcome: 2.4 Clinical adverse events.
2. Comparison 2: Ivermectin versus thiabendazole. Adverse events (clinical and laboratory).
| Trial | Assessment method | Timing | Ivermectin | Thiabendazole |
| Adenusi 2003 | "Voluntary spontaneous complaints by subjects and also by interviews conducted using a standard questionnaire based on the common adverse effects of either drug reported in the literature" | 7 days post‐treatment | Evaluated for AEs 122 patients:
|
Evaluated for AEs 109 patients:
|
| Bisoffi 2011 | "reported by patients" | Days 1 and 2 of treatment and during scheduled and unscheduled visits | Evaluated for AEs 115 patients:
|
Evaluated for AEs 108 patients:
|
| Gann 1994 | "To assess drug safety, we took patient histories, did physical examination and ran complete blood cell counts and serum chemistries (including liver function test)" | Before and on day 7 after treatment | Evaluated for AEs 34 patients:
|
Evaluated for AEs 19 patients:
|
Abbreviations: AEs: adverse events.
In ivermectin group, adverse events frequently described were fatigue (13%) and headache (9%) (Adenusi 2003); dizziness and drowsiness (10%) (Bisoffi 2011); and itching (12%) and lightheadedness (9%) (Gann 1994).
In thiabendazole group, adverse events frequently described were fatigue (50%), nausea (45%), anorexia (36%) and dizziness (26%) (Adenusi 2003); dizziness (53%), nausea and vomiting (Bisoffi 2011); disorientation (89%), fatigue (79%) and nausea (68%) (Gann 1994).
Adverse analytical changes
In Gann 1994, a modest elevation of transaminases was reported to cause hepatotoxicity (Table 4).
Comparison 3: Single dose versus double dose ivermectin
Two trials assessed single (200 μg/kg) versus double doses (200 μg/kg for two consecutive days) of ivermectin (Gann 1994; Suputtamongkol 2011). Taking double doses of ivermectin was not associated with higher cure (RR 1.02, 95% CI 0.94 to 1.11; 94 participants; two trials; Analysis 3.1).
3.1. Analysis.
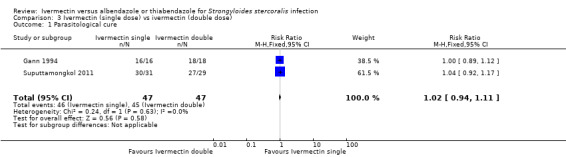
Comparison 3 Ivermectin (single dose) vs ivermectin (double dose), Outcome 1 Parasitological cure.
Discussion
Summary of main results
We undertook this Cochrane Review to assess the effectiveness of ivermectin compared with albendazole and thiabendazole in the parasitological cure of chronic strongyloidiasis.
The results suggest that there is evidence of low to moderate quality that ivermectin is superior in terms of efficacy than albendazole but, given the low overall incidence of adverse effects, meta‐analyses may be underpowered to confidently detect differences in the incidence of adverse events between both treatments (see Table 1). There is evidence of low to moderate quality that ivermectin appears to be as effective as thiabendazole, and presents less adverse events (see Table 2).
Subgroup analyses showed no differences in the efficacy of ivermectin according to type of population (endemic versus non endemic) neither for the comparison with albendazole nor thiabendazole.
We found no difference in the parasitological cure according to dosage (single dose or double doses) of ivermectin, although this result is based on only two trials with few patients.
Dizziness, nausea and disorientation were the most frequent adverse events reported in the included trials. Although albendazole and thiabendazole belong to the same drug family (benzimidazoles), they have different effects and different adverse events. In the current review, adverse events were generally poorly assessed. The most frequent abnormal laboratory test in patients that received benzimidazoles was hepatotoxicity (increase in transaminase levels). However, the clinical significance of this effect was no serious and all patients recover the normal levels in approximately three weeks.
Overall completeness and applicability of evidence
These findings are of importance for clinical perspectives. The results of this Cochrane Review don't allow formulation of clear public health conclusions, due to the low quality of evidence on the efficacy of treatment for strongyloides and the scarce data on safety. To date, no public health strategy has been developed to control strongyloidiasis. However since 1989, the WHO Onchocerciasis Control Programme has fought against onchocerciasis by means of mass administration of ivermectin and vector control initiatives. Similarly, since 2000, albendazole either with ivermectin or diethylcarbamazine citrate has been the cornerstone of the WHO Global Programme to Eliminate Lymphatic Filariasis. In those areas where mass treatment with ivermectin has been used to control onchocerciasis or lymphatic filariasis, the prevalence of infection with soil‐transmitted helminth parasites, has been reduced, most or all of which are deemed to be a major cause of the morbidity arising from childhood nutrition and growth. This could have impact on the incidence of strongyloidiasis in endemic areas, but there is no clear data on this.
There is no report on resistance to ivermectin which is a favourable factor to be used in mass community treatment. The WHO recommends double therapy with ivermectin and albendazole in endemic areas with coinfection of soil‐transmitted helminthiasis and lymphatic filariasis; and triple therapy with ivermectin, albendazole and praziquantel in schistosomiasis‐endemic areas. Thiabendazol seems to be as effective as ivermectin but is not produced in a lot of countries. Being albendazole less effective than ivermectin, it is considered a better alternative treatment for strongyloidiasis than thiabendazole.
All trials included patient with chronic strongyloidiasis. We have no evidence about the impact of ivermectin on other clinical stages (acute strongyloidiasis or hyperinfection syndrome). The more effective dose of ivermectin (single or double) is a question that remains unanswered and deserves further rigorous research. Five out of seven trials included only immunocompetent patients and only two trials included an unknown proportion of immunocompromised patients. It is known that immunocompromised people are the most vulnerable population at risk for developing fatal illness. Unfortunately the review provides little information about the treatment effects on this vulnerable population.
This Cochrane Review does not provide information about the ideal doses for different ages. We cannot answer the question as to the benefit of ivermectin in very young or very old people as most of the trials did not include information about effectiveness and age.
The effect of ivermectin in preventing new infections is not assessed. The trials included in this systematic review were not primarily designed to evaluate the effectiveness of ivermectin in preventing new infections of strongyloidiasis and this outcome was not commonly reported.
Quality of the evidence
Many trials did not adequately report the trial characteristics that are important to evaluate the quality of the evidence. Most trials did not explain if, or how, the sample size was predetermined and many had small sample sizes. Almost none of the trials used an adequate method of allocation concealment nor blindness. However we have considered that lack of blindness has a low risk of bias because the measurement of the outcome (parasitological cure) was done objectively. Also, there was insufficient information to assess the attrition bias of the trials included; we classified four of the seven included trials as having an unclear risk of bias.
Potential biases in the review process
Publication bias is a major threat to the validity of systematic reviews. To minimize the risk of publication bias, we conducted a comprehensive search across numerous clinical trial databases. Nonetheless, as for any systematic review, we cannot rule out the influence of publication bias. Unfortunately given the small number of included trials we were impeded to reliably assess the presence of publication bias.
Agreements and disagreements with other studies or reviews
We have not identified any trials similar to this Cochrane Review. A systematic review was published on 2009 (Santiago 2009) about prophylaxis for strongyloidiasis hyperinfection which objective was to determine patterns of prophylaxis in hyperinfection syndrome in immunosuppressed rheumatology patients. Another systematic review was published on 2013 about case reports and short cases of hyperinfection syndrome (HS) and disseminated strongyloidiasis (DS) described 244 cases treated with different drugs, administration route and duration. Similar fatality rate was observed between patients with DS (68.5%) and HS (60%) (Buonfrate 2013).
Authors' conclusions
Implications for practice.
More people are cured with ivermectin than with albendazole for chronic strongyloidiasis, and it does not have more adverse effects. Ivermectin results in similar cure rates when compared to thianbendazole, but there are more adverse effects with thiabendazole. The most effective dose of ivermectin (single or double) is a question that remains still unanswered and deserves further research.
