Abstract
Purpose
To explore whether, and to what extent, minor consent influences adolescent vaccine delivery in the United States.
Methods
A telephone survey was completed by 263 professionals with responsibilities for adolescent health care and/or vaccination in 43 states. Measures included perceived frequency of unaccompanied minor visits and perceived likelihood of vaccine delivery to unaccompanied minors in hypothetical scenarios that varied by adolescent age, vaccine type, visit type, and clinical setting.
Results
Among the 76 respondents most familiar with private primary care clinics, 47.1% reported perceptions that 17-year-old patients often present without a parent/legal guardian. Among the 104 respondents most familiar with public primary care clinics, 56.7% reported that 17-year-old patients often present alone. In response to hypothetical scenarios, approximately 30% of respondents familiar with private clinics and 50% of respondents familiar with public clinics reported perceptions that unaccompanied 17-year-old adolescents would not receive influenza, Tdap, or human papillomavirus vaccines during routine check-ups because they could not provide consent. Perceived likelihood of unaccompanied minors receiving vaccines when seen for confidential services in primary care, sexually transmitted disease, and Title X/family planning clinics varied significantly by vaccine type and clinical setting. On average, respondents reported that they would support minors having the ability to self-consent for vaccines at age 14.
Conclusions
The inability of minors to consent for vaccines is likely one barrier to vaccination. Interventions to increase adolescent vaccination should consider strategies that increase the ability of unaccompanied minors, particularly older minors, to receive vaccines within the context of legal, ethical, and professional guidelines.
Keywords: Vaccination, Adolescents, Adolescent health services, Informed consent by minors, Confidentiality, Privacy
There have been substantial changes in recommendations for routine vaccination among adolescents, including new recommendations for pertussis (TdaP), meningococcal (MCV4), human papillomavirus (HPV), and influenza vaccines [1,2]. Although the proportion of adolescents who receive recommended vaccines has steadily increased, levels of adolescent vaccination coverage are suboptimal and below levels of coverage for recommended vaccines among young children [3–6]. In 2011, vaccination coverage among adolescents ages 13–17 was 78% for Tdap and 71% for MCV4; 53% of females in this age group received at least one HPV vaccination and only 35% completed the three-dose vaccine series [7]. In contrast, at least 90% of children 19–35 months of age have received at least one dose of measles/mumps/rubella and varicella vaccine, as well as three doses of DTP/DT/Tdap, Haemophilus influenza type B, hepatitis B, and pneumococcal conjugate vaccine [8,9].
Multiple barriers to high rates of adolescent vaccination coverage have been described [10–14], but whether issues related to minor consent may act as a barrier to receipt of vaccines is not known [15,16]. During adolescence, there are circumstances in which minors may be permitted to provide their own informed consent for health care services and parental consent is not required. The informed consent requirements for minors vary by state and are guided by a combination of state and federal laws, the mature minor doctrine, and recommendations of professional organizations [17–23]. Circumstances in which a minor may consent for his or her own care typically include when a minor is emancipated or has another “status” that supports independent consent (e.g., married, homeless), or is receiving services related to sensitive health concerns (e.g., sexually transmitted diseases [STDs], pregnancy prevention, pregnancy, substance use, or mental health issues). State laws are generally silent on the specific issues of minor consent and vaccination. In a few states, however, minor consent laws specifically allow minors to consent to services for the prevention (as well as diagnosis and treatment) of STDs [15,23]. These laws have been or could be interpreted to allow HPV vaccination based on a minor’s consent [15]. It is within this context of varying circumstances that practitioners make decisions about whether unaccompanied minors can provide their own consent to receive vaccinations.
To better understand whether issues related to minor consent may act as a barrier to adolescent vaccination, we investigate perceived frequency of unaccompanied minor visits to health care settings, and providers’ perceptions of the extent to which minor consent issues influence delivery of vaccine by adolescent age, vaccine type, and clinical setting. Finally, we assessed whether key stakeholders would support minor consent for vaccines.
Methods
We conducted a telephone interview survey of professionals across the United States with direct responsibilities related to adolescent health care or vaccinations. The study was approved by our Institutional Review Board.
Survey sample
In this exploratory study, we were interested in the perspective of representatives from diverse clinic settings in which adolescent vaccines are delivered. Adolescent vaccines are delivered in public and private primary care clinics, STD clinics, and family planning clinics; furthermore, immunization program managers may be involved with vaccine delivery programs in all sites. We therefore used a purposive sampling design to survey medical providers familiar with adolescent vaccine practices in public or private health care settings, immunization program managers, STD program managers, and Title X/family planning program managers.
