Abstract
Mice incapable of synthesizing the myelin lipid sulfatide form paranodes that deteriorate with age. Similar instability also occurs in mice that lack contactin, contactin-associated protein or neurofascin155 (Nfasc155), the proteins that cluster in the paranode and form the junctional complex that mediates myelin-axon adhesion. In contrast to these proteins, sulfatide has not been shown to be enriched in the paranode nor has a sulfatide paranodal binding partner been identified; thus, it remains unclear how the absence of sulfatide results in compromised paranode integrity., Using an in situ extraction procedure, it has been reported that the absence of the myelin sphingolipids, galactocerebroside and sulfatide, increased the susceptibility of Nfasc155 to detergent extraction. Here, employing a similar approach, we demonstrate that in the presence of galactocerebroside but in the absence of sulfatide Nfasc155 is susceptible to detergent extraction. Furthermore, we use this in situ approach to show that stable association of myelin-associate glycoprotein (MAG) with the myelin membrane is sulfatide dependent while the membrane associations of myelin/oligodendrocyte glycoprotein, myelin basic protein and cyclic nucleotide phosphodiesterase are sulfatide independent. These findings indicate that myelin proteins maintain their membrane associations by different mechanisms. Moreover, the myelin proteins that cluster in the paranode and require sulfatide mediate myelin-axon adhesion. Additionally, the apparent dependency on sulfatide for maintaining Nfasc155 and MAG associations is intriguing since the fatty acid composition of sulfatide is altered and paranodal ultrastructure is compromised in multiple sclerosis. Thus, our findings present a potential link between sulfatide perturbation and myelin deterioration in multiple sclerosis.
Keywords: sulfatide, neurofascin, membrane rafts, myelin
INTRODUCTION
The closest apposition between the myelin sheath and the axon occurs at the paranode and is maintained by a myelin-axon junctional complex [1–3] that is formed by the binding of the axonal heterocomplex of contactin-associated protein (Caspr) and contactin with the myelin protein neurofascin155 (Nfasc155) [4–7]. In mice that lack Caspr, contactin or Nfasc155, paranodal junctions are compromised; nodal and paranodal protein membrane domains are not properly established or maintained; and axonal conduction is significantly impaired [8–11]. Interestingly, similar abnormalities also occur in mice that contain a mutation in the gene that encodes cerebroside sulfotransferase (CST), the enzyme responsible for the sulfation of galactocerebroside [12], and thus, are incapable of synthesizing the myelin sphingolipid sulfatide [13–16].
Since sulfatide is concentrated in the outer leaflet of the myelin membrane bilayer [17–18], it is fortuitously positioned to mediate myelin-axon interactions; however, no axolemmal binding partner has been identified. As an alternative to serving as an axonal ligand, sulfatide may indirectly mediate myelin-axon interactions by ensuring the proper establishment and maintenance of myelin protein domains that are involved in these interactions. In support of this possibility, Maggio and colleagues have demonstrated that myelin sphingolipids mediate lateral segregation of membrane constituents in lipid monolayers suggesting that the sphingolipids potentially regulate cell membrane organization [19–22]. Moreover, we have shown that Nfasc155 initially clusters in the paranodes of the CST “knockout” (KO) mice but these clusters begin to deteriorate by 30 days of age even though levels of Nfasc155 remain constant in the mutant animals [15–16]. Therefore, we proposed that the myelin sphingolipid sulfatide is essential for maintaining proper membrane organization of Nfasc155 domains and ultimately paranode structure.
In support of this hypothesis, Schafer et al. [23] demonstrated that Nfasc155 paranodal clusters were stabilized during optic nerve development coinciding with the formation of membrane rafts, small membrane domains involved in signaling, adhesion, trafficking and sorting [24]. Moreover, Schafer et al. [23] used a biochemical approach to demonstrate that Nfasc155 does not associate with rafts in mice that are incapable of synthesizing the myelin galactolipids, galactocerebroside and sulfatide. Here, we have modified this in situ extraction approach to determine whether the absence of sulfatide, but the presence of normal levels of galactocerebroside [13], would result in compromised myelin membrane associations of Nfasc155 and other prominent myelin proteins.