For patients with some underlying immunosuppressive disorder, or in patients who are very young or very old, current data are insufficient to make a conclusive statement as regards appropriate management.
Implications for research.
Well‐designed trials may help investigate the effect of different doses (single, double or multiple doses) and regimens of ivermectin to identify appropriate doses for treatment and prophylaxis in different group of patients to facilitate adherence.
The single most important problem posed by strongyloidiasis is its potential to produce a hyperinfection syndrome in vulnerable population. Future trials could focus in such population. We are unable to comment on the effects of ivermectin in other syndromes, specially in the high‐risk groups for hyperinfection syndrome.
Acknowledgements
The editorial base for the CIDG is funded by UKaid from the UK Government for the benefit of developing countries.
We are grateful to Harriet MacLehose, Vittoria Lutje and CIDG editorial staff. Also, we thank Paul Garner, Reive Robb, Anne‐Marie Stephani, Xavier Bonfil and Marta Roque for helping us to complete this Cochrane Review version.
We acknowledge everyone who responded to queries regarding ivermectin trials for strongyloidiasis.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | strongyloid* | STRONGYLOIDES | STRONGYLOIDES | STRONGYLOIDIASIS | strongyloid* |
| 2 | thiabendazole | STRONGYLOIDES STERCORALIS | STRONGYLOIDES STERCORALIS | STRONGYLOIDIASIS‐INFECTION | thiabendazole |
| 3 | albendazole | strongyloid* | strongyloid* | strongyloides NEAR infection$ | albendazole |
| 4 | mebendazole | strongyloides infection* | strongyloides infection* | strongyloides NEAR stercoralis | mebendazole |
| 5 | anthelmint* | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | anthelmint* |
| 6 | ivermectin* | thiabendazole | thiabendazole | THERAPY | 2 or 3 or 4 or 5 |
| 7 | 2 or 3 or 4 or 5 or 6 | albendazole | albendazole | TREATMENT | 1 and 6 |
| 8 | 1 and 7 | mebendazole | mebendazole | thiabendazole | — |
| 9 | — | anthelmint$ | anthelmint$ | albendazole | — |
| 10 | — | ivermectin* | ivermectin* | mebendazole | — |
| 11 | — | 6 or 7 or 8 or 9 or 10 | 6 or 7 or 8 or 9 or 10 | anthelmint$ | — |
| 12 | — | 5 and 11 | 5 and 11 | ivermectin$ | — |
| 13 | — | — | — | 6 or 7 or 8 or 9 or 10 or 11 or 12 | — |
| 14 | — | — | — | 5 and 13 | — |
aCIDG Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by the Cochrane Collaboration (Lefebvre 2011); upper case: MeSH or EMTREE heading; lower case: free text term.
Data and analyses
Comparison 1. Ivermectin versus albendazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological cure | 4 | 478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.55, 2.08] |
| 2 Parasitological cure (type of population) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Endemic | 3 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.50, 2.04] |
| 2.2 Non‐endemic | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.28, 3.80] |
| 3 Parasitological cure (doses of ivermectin) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Ivermectin (single dose) versus albendazole | 4 | 434 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.57, 2.16] |
| 3.2 Ivermectin (double doses) versus albendazole | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.96, 2.02] |
| 4 Parasitological cure (sensitivity analysis) | 2 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.58, 2.27] |
| 5 Clinical adverse events | 4 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.09] |
Comparison 2. Ivermectin versus thiabendazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological cure | 3 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.96, 1.20] |
| 2 Parasitological cure (type of population) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Endemic | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.94, 1.22] |
| 2.2 Non‐endemic | 2 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.90, 1.29] |
| 3 Parasitological cure (doses of ivermectin) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Ivermectin (single dose) versus thiabendazole | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.22] |
| 3.2 Ivermectin (double doses) versus thiabendazole | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.21] |
| 4 Clinical adverse events | 3 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.50] |
Comparison 3. Ivermectin (single dose) vs ivermectin (double dose).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitological cure | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adenusi 2003.
| Methods | RCT. Length of follow‐up: 4 weeks post‐treatment. |
|
| Participants | Number randomized: 252 participants (216 participants completed the trial: 21 treatment withdrawals and 15 losses to follow‐up). Inclusion criteria: aged 5 to 66 years old, with uncomplicated intestinal strongyloidiasis and whose stools were positive of S. stercoralis larvae during a survey on intestinal helminths. Exclusion criteria: they had no received any form of anti‐filarial therapy and any other antihelmintic treatment in the 6 months and 72 hours respectively, preceding the trial. Only subjects with no allergic diathesis, disseminated strongyloidiasis, severe renal, hepatic, haematological (haemoglobin level under 5 g/dL) or cardiovascular functions participated in the trial. Potentially childbearing women not using contraceptives and subjects in which the parasite was detected in stool samples more than 30 days before commencement of the trial were excluded. Patients were recruited through a community survey and signs and symptoms such as epigastric pain, urticaria, and diarrhoea were recorded. |
|
| Interventions | Ivermectin 200 μg/kg single dose (N = 126) versus thiabendazole 50 mg/kg/day for 3 days (N = 126). | |
| Outcomes | Drug efficacy: negative stool test at 7, 21 and 30 days. A subject was considered parasitologically cured, if all 3 post‐treatment stool samples tested negative for S. stercoralis. All patients who did not provide all 3 follow‐up stool samples were excluded from the analysis of drug efficacy. Clinical adverse events were investigated through voluntary spontaneous complaints and also by interviews conducted using a standard questionnaire within 7 days post‐treatment. |
|
| Notes | Diagnostic method: Baermann. Place: Yewa South, Nigeria‐Africa. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment regimens were randomized from a list for the sequential allocation of the drugs, prepared in advance. |
| Allocation concealment (selection bias) | High risk | We do not know how allocation was concealed. Probably not done. |
| Blinding of participants and personnel (performance bias) Cure overall | Low risk | No blinding of participants and personnel but we don't believe this will introduce bias. |
| Blinding of participants and personnel (performance bias) Adverse events overall | High risk | No blinding of participants and personnel and the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Cure overall | Low risk | No blinding, but the we judge that the outcome measurement is unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Adverse events overall | High risk | No blinding and the outcome measurement are likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 36/252 participants (14.3%) lost during follow‐up period (13/126: 9 lost to follow‐up and 4 incomplete treatment in the ivermectin arm and 23/126: 9 lost to follow‐up and 17 incomplete treatment in the thiabendazole arm). No reasons for missing data provided. Per‐protocol analysis. |
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available but it is clear that the published report include all expected outcomes. |
| Other bias | Low risk | The trial appears to be free of other sources of bias. |
Bisoffi 2011.