We did not aim for a representative sample of providers. We intended to interview four medical providers in each state. Names of medical providers familiar with adolescent vaccine practices were solicited from the leadership of state American Academy of Pediatrics chapters and regional Society for Adolescent Health and Medicine chapters; nondiscriminative snowball sampling allowed us to contact multiple potential participants.
For public health officials, we intended to interview one immunization, one STD, and one Title X/family planning program manager in each Centers for Disease Control and Prevention–recognized state and jurisdiction. There are five jurisdictions for immunization programs, six jurisdictions for STD programs, and one jurisdiction for Title X/family planning. Lists of immunization and STD program managers were provided by the Centers for Disease Control and Prevention. Contact information for Title X grantees was obtained from the Office of Population Affairs. Program managers were allowed to designate proxy respondents if they felt a staff member would be more familiar with vaccine practices in their clinic settings.
Potential study participants were solicited via email with limited telephone and facsimile follow-up; contact information for those interested in participating was provided to the University of North Carolina Survey Research Unit. A minimum of 12 telephone call attempts were made by the Survey Research Unit. Respondents who provided verbal informed consent were interviewed upon initial contact when possible; otherwise, appointment times accommodated respondents’ schedules. Respondents were not compensated.
Interview surveys
Data collection occurred between February and April 2009 using Blaise computer-assisted interviewing system. After respondents were queried about demographic characteristics, interviewers determined whether respondents were most familiar with vaccine practices in private primary care, public primary care, STD, or Title X/family planning clinics in their state; skip patterns in the survey directed respondents to questions relevant to the settings with which they were most familiar. Respondents were instructed that the term “parent” would be used to represent “parent or legal guardian” for the entire survey, and that the survey was intended to assess vaccine practices for cognitively normal adolescents. On average, each interview lasted 20 minutes.
Measures
Sociodemographic characteristics included gender, age, and professional role (medical provider; immunization, STD, or Title X/family planning program manager).
Perceived frequency of unaccompanied minor visits in public and private primary care clinics was measured by asking respondents their perception of how often 17-, 15-, and 12-year-old adolescents visit primary care clinics for medical care without a parent in the building. Response options included often, sometimes, rarely, never, and don’t know.
Extent to which minor consent issues influence delivery of vaccine was investigated by a series of hypothetical scenarios. Respondents were asked how likely it would be for adolescents of differing ages (e.g., 17, 15, 12 years) to be vaccinated in their state within the context of hypothetical scenarios during which an adolescent was medically eligible, the specific vaccine (e.g., influenza, Tdap, HPV) was available in the clinical site at no cost, the adolescent agreed to be vaccinated, and a parent was not available even by phone to provide consent; response options included all, most, or some or none of the time. Respondents received scenarios linked to the clinical site with which they were most familiar. Because adolescents may seek routine or confidential health care within primary care clinics (which may influence clinicians’ behaviors in terms of delivery of vaccine), respondents familiar with primary care clinics were asked to respond to scenarios representing each of these situations separately. In scenarios for respondents most familiar with STD and Title X/family planning clinics, we used adolescents presenting alone for STD (or family planning) services, and clarified that there was no known or suspected history of sexual abuse.
Age at which respondents would support minor consent for influenza, Tdap, and HPV vaccines was measured by the following: “At what age would you support efforts to allow minors to consent for their own (specifically named) vaccinations?” Response options were listed as younger than 11, by 1-year intervals between 11 and 21 years of age, and older than 21.
Analysis
Descriptive statistics identified the sociodemographic characteristics of respondents and their responses to the survey questions. Generalized estimating equations for the generalized linear model were used to estimate difference in distribution of perceived visit frequency by age group and clinical setting while controlling for correlated responses by individuals. For each scenario presented, response categories representing the respondents’ perceived likelihood of the minor receiving each vaccine measured on a 5-point Likert scale were collapsed to a dichotomous response of none of the time versus all, most, or some of the time; don’t know and refuse responses were recoded as missing. We tested for three issues across scenarios: (1) likelihood of receiving different vaccines within each age group; (2) likelihood of receiving a specific vaccine across different age groups; and (3) likelihood of receiving a specific vaccine at a certain age by type of primary care sought (e.g., routine or confidential). Again, generalized estimating equations were used to estimate differences across scenarios while controlling for correlated responses. For each comparison, a score test was used to test the overall significance, followed by pairwise comparisons. Mean age of support for minor consent was calculated based on frequency distributions after recoding “younger than age 11” to age 10. Statistical tests were found significant at p < .05. All analyses were performed in SAS v.9.2 (SAS Institute Inc., Cary, NC).
Results
Sample
The sampling plan called for interviews of 366 professionals. Using referral sampling techniques, more than 800 e-mails were sent soliciting participation and/or referrals and 287 professionals responded with interest in participating. Interviews were completed by 263 participants in 43 states.
Most respondents were female (69.2%), the parent of a current or former adolescent (68.4%), and provided some direct clinical care (74.1%); their mean age was 50 years (range 26–79). The largest professional group was medical providers (n = 130, 49.4%), who were evenly divided between those who reported that they were most familiar with private versus public clinic settings. Public health professionals from state or jurisdiction immunization (n = 52/55), STD (n = 42/56), and Title X/family planning (n = 39/51) programs made up the remaining 50.6% respondents. On average, respondents reported 6–10 years of experience in their current position, and those with direct clinical care reported 16–20 years of clinical experience.
Perceived frequency of unaccompanied minor adolescent visits to primary care clinics
Seventy-six (28.9%) of all respondents reported they were most familiar with private primary care clinics. Nearly half (47.1%) of these respondents reported that 17-year-old patients often present for care without a parent in the building, and 47.1% reported that they sometimes present alone (Table 1). The distribution of perceived frequency of unaccompanied visits varied significantly by age of adolescent (p < .001). The vast majority (88.9%) reported that 12-year-old patients rarely or never seek care in private primary care clinics without the presence of a parent or guardian.
Table 1.
Respondents’ perceptions of the frequency of minors presenting alone to private and public primary care clinics, by age
| Clinical setting | 12 year olds |
15 year olds |
17 year olds |
|---|---|---|---|
| Number (%) | Number (%) | Number (%) | |
| Private primary care (n = 76)a | |||
| Often seeks care alone | 1 (1.4) | 8 (11.4) | 33 (47.1)b |
| Sometimes seeks care alone | 7 (9.7) | 32 (45.7) | 33 (47.1) |
| Rarely/never seeks care alone | 64 (88.9) | 30 (42.9) | 4 (5.7) |
| Missing/unknown | 4 | 6 | 6 |
| Public primary care (n = 104)a | |||
| Often seeks care alone | 8 (8.2) | 26 (26.8) | 55 (56.7)b |
| Sometimes seeks care alone | 25 (25.5) | 44 (45.4) | 30 (30.9) |
| Rarely/never seeks care alone | 65 (66.3) | 27 (27.8) | 12 (12.4) |
| Missing/unknown | 6 | 7 | 7 |
Number of respondents most familiar with specified clinic setting.
Significant difference (p < .001) in distribution of frequencies reported across all age groups for clinic setting (i.e., 12 vs. 15; 12 vs. 17; and 15 vs. 17).
A total of 104 (39.5%) respondents reported that they were most familiar with public primary care clinics. When these respondents were asked about 17-year-old patients, 56.7% reported that they often present for care without a parent in the building and 30.9% reported that they sometimes present alone (Table 1). Perceived likelihood of unaccompanied minors presenting for care varied significantly by age of adolescent (p < .001). The majority (66.3%) reported that 12-year-old patients rarely or never seek care in public primary care clinics alone.
Private primary care clinics, minor consent, and vaccine delivery
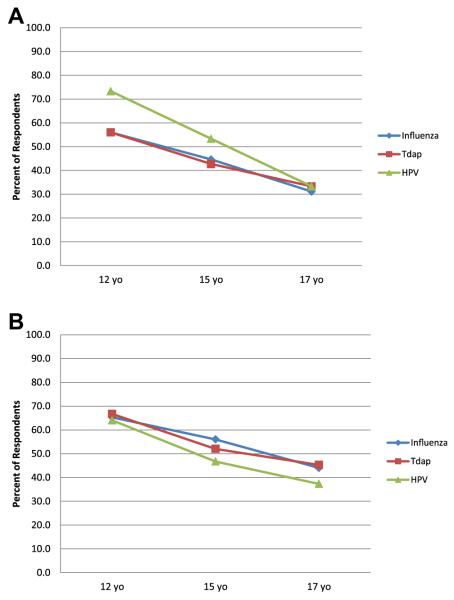
Respondents’ most familiar with private primary care clinics (n = 76) were asked in hypothetical scenarios the likelihood that patients of differing ages (17, 15, 12) would be vaccinated in their state if the patient was medically eligible for a specific vaccine (influenza, Tdap, HPV), which was available on site at no cost, and a parent was not available even by phone for consent. Approximately 30% of respondents reported that 17-year-old patients seen for a routine check-up and found to be eligible for HPV, influenza, and Tdap vaccines would not receive the respective vaccinations if a parent was not present (Figure 1A). The proportions of respondents reporting an unaccompanied adolescent would not receive vaccines increased as adolescent age decreased (p < .01), although there was variation by vaccine type. Seventy-three percent of respondents reported that unaccompanied 12-year-old patients would not receive HPV vaccine, whereas 56.0% reported that they would not receive influenza or Tdap (p < .01).