As demonstrated by Schafer et al. [23], immunocytochemistry provides a valid method to assess the distribution of membrane associated proteins that form clusters (e.g. Nfasc155); however, this approach is not useful in analyzing non-clustering proteins. Therefore, we have also employed western blot analysis to compare the relative levels of myelin proteins prior to and following in situ extraction providing insight into the mechanisms that regulate stability of membrane associations of non-clustered myelin proteins in the CST wild type (WT) and KO mice. Together, our findings demonstrate that the myelin adhesion proteins Nfasc155 high (Nfasc155H) [16] and myelin-associated glycoprotein (MAG) [25] are significantly more susceptible to extraction in the absence of sulfatide whereas myelin proteins not implicated in myelin-axon adhesion are either not susceptible to detergent extraction or their extraction susceptibility is independent of sulfatide. Interestingly, Nfasc155 and MAG are paranodal adhesion proteins [26–28] consistent with our hypothesis that sulfatide regulates paranode integrity and adhesion by stabilizing cis membrane associations. These findings are intriguing since, in MS, the fatty acid composition of sulfatide is altered [29] and paranodal ultrastructure is compromised [30] consistent with a role of sulfatide in paranode maintenance. Thus, our findings present a potential link between sulfatide perturbation and myelin deterioration in MS.
MATERIALS AND METHODS
Animals
All mice used in this study were generated and genotyped as previously described [13; 31]. Although the myelin paranode degenerates with age in the CST KO mice, minimal paranodal ultrastructural disruption is observed in 15 day old mutants as paranodal loops are tightly packed with adjacent loops and oriented toward the axolemma [15]. Since paranodal structure is grossly normal in the 15 day old CST KO mice, all extraction studies were conducted at this age.
Antibodies
The primary neurofascin antibodies used for the quantitative immunocytochemical analysis have been previously characterized and are known as FIGQY (1:200) [32], NF-C1 (1:500) [26] and CT-NF (1:500) [11]. The FIGQY antibody was provided by Dr. Matt Rasband (Baylor Medical College); the NF-C1 antibody was provided by Dr. Peter Brophy (University of Edinburgh, Scotland); the CT-NF antibody was provided by Dr. Manzoor Bhat (University of Texas-San Antonio). These antibodies are directed against the C terminus of the protein and recognize both glial (Nfasc155) and neuronal (Nfasc186) isoforms.
The primary antibodies used for western blot analyses are as follows: FIGQY anti-pan neurofascin (1:4,000) [32], anti-Caspr (1:5,000, M. Bhat) [8], anti-2’,3’, cyclic nucleotide 3’phosphodiesterase (CNP, 1:5,000, Sternberger Monoclonals Inc.), anti-extracellular signal-regulated kinase 2 (ERK2, 1:10,000, Santa Cruz Biotechnologies), anti-β-actin (1:10,000, Sigma-Aldrich), anti-pan myelin-associated glycoprotein (MAG, 1:2,000, Millipore), anti-myelin/oligodendrocyte glycoprotein (MOG, 1:2,000, Millipore), anti-myelin basic proteins (MBPs, 1:100, Millipore), anti-contactin (1:3000, R&D Systems), anti-β-III tubulin (1:2000, Millipore), anti-flotillin-1 (1:2000, BD Biosciences Pharmingen) and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:10,000, Millipore).
In situ detergent extraction for immumocytochemical analysis
The in situ protein extraction was performed as described by Schafer et al. [23]; however, the approach was modified for spinal cord samples. Cervical spinal cords from15 day old CST WT (n=4) and littermate KO (n=5) mice were harvested, weighed using a Mettler top load balance, and immediately incubated in 3ml of extraction buffer consisting of 20 mM Tris-HCl (pH 8.0), 10 mM EDTA, 150 mM NaCl, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) with or without detergent at 4°C for one hour. The weight of the WT cervical spinals cords ranged between 0.0453g and 0.0549g with an average weight of 0.0487g±0.0043g. The weight of the KO cervical spinal cords ranged between 0.0466g and 0.0527g with an average weight of 0.0491g±0.0.0038g. The two detergents utilized in this study were Triton X-100 (1%; non-ionic) (ICN Biomedicals, Inc., Aurora, OH) and CHAPS (cholamidopropyl dimethylammonio-1-propane-sulfonate) (1%; zwitterionic) (Sigma-Aldrich). These detergents were chosen as they are the most commonly used detergents for protein extraction studies [23; 33].
Immunocytochemical analysis
Following detergent incubation the spinal cords were immersion fixed in 4% paraformaldehyde in 0.1 M Millonigs (pH 7.3; 4°C) for 1 hour and immunolabeled as previously described [15]. Immunolabeled sections were analyzed using a Leica TCS-SP2 AOBS laser scanning confocal microscope housed in the Virginia Commonwealth University Microscopy Facility. Maximum intensity projection images (compiled from eight optical sections spanning 3.0 microns through focus) were collected from both CST WT and KO spinal cord sections. A minimum of six maximum projection images were collected for each tissue section; a minimum of 2 sections per mouse was analyzed. Confocal images were collected using a pin hole diameter of one Airy unit, a scan resolution of 1024 X 1024 and a scan zoom of 2. The objective lens used for image capture was a 63X/1.4 NA oil immersion lens. To ensure that similar regions of the cervical cord were sampled, the cerebellum was used to discern ventral and dorsal columns. All images were collected from the cervical ventral columns. The posterior border of the cerebellum was used as the most anterior extent of the spinal cord and thus served as the starting point for image collection as previously described [16]. Initial penetration studies revealed that the depth of detergent penetration following 1 hour of incubation was never less than 150 µm [24]; therefore, all quantitative analyses of Nfasc155 clusters were limited to the peripheral most 119.5 µm of the ventral column of the cervical cord corresponding to the width of the microscopic field.