| Methods | RCT. Length of follow‐up: 16 to 24 weeks post‐treatment. |
|
| Participants | Number randomized: 223 Inclusion criteria: aged 5 to 85 years old. Eligible patients were male and female subjects older than 5 years and weighing > 15 kg. They were travellers, immigrants residents, and autochthonous residents living in Italy. They had no have a diagnosis of strongyloidiasis established by IFAT. Exclusion criteria: pregnancy or breastfeeding; CNS diseases; disseminated strongyloidiasis: immunodeficiency (malignancies, chemotherapy or other immunosuppressive treatments); planned travel to endemic countries before follow‐up; lack of informed consent. HIV positive subjects were excluded if CD4 count was lower than 400/µL. Baseline signs and symptoms (not reported) were recorded. |
|
| Interventions | Ivermectin 200 μg/kg single dose (N = 115) versus thiabendazole two daily doses of 25 mg/kg/day for 2 days (N = 108). | |
| Outcomes | Drug efficacy: cure at Time 2 (Time 2: 4 to 6 months after recruitment), defined as follows: negative stool agar culture and negative IFAT or decrease of two or more antibody titres. Adverse events reported by the patients. |
|
| Notes | Diagnostic method: stool agar culture and IFAT. Place: Italian travellers attended at Sacro‐Cuore Hospital, Verona‐Italy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization list was computer‐generated by a biostatistician who was not directly involved in the trial and handled to a nurse who was not involved in the trial. The patients received an unique ID number. |
| Allocation concealment (selection bias) | Low risk | The list was kept in a locked drawer. As randomization was not in blocks, there was no way for the investigator to guess in advance the next assignment treatment. |
| Blinding of participants and personnel (performance bias) Cure overall | Low risk | No blinding of participants and personnel, but we don't believe this will introduce bias. |
| Blinding of participants and personnel (performance bias) Adverse events overall | High risk | No blinding, and the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Cure overall | Low risk | Blinding of laboratory staff was ensured: the laboratory personnel performing the analyses (stool culture, serology) had no direct contact with the investigators and no information as regards the drug administered to the patients. |
| Blinding of outcome assessment (detection bias) Adverse events overall | High risk | No blinding, and the outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25/223 participants (11.2%) lost during follow‐up. Compliance to follow‐up was higher for ivermectin (106/115 or 92.2%) than thiabendazole (92/108 or 85.2%). No reasons for missing data provided. Per‐protocol analysis. |
| Selective reporting (reporting bias) | Low risk | The trial protocol is available and all of the trial's pre‐stated outcomes that are of interest in the review have been reported. |
| Other bias | Unclear risk | Recruitment was concluded before the required sample size was obtained. The reason was the important difference in tolerability observed between the two arms. This was not an explicitly defined criteria for the early conclusion of the trial (all observed adverse events were mild to moderate). |
Datry 1994.
| Methods | RCT. Lenght of follow‐up: 12 weeks. |
|
| Participants | Number randomized: 60 participants Inclusion criteria: adults and children (5 to 70 years). Patients were admitted to the trial if S. stercoralis had been detected in a stool sediment within 30 days preceding the trial. Exclusion criteria: they did not show any indication of disseminated strongyloidiasis, acute or serious illness, or any marked abnormality of liver, renal, hematopoietic or cardiovascular function, and had not received any other antifilarial drug in the 6 months, or other antihelmintic treatment in the 72 hours preceding the trial. Potentially child‐bearing women who were not using contraceptives were excluded, also. Baseline signs and symptoms such as pruritus was recorded (the trial refers that clinical outcome was favourable in all the patients who were cured, except for one who complained of persistent pruritus, which was not related to strongyloidiasis). |
|
| Interventions | Ivermectin 150 to 200 μg/kg in a single dose (N = 32) and albendazole 400 mg/day for 3 days (N = 28). | |
| Outcomes | Drug efficacy: negative stool samples (7, 30, and 90 days). Tolerance was evaluated at each visit by a thorough questionnaire and physical examination. Hepatic, renal and haematological functions were investigated before treatment and on day 7. | |
| Notes | Diagnostic method: smear examination, Kato thick smears, formalin‐ether concentrations, and Baermann. Place: residents in France coming from sub‐Saharan Africa, Caribbean, south‐east Asia, and Latin America. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were randomly assigned to either ivermectin or albendazole treatment". We do not know how the randomization was done. |
| Allocation concealment (selection bias) | High risk | We do not know how allocation was concealed. Probably not done. |
| Blinding of participants and personnel (performance bias) Cure overall | Low risk | No blinding of participants and personnel but review authors don't believe this will introduce bias. |
| Blinding of participants and personnel (performance bias) Adverse events overall | High risk | No blinding, and the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Cure overall | Low risk | This was an open‐label trial. Therefore blinding of laboratory staff was ensured (the laboratory personnel performing the analyses (stool culture, serology) had no direct contact with the investigators and no information as regards the drug administered to the patient. |
| Blinding of outcome assessment (detection bias) Adverse events overall | High risk | No blinding, and the outcome is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/60 patients were not included in the analysis (4 belonged to the albendazole group and 3 to the ivermectin group). The reasons for missing data were inadequate follow‐up (4 patients) and inclusion faults (diagnosis of S. stercoralis infection more than 30 days before enrolment (two) and concomitant anthelmintic therapy (one). Per‐protocol analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol provided, but given the outcomes nominated in the methods section, all pre‐specified outcomes were reported. |
| Other bias | Low risk | The trial appears to be free of other sources of bias. |
Gann 1994.
| Methods | RCT. Lenght of follow‐up: 12 weeks. |
|
| Participants | Number randomized: 53 participants. Inclusion criteria: aged 5 to 70 years old. People suspected of having strongyloidiasis were identified through neighbourhood clinics, schools, primary care physicians and hospital laboratories in Lowell. Patients were suspected of being infected on the basis of symptoms, eosinophilia, or positive serology. Baseline signs and symptoms such as epigastric pain, diarrhoea, losing weight, urticaria and cough were recorded. Inclusion criteria: Non‐pregnant 5‐ to 70‐year‐old patients with stool tests positive for S. stercoralis. Exclusion criteria: people with no signs of hyperinfection or major concurrent illness. |
|
| Interventions | Ivermectin 200 μg/kg single dose (N = 16). Ivermectin 200 μg/kg for 2 consecutive days (N = 18) or thiabendazole 25 mg/kg twice per day for 3 days (N = 19). | |
| Outcomes | Drug efficacy: negative stool samples (7, 30, 90, and 180 days). Subsequent stool examinations done 10 and 22 months after treatment. To assess adverse reactions and encourage total compliance, patients were contacted daily during treatment by a Cambodian‐speaking research assistant. To assess drug safety, we took patient histories, did physical examinations, and ran complete blood cell counts and serum chemistries (including liver function tests) shortly before and on day 7 after treatment. | |
| Notes | Diagnostic method: Baermann. Place: Southeast Asian refugees living in Lowell, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned (using Social Security number and a random number table). |
| Allocation concealment (selection bias) | High risk | No information was supplied regarding concealment. Probably not done. |
| Blinding of participants and personnel (performance bias) Cure overall | Low risk | No blinding of participants and personnel but we don't believe this will introduce bias. |
| Blinding of participants and personnel (performance bias) Adverse events overall | High risk | No blinding, and the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Cure overall | Low risk | Laboratory personnel conducting stool and blood testing were blinded to the patients treatment group. |
| Blinding of outcome assessment (detection bias) Adverse events overall | High risk | No blinding, and the outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/16 (6%), 0/18 (0%), and 0/19 (0%) lost during 3 months of follow‐up period. Per‐protocol analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol provided, but given the outcomes nominated in the methods section, all pre‐specified outcomes were reported. |
| Other bias | Low risk | No additional biases were identified. |
Marti 1996.
| Methods | RCT. Lenght of follow‐up: 3 weeks. |
|
| Participants | Number randomized: 417 participants. Inclusion criteria: schoolchildren aged 9 to 22 years old. Inclusion criteria: any individual with demonstrated first or third‐stage larvae of S. stercoralis on stool sample was included in the trial. Exclusion criteria: consent not given; fever or other signs of acute illness; severe neurologic disorders; severe liver disorders; and pregnancy. Baseline signs and symptoms such as cough, abdominal distention, diffuse itching, urticaria, and larva migrans were recorded. |
|
| Interventions | Ivermectin 200 /kg single dose (N = 208) versus albendazole 400 mg/day for 3 days (N = 209). | |
| Outcomes | Drug efficacy: negative stool samples (3 and 21 days). Symptoms of strongyloidiasis and adverse effects of the two drugs according to the literature were detailed beforehand. The list was translated from English into Kiswahili and back to English to ensure correct interpretation of the findings. The interviews were carried out in Kiswahili by a medical assistant of the Ministry of Health. Special symptoms were recorded on a separate sheet, where the findings of a thorough clinical examination were also recorded. | |
| Notes | Kato‐Katz smear and Baermann. Place: Zanzibar. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomized list for the sequential allocation of the drugs was prepared in advance. |
| Allocation concealment (selection bias) | High risk | We do not know how allocation was concealed. Probably not done. |
| Blinding of participants and personnel (performance bias) Cure overall | Low risk | No blinding of participants and personnel but we don't believe this will introduce bias. |
| Blinding of participants and personnel (performance bias) Adverse events overall | High risk | No blinding, and the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Cure overall | Low risk | No blinding, but we judge that the outcome measurement is unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Adverse events overall | High risk | No blinding, and the outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 116/ 417 (28%) lost during follow‐up period (56/208 (27%):45 incomplete questionnaire or treatment and 11 incomplete follow‐up in the ivermectin arm and 60/209 (29%): 39 incomplete questionnaire or treatment and 21 incomplete follow‐up in the albendazole arm). Per‐protocol analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol provided, but given the outcomes nominated in the methods section, all pre‐specified outcomes were reported. |
| Other bias | Low risk | No additional biases were identified. |
Suputtamongkol 2008.
| Methods | RCT. Leght of follow‐up: 2 to 3 weeks. |
|
| Participants | Number randomized: 42 participants. Inclusion criteria: Adult people (included immunocompromised patients: 5 immunosuppressive drugs users, 3 AIDS/HIV patients, 2 hematological malignancy patients). Aged 22 to 87 years old, were recruited if characteristic rhabditiform larvae of S. stercoralis were present on faecal microscopy. Baseline signs and symptoms such as abdominal pain, diarrhoea and nausea/vomiting were recorded. Exclusion criteria: a history of allergic reaction to either trial medication, treatment in the month prior to the trial with any drug known to have anti‐Strongyloides activity, pregnancy or lactation, and any suggestion of disseminated strongyloidiasis. |
|
| Interventions | Ivermectin 200 μg/kg single oral dose (parenteral veterinary preparation) (N = 21) and albendazole 800 mg daily for 7 days (N = 21). | |
| Outcomes | Drug efficacy: negative stool samples 7 days. Adverse events were reported. | |
| Notes | Diagnostic method: smear examination and formol‐ether concentration. Place: Thailand, Siriraj Hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were randomly allocated. We do not know how the randomization was done. |
| Allocation concealment (selection bias) | High risk | We do not know how allocation was concealed. Probably not done. |
| Blinding of participants and personnel (performance bias) Cure overall | Low risk | No blinding of participants and personnel but we don't believe this will introduce bias. |
| Blinding of participants and personnel (performance bias) Adverse events overall | High risk | No blinding of participants and personnel and the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Cure overall | Low risk | No blinding, but we judge that the outcome measurement is unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Adverse events overall | High risk | No blinding, and the outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five patients were lost from the albendazole group and three from the ivermectin group, during median follow‐up periods of 13 days (range 6 to 85 days) and 19 days (3 to 117 days), respectively. Per‐protocol analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol provided, but given the outcomes nominated in the methods section, all pre‐specified outcomes were reported. |
| Other bias | Low risk | No additional biases were identified. |
Suputtamongkol 2011.
| Methods | RCT. Leght of follow‐up: seven hospital visits (baseline, 2 weeks, 1, 3, 6 and 9 months, and 1 year after treatment. |
|
| Participants | Number randomized: 100 participants. Inclusion criteria: aged 23 to 81 years (included immunocompromised patients: 10 AIDS/HIV patients, 32 immunosuppressive drugs user patients, 9 hematological malignancy patients) recruited if characteristic rhabditiform larvae of S. stercoralis were present on fecal microscopy. Baseline signs and symptoms such as abdominal pain, diarrhoea, and nausea/vomiting were recorded. Exclusion criteria: history of allergic reaction to either trial medication, treatment within the month prior to the trial with any drug known to have anti‐strongyloides activity, pregnancy or lactation and any suggestion of disseminated strongyloidiasis. |
|
| Interventions | Three arms: ivermectin 200 μg/kg single oral dose (N = 32), ivermectin two oral doses of 200 μg/kg given 2 weeks apart (N = 32), and albendazole 800 mg daily for 7 days (N = 36). | |
| Outcomes | Drug efficacy: cure was defined as clinical improvement and the absence of rhabditiform larvae in the stool at day 14 of treatment and through the follow‐up period. Adverse events and laboratory abnormalities were reported. | |
| Notes | Diagnostic method: direct smear, formol‐ether concentration method and modified Koga agar plate culture. Place: Thailand, Siriraj Hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated, simple, random allocation sequences were prepared for 3 trial groups by the investigator team. |
| Allocation concealment (selection bias) | Low risk | These were sealed in an opaque envelope and numbered. The investigator (YS) assigned trial participants to their respective treatment group after opening the sealed envelope. |
| Blinding of participants and personnel (performance bias) Cure overall | Low risk | No blinding of participants and personnel but we don't believe this will introduce bias. |
| Blinding of participants and personnel (performance bias) Adverse events overall | High risk | No blinding of participants and personnel and the outcome is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Cure overall | Low risk | No blinding, but we judge that the outcome measurement is unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Adverse events overall | High risk | No blinding, and the outcome measurement is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six patients were excluded in the albendazole group (3 deaths before treatment and 3 lost to follow‐up), one patient was lost to follow‐up in one arm of the ivermectin, an one patient was lost to follow‐up in the other arm of ivermectin. Per‐protocol analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol provided, but given the outcomes nominated in the methods section, all pre‐specified outcomes were reported. |
| Other bias | Low risk | No additional biases were identified. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Archibald 1993 | Participants not randomized, no control group. |
| Beus 1989 | Participants not randomized. |
| Bezjak 1968 | Participants not randomized, no control group. |
| Chanthavanich 1989 | Ivermectin is not a control group. |
| Chaun 1967 | Participants not randomized, no control group. |
| Franz 1963 | Participants not randomized, no control group. |
| Grove 1982 | Participants not randomized, no control group. |
| Gupte 1975 | Participants not randomized, no control group. |
| Huapaya 2003 | Participants not randomized, no control group. |
| Igual‐Adell 2004 | Participants not randomized. |
| Krubwa 1974 | Participants not randomized. |
| Marcos 2005 | Participants not randomized, no control group. |
| Mojon 1987 | Participants not randomized, no control group. |
| Nampijja 2012 | Trial with different intervention. Ivermectin is not a control group. |
| Naquira 1989 | No control group. |
| Nimura 1992 | Participants not randomized, no control group. |
| Nontasut 2005 | Probably a non‐randomized design. |
| Oyakawa 1991 | Participants not randomized, no control group. |
| Pitisuttithum 1995 | Ivermectin is not a control group. |
| Portugal 2002 | Trial used participants who tested negative for S. stercoralis at baseline. |
| Pungpak 1987 | Participants not randomized. |
| Reynoldson 1997 | Did not meet our inclusion criteria: different interventions of interest. |
| Rossignol 1983 | Did not meet our inclusion criteria: different interventions of interest. |
| Salazar 1994 | Probably a non‐randomized design. |
| Schaffel 2000 | No control group. |
| Shikiya 1990 | Participants not randomized. |
| Shikiya 1991 | Participants not randomized. |
| Shikiya 1992 | Participants not randomized. |
| Shikiya 1994 | Participants not randomized. |
| Singthong 2006 | Ivermectin is not a control group. |
| Steinmann 2008 | Did not fit with our inclusion criteria: different interventions of interest. |
| Toma 2000 | Participants not randomized. |
| Whitworth 1991 | Cross‐sectional trial. |
| Xiao 2013 | Not a RCT. |
| Yap 2013 | Ivermectin is not a control group. Cohort trial. |
| Zaha 2004 | Participants not randomized. |
Characteristics of ongoing studies [ordered by study ID]
NCT01570504.
| Trial name or title | Multiple versus single dose of ivermectin for the treatment of strongyloidiasis (STRONGTREAT) Trial registration number (EudraCT number): 2011‐002784‐24 |
| Methods | Randomized, open‐label, multi centre Phase III clinical trial on multiple versus single dose of ivermectin for the treatment of strongyloidiasis |
| Participants | Inclusion criteria: male and female patients older than 5 years and weighting > 15 kg. Current residence in non‐endemic areas. Either direct diagnosis of S. stercoralis infection and positive serology at any titre or positive serology at "high" titre, irrespective of results of direct tests. Exclusion criteria: pregnant or lactating women; subjects suffering from CNS diseases; disseminated strongyloidiasis; immunocompromised patients; lack of informed consent; previous treatment with ivermectin (in the last year). |
| Interventions | Experimental: ivermectin multiple doses (a dose of 200 μg/kg of ivermectin given on days 1,2, 15 and 16). Active comparator: 1 dose ivermectin (a single 200 μg/kg dose of ivermectin). |
| Outcomes | Primary outcome: clearance of strongyloides infection (clearance of infection is defined by negative stool agar/charcoal culture ‐ direct examination of three faecal samples for S. stercoralis and negative serology or decrease in titre below a defined cutoff. Secondary outcome: all‐cause mortality during the 12 months of follow‐up. Patients with partial response to treatment at T2. Patients with adverse reactions. Time Frame: from 1st to 5th day of treatment and from 15th to 19th day (or 72 hours from treatment completion). Patients with increase in blood ALT over cutoff value. Patients with decrease in WBC count below cutoff value. Average difference in blood ALT and WBC count at day 17, compared with baseline. Average difference in blood eosinophil count at T2, compared with baseline. |
| Starting date | March 2013 |
| Contact information | Dora Buonfrate, MD (dora.buonfrate@sacrocuore.it) +39 601 3563 |
| Notes | Funders: European Comission‐Framework VII |
Differences between protocol and review
The analysis of the two types of benzimidazoles (albendazole & thiabendazole) was initially planned as a subgroup analysis within a broad comparison that did not distinguish between both drugs. Given the high heterogeneity between both drugs, we converted the subgroup analysis into two main comparisons. This change was agreed with the CIDG editors.
We changed the 'Risk of bias' tool during preparation of the review to reflect the changes suggested in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We could not explore publication bias using funnel plots because there was an insufficient number of trials in the comparisons (ivermectin versus albendazole: four trials and ivermectin versus thiabendazole: three trials).
As the risk of bias of the trials was similar we could not conduct sensitivity analyses.
We have added new authors have been added to the review team: Maria N Plana (MNP) and Jose A Perez‐Molina.
Contributions of authors
Cesar Henriquez is the guarantor of the Cochrane Review. All review authors contributed substantially to either the conception or conduct of the review; helped draft the manuscript and critically revised it; approved the final version to be published; and are accountable for the integrity and accuracy of the work.
Specific contributors of review authors are as follows:
Cesar Henriquez‐Camacho: Background, Objectives, Methods, interpretation of Results, and Discussion.
Eduardo Gotuzzo: Background and Methods.
Angelica Terashima: Background.
Juan Echevarria: Background and Methods.
A. Clinton White Jr: Background, Methods and Discussion.
Frine Samalvides: Methods.
Jose A Perez‐Molina: Background, Methods, interpretation of results and Discussion.
Maria N Plana: Methods, interpretation of results and Discussion.
Sources of support
Internal sources
Instituto de Medicina Tropical Alexander von Humboldt, Lima, Peru.
Hospital Clinico San Carlos, Madrid, Spain.
Hospital Universitario Fundación Alcorcón, Spain.
Hospital Ramon y Cajal, IRYCIS, Madrid, Spain.
Universidad Rey Juan Carlos, Madrid, Spain.
External sources
CIBER Epidemiologia y Salud Publica (CIBERESP), Madrid, Spain.
Declarations of interest
We declare that we have no conflicts of interest.
Unchanged
References
References to studies included in this review
Adenusi 2003 {published data only}
- Adenusi A, Oke A, Adenusi O. Comparison of ivermectin and thiabendazole in the treatment of uncomplicated human Strongyloides stercoralis infection. African Journal of Biotechnology 2003;2(11):466‐9. [Google Scholar]
Bisoffi 2011 {published and unpublished data}
- Bisoffi Z, Buonfrate D, Angheben A, Boscolo M, Anselmi M, Marocco S, et al. Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis. PLoS Neglected Tropical Diseases 2011;5(7):e1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Datry 1994 {published data only}
- Datry A, Hilmarsdottir I, Mayorga‐Sagastume R, Lyagoubi M, Gaxotte P, Biligui S, et al. Treatment of Strongyloides stercoralis infection with ivermectin compared with albendazole: results of an open study of 60 cases. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(3):344‐5. [DOI] [PubMed] [Google Scholar]
Gann 1994 {published data only}
- Gann PH, Neva FA, Gam AA. A randomized trial of single‐ and two‐dose ivermectin versus thiabendazole for treatment of strongyloidiasis. Journal of Infectious Diseases 1994;169(5):1076‐9. [DOI] [PubMed] [Google Scholar]
Marti 1996 {published data only}
- Marti H, Haji H, Savioli L, Chwaya H, Mgeni A, Ameir J, et al. A comparative trial of a single‐dose Ivermectin versus three days of Albendazole for treatment of Strongyloides stercoralis and other soil‐transmitted helminth infections in children. American Journal of Tropical Medicine and Hygiene 1996;55(5):477‐81. [DOI] [PubMed] [Google Scholar]
Suputtamongkol 2008 {published data only}
- Suputtamongkol Y, Kungpanichkul N, Silpasakorn S, Beeching N. Efficacy and safety of a single‐dose veterinary preparation of ivermectin versus 7‐day high‐dose albendazole for chronic strongyloidiasis. International Journal of Antimicrobial Agents 2008;31(1):46‐9. [DOI] [PubMed] [Google Scholar]
Suputtamongkol 2011 {published data only}
- Suputtamongkol Y, Premasathian N, Bhumimuang K, Waywa D, Nilganuwong S, Karuphong E, et al. Efficacy and safety of single and double doses of ivermectin versus 7‐day high dose albendazole for chronic strongyloidiasis. PLoS Neglected Tropical Diseases 2011;5(5):e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Archibald 1993 {published data only}
- Archibald L, Beeching N, Gill G, Bailey J, Bell D. Albendazole is effective treatment for chronic strongyloidiasis. Quarterly Journal of Medicine 1993;86(3):191‐5. [PubMed] [Google Scholar]
Beus 1989 {published data only}
- Beus A. A comparative study of thiabendazole and mebendazole in strongyloidiasis [Poredbeno ispitivanje tiabendazola i mebendazola u strongiloidozi [Croatian]]. Liječnički Vjesnik 1989;111(3):98‐101. [PubMed] [Google Scholar]
Bezjak 1968 {published data only}
- Bezjak B. A clinical trial of thiabendazole in strongyloidiasis. American Journal of Tropical Medicine and Hygiene 1968;17(5):733‐6. [DOI] [PubMed] [Google Scholar]
Chanthavanich 1989 {published data only}
- Chanthavanich P, Nontasut P, Prarinyanuprp V, Sa‐Nguankiat S. Repeated doses of albendazole against strongyloidiasis in Thai children. Southeast Asian Journal of Tropical Medicine and Public Health 1989;20(2):221‐6. [PubMed] [Google Scholar]
Chaun 1967 {published data only}
- Chaun H. The treatment of chronic strongyloidiasis with thiabendazole. Transactions of the Royal Society of Tropical Medicine and Hygiene 1967;61(6):812‐6. [DOI] [PubMed] [Google Scholar]
Franz 1963 {published data only}
- Franz K. Clinical trials with thiabendazole against human strongyloidiasis. American Journal Tropical Medicine and Hygiene 1963;14:211‐4. [DOI] [PubMed] [Google Scholar]
Grove 1982 {published data only}
- Grove DI. Treatment of strongyloidiasis with thiabendazole: an analysis of toxicity and effectiveness. Transaction of the Royal Society of Tropical Medicine and Hygiene 1982;76(1):114‐8. [DOI] [PubMed] [Google Scholar]
Gupte 1975 {published data only}
- Gupte S. Treatment of strongyloidiasis. Indian Pediatrics 1975;12(7):611. [PubMed] [Google Scholar]
Huapaya 2003 {published data only}
- Huapaya P, Epinoza Y, Huiza A, Sevilla C, Vildósola H. Treatment of Strongyloides stercoralis with ivermectin and thiabendazole [Tratamiento de Strongylodes stercoralis con ivermectina y tiabendazole]. Anales de la Facultad de Medicina 2003;64(2):89‐93. [Google Scholar]
Igual‐Adell 2004 {published data only}
- Igual‐Adell R, Oltra‐Alcaraz C, Soler‐Company E, Sánchez‐Sánchez P, Matogo‐Oyana J, Rodríguez‐Calabuig D. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opinion on Pharmacotherapy 2004;5(12):2615‐9. [DOI] [PubMed] [Google Scholar]
Krubwa 1974 {published data only}
- Krubwa F, Gatti F, Lontie M, Nguete K, Vandepitte J, Thienpont D. Quarterly administration of mebendazole to suburban school children [Administration trimestrielle de Mebendazole en milieu scolaire suburbain]. Medecine Tropicale 1974;34(5):679‐87. [Google Scholar]
Marcos 2005 {published data only}
- Marcos L, Terashima A, Samalvides F, Alvarez H, Lindo F, Tello R, et al. Thiabendazole for control of Strongyloides stercoralis infection in a hyperendemic area of Peru [Tiabendazol para el control de la infección por Strongyloides stercoralis en una zona hiperendémica en el Perú]. Revista Gastroenterología del Perú 2005;25(4):341‐8. [PubMed] [Google Scholar]
Mojon 1987 {published data only}
- Mojon M, Nielsen PB. Treatment of Strongyloides stercoralis with albendazole. A cure rate of 86 per cent. Zentralblatt für Bakteriologie, Mikrobiologie, und Hygiene 1987;263(4):619‐24. [DOI] [PubMed] [Google Scholar]
Nampijja 2012 {published data only}
- Nampijja M, Apule B, Lule S, Akurut H, Muhangi L, Webb EL, et al. Effects of maternal worm infections and anthelminthic treatment during pregnancy on infant motor and neurocognitive functioning. Journal of the International Neuropsychological Society 2012;18(6):1019‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naquira 1989 {published data only}
- Naquira C, Jimenez G, Guerra J, Bernal R, Nalin D, Neu D, et al. Ivermectin for human strongyloidiasis and other intestinal helminths. American Journal of Tropical Medicine and Hygiene 1989;40(3):304‐9. [DOI] [PubMed] [Google Scholar]
Nimura 1992 {published data only}
- Nimura S, Hirata T, Zaha O, Nakamura H, Kouchi A, Uehara T, et al. Clinical study of albendazole therapy for strongyloidiasis [Japanese]. Kansenshōgaku Zasshi 1992;66(9):1231‐5. [DOI] [PubMed] [Google Scholar]
Nontasut 2005 {published data only}
- Nontasut P, Muennoo C, Sa‐nguankiat S, Fongsri S, Vichit A. Prevalence of strongyloides in northern Thailand and treatment with ivermectin vs albendazole. Southest Asian Journal of Tropical Medicine and Public Health 2005;36(2):442‐4. [PubMed] [Google Scholar]
Oyakawa 1991 {published data only}
- Oyakawa T, Kuniyoshi T. Arakaki T, Higashionna A, Shikiya K, Sakugawa H, et al. New trial with thiabendazole for treatment of human strongyloidiasis [Japanese]. Kansenshōgaku Zasshi 1991;65(3):304‐10. [DOI] [PubMed] [Google Scholar]
Pitisuttithum 1995 {published data only}
- Pitisuttithum P, Supanaranond W, Chindanond D. A randomized comparative study of albendazole and thiabendazole in chronic strongyloidiasis. Southeast Asian Journal of Tropical Medicine and Public Health 1995;26(4):735‐8. [PubMed] [Google Scholar]
Portugal 2002 {published data only}
- Portugal R, Schaffel R, Almeida L, Spector N, Nucci M. Thiabendazole for the prophylaxis of strongyloidiasis in immunosuppressed patients with hematologic diseases: a randomized, double‐blind placebo‐controlled study. Haematologica 2002;87(6):663‐4. [PubMed] [Google Scholar]
Pungpak 1987 {published data only}
- Pungpak S, Bunnag D, Chindanond D, Radmoyos B. Albendazoles in the treatment of strongyloidiasis. Southeast Asian Journal of Tropical Medicine and Public Health 1987;18(2):207‐10. [PubMed] [Google Scholar]
Reynoldson 1997 {published data only}
- Reynoldson J, Behnke J, Pallant L, Macnish M, Gilbert F, Giles S, et al. Failure of pyrantel in treatment of human hookworm infections (Ancylostoma duodenale) in the Kimberley region of north west Australia. Acta Tropica 1997;68(3):301‐12. [DOI] [PubMed] [Google Scholar]
Rossignol 1983 {published data only}
- Rossignol JF, Maisonneuve H. Albendazole: placebo‐controlled study in 870 patients with intestinal helminthiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 1983;77(5):707‐11. [DOI] [PubMed] [Google Scholar]
Salazar 1994 {published data only}
- Salazar S, Berk S, Howe D, Berk S. Ivermectin vs thiabendazole in the treatment of strongyloidiasis. Infections in Medicine 1994;11(1):50‐9. [Google Scholar]
Schaffel 2000 {published data only}
- Schaffel R, Nucci M, Portugal R, Castro M, Ferrerira S, Almeida L, et al. Thiabendazole for the treatment of strongyloidiasis in patients with hematologic malignancies. Clinical of Infectious Diseases 2000;31(3):821‐2. [DOI] [PubMed] [Google Scholar]
Shikiya 1990 {published data only}
- Shikiya K, Kuniyoshu T, Higashionna A, Arakaki T, Oyakawa T, Kadena K, et al. Treatment of strongyloidiasis with mebendazole and its combination with thiabendazole [Japanese]. Kansenshōgaku Zasshi 1990;64(11):1408‐15. [DOI] [PubMed] [Google Scholar]
Shikiya 1991 {published data only}
- Shikiya K, Kinjo N, Ikema M, Yamashiro A, Uechi H, Oyakawa T, et al. Comparison of efficacy of powder and tablet of mebendazole in the treatment of strongyloidiasis. Kansenshōgaku Zasshi 1991;65(6):681‐6. [DOI] [PubMed] [Google Scholar]
Shikiya 1992 {published data only}
- Shikiya K, Zaha O, Niimura S, Nakamura H, Nakayoshi T, Kochi A, et al. Clinical study of eradicated and resistant patients to treatment with ivermectin for strongyloidiasis [Japanese]. Kansenshōgaku Zasshi 1992;66(7):935‐43. [DOI] [PubMed] [Google Scholar]
Shikiya 1994 {published data only}
- Shikiya K, Zaha O, Niimura S, Uehara T, Ohshiro J, Kinjo F, et al. Clinical study on ivermectin against 125 strongyloidiasis patients [Japanese]. Kansenshōgaku Zasshi 1994;68(1):13‐20. [DOI] [PubMed] [Google Scholar]
Singthong 2006 {published data only}
- Singthong S, Intapan P, Wongsaroji T, Maleewong W. Randomized comparative trial of two high‐dose albendazole regimens for uncomplicated human strongyloidiasis. Southeast Asian Journal of Tropical Medicine and Public Health 2006;37(Suppl 3):32‐4. [PubMed] [Google Scholar]
Steinmann 2008 {published data only}
- Steinmann P, Zhou XN, Du ZW, Jiang JY, Xiao SH, Wu ZX, et al. Tribendimidine and albendazole for treating soil‐transmitted helminths, Strongyloides stercoralis and Taenia spp.: Open‐label randomized trial. PLoS Neglected Tropical Diseases 2008;2(10):e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toma 2000 {published data only}
- Toma H, Sato Y, Shiroma Y, Kobayashi J, Shimabukuro I, Takara M. Comparative studies on the efficacy of three anthelminthics on treatment of human strongyloidiasis in Okinawa, Japan. Southeast Asian Journal of Tropical Medicine and Public Health 2000;31(1):147‐51. [PubMed] [Google Scholar]
Whitworth 1991 {published data only}
- Whitworth JA, Morgan D, Maude GH, McNicholas AM, Taylor DW. A field study of the effect of ivermectin on intestinal helminths in man. Transaction Royal Society of Tropical Medicine and Hygiene 1991;85(2):232‐4. [DOI] [PubMed] [Google Scholar]
Xiao 2013 {published data only}
- Xiao SH, Utzinger J, Tanner M, Keiser J, Xue J. Advances with the Chinese anthelminthic drug tribendimidine in clinical trials and laboratory investigations. Acta Tropical 2013;126(2):115‐26. [DOI] [PubMed] [Google Scholar]
Yap 2013 {published data only}
- Yap P, Du ZW, Wu FW, Jiang JY, Chen R, Zhou XN, et al. Rapid re‐infection with soil‐transmitted helminths after triple‐dose albendazole treatment of school‐aged children in Yunnan, People's Republic of China. American Journal of Tropical Medicine and Hygiene 2013;89(1):23‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaha 2004 {published data only}
- Zaha O, Hirata T, Uchima N, Kinjo F, Saito A. Comparison of anthelmintic effects of two doses of ivermectin on intestinal strongyloidiasis in patients negative or positive for anti‐HTLV‐1 antibody. Journal of Infection and Chemotherapy 2004;10(6):348‐51. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01570504 {published data only}
- NCT01570504. Multiple Versus Single Dose of Ivermectin for the Treatment of Strongyloidiasis (STRONGTREAT). https://clinicaltrials.gov/ct2/show/NCT01570504 (accessed 24 August 2015). [NCT01570504]
Additional references
Angheben 2011
- Angheben A, Mistretta M, Gobbo M, Bonafini S, Lacovazzi T, Sepe A, et al. Acute strongyloidiasis in Italian tourists returning from Southeast Asia. Journal of Travel Medicine 2011;18(2):138‐40. [DOI] [PubMed] [Google Scholar]
Berk 1987
- Berk S, Verghese A, Alvarez S, Hall K, Smith B. Clinical and epidemiologic features of strongyloidiasis. A prospective study in rural Tennessee. Archives of Internal Medicine 1987;147(7):1257‐61. [PubMed] [Google Scholar]
Bethony 2006
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil‐transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 2006;367(9521):1521‐32. [DOI] [PubMed] [Google Scholar]
Bisoffi 2013
- Bisoffi Z, Buonfrate D, Montresor A, Requena‐Méndez A, Muñoz J, Krolewiecki AJ, et al. Strongyloides stercoralis: a plea for action. PLoS Neglected Tropical Diseases 2013;7(5):e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisoffi 2014
- Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Neglected Tropical Diseases 2014;8(1):e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boken 1993
- Boken DJ, Leoni PA, Preheim LC. Treatment of Strongyloides stercoralis hyperinfection syndrome with thiabendazole administered per rectum. Clinical Infectious Diseases 1993;16(1):123‐6. [DOI] [PubMed] [Google Scholar]
Buonfrate 2012
- Buonfrate D, Angheben A, Gobbi F, Munoz J, Requena‐Mendez A, Gotuzzo E, et al. Imported strongyloidiasis: epidemiology, presentations, and treatment. Current Infectious Disease Reports 2012;14(3):256‐62. [DOI] [PubMed] [Google Scholar]
Buonfrate 2013
- Buonfrate D, Requena‐Mendez A, Angheben A, Muñoz J, Gobbi F, Ende J, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infectious Diseases 2013;13:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 1991
- Campbell WC. Ivermectin as an anti parasitic agent for use in humans. Annual Review of Microbiology 1991;45:445‐74. [DOI] [PubMed] [Google Scholar]
CDC 2013
- Centers for Disease Control and Prevention. Strongyloides. http://www.cdc.gov/parasites/strongyloides/health_professionals/ (accessed 24 August 2015).
Cook 1992
- Cook GC. Use of antiprotozoan and anthelmintic drugs during pregnancy: side‐effects and contra‐indications. Journal of Infection 1992;25(1):1‐9. [DOI] [PubMed] [Google Scholar]
Coovadia 1993
- Coovadia YM, Rajput MC, Bhana RH. Disseminated strongyloidiasis in a diabetic patient. Tropical and Geographical Medicine 1993;45(4):179‐80. [PubMed] [Google Scholar]
Courouble 2004
- Courouble G, Rouet F, Herrmann‐Storck C, Nicolas M, Candolfi E, Deloumeaux J, et al. Epidemiologic study of the association between human T‐cell lymphotropic virus type 1 and Strongyloides stercoralis infection in female blood donors (Guadeloupe, French West Indies). West Indian Medical Journal 2004;53(1):3‐6. [PubMed] [Google Scholar]
Crump 2011
- Crump A, Ōmura S. Ivermectin, 'wonder drug' from Japan: the human use perspective. Proceedings of the Japan Academy 2011;87(2):13‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
dos Santos 2009
- dos Santos AR, Falcão CA, Muzitano MF, Kaiser CR, Rossi‐Bergmann B, Férézou J. Ivermectin‐derived leishmanicidal compounds. Bioorganic and Medicinal Chemistry 2009;17(2):496‐502. [DOI] [PubMed] [Google Scholar]
Fardet 2007
- Fardet L, Généreau T, Poirot JL, Guidet B, Kettaneh A, Cabane J. Severe strongyloidiasis in corticosteroid treated patients: case series and literature review. Journal of Infection 2007;54(1):18‐27. [DOI] [PubMed] [Google Scholar]
Freedman 1991
- Freedman D. Experimental infection of human subjects with Strongyloides species. Reviews of Infectious Diseases 1991;13(6):1221‐6. [DOI] [PubMed] [Google Scholar]
Geary 2005
- Geary TG. Ivermectin 20 years on: maturation of a wonder drug. Trends in Parasitology 2005;21(11):530‐2. [DOI] [PubMed] [Google Scholar]
Heukelbach 2004
- Heukelbach J, Winter B, Wilcke T, Muehlen M, Albrecht S, Oliviera FA, et al. Selectivemass treatment with ivermectin to control intestinal helminthiasis and parasitic skin diseases in a severely affected population. Bulletin of the World Health Organization 2004;82(8):563‐71. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirata 2006
- Hirata T, Uchima N, Kishimoto K, Zaha O, Kinjo N, Hokama A, et al. Impairment of host immune response against Strongyloides stercoralis by human T cell lymphotropic virus type 1 infection. American Journal of Tropical Medicine and Hygiene 2006;74(2):246‐9. [PubMed] [Google Scholar]
Horton 2000
- Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitology 2000;121(Suppl):S113‐32. [DOI] [PubMed] [Google Scholar]
Keiser 2004
- Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clinical Microbiology Reviews 2004;17(1):208‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacey 1990
- Lacey E. Mode of action of benzimidazoles. Parasitology Today 1990;6(4):112‐5. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Liu 1996
- Liu LX, Weller PF. Antiparasitic drugs. New England Journal of Medicine 1996;334(18):1178‐84. [DOI] [PubMed] [Google Scholar]
Maguire 2005
- Maguire J. Intestinal nematodes. In: Mandell GL, Bennett JE, Dolin R editor(s). Principles and Practice of Infectious Diseases. 6th Edition. Vol. 2, Philadelphia: Elsevier, 2005:3260‐7. [Google Scholar]
Marty 2005
- Marty FM, Lowry CM, Rodriguez M, Milner DA, Pieciak WS, Sinha A, et al. Treatment of human disseminated strongyloidiasis with a parenteral veterinary formulation of ivermectin. Clinical Infectious Diseases 2005;41(1):e5‐8. [DOI] [PubMed] [Google Scholar]
Mejia 2012
- Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Current Opinion in Infectious Disease 2012;25(4):458‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merck 2007
- Merck, Co. Inc. Tablets: Stromectol® (ivermectin) [NDA 50‐742/S‐022]. 2007. www.fda.gov/medwatch/safety/2008/Sep_PI/Stromectol_PI.pdf (accessed February 2009).
Miller 2008
- Miller MA, Church LW, Salgado CD. Strongyloides hyperinfection: a treatment dilemma. American Journal of the Medical Sciences 2008;336(4):358‐61. [DOI] [PubMed] [Google Scholar]
Moncayo 2008
- Moncayo AL, Vaca M, Amorim L, Rodriguez A, Erazo S, Oviedo G, et. al. Impact of long‐term treatment with ivermectin on the prevalence and intensity of soil‐transmitted helminth infections. PLoS Neglected Tropical Diseases 2008;2(9):e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montes 2010
- Montesa M, Sawhney C, Barros N. Strongyloides stercoralis: there but not seen. Current Opinion in Infectious Diseases 2010;23(5):500‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nahmias 1994
- Nahmias J, Goldsmith R, Soibelman M, el‐On J. Three‐ to 7‐year follow‐up after albendazole treatment of 68 patients with cystic echinococcosis (hydatid disease). Annals of Tropical Medicine and Parasitology 1994;88(3):295‐304. [DOI] [PubMed] [Google Scholar]
Nucci 1995
- Nucci M, Portugal R, Pulcheri W, Spector N, Ferreira SB, Castro M, et al. Strongyloidiasis in patients with hematologic malignancies. Clinical Infectious Diseases 1995;21(3):675‐7. [DOI] [PubMed] [Google Scholar]
Nuesch 2005
- Nuesch R, Zimmerli L, Stockli R, Gyr N, Christoph Hatz FR. Imported strongyloidosis: a longitudinal analysis of 31 cases. Journal of Travel Medicine 2005;12(2):80‐4. [DOI] [PubMed] [Google Scholar]
Olsen 2009
- Olsen A, Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, et al. Strongyloidiasis‐the most neglected of the neglected tropical diseases?. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(10):967‐72. [DOI] [PubMed] [Google Scholar]
Ottesen 1994
- Ottesen EA, Campbell WC. Ivermectin in human medicine. Journal of Antimicrobial Chemotherapy 1994;34(2):195‐203. [DOI] [PubMed] [Google Scholar]
Ottesen 2008
- Ottesen EA, Hooper PJ, Bradley M, Biswas G. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Neglected Tropical Diseases 2008;2(10):e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pacanowski 2005
- Pacanowski J, Santos MD, Roux A, Maignan C, Guillot J, Lavarde V, et al. Subcutaneous ivermectin as a safe salvage therapy in Strongyloides stercoralis hyperinfection syndrome: a case report. American Journal of Tropical Medicine and Hygiene 2005;73(1):122‐4. [PubMed] [Google Scholar]
Patel 2008
- Patel G, Arvelakis A, Sauter BV, Gondolesi GE, Caplivski D, Huprikar S. Strongyloides hyperinfection syndrome after intestinal transplantation. Transplant Infectious Disease 2008;10(2):137‐41. [DOI] [PubMed] [Google Scholar]
Ramanathan 2008
- Ramanathan R, Burbelo P, Groot S, Iadarola MJ, Neva FA, Nutman TB. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. Journal of Infectious Diseases 2008;198(3):444‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Requena‐Méndez 2013
- Requena‐Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Neglected Tropical Diseases 2013;7(1):e2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salluh 2005
- Salluh JI, Feres GA, Velasco E, Holanda GS, Toscano L, Soares M. Successful use of parenteral ivermectin in an immunosuppressed patient with disseminated strongyloidiasis and septic shock. Intensive Care Medicine 2005;31(9):1292. [DOI] [PubMed] [Google Scholar]
Santiago 2009
- Santiago M, Leitão B. Prevention of strongyloides hyperinfection syndrome: a rheumatological point of view. European Journal of Internal Medicine 2009;20(8):744‐8. [DOI] [PubMed] [Google Scholar]
Schär 2013
- Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Neglected Tropical Diseases 2013;7(7):e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Streit 2008
- Streit A. Reproduction in Strongyloides (Nematoda): a life between sex and parthenogenesis. Parasitology 2008;135(3):285‐94. [DOI] [PubMed] [Google Scholar]
Tarr 2003
- Tarr PE, Miele PS, Peregoy KS, Smith MA, Neva FA, Lucey DR. Case report: Rectal administration of ivermectin to a patient with Strongyloides hyperinfection syndrome. American Journal of Tropical Medicine and Hygiene 2003;68(4):453‐5. [PubMed] [Google Scholar]
Ten Hove 2009
- Hove R, Esbroeck M, Vervoort T. Molecular diagnostics of intestinal parasites in returning travellers. European Journal of Clinical Microbiology of Infectious Diseases 2009;28(9):1045‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
The Medical Letter 2013
- The Medical Letter. Drugs for parasitic infections. The Medical Letter. 3rd Edition. New York: The Medical Letter Inc., 2013:e20. [Google Scholar]
Traore 2012
- Traore MO, Sarr MD, Badji A, Bissan Y, Diawara L, et al. Proof‐of‐principle of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: final results of a study in Mali and Senegal. PLoS Neglected Tropical Diseases 2012;6(9):e1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Doorn 2007
- Doorn HR, Koelewijn R, Hofwegen H, Gilis H, Wetsteyn JC, Wismans PJ, et al. Use of enzyme‐linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. Journal of Clinical Microbiology 2007;45(2):438‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walzer 1982
- Walzer PD, Milder JE, Banwell JG, Kilgore G, Klein M, Parker R. Epidemiologic features of Strongyloides stercoralis infection in an endemic area of the United States. American Journal of Tropical Medicine and Hygiene 1982;31(2):313‐9. [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. Preventive chemotherapy in human helminthiasis: coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: WHO, 2006. [Google Scholar]
WHO 2015
- World Health Organization. Model List of Essential Medicines. 19th List. Essential medicines and health products April 2015.
Yori 2006
- Yori PP, Kosek M, Gilman RH, Cordova J, Bern C, Chavez CB, et al. Seroepidemiology of strongyloidiasis in the Peruvian Amazon. American Journal of Tropical Medicine and Hygiene 2006;74(1):97‐102. [PMC free article] [PubMed] [Google Scholar]
Zago‐Gomes 2002
- Zago‐Gomes MP, Aikawa KF, Perazzio SF, Gonçalves CS, Pereira FE. Prevalence of intestinal nematodes in alcoholic patients. Revista da Sociedade Brasileira de Medicina Tropical 2002;35(6):571‐4. [DOI] [PubMed] [Google Scholar]
Zaha 2000
- Zaha O, Hirata T, Kinjo F, Saito A. Strongyloidiasis‐‐progress in diagnosis and treatment. Internal Medicine 2000;39(9):695‐700. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Henriquez‐Camacho 2012
- Henriquez‐Camacho CAJ, Gotuzzo E, Echevarria J, White Jr AC, Terashima A, Samalvides F, et al. Ivermectin versus benzimidazoles for treating strongyloides infection. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007745.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