Figure 1.
Proportion of respondents who reported that unaccompanied minors would not likely receive vaccine in private primary care clinics within the context of hypothetical scenarios, by reason for visit, age, and vaccine (n = 76).a (A) Routine check-up.b,c (B) Confidential sexually transmitted disease (STD) testing.c aRespondents are those who reported they were most familiar with practices in private primary care clinic settings; for hypothetical scenarios, respondents were asked how likely it would be for cognitively normal adolescents of differing ages (e.g., 17, 15, 12 years) to be vaccinated in their state during a routine (or confidential) visit if medically eligible, the specific vaccine (e.g., influenza, Tdap, human papillomavirus [HPV]) was available in the clinical site at no cost, and a parent/legal guardian was not available even by phone for consent. bSignificant differences in likelihood of respondents reporting that a 12-year-old unaccompanied minor would receive HPV versus Tdap (p = .002), and HPV versus influenza (p < .001). cSignificant differences in likelihood of respondents reporting an unaccompanied minor would receive each vaccine by age (12 vs. 15; 15 vs. 17; and 12 vs. 17; all p < .05).
When queried about hypothetical scenarios during which an adolescent presented to a private primary care clinic requesting confidential STD testing, approximately 40% of respondents reported that 17-year-old patients presenting alone would not receive HPV, influenza, or Tdap even if they were medically eligible and free vaccines were available (Figure 1B). As with the scenario for routine visits, the proportions of respondents reporting an unaccompanied minor would not receive vaccines increased as adolescent age decreased (p < .05).
Respondents reported that 15- and 17-year-old unaccompanied adolescents seeking confidential services in private primary care clinics were less likely to receive Tdap and influenza vaccines than if they were seeking routine care (p = .05); reason for visit did not influence likelihood of receiving HPV.
Public primary care clinics, minor consent, and vaccine delivery
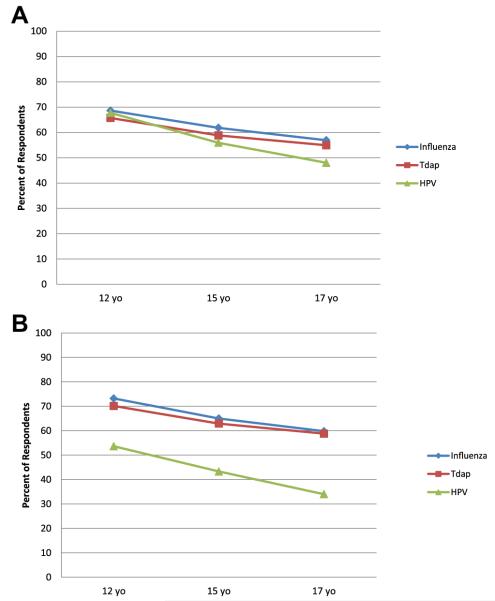
When respondents most familiar with public primary care clinics (n = 104) responded to hypothetical scenarios, 48%–57% reported that unaccompanied 17-year-old patients seen for a routine check-up and found to be eligible for HPV, influenza, and Tdap vaccines would not receive any of these vaccinations (Figure 2A). The proportions of respondents reporting an adolescent would not receive vaccines if they were at the clinic without a parent increased as adolescent age decreased (p < .05), and approximately 67% reported that unaccompanied 12-year-old patients would not receive any vaccines. The only variation by vaccine type was at age 17, when respondents reported adolescents were significantly less likely to receive influenza as compared to HPV vaccine (p = .01).
Figure 2.
Proportion of respondents who reported that unaccompanied minors would not likely receive vaccine in public primary care clinics within the context of hypothetical scenarios, by reason for visit, age, and vaccine (n = 104).a (A) Routine check-up.b,c (B) Confidential sexually transmitted disease (STD) testing.c,d aRespondents are those who reported they were most familiar with practices in public primary care clinic settings; for hypothetical scenarios, respondents were asked how likely it would be for cognitively normal adolescents of differing ages (e.g., 17, 15, 12 years) to be vaccinated in their state during a routine (or confidential) visit if medically eligible, the specific vaccine (e.g., influenza, Tdap, human papillomavirus [HPV]) was available in the clinical site at no cost, and a parent/legal guardian was not available even by phone for consent. bSignificant differences in likelihood of respondents reporting that a 17-year-old unaccompanied minor would receive HPV versus influenza (p = .01). cSignificant differences in likelihood of respondents reporting an unaccompanied minor would receive each vaccine by age (12 vs. 15; 15 vs. 17; and 12 vs. 17; all p < .05). dSignificant differences in likelihood of respondents reporting that an unaccompanied minor at each age (12, 15, and 17) would receive HPV versus influenza, and HPV versus Tdap (p < .001).
A difference in pattern was noted when respondents were asked about unaccompanied adolescents presenting to public primary care clinics for confidential STD testing (Figure 2B). In this scenario, the absence of a parent was less of a barrier to receiving HPV vaccine as compared with receiving influenza or Tdap vaccine (p < .001). Nonetheless, 34.0% of 17-year-old and 53.6% of 12-year-old unaccompanied patients would not receive HPV vaccine.
STD clinics
In response to scenarios that provided the hypothetical context that all vaccines were available on site and free, STD clinic program managers and those respondents most familiar with practices in STD clinics were asked to report the likelihood that unaccompanied minors of varying ages presenting for STD services would receive HPV, hepatitis B, influenza, and Tdap vaccines if they were found to be medically eligible. A significantly higher proportion of respondents reported a 12-year-old adolescent would not receive vaccines if they were at the clinic without a parent compared with 15 or 17 year olds (p < .05) (Figure 3). Regardless of the age of the patient, respondents were significantly less likely to report that influenza and Tdap vaccines would be administered to unaccompanied minors when compared with hepatitis B and HPV vaccines (p < .05).
Figure 3.
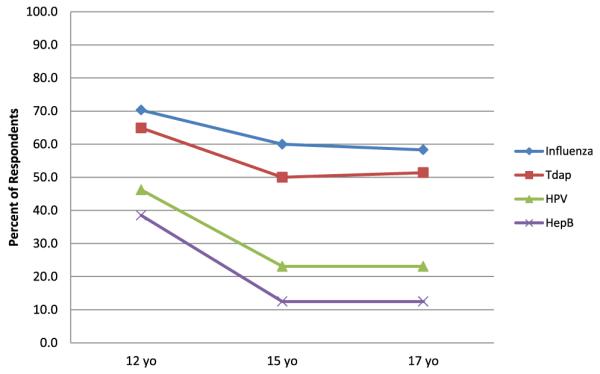
Proportion of respondents who reported that unaccompanied minors would not likely receive vaccine in sexually transmitted disease (STD) clinics within the context of hypothetical scenarios, by age and vaccine (n = 40).a,b,c aRespondents included STD program managers and those who reported they were most familiar with practices in STD clinics; for hypothetical scenarios, respondents were asked how likely it would be for cognitively normal adolescents of differing ages (e.g., 17, 15, 12 years) presenting for STD services to be vaccinated in their state if the adolescent was medically eligible, the specific vaccine (e.g., influenza, Tdap, human papillomavirus [HPV], hepatitis [Hep] B) was available in the clinic at no cost, and a parent was not available even by phone for consent. bSignificant differences in likelihood of respondents reporting that a 12-year-old unaccompanied minor would receive HPV versus Tdap, HPV versus influenza, Hep B versus Tdap, Hep B versus influenza (p < .05); Significant differences in likelihood of respondents reporting that 15- and 17-year-old unaccompanied minors would receive HPV versus Tdap, HPV versus influenza, Hep B versus Tdap, Hep B versus influenza (p < .01). cSignificant differences in likelihood of respondents reporting an unaccompanied minor would receive HPV or Heb B vaccine by age groups 12 versus 15, and 12 versus 17 (p < .01) and for Tdap p < .05.
Title X/family planning clinics
Similarly, Title X/family planning program managers and those respondents most familiar with practices in family planning clinics were asked to report the likelihood that unaccompanied minors of varying ages presenting for family planning services in a hypothetical scenario would receive vaccines if they were found to be medically eligible for free, available vaccines. The proportion of respondents who reported that 12-, 15-, and 17-year-old patients would not likely receive hepatitis B or HPV vaccine ranged from 46.0% to 29.7% (Figure 4). The proportion of respondents who reported that 12-, 15-, and 17-year-old patients would not likely receive influenza or Tdap vaccine ranged from 63.2% to 54.1%. Respondents reported that 15- and 17-year-old patients were significantly less likely to receive influenza and Tdap as compared with HPV and hepatitis B vaccines (p < .01).
Figure 4.
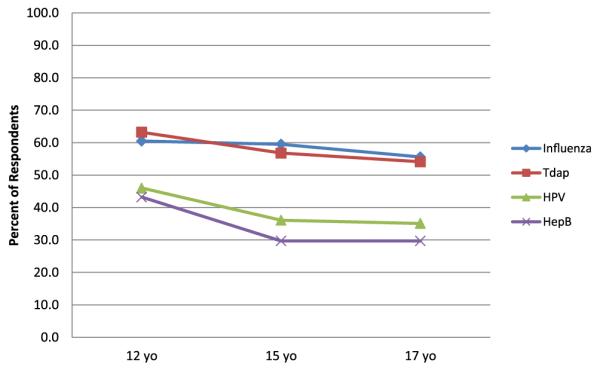
Proportion of respondents who reported unaccompanied minors would not likely receive vaccine in Title X/family planning clinics within the context of hypothetical scenarios, by age and vaccine (n = 38).a,b aRespondents include Title X/family planning program managers and those who reported they were most familiar with practices in these clinic settings; for hypothetical scenarios, respondents were asked how likely it would be for cognitively normal adolescents of differing ages (e.g., 17, 15, 12 years) presenting for family planning services to be vaccinated in their state if the adolescent was medically eligible, the specific vaccine (e.g. influenza, Tdap, human papillomavirus [HPV], hepatitis [Hep] B) was available in the clinic at no cost, and a parent was not available even by phone for consent. bSignificant differences in likelihood of respondents reporting that 15- and 17-year-old unaccompanied minor would receive HPV versus Tdap, HPV versus influenza, Hep B versus Tdap, Hep B versus influenza (p < 001).
Age of support for minor consent
When respondents were asked at what age they would support efforts to allow minors to consent for their own vaccinations, responses ranged from younger than 11 to 18 years for Tdap and influenza and 11–18 years for HPV. Respondents reported they would, on average, support efforts to allow minors to consent for their own vaccination at the following ages: 13.8 (standard deviation [SD] 2.1) years old for HPV; 14.1 (SD 2.2) years old for influenza; 14.3 (SD 2.2) years old for Tdap.
Discussion
The majority of key stakeholders who participated in this study perceive that older minors sometimes or often present alone to primary care clinic settings. In response to hypothetical scenarios, they also report perceptions that many unaccompanied minors would not receive vaccinations even if they were due for vaccines, medically eligible, agreed to be vaccinated, and the vaccines were available at no cost. These results add to our understanding of whether and how the inability of minors to consent for vaccines may act as a barrier to adolescent vaccination, and suggest that this is an area that warrants further investigation.
Perceptions that older adolescents are more likely than younger adolescents to visit clinic settings without their parents are not surprising. Older adolescents may be asked by parents to visit clinics alone (e.g., when parents have to work), and have the skills to use public or motor vehicle transportation. Older adolescents are also more likely to engage in sexual behaviors that place them at risk for STDs and pregnancy [24,25] and to seek health care for confidential services [26]. Interventions to increase adolescent vaccination rates may be strengthened by considering issues related to the ability of unaccompanied older minors to receive vaccines within the context of legal, ethical, and professional guidelines [15,16,23]. Younger minors visit clinic settings without a parent less frequently than older minors, although some do. Strategies developed to increase vaccination of older minors could be evaluated to determine their appropriateness for younger minors, depending on the setting and other circumstances.
Based on respondents’ perceptions of what would happen in hypothetical scenarios, there may be variation in the extent to which an unaccompanied minor’s ability to consent to vaccines influences vaccine delivery by vaccine type and clinical setting. For example, respondents perceived fewer minor consent–related barriers to receipt of HPV vaccine by unaccompanied minors of any age if they presented to public primary care clinics for confidential STD testing, STD clinics, or Title X/family planning clinics, both as compared with Tdap or influenza vaccine and with other clinical settings. This variation may be related to interpretation of state laws allowing minors to consent to STD-related health care in a way that permits minor consent for STD-related vaccination [23]. Variations in interpretation of state minor consent laws or implementation through clinic policies and procedures may also exist in private versus public settings. Based on respondents’ perceptions, if unaccompanied 17-year-old adolescents presented for routine care in private primary care clinics, one-third would not be able to get Tdap and influenza vaccines because they could not provide consent. If same-aged adolescents presented to public primary care, STD, or Title X/family planning clinics, respondents reported at least half would not be able to receive these vaccines (even if available free) because they could not consent. Strategies that all clinics use to be able to deliver vaccines to adolescents in the absence of the physical presence of a parent are worth exploring. These may include obtaining parental consent for routine care and/or vaccines in advance of actual visits or strategies to obtain real-time consent with use of new technology (e.g., text messaging).
Clearly, minor consent-related barriers must be placed within the context of many other potential barriers to adolescent vaccination [27]. Compared with younger children, adolescents use health care less frequently and therefore have fewer opportunities to receive vaccines [28,29], whether or not a parent is present. Approximately 20% of adolescents are disconnected from routine care [24], and it is particularly unfortunate to miss any opportunities to vaccinate these young people. Vaccines are not widely available in all primary care clinics, and specialized clinics such as STD and Title X/family planning clinics may only carry vaccines linked to reproductive health care. Vaccine costs can represent a substantial barrier to vaccination. Further research will be needed to more fully understand the extent to which minor consent issues influence actual vaccination practices and to place these issues within the context of multiple other barriers.
Limitations to this study include a purposefully selected rather than a representative sample of medical providers, although we did achieve near saturation sampling for STD clinic, Title X/family planning, and immunization program manager subsamples. We did not collect reasons for refusal and are unable to determine how this may have affected our results. We assessed respondents’ perceptions of vaccine practices and used hypothetical scenarios in our study; perceptions and responses to hypothetical scenarios may not reflect actual practices. We did not collect data that allowed us to estimate actual frequencies or quantify our results, which would be an important component of future research. Furthermore, we did not ask about efforts that may be used to contact parents of unaccompanied minors to encourage vaccination at later dates.
Despite these limitations, our results represent an initial step toward understanding the potential specific influence of minor consent on adolescent vaccine delivery. Future research is needed to verify that minor consent is in fact a barrier to actual adolescent vaccine delivery and to quantify the extent to which this may be true. Our respondents reported that they, on average, would support efforts to allow minors to consent for their own vaccinations at approximately 14 years of age, but it is not clear whether this is appropriate to consider, if or under what conditions parents and adolescents might also be in support [30], or whether this would be an effective strategy for increasing adolescent vaccination. Finally, efforts to understand state minor consent laws and policies, existing practices and procedures used for implementing state laws, and their impact on adolescent vaccine delivery will need to continue.
IMPLICATIONS AND CONTRIBUTION.
This exploratory research suggests older adolescents are frequently seen in clinic settings without parents or legal guardians. Interventions to increase adolescent vaccination should consider strategies that increase the ability of unaccompanied minors, particularly older minors, to receive vaccines within the context of legal, ethical, and professional guidelines.
Acknowledgments
Funding support was provided by North Carolina Division of Public Health and the Centers for Disease Control and Prevention (principal investigator, Carol A. Ford).
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors, and do not necessarily represent the views of the Centers for Disease Control and Prevention or the U.S. Department of Health and Human Services.
Potential Conflict of Interest: Abigail English was a co-investigator on a research project funded by GlaxoSmithKline, 2006–2009 and has spoken at Merck & Co., Inc. for Grand Rounds, 2012. Carol A. Ford was a co-investigator on a research project funded by GlaxoSmithKline, 2006–2009.
References
- [1].Centers for Disease Control and Prevention Immunization of adolescents: Recommendations of the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, the American Academy of Family Physicians, and the American Medical Association. MMWR. 1996;45:1–16. [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention General recommendations on immunizations: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2011;60 [Google Scholar]
- [3].McCauley MM, Stokley S, Stevenson J, Fishbein DB. Adolescent vaccination: Coverage achieved by ages 13-15 years, and vaccinations received as recommended during ages 11-12 years, National Health Interview Survey 1997-2003. J Adolesc Health. 2008;43:540–7. doi: 10.1016/j.jadohealth.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [4].Centers for Disease Control and Prevention Vaccination coverage among adolescents aged 13–17 years–United States 2007. MMWR. 2008;57:1100–3. [PubMed] [Google Scholar]
- [5].Dorell C, Stokley S, Yankey D, Cohn A. National, state, and local area vaccination coverage among adolescents aged 13 to 17 years – United States, 2009. MMWR. 2010;59:1018–23. [PubMed] [Google Scholar]
- [6].National and state vaccination coverage among adolescents aged 13 through 17 years–United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:1117–23. [PubMed] [Google Scholar]
- [7].National and state vaccination coverage among adolescents aged 13-17 years–United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:671–7. [PubMed] [Google Scholar]
- [8].National, state, and local area vaccination coverage among children aged 19-35 months–United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:689–96. [PubMed] [Google Scholar]
- [9].Centers for Disease Control and Prevention National and State Vaccination Coverage Among Children Aged 19–35 Months — United States, 2010. MMWR. 2011;60:1157–63. [PubMed] [Google Scholar]
- [10].Szilagyi PG, Rand CM, McLaurin J, et al. Delivering adolescent vaccinations in the medical home: A new era? Pediatrics. 2008;121(Suppl 1):S15–24. doi: 10.1542/peds.2007-1115C. [DOI] [PubMed] [Google Scholar]
- [11].Lindley MC, Boyer-Chu L, Fishbein DB, et al. The role of schools in strengthening delivery of new adolescent vaccinations. Pediatrics. 2008;121(Suppl 1):S46–54. doi: 10.1542/peds.2007-1115F. [DOI] [PubMed] [Google Scholar]
- [12].Broder KR, Cohn AC, Schwartz B, et al. Adolescent immunizations and other clinical preventive services: A needle and a hook? Pediatrics. 2008;121(Suppl 1):S25–34. doi: 10.1542/peds.2007-1115D. [DOI] [PubMed] [Google Scholar]
- [13].Freed GL. Lessons from across the pond: What the US can learn from European immunization programs. Vaccine. 2007;25:6148–57. doi: 10.1016/j.vaccine.2007.05.050. [DOI] [PubMed] [Google Scholar]
- [14].Kharbanda EO, Lee GM, Koenigs L. Financing vaccines for adolescents: A position paper of the Society for Adolescent Health and Medicine. J Adolesc Health. 2011;48:320–1. doi: 10.1016/j.jadohealth.2010.12.025. [DOI] [PubMed] [Google Scholar]
- [15].English A, Shaw FE, McCauley MM, Fishbein DB. Legal basis of consent for health care and vaccination for adolescents. Pediatrics. 2008;121(Suppl 1):S85–7. doi: 10.1542/peds.2007-1115J. [DOI] [PubMed] [Google Scholar]
- [16].Ford CA, English A, Davenport AF, Stinnett AJ. Increasing adolescent vaccination: Barriers and strategies in the context of policy, legal, and financial issues. J Adolesc Health. 2009;44:568–74. doi: 10.1016/j.jadohealth.2008.11.015. [DOI] [PubMed] [Google Scholar]
- [17].English A. Treating adolescents: Legal and ethical considerations. In: Farrow J, editor. The medical clinics of North America. WB Saunders Company; Philadelphia: 1990. pp. 1097–112. [DOI] [PubMed] [Google Scholar]
- [18].Hofmann A. A rational policy toward consent and confidentiality in adolescent health care. J Adolesc Health Care. 1980;1:9–17. doi: 10.1016/s0197-0070(80)80003-9. [DOI] [PubMed] [Google Scholar]
- [19].Council on Scientific Affairs AMA Confidential health services for adolescents. JAMA. 1993;269:1420–4. [PubMed] [Google Scholar]
- [20].Gans J. Policy compendium on confidential health services for adolescents. American Medical Association; Chicago: 1993. [Google Scholar]
- [21].Ford C, English A, Sigman G. Confidential Health Care for Adolescents: Position Paper of the Society for Adolescent Medicine. J Adolesc Health. 2004;35:1. [PubMed] [Google Scholar]
- [22].English A, Ford CA. The HIPAA Privacy Rule and adolescents: Legal questions and clinical challenges. Perspect Sex Reprod Health. 2004;36:80–6. doi: 10.1363/psrh.36.80.04. [DOI] [PubMed] [Google Scholar]
- [23].English A, Bass L, Boyle AD, Eshragh F. State minor consent laws: A summary. 3rd ed Chapel Hill, NC: 2010. [Google Scholar]
- [24].Marcell AV, Matson P, Ellen JM, Ford CA. Annual physical examination reports vary by gender once teenagers become sexually active. J Adolesc Health. 2011;49:47–52. doi: 10.1016/j.jadohealth.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Eaton DK, Kann L, Kinchen S, et al. Youth risk behavior surveillance - United States, 2009. MMWR Surveill Summ. 2010;59:1–142. [PubMed] [Google Scholar]
- [26].Sugerman S, Halfon N, Fink A, et al. Family planning clinic clients: Their usual health care providers, insurance status, and implications for managed care. J Adolesc Health. 2000;27:25–33. doi: 10.1016/s1054-139x(99)00126-3. [DOI] [PubMed] [Google Scholar]
- [27].Middleman AB. Adolescent immunizations: Policies to provide a shot in the arm for adolescents. J Adolesc Health. 2007;41:109–18. doi: 10.1016/j.jadohealth.2007.04.004. [DOI] [PubMed] [Google Scholar]
- [28].Irwin CE, Jr, Adams SH, Park MJ, Newacheck PW. Preventive care for adolescents: Few get visits and fewer get services. Pediatrics. 2009;123:e565–72. doi: 10.1542/peds.2008-2601. [DOI] [PubMed] [Google Scholar]
- [29].Rand CM, Shone LP, Albertin C, et al. National health care visit patterns of adolescents: Implications for delivery of new adolescent vaccines. Arch Pediatr Adolesc Med. 2007;161:252–9. doi: 10.1001/archpedi.161.3.252. [DOI] [PubMed] [Google Scholar]
- [30].Kennedy A, Stokley S, Curtis CR, Gust D. Limited awareness of vaccines recommended for adolescents and other results from two national consumer health surveys in the United States. J Adolesc Health. 2012;50:198–200. doi: 10.1016/j.jadohealth.2011.04.017. [DOI] [PubMed] [Google Scholar]