The number of paired Nfasc155 clusters was counted per image and the average number of clusters per image was determined for each mouse. Two sets of criteria were used to identify pairs of neurofascin-containing paranodes as previously described [34]. Briefly, all paired rectangular clusters (corresponding to two adjacent paranodes) separated by a small region void of labeling (corresponding to the node of Ranvier) were identified as a single paranodal pair. Since the antibodies used in this study label both Nfasc155, the glial form of neurofascin, and Nfasc186, the neuronal form of neurofascin, extended rectangular complexes, lacking a negatively labeled node of Ranvier, were also observed. For identification of Nfasc155/Nfasc186 complex clusters, solid rectangular clusters ranging between 5 and 15 microns with well demarcated ends were also identified as a single paranodal pair. To confirm that the FIGQY, NF-C1or CT-NF positive clusters in singly labeled tissue sections corresponded with the nodal/paranodal region, an adjacent section from each mouse was also double labeled with either the FIGQY or NF-C1 antibody in conjunction with a pan sodium channel antibody (1:200; Sigma-Aldrich).
In situ detergent extraction for western blot analysis
For accurate analysis of protein extraction by western blot approaches, complete penetration of the detergent is required. As previously described, the one hour incubation of the intact spinal cord provided limited penetration. Limited penetration was an asset for the immunohistochemical analysis as it provided an internal control; however, it was not compatible for western blot studies. Therefore, to ensure complete tissue penetration, the ventral columns of the spinal cords were harvested using an American Optical dissecting microscope at 15X. The isolated columns from the WT mice weighed between 0.0142g and 0.0187g with an average weight of 0.0167g±0.0021g. The isolated columns from the KO mice weighed between 0.0130g and 0.0201g with an average weight of 0.0173g ±0.0033g. The isolated columns were quickly rinsed in PBS at 4°C and immediately incubated in 3ml of extraction buffer (4°C) with or without detergent for 2 hours at 4°C. The optimal incubation time of two hours was determined by analyzing levels of two cytoplasmic proteins ERK2 and GAPDH following incubation of varying times (1 hour, 2 hours, 4 hours, 12 hours and 24 hours (not shown)) in the extraction buffer with or without detergent. Following incubation, the spinal cord was removed from the extraction buffer; the extraction buffer was discarded and the cord was rinsed in ice cold PBS and homogenized as described below. To confirm preservation of ventral column integrity, western blot analysis of β-actin was conducted and silver stained gels were used to compare qualitatively total protein profiles of WT and KO tissues that were incubated with and without detergent.
Western blot analyses
Following the two hour incubation in the extraction buffer (4°C), the spinal cord ventral columns were homogenized in 100 µl of 1% sodium dodecyl sulfate (SDS) in PBS with 5 µl of protease inhibitor cocktail (Sigma) for one minute. Homogenates were aliquoted and stored at −80°C. Protein concentrations were determined with the Micro BCA Protein Assay kit (Thermo Scientific, Rockford, IL) and proteins were diluted to 2 µg/µl with 1% SDS and then to 1 µg/µl with 2X Laemmeli Sample Buffer (Bio-Rad, Hercules, CA) containing 5% β-mercaptoethanol. 10% polyacrylamide gels were used for separation and proteins were transferred to nitrocellulose. Optimal protein transfer required 2 hours at 100 Volts using the TG Transfer buffer (Bio-Rad). Magic Mark XP Western Protein Standard (Invitrogen, Carlsbad, CA) was used as a molecular mass indicator. Membranes were washed in PBS, blocked in 5% dry milk in PBS with 0.05% Tween-20, and incubated with primary antibodies overnight at 4°C. Membranes were washed, blocked, incubated with the appropriate horseradish peroxidase conjugated secondary antibody (1:10,000 Santa Cruz Biotechnologies, Santa Cruz, CA) for two hours at room temperature, washed in PBS-Tween-20 (PBST) followed by PBS, and visualized with chemiluminescent substrate (Millipore, Billerica, MA) and CL-Xposure film (Thermo Scientific). In parallel to the analyses of the glycosylated neurofascins, these proteins were also analyzed following deglycosylation with PNGaseF as previously described [16], which provided better separation of the isoforