Abstract
Objective
This double blind, randomized, controlled trial evaluated 12 months high dose vitamin D2 supplementation for improving insulin sensitivity, secretion and glycemic status.
Methods
African American men with prediabetes (A1C 5.7 – 6.4%), hypovitaminosis D (25OHD 5 – 29 ng/ml), and prevalent medical problems were supplemented with vitamin D3 (400 IU/day) and then randomized to weekly placebo or vitamin D2 (50,000 IU). The primary outcome was the change in oral glucose insulin sensitivity (OGIS, from oral glucose tolerance test) after 12 months of treatment. Secondary outcomes included other glycemic indices, A1C and incident diabetes.
Results
Baseline characteristics were similar in vitamin D-supplemented (n = 87) and placebo (n = 86) subjects completing the trial with average concentrations 14.4 ng/ml, 362 and 6.1% for 25OHD, OGIS and A1C, respectively. After 12 months vitamin D-supplemented group had a change in serum 25OHD +35 vs +6 ng/ml for placebo, p<0.001; OGIS +7.8 vs −16.0 for placebo, p = 0.026; and A1C −0.01 vs +0.01% for placebo, p = 0.66; while 10% in both groups progressed to diabetes. A post hoc analysis of participants with baseline impaired fasting glucose showed that more subjects in the vitamin D subgroup (31.6%) than placebo (8.3%) returned to normal glucose tolerance, but the difference did not reach significance (p=0.13).
Conclusion
The trial does not provide evidence that 12 months of high-dose D2 repletion improves clinically relevant glycemic outcomes in subjects with prediabetes and hypovitaminosis D (NCT01375660).
Keywords: Vitamin D supplement, prediabetes, African American
INTRODUCTION
Vitamin D deficiency contributes to health disparities and disease burden in African American men (AAM) but controversy remains on whether repletion improves outcomes. African Americans have increased risk for type 2 diabetes (T2DM) and hypovitaminosis D with the prevalence of 18% and 30% for diabetes and vitamin D deficiency, respectively, compared to 8.3% and 8.1%, respectively, in the US general population (1,2). African American men are ordinarily underrepresented in clinical trials (3,4). Addressing disparities in health care is one of the missions of the Veterans Health Administration (VHA). The VHA is America’s largest integrated health care system, with more than 1,700 sites of care that delivers state of the art health care and serves clinical, educational and scientific missions (5). Prevention of diabetes constitutes one of the priorities for the VHA. Lifestyle modification is the best existing approach to diabetes prevention but is difficult to achieve and maintain over long term (6).
The search for novel approaches to prevent diabetes continues (7) and among them vitamin D supplements may play a role (8–10). A recent meta-analysis of 35 randomized controlled trials (43,407 patients, mixed populations) has shown no overall effect of vitamin D3 on glucose homeostasis or diabetes prevention (11). The Institute of Medicine has recommended a conservative approach to vitamin D supplementation and called for more research especially randomized clinical trials (12).
None of the previous longer-term trials used high dose vitamin D2 (ergocalciferol), enrolled participants with prevalent medical problems or substantial number of African American men (10,11). The objective of this randomized trial was to determine the efficacy and safety of 12 months treatment with vitamin D2 for improving glucose homeostasis in AAM veterans with dysglycemia and hypovitaminosis D. We dedicated the study to the population underrepresented in clinical trials (i.e. AAM) and used convenient weekly vitamin D2 supplement.
METHODS
Study Design and Subjects
This was a double blind randomized placebo-controlled trial “D vitamin Intervention in Veteran Administration (DIVA)”. The primary objective was to determine whether high dose of vitamin D supplementation (designed to raise 25OHD into normal range) would improve oral glucose insulin sensitivity in African American men with dysglycemia and hypovitaminosis D. The eligible participants were randomized to placebo or vitamin D (1:1 ratio) with stratification according to age, 35–65 or 66–85 years, and presence of medical and psychiatric conditions to avoid possible age- and medical condition-related differences in primary outcome. The participants came for initial assessment including 3-hour oral glucose tolerance test (OGTT), for follow-up every 3 months and final 3-hour OGTT after 12 months of treatment. The study was approved by the University of Illinois Institutional Review Board and by the Jesse Brown VA Medical Center (JBVAMC) Research and Development Committee. The study was registered at clinicaltrials.gov as NCT01375660.
We recruited subjects among AAM veterans coming for medical care to JBVAMC in Chicago using flyers, information sheets, prescreening of electronic medical records, and letters to JBVAMC patients and doctors. The main inclusion criteria were as follows: AAM veteran, age 35–85 years, BMI 28–39 kg/m2, fasting glucose 95–125 mg/dl and/or A1C 5.7 – 6.4% (38.8 – 46.5 mmol/mol), 25OHD 5.0 – 29 ng/ml. The criteria for the diagnoses of prediabetes and diabetes were based on the American Diabetes Association recommendations (6). Participants who were diagnosed with diabetes during screening or intervention (A1C 6.5 – 6.9% or 47.5 – 51.9 mmol/mol) were allowed in the study if they did not need to take anti-diabetic medications and A1C remained <7% (<53 mmo/mol). The main exclusion criteria were as follows: diabetes and medical conditions that would be expected to interfere with the study or increase risk to the subject, such as kidney stones, hyperparathyroidism, sarcoidosis, hypercalcemia, and chronic kidney disease beyond stage 3a (eGFR <45 mls/min/1.73m2). Additional recorded diagnoses included hypertension, hyperlipidemia, arthritis, cardiovascular disease, cancer and psychiatric problems as well as medications. The age-adjusted Charlson index, a validated index of chronic disease prognosis was calculated based on the previously published formula (13) and included one point for myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic obstruction pulmonary disease, connective tissue disease, peptic ulcer disease, uncomplicated diabetes and mild liver disease; two points for complicated diabetes, moderate to severe chronic kidney disease, hemiplegia, leukemia, lymphoma, solid tumor without metastasis; three points for moderate to severe liver disease and 6 points for metastatic solid tumors or AIDS. Age adjustment added 1 point for every 10 years above age 40. Total 2,067 subjects were prescreened, 205 randomized, and 173 had final OGTT (Fig. 1). The subjects provided written informed consent prior to participation.
Fig. 1.
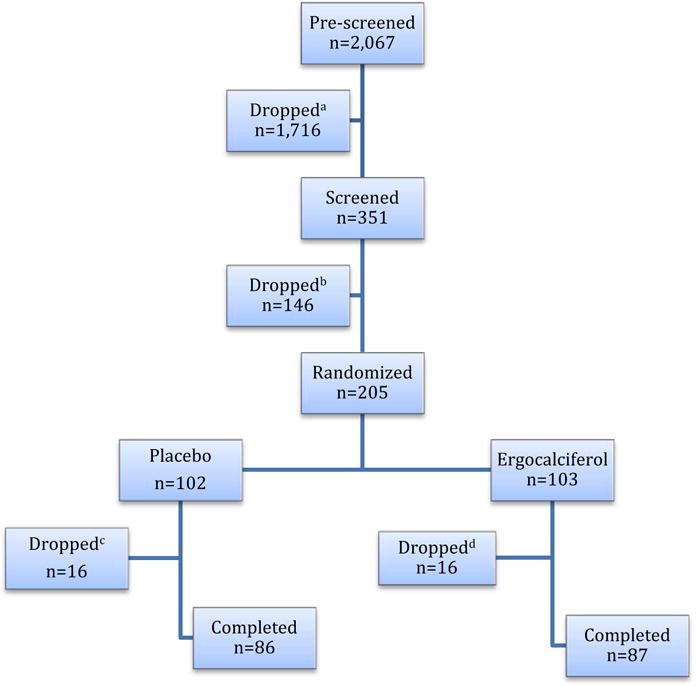
Flow diagram of the trial. aIneligible n=1716: 927 T2DM, 309 BMI<28 or >40 kg/m2, 86 A1C<5.7%, 86 GFR<45 ml/min/1.73m2, 120 on vitamin D supplements, 137 advanced chronic conditions, 51 ineligible sex or race; bDropped n=146: 17 ineligible: 5 A1C<5.7%, 5 A1C>6.9%, 3 GFR<45 ml/min/1.73m2, 4 poor venous access, and 129 lost interest; cDropped n=16: 6 personal issues and lost interest, 2 moved out of state, 2 could not be reached, 6 medical issues (3 depressive disorder and/or substance dependence, 1 hypercalcemia, 1 tumor of the bladder, 1 angioedema due to ACE inhibitor); |dDropped n=16: 4 personal issues and lost interest, 3 moved out of state, 1 could not be reached, 8 medical issues (3 depressive disorder and/or substance dependence, 1 newly diagnosed T2DM requiring medications, 2 liver problems, 1 congestive heart failure, 1 hypokalemia).
All subjects received supplementation with cholecalciferol (D3) 400 IU as multiple vitamins from JBVAMC pharmacy since it was regarded unethical to withhold supplementation from those with hypovitaminosis D. In addition, the subjects were instructed to take either weekly ergocalciferol (D2) 50,000 IU (Pliva Co) or identically looking soy oil-containing placebo (both encapsulated by the research pharmacy). The research pharmacist randomized the subjects using a computer-generated code, adjusted doses and was the only person who knew the allocation and serum 25OHD during the study. Dose adjustments were done at 3, 6, and 9-month visits to maintain serum 25OHD concentrations of 40 – 100 ng/mL and serum calcium within normal range. Compliance was monitored by pill count at each visit. The subjects were advised to maintain their usual diet and physical activity. Season of serum 25OHD sampling was not taken into account since the study lasted 12 months.
The 3-hour OGTT was performed after an overnight fast. A baseline and post-Glucola (75g glucose) venous blood samples were obtained at 10, 20, 30, 60, 90, 120, 150 and 180 minutes for glucose, insulin, and C-peptide measurements. Glucose tolerance status was defined using the ADA criteria for normal and impaired tolerance including normal glucose tolerance (NGT), impaired fasting glucose (IFG), impaired glucose tolerance (IFG), both (IFG and IGT), and diabetes (14).
Blood samples were used for measuring A1C, 25OHD, glucose, calcium, insulin, and C-peptide and chemistry in the clinical laboratory applying laboratory standards of care and references. The analytical methods included ion-exchange high performance liquid chromatography (TOSOH G8 analyzer) for A1C, hexokinase and spectrophotometric assay (Siemens Vista 1500 Chemistry analyzer) for glucose and calcium, chemiluminescent immunoassay (Siemens ADVIA Centaur XP Chemistry analyzer) for insulin, immunochemiluminometric assay (ICMA, DiaSorin LIAISON analyzer) for 25OHD, and chemiluminescent immunoassay (Siemens Immulite 2000 analyzer) for C-peptide.
Calculations of glycemic indices
Calculations for glycemic indices were based on OGTT under dynamic, i.e. postprandial conditions. Insulin sensitivity was assessed by two methods: 1) Oral Glucose Insulin Sensitivity (OGIS, the primary outcome) based on modeling provided online http://webmet.pd.cnr.it/ogis/ in ml*min−1*m−2 (15) and 2) Matsuda composite based on formula 104/Square Root of [(fasting glucose × fasting insulin) × (mean glucose × mean insulin)] (16). Insulin secretion was assessed by two methods: 1) Insulinogenic index-30 [(insulin at 30 min – fasting insulin)/(glucose at 30 min – fasting glucose)] (17,18) and 2) C-peptidogenic index-30 [(C-peptide at 30 min – fasting C-peptide)/(glucose at 30 min – fasting glucose)] (18–20). All formulas had been validated against the glucose clamp with and without tracers (15,16,18–22).
Statistical Analyses
The sample size for the study was calculated according to general recommendations and previously published data to achieve 80% power and significance level (alpha) of 0.050 using a two-sided two-sample unequal-variance t-test (23). Before statistical analysis, normal distribution and homogeneity of the variances were tested. Baseline characteristics were compared with independent t tests for continuous variables or chi-square tests for categorical variables. Between-group changes (final minus baseline) were analyzed for each outcome variable by two-way ANOVA with treatment (between), time (within) and treatment × time interaction used as independent variables. Efficacy of vitamin D supplementation was analyzed as the intention-to-treat (ITT) based on original random assignment of the groups. To verify primary analysis secondary analysis was performed using data from participants with vitamin D deficiency (25OHD <20ng/ml) and those with prediabetes. Hypotheses were specified a priori, therefore, adjustments for multiple comparisons were not made (24).
Association between main outcome OGIS and clinically relevant vitamin D-related characteristics (25) was evaluated for the whole group by simple linear and stepwise multiple linear regression analyses with OGIS response (final minus baseline) as the dependent variable and important predictors as the independent variables (baseline age, BMI, A1C, fasting glucose, insulin, C-peptide, 25OHD, OGIS, 2-hour glucose, and group assignment). Values were represented as means and standard deviations (SD) unless specified. The significance was determined at p <0.05 (two tailed). All statistical analyses were performed using SAS 9.2 statistical software (SAS Institute, NC).
RESULTS
The data on screening, randomization, attrition and completion rates are reviewed in Fig. 1. The baseline characteristics were similar in placebo vs vitamin D group (Table 1). Likewise, baseline characteristics were similar between 173 subjects who completed and 32 subjects who discontinued the study (data not shown). Compliance was similar in both groups (77% and 76% in placebo and vitamin D groups, respectively, p=0.736). Disease burden was relatively high, average number of medical conditions was 4 (range 0 – 8) and average number of medications was 6.5 (range 0 – 17) per person. The mean [SD] dose of D2 was 62,762 [10,772], range 50,000 – 88,460 IU per week. There was a rise of serum 25OHD in both groups and at 12 months 76% of vitamin D group subjects reached 25OHD of 30 ng/dl or higher, range 12 – 109 ng/dl) (Table 1).
Table 1.
Baseline Characteristics and 25OHD Change over 12-month Trial Period1
| Characteristics | Placebo, n=86 | Vitamin D, n=87 | P value |
|---|---|---|---|
|
| |||
| Baseline Characteristics | |||
| Age (y) | 59.8 ± 6.0 | 58.2 ± 6.0 | 0.204 |
| Body weight (kg) | 101.2 ± 9.3 | 102.6 ± 10.2 | 0.428 |
| BMI (kg/m2) | 31.5 ± 2.4 | 32.4 ± 2.9 | 0.072 |
| SBP (mmHg) | 133 ± 13 | 136 ± 13 | 0.272 |
| DBP (mmHg) | 78 ± 11 | 80 ± 9 | 0.408 |
| Creatinine (mg/dl) | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.192 |
| Fasting glucose (mg/dl) | 97.7 ± 10.3 | 98.3 ± 9.3 | 0.772 |
| 2-hour glucose (mg/dl) | 129.7 ± 34.6 | 131.9 ± 31.8 | 0.749 |
| Fasting insulin (μU/ml) | 18.4 ± 9.4 | 19.5 ± 10.7 | 0.685 |
| Fasting C-peptide (ng/ml) | 3.0 ± 1.2 | 2.8 ± 1.0 | 0.335 |
| 25OHD status, n [%] | |||
| 25OHD <10 ng/ml | 24 [27.9] | 20 [22.7] | 0.687 |
| 25OHD 10–19 ng/ml | 51 [59.3] | 52 [59.1] | 0.912 |
| 25OHD 20–29 ng/ml | 11 [12.8] | 15 [18.2] | 0.798 |
| Glycemic status | |||
| Based on A1C | |||
| Prediabetes | 77 [89.5] | 69 [79.3] | 0.875 |
| Diabetes | 9 [10.5] | 18 [20.7] | 0.068 |
| Based on OGTT | |||
| NGT | 48 [55.7] | 35 [40.2] | 0.088 |
| IFG | 12 [14.0] | 19 [21.8] | |
| IGT | 6 [7.0] | 16 [18.4] | |
| IFG & IGT | 11 [12.8] | 9 [10.4] | |
| Diabetes | 9 [10.5] | 8 [9.2] | |
| Medical problems, n [%] | |||
| Hypertension | 59 [68.6] | 61 [69.3] | 0.873 |
| Hyperlipidemia | 51 [59.3] | 43 [48.9] | 0.543 |
| Cardiovascular2 | 11 [12.8] | 15 [17.1] | 0.465 |
| Arthritis | 34 [39.5] | 31 [35.2] | 0.784 |
| Cancer | 9 [10.5] | 14 [15.9] | 0.367 |
| Psychiatric2 | 66 [76.7] | 64 [72.7] | 0.769 |
| All co-morbidities/person | 3.8 [1.4] | 4.0 [1.6] | 0.459 |
| Number of meds/person | 5.9 [3.0] | 6.8 [2.9] | 0.119 |
| Charlson index3 | 2.1 [1.1] | 2.3 [1.2] | 0.251 |
| 25OHD change (ng/ml) | |||
| Baseline | 14.0 ± 4.8 | 14.7 ± 4.7 | 0.393 |
| 3 mo | 22.0 ± 6.5 | 35.3 ± 10.0 | <0.001 |
| 6 mo | 20.3 ± 6.1 | 40.0 ± 11.7 | <0.001 |
| 9 mo | 21.7 ± 6.9 | 43.3 ± 13.7 | <0.001 |
| 12 mo (final) | 19.9 ± 7.3 | 48.1 ± 18.4 | <0.001 |
Abbreviations: A1C, glycosylated hemoglobin; DBP, diastolic blood pressure; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; OGTT, oral glucose tolerance test; SBP, systolic blood pressure; 25OHD, serum 25-hydroxyvitamin D.
Values are Mean ± SD or n [%]; P values from independent t tests (continuous) or chi-square tests (categorical) between groups.
Cardiovascular problems: coronary artery disease, stroke, peripheral vascular disease and congestive heart failure and Psychiatric problems: depression, post-traumatic stress disorder, substance use and other psychiatric conditions.
Charlson index of chronic disease
Table 2 summarized results for comparisons within the groups (final vs baseline) and between the groups (placebo vs vitamin D) for measured glycemic indices. Changes in body weight, BMI, blood pressure, circulating glucose, insulin, and C-peptide were not different within or between the groups (data not shown). There was no difference between the groups in A1C, incident diabetes or reversal to normal glycemia despite of improved insulin sensitivity in vitamin D group compared to placebo. The analysis of subjects with baseline 25OHD <20 ng/ml or prediabetes (n = 146) did not change the results. A post hoc analysis of participants with baseline impaired fasting glucose showed that more subjects in the vitamin D subgroup (31.6%) than placebo (8.3%) returned to normal glucose tolerance, but the difference did not reach significance (p=0.13) (Table 2). Similarly, in the subgroup with baseline impaired fasting glucose and reverting to normal glucose tolerance at study completion, insulin secretion appeared improved in vitamin D-supplemented vs placebo group. The changes in Insulinogenic Index-30 and C-peptidogenic index-30 were (mean [SD]) as follows: +4.1 [4.8] vs −0.12 [1.12] (p=0.06) and +76.3 [67.8] vs −5.4 [16.4] (p=0.016) in vitamin D-supplemented vs placebo subgroups, respectively, suggesting possibility of vitamin D contribution to improvement of insulin secretion in early stages of dysglycemia.
Table 2.
Comparison of baseline, final and change (final minus baseline) measures of main outcomes within placebo and vitamin D treatment groups, and between groups1
| Index | Placebo, n=86 | Vitamin D, n=87 | P value2 |
|---|---|---|---|
|
| |||
| Insulin sensitivity | |||
| OGIS | |||
| Baseline | 369.00 ± 66.27 | 355.55 ± 64.10 | 0.277 |
| Final | 353.00 ± 63.673 | 363.37 ± 58.60 | 0.455 |
| Change (final – baseline) | −16.00 ± 55.83 | 7.82 ± 56.02 | 0.026 |
| Matsuda composite | |||
| Baseline | 3.15 ± 1.38 | 2.99 ± 1.45 | 0.596 |
| Final | 3.28 ± 1.69 | 3.43 ± 1.83 | 0.694 |
| Change (final – baseline) | 0.13 ± 1.43 | 0.44 ± 1.51 | 0.389 |
| Insulin secretion | |||
| Insulinogenic index-30 | |||
| Baseline | 2.06 ± 1.16 | 1.70 ± 0.96 | 0.134 |
| Final | 2.02 ± 1.24 | 1.98 ± 1.42 | 0.894 |
| Change (final – baseline) | −0.03 ± 1.10 | 0.26 ± 1.03 | 0.340 |
| C-peptidogenic index-30 | |||
| Baseline | 37.59 ± 16.83 | 32.55 ± 14.43 | 0.148 |
| Final | 37.19 ± 18.03 | 38.01 ± 22.90 | 0.876 |
| Change (final – baseline) | −0.64 ± 15.84 | 5.32 ± 17.47 | 0.220 |
| A1C (%) | |||
| Baseline | 6.08 ± 0.20 | 6.14 ± 0.26 | 0.142 |
| 6 mo | 6.14 ± 0.28 | 6.12 ± 0.31 | 0.707 |
| Change (6 mo – baseline) | 0.05 ± 0.20 | −0.03 ± 0.18 | 0.031 |
| Final 12 mo | 6.09 ± 0.26 | 6.14 ± 0.30 | 0.356 |
| Change (12 mo – baseline) | 0.01 ± 0.21 | −0.01 ± 0.18 | 0.663 |
| Glycemic status change from baseline to 12 mo (n [%]) | |||
| Based on A1C | |||
| Incident diabetes | 9 [10.5] | 9 [10.2] | 0.869 |
| From prediabetes to normal | 6 [7] | 8 [9.1] | 0.786 |
| Based on OGTT | |||
| Incident diabetes | 3 [3.9] | 3 [3.8] | 0.878 |
| From IFG to NGT | 1 [8.3] | 6 [31.6] | 0.132 |
| From IGT to NGT | 2 [33.3] | 5 [31.3] | 0.848 |
Values are Mean ± SD or n [%]. Abbreviations: OGIS, oral glucose insulin sensitivity; NGT, IFG, IGT as in Table 1.
P values from independent t tests (continuous) or chi-square tests (categorical) between 2 groups
P=0.036 from independent t tests (continuous) for within the group comparison.
The univariate regression analysis for the whole group (n = 173) showed positive correlation between changes in OGIS (Δ-OGIS) and several baseline characteristics including fasting glucose (r=0.18, p=0.017), glucose at 2-hour of OGTT (r=0.19, p=0.013), group assignment (r=0.16, p=0.037) and negative correlation of Δ-OGIS with baseline OGIS (r=−0.45, p<0.001). The stepwise multiple linear regression analysis showed that baseline OGIS was the only independent predictor of OGIS response (Δ-OGIS) and the model accounted for 20% of OGIS response variance (Table 3).
Table 3.
Predictors of oral glucose insulin sensitivity (OGIS) response (final minus baseline)1
| Predictors2 | Regression coefficient | SE | 95% CI | P value |
|---|---|---|---|---|
|
| ||||
| Body weight | −0.027 | 0.411 | −0.839, 0.786 | 0.948 |
| A1C | −10.69 | 18.69 | −47.61, 26.21 | 0.568 |
| Fasting glucose | 0.023 | 0.389 | −0.745, 0.791 | 0.953 |
| 2-hour glucose | −0.137 | 0.127 | −0.388, 0.114 | 0.284 |
| OGIS | −0.445 | 0.081 | −0.604, −0.286 | <0.0001 |
| Group3 | 17.08 | 9.741 | −2.154, 36.31 | 0.081 |
The stepwise multiple linear regression analysis for the whole group (n = 173). Adjusted R2 for the model 0.20.
Predictors are values at baseline.
Group: placebo vs vitamin D-supplemented group
There was no side effects deemed related to vitamin D treatment. One subject was diagnosed with hypercalcemia (10.2 mg/dl) at three months after initiation of treatment, was dropped from the study and subsequently revealed to belong to a placebo group. At final visit three subjects (one in placebo and two in vitamin D group) had elevated calcium while 25OHD was in normal range (serum calcium 10.2 – 10.4 with reference range 8.5 − 10.1 mg/dl).
Based on the results that the primary outcome of the study (i.e. OGIS) improved and a secondary but more clinically relevant outcome (A1C) did not improve, we calculated the sample size based on our data for Δ-A1C. Calculation showed that 1502 subjects for each group were needed to achieve 80% power to reject the null hypothesis of equal means based on the population mean difference μ1 – μ2 = (0.01 − [−0.01]) = 0.02, standard deviations of 0.21 for group 1 and 0.18 for group 2, and a significance level (alpha) of 0.050 using a two-sided two-sample unequal-variance t-test.
DISCUSSION
The results showed that high-dose vitamin D2 supplementation for a year did not improve A1C or prevents diabetes in subjects with prediabetes. The results were in line with previously reviewed (10) and recently published trials and a meta-analysis (11,26,27). Two of published trials were using high dose vitamin D3 (≥20,000 IU/week) for 1 year in subjects with prediabetes (26,27). Both demonstrated no effect of vitamin D3 supplementation on insulin sensitivity and secretion or A1C. Similarly, the present study revealed no effect of D2 on A1C despite small but significant improvement of insulin sensitivity measured by OGIS. The OGIS index using physiological principles for empirical compartment-based modeling was validated against intravenous glucose clamp (15). Since introduction in 2001 OGIS was utilized in observational studies and randomized controlled trials (22,23,28–32). It performed the best in comparison to other indices of insulin sensitivity (i.e. HOMA-IR, QUICKI, Matsuda composite, Stumvoll, and Gutt) (23,28) conceivably contributing to the discordance in the results of the present and previous studies. The differences in the demographic characteristics and comorbidities, all well-known determinants of glycemic status (1,6,10,14,25), might have added to the inconsistencies in the results. Furthermore, a novel approach for localizing the association of vitamin D status with insulin resistance to one region of the circulating 25OHD was suggested (25). The concept of “sensitivie range” was based on the expectation that the effect of vitamin D on glycemic control was small (about 2%), easily obscured by other factors (e.g. obesity) and, as for other nutrients, to be sigmoidal in shape, with response reaching a plateau at some point within the plausible intake range. By analyzing data from 4,116 subjects with 25OHD ranging 4 – 144 ng/ml, the authors concluded that the “sensitive range” for 25OHD was 16 – 36 ng/ml. In this response region, the absolute magnitude of interactions between vitamin D and metabolic parameters was 2 to 7 times greater than those observed for the wider range (25). Corroborating these results, we observed small effect of vitamin D supplementation with the response of OGIS about 2% (mean Δ-OGIS was 8 in vitamin D-supplemented group while population mean OGIS was 350 ml*min−1*m−2).
There were several strengths of the trial. The trial addressed disparity of health research by being dedicated to African American men with prevalent chronic conditions. The trial was of long duration, used convenient weekly D2 dosing and measured validated sensitive dynamic glycemic indices. Lastly, the results generated the hypothesis that vitamin D supplementation might be predominantly efficacious in early stages of dysglycemia, i.e. impaired fasting glucose. There were several limitations of the study. The study enrolled a single race and gender, used surrogate markers of glucose homeostasis, was underpowered to show changes in A1C or diabetes prevention, and although the subjects were randomized, possible residual confounding by diet, physical activity, and medical problems could remain.
CONCLUSION
In conclusion, the trial does not provide evidence that 12-month high-dose D2 repletion improves clinically relevant glycemic outcomes in subjects with prediabetes and hypovitaminosis D. Further trials powered for diabetes prevention and identifying populations that can benefit from vitamin D supplementation are warranted.
Acknowledgments
The authors’ responsibilities were as follows – EB designed the study, researched the literature, obtained institutional review board approval, carried out the study, wrote the paper; BM, AA, YE, and IC: conducted research including participant recruitment, testing, data and sample collection, and analyses; SK: helped designing the study and writing the paper; EB had primary responsibility for final content.
The authors thank Brian Glovack, PharmD and Michael Pacini, PharmD for maintaining drug inventory and dose adjustments, Bharathi Reddivari for recruiting and following up subjects, Hajwa Kim for help with statistical analysis. The authors also thank Hiba Mohiuddin, Nathaniel Chertok, Emily Lelchuk, Chizelle Onochie, Hassan Zaidi, Karthik Cherukupally, Shweta Kurma for recruiting subjects and collecting data.
Supported by a Merit Review grant from the Department of Veterans Affairs and in part by NIH grant for University Clinical and Translational Research Center
Abbreviations
- A1C
glycosylated hemoglobin
- AUC
area under the curve
- BMI
body mass index
- D2
ergocalciferol
- D3
cholecalciferol
- eGFR
estimated Glomerular Filtration Rate
- HOMA-IR
Homeostatic model assessment-insulin resistance
- IGT
impaired glucose tolerance
- IFG
impaired fasting glucose
- NGT
normal glucose tolerance
- OGIS
oral glucose insulin sensitivity index
- OGTT
oral glucose tolerance test
- QUICKI
Quantitative Insulin-Sensitivity Check Index
- VHA
Veterans Health Administration
- 25OHD
serum 25-hydroxyvitamin D concentration
Footnotes
The contents of this article do not represent the views of the Department of Veterans Affairs or the US Government.
Trial registry: clinicaltrials.gov as NCT 01375660
All authors have no relevant conflicts of interest to disclose. All authors reviewed and edited the manuscript.
Contributor Information
Elena Barengolts, Email: ebarengolts@gmail.com.
Buvana Manickam, Email: buvana.manickam@gmail.com.
Yuval Eisenberg, Email: eisenbe1@uic.edu.
Arfana Akbar, Email: Arfana.Akbar@va.gov.
Subhash Kukreja, Email: skukreja@uic.edu.
Irina Ciubotaru, Email: iciubot1@uic.edu.
References
- 1.American Diabetes Association: Data and statistics about diabetes. Available at: http://professional.diabetes.org/admin Fast Facts. Accessed October 15, 2014.
- 2.Second National Report of Biochemical indicators of diet and nutrition in the U.S. population 2012. Centers for Disease Control and Prevention; 2012. Available at: http://www.cdc.gov/nutritionreport/pdf/Nutrition_Book_complete508_final.pdf Accessed October 15, 2014. [Google Scholar]
- 3.Kibler JL, Brisco K. Evaluation of a brief questionnaire for assessing barriers to research participation. Ethn Dis. 2006;16:547–550. [PubMed] [Google Scholar]
- 4.Byrd GS, Edwards CL, Kelkar VA, et al. Recruiting intergenerational African American males for biomedical research Studies: a major research challenge. J Natl Med Assoc. 2011;103:480–487. doi: 10.1016/s0027-9684(15)30361-8. [DOI] [PubMed] [Google Scholar]
- 5.Veteran Administration. Available at: http://www.va.gov/explore. Accessed October 15, 2014.
- 6.Executive summary: standards of medical care in diabetes–2014. Diabetes Care. 2014;37(Suppl 1):S5–13. doi: 10.2337/dc14-S005. [DOI] [PubMed] [Google Scholar]
- 7.Chiasson JL. Pharmacological and surgical intervention for the prevention of diabetes. Nestle Nutr Workshop Ser Clin Perform Programme. 2006;11:31–39. doi: 10.1159/000094404. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 9.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barengolts E. Vitamin D role and use in pre-diabetes. Endocr Pract. 2010;16:476–485. doi: 10.4158/EP09195.RA. [DOI] [PubMed] [Google Scholar]
- 11.Seida JC, Mitri J, Colmers IN, et al. Effect of vitamin d3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:3551–3560. doi: 10.1210/jc.2014-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.American Diabetes Association. Standards of medical care in diabetes 2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24:539–548. doi: 10.2337/diacare.24.3.539. [DOI] [PubMed] [Google Scholar]
- 16.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic glucose clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 17.Kosaka K, Hagura R, Kuzuya T. Insulin responses in equivocal and definite diabetes, with special reference to subjects who had mild glucose intolerance but later developed definite diabetes. Diabetes. 1977;26:944–952. doi: 10.2337/diab.26.10.944. [DOI] [PubMed] [Google Scholar]
- 18.Bergstrom RW, Wahl PW, Leonetti DL, Fujimoto WY. Association of fasting glucose levels with a delayed secretion of insulin after oral glucose in subjects with glucose intolerance. J Clin Endocrinol Metab. 1990;71:1447–1453. doi: 10.1210/jcem-71-6-1447. [DOI] [PubMed] [Google Scholar]
- 19.Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and C-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract. 2006;72:298–301. doi: 10.1016/j.diabres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Okuno Y, Sakaguchi K, Komada H, et al. Correlation of serum CPR to plasma glucose ratio with various indices of insulin secretion and diseases duration in type 2 diabetes. Kobe J Med Sci. 2013;59:E44–53. [PubMed] [Google Scholar]
- 21.Abdul-Ghani MA, Matsuda M, Jani R, et al. The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2008;295:E401–406. doi: 10.1152/ajpendo.00674.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatunic M, Finucane FM, Norris S, Pacini G, Nolan JJ. Glucose metabolism after normalization of markers of iron overload by venesection in subjects with hereditary hemochromatosis. Metabolism. 2010;59:1811–1815. doi: 10.1016/j.metabol.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Nagpal J, Pande JN, Bhartiaet A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabetes Med. 2009;26:19–27. doi: 10.1111/j.1464-5491.2008.02636.x. [DOI] [PubMed] [Google Scholar]
- 24.Schultz KF, Grimes DA. Multiplicity in randomized trials I: endpoints and treatments. Lancet. 2005;365:1591–1595. doi: 10.1016/S0140-6736(05)66461-6. [DOI] [PubMed] [Google Scholar]
- 25.Heaney RP, French CB, Nguyen S, et al. A novel approach localizes the association of vitamin D status with insulin resistance to one region of the 25-hydroxyvitamin D continuum. Adv Nutr. 2013;4:303–310. doi: 10.3945/an.113.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson MB, Duran P, Lee ML, Friedman TC. High-dose vitamin D supplementation in people with prediabetes and hypovitaminosis D. Diabetes Care. 2013;36:260–266. doi: 10.2337/dc12-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sollid ST, Hutchinson MY, Fuskevåg OM, et al. No effect of high-dose vitamin D supplementation on glycemic status or cardiovascular risk factors in subjects with prediabetes. Diabetes Care. 2014;37:2123–2031. doi: 10.2337/dc14-0218. [DOI] [PubMed] [Google Scholar]
- 28.Kanauchi M, Kanauchi K, Inoue T, Kimura K, Saito Y. Surrogate markers of insulin resistance in assessing individuals with new categories “prehypertension” and “prediabetes”. Clin Chem Lab Med. 2007;45:35–39. doi: 10.1515/CCLM.2007.015. [DOI] [PubMed] [Google Scholar]
- 29.Kautzky-Willer A, Krssak M, Winzer C, et al. Increased intramyocellular lipid concentration identifies impaired glucose metabolism in women with previous gestational diabetes. Diabetes. 2003;52:244–251. doi: 10.2337/diabetes.52.2.244. [DOI] [PubMed] [Google Scholar]
- 30.Rask E, Olsson T, Soderberg S, et al. Insulin secretion and incretin hormones after oral glucose in non-obese subjects with impaired glucose tolerance. Metabolism. 2004;53:624–631. doi: 10.1016/j.metabol.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55:1430–1435. doi: 10.2337/db05-1200. [DOI] [PubMed] [Google Scholar]
- 32.Ludvik B, Hanefeld M, Pacini G. Improved metabolic control by Ipomoea batatas (Caiapo) is associated with increased adiponectin and decreased fibrinogen levels in type 2 diabetic subjects. Diabetes Obes Metab. 2008;10:586–592. doi: 10.1111/j.1463-1326.2007.00752.x. [DOI] [PubMed] [Google Scholar]


