Summary
The presence of subclinical hemopoietic clones driven by leukemia-associated mutations is commonly observed in old age, but also occurs in younger people. We recently found that some leukemia-initiating mutations appear particularly able to found hemopoietic clones in advanced old age. We discuss the biological and clinical implications of these findings.
Introduction
The somatic evolutionary history of clonal blood disorders can be traced back for years or even decades1 and the incidences of many of these disorders including myeloid malignancies such as acute myeloid leukemia (AML), myeloproliferative disorders (MPD) and particularly myelodysplastic syndromes (MDS) rise with age. Although this age-related rise in incidence is largely attributable to the stochastic accrual of somatic mutations with time rather than exposure to external mutagens, this alone cannot, for example, explain why MDS becomes commoner than AML only after the seventh decade. Furthermore, much of the age-related increase in the incidence of AML beyond the age of 60 years represents progression from MDS. However, bar for the fact that different myeloid malignancies are associated with different somatic mutations, there is little understanding of why MDS are so strongly associated with old age. Here we propose an explanation for this association that takes into account recent developments pertaining to the phenomenon of age-related clonal hemopoiesis2–4, which could have important therapeutic implications for myeloid malignancies and possibly other cancers.
Clonal hemopoiesis as a bottleneck for leukemogenesis
Cancer develops through the serial acquisition of somatic mutations over time in cells that undergo selection at a clonal and sub-clonal level. This process closely resembles Darwinian evolution, with mutant stem cells behaving as the unit of selection1. However, in contrast to the natural selection of animal species, cancer evolution is critically dependent on the acquisition of somatic mutations during the lifetime of an individual. This directly links the passage of time to ageing, a process that does not only increase the likelihood of mutation acquisition, but also has profound effects on cells and tissue microenvironments.
The essential first step in myeloid leukemogenesis is the foundation of a clone of pre-leukemic cells. Leukemic progression then relies on a cell belonging to this clone acquiring additional co-operating mutations and giving rise to a new sub-clone(s), which can in turn acquire further mutations en route to the development of a disorder such as AML or MDS. The abiding characteristic of founder mutations is their ability to impart a growth advantage on an HSC, which then outgrows its peers at a rate influenced by the particular mutation. As clonal size directly affects the probability that progeny with one mutation will acquire additional mutations, the ability of founder mutations to generate large clones and the speed with which they do this will influence the likelihood of developing neoplasia in a person’s lifetime.
Significant insights into the clonal evolution of myeloid malignancies were derived from recent studies showing that founder clones driven by myeloid leukemia-associated mutations are relatively common in the blood of hematologically healthy individuals and become commoner with age. This phenomenon, referred to as age-related clonal hemopoiesis (ARCH), is most commonly driven by mutations in one of a small number of genes including DNMT3A, TET2, JAK2, ASXL1, SF3B1 & SRSF22–4. Importantly, these mutations were previously shown to have the characteristics of leukemia-initiating mutations1,5, indicating that ARCH is the likely precursor of most myeloid neoplasms, even though it evidently only culminates to neoplasia in a minority of cases. ARCH therefore represents a bottleneck in leukemogenesis, beyond which different paths towards neoplasia are opened. Onward progression towards neoplasia can ensue either through simple clonal expansion (accumulation) or more likely via the accrual of collaborating mutations (molecular synergy). As clonal size increases, so will the likelihood of acquiring such mutations, affording this stochastic process with a degree of determinism. Recently, clonal expansions were shown to be abundant in sun-exposed epidermis, most often driven by mutations in NOTCH genes, but also genes such FAT1, TP53, FGFR3 and others6, indicating that clonal expansions are not unique to the hemopoietic system. As with ARCH, it appears that these clones only evolve to cancer infrequently.
Ageing and clonal hemopoiesis
We recently found that recurrent point mutations affecting spliceosome genes SF3B1 and SRSF2 were associated with clonal hemopoiesis in individuals aged 70 years or over, but not in younger people3. These mutations, present in circa 40-50% of cases of MDS and a significant proportion of de novo AML in the elderly, are known to exhibit characteristics of early/initiating mutations5 and to influence the disease phenotype and patient outcomes. In our study of more than 4000 individuals, we did not identify SF3B1 or SRSF2 mutant clones in blood DNA in anyone younger than 70-years-old. Also the prevalence of such clones rose sharply beyond this age, reaching 8.3% in those over 90 years, with some individuals harboring more than one independent mutation. Like most ARCH-associated mutations, those affecting SF3B1 and SRSF2 are single nucleotide substitutions and therefore expected to be acquired with a similar likelihood and age-distribution as other mutations. Therefore, our observations suggest that these mutations are particularly able to impart a fitness advantage to HSCs in an ageing hemopoietic system. This offers an explanation for the observation that MDS associated with these mutations are seen in significantly older patients than other MDS. Age-related clonal selection pressures may also influence the age-distribution of ARCH driven by mutations in other spliceosome genes (e.g. U2AF1 – suggested by a re-analysis of age-linked data from Papaemmanuil et al5) and possibly other non-spliceosome genes. For example, we observed that the prevalence of ARCH associated with DNMT3A R882 mutations rises with age up to the seventh decade, but then remains relatively stable3. The same distribution was described by Jaiswal et al2; however they also found that the incidence of ARCH due to other DNMT3A mutations (non-R882) continued to rise beyond the age of 70. Therefore age-related changes in hemopoiesis appear able to affect the extent of fitness advantage imparted to HSCs by different mutations.
The hallmarks of ageing in haemopoiesis include a reduction in red cell production, a decline in responsiveness to external stimuli and a rise in the incidence of myeloid malignancies7. Age-related cell-autonomous changes in HSCs including loss of epigenetic marks and a reduction in regenerative potential can explain some of these observations7. As well as affecting HSCs themselves, ageing has a significant impact on the hemopoietic microenvironment, the neuroendocrine system and the immune system (reviewed by Geiger et al7). This dynamic relationship between ageing HSCs and their environment is reminiscent of how a changing environment alters selection pressures on free-living organisms. So whilst it is clear that somatic mutations can drive clonal expansion, the synchronous accretion of mutations and age-related changes in hemopoiesis, raises the possibility that the latter may also play a significant role in clonal selection. In fact stochastic mathematical models of hemopoietic evolution predict that non-cell autonomous processes rather than somatic mutations are rate-limiting for the development of hemopoietic cell clones8. Such a model is supported by our findings that in individuals with clonal hemopoiesis associated with mutant SF3B1 or SRSF2, a significant proportion harbored more than one apparently independent clone with mutations in these genes. Such convergent evolution alludes to a new environmental pressure operating in aged hemopoiesis. Equivalent observations have been made in murine models, where the aged, but not the young, hematopoietic microenvironment was shown to favor oligo- or monoclonal hemopoiesis rather than polyclonality7, a finding that mirrors the observation that elderly humans can harbor hemopoietic clones without identifiable driver mutations4. We propose that these observations are evidence of an important interaction between hemopoietic ageing and HSC fitness, which influences the development of clonal hemopoiesis driven by particular types of mutations. As these mutations accrue over many decades prior to the onset of “ageing”, a sudden rise in clonal hemopoiesis and associated clonal disorders driven by such mutations is observed in the elderly.
Drivers, passengers and the environment
Cancer-associated mutations are defined as ‘drivers’ when they impart fitness and drive the growth of the malignant clone and ‘passengers’ when their effects on cellular fitness are neutral. However, if the ability of mutations to impart “fitness” is altered by the prevailing environment, as predicted by mathematical models8, this will need to be taken into account when classifying them. The most striking example of this pertains to somatic loss-of-function mutations in the X-linked gene PIGA, which underlie the rare clonal disorder paroxysmal nocturnal hemoglobinuria (PNH). These mutations do not ordinarily impart a fitness advantage to HSCs, but do so in the context of disorders such as aplastic anemia, an autoimmune process attacking surface antigens on HSCs. Loss of PIGA disrupts the synthesis of phospholipid anchors and stops the expression of a set of surface proteins/antigens, giving a clonal advantage to the host HSC. In many PNH patients clonal expansion continues through the acquisition of bona fide leukemogenic mutations such as those affecting JAK2, TET2 and ASXL19 and some go on to develop MDS or AML. We propose that similar selection processes operate in an ageing hemopoietic environment inviting a “clonal escape” through the acquisition of particular somatic mutations. The identification of multiple independent ARCH clones driven by mutant SF3B1 or SRSF2 in several individuals aged 70 years or older3 is highly reminiscent of the multiple clones driven by independent PIGA mutations in PNH patients, indicating that the environment is a key determinant of clonal fitness and evolutionary fate in both these contexts (Figure 1).
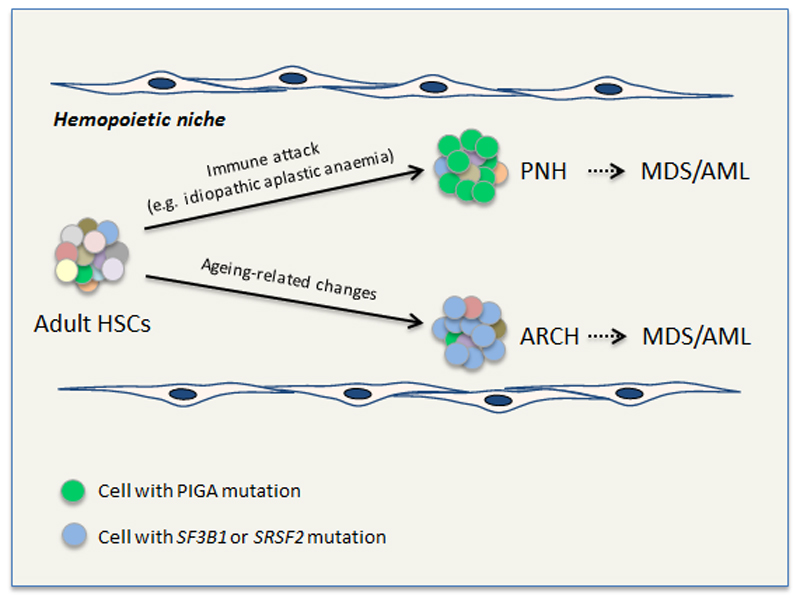
Figure 1. Model for clonal selection to overcome acquired changes in the hemopoietic microenvironment.
Hemopoietic stem cells (HSCs) acquire genetic mutations over time, generating a mosaic of adult HSCs with different mutations, depicted here in different colors. If/when a significant change in the prevailing hemopoietic microenvironment develops, cells with a relative fitness advantage in the new environment (imparted on them by their individual mutation cargo) outgrow their peers. This paradigm is well-established in paroxysmal nocturnal hemoglobinuria (PNH) where loss of PIGA is thought to enable cells to evade immunological attack. We propose that an equivalent selection process operates in an aged hemopoietic niche to drive clonal expansion of HSCs with mutations in SF3B1, SRSF2 and possibly other genes. A key piece of evidence supporting equivalence between the two processes comes from the fact that in many cases multiple clones with independent mutations develop in the same individual (affecting PIGA in PNH and SF3B1/SRSF2 in age-related clonal hemopoiesis, ARCH). Both PNH and ARCH can progress to a neoplastic disorder such as MDS or AML in a proportion of cases.
Age-related changes as potential therapeutic targets
We have outlined the observations supporting the premise that the ageing microenvironment is permissive to the outgrowth of ARCH clones driven by particular spliceosome gene mutations. As these clones are the likely precursors of MDS and AML, their identification could offer opportunities to intervene and reverse clonal expansion at a premalignant stage, thus reducing the likelihood of leukemic progression. Additionally, such interventions may be effective against established disease, particularly in non-acute syndromes such as MDS.
Future research efforts in this field should concentrate on elucidating which of the many cellular, molecular and immunological effects of ageing underlie the changing selection pressures on HSCs, as these would offer rational therapeutic targets. A plethora of age-related changes affect the hematopoietic niche, but particular ones put themselves forward as candidates for such phenomena, including the rise in the levels of the cytokines Interleukin-6 (IL-6), Interleukin-1β (IL1β), Rantes/Ccl5, the reduction of in levels of CXCL12 and alterations in the sympathetic nervous system within the bone marrow (BM) niche7. In recent studies IL-6 secretion by BCR-ABL mutant cells in a mouse model of chronic myeloid leukemia (CML) was shown to act on normal stem and progenitor cells imparting upon them a leukemic-like phenotype and gene signature. IL-6 inhibition with an anti-IL-6 antibody, reversed this phenotype and eliminated the bulk of disease whilst similar observations were made for normal human blood progenitor cells exposed to CML cells in-vitro10. In another study, IL1β produced by JAK2 V617F mutant cells led to loss of nestin-positive mesenchymal stem cells (MSCs) via BM neural damage. In turn, depletion of nestin-positive cells and their production of CXCL12 expanded mutant HSCs and accelerated progression of the myeloproliferative neoplasm (MPN)10. Treatment using β3-adrenergic agonists restored the sympathetic regulation of nestin-positive MSCs, preventing the loss of these cells and blocking MPN progression. Rantes/Ccl5 is another cytokine whose levels rise with age and which influences haematopoietic stem cell fate7. Forced overexpression of Rantes was shown to act on BM progenitor cells to drive a myeloid differentiation bias at the expense of T-lymphopoiesis. This age-mediated myeloid bias, characteristic of hemopoietic ageing was reversed by exposing old cells to a young milieu7.
Age-related innate and adaptive immune attrition is another significant age-related process with potential effects on clonal selection and a possible role in the phenomenon of late-onset ARCH. This may be particularly pertinent to the expansion of clones associated with spliceososome mutations, as these could be associated with the formation of neo-antigens secondary to aberrant splicing. Such neo-antigens may elicit an immune response followed by immune clearance in healthy young subjects. Any failure to do so as a result of immuno-senescence would enable a clonal escape and expansion in elderly individuals. Interestingly, significant reductions in the diversity of T-cell receptor repertoires7 as well as the number of naïve T-cells are observed after the age of 70 years, which is also the age after which spliceosome-driven ARCH is observed3.
Whilst the above may be speculative, a number of possible avenues of investigation could provide some answers as to the identity of the particular changes facilitating the emergence of ARCH in association with spliceosome gene mutations in old age. One possible approach is to study the characteristics of the rare young individuals with mutant SF3B1/SRSF2-driven MDS5 and look for possible genetic or environmental differences (e.g. cytokine levels associated with genetic variants). Also, the recent development of mouse models carrying the relevant mutations offers opportunities to study the role of an ageing environment in the emergence of spliceosome mutant clonal hemopoiesis and possibly clonal hemopoiesis driven by other types of somatic mutations.
Acknowledgements
TM is funded by a Wellcome Trust Clinician Scientist Fellowship (WT098051). G.S.V. is funded by a Wellcome Trust Senior Fellowship in Clinical Science (WT095663MA), and work in his laboratory is also funded by Bloodwise (Leukaemia Lymphoma Research) and the Kay Kendall Leukaemia Fund. We thank Dr Elli Papaemmanuil for access to age-linked mutational data from Papaemmanuil et al5. G.S.V. is a consultant for and holds stock in KYMAB and receives an educational grant from Celgene.
References
- 1.Potter NE, Greaves M. Cancer: Persistence of leukaemic ancestors. Nature. 2014;506:300–301. doi: 10.1038/nature13056. [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal S, et al. Age-related clonal hematopoiesis associated with adverse outcomes. The New England journal of medicine. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKerrell T, et al. Leukemia-associated somatic mutations drive distinct patterns of age-related clonal hemopoiesis. Cell Rep. 2015;10:1239–1245. doi: 10.1016/j.celrep.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genovese G, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. The New England journal of medicine. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papaemmanuil E, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122:3616–3627. doi: 10.1182/blood-2013-08-518886. quiz 3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martincorena I, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348:880–886. doi: 10.1126/science.aaa6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nature reviews Immunology. 2013;13:376–389. doi: 10.1038/nri3433. [DOI] [PubMed] [Google Scholar]
- 8.Rozhok AI, Salstrom JL, DeGregori J. Stochastic modeling indicates that aging and somatic evolution in the hematopoetic system are driven by non-cell-autonomous processes. Aging. 2014;6:1033–1048. doi: 10.18632/aging.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen W, et al. Deep sequencing reveals stepwise mutation acquisition in paroxysmal nocturnal hemoglobinuria. The Journal of clinical investigation. 2014;124:4529–4538. doi: 10.1172/JCI74747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendez-Ferrer S, Garcia-Fernandez M, de Castillejo CL. Convert and conquer: the strategy of chronic myelogenous leukemic cells. Cancer Cell. 2015;27:611–613. doi: 10.1016/j.ccell.2015.04.012. [DOI] [PubMed] [Google Scholar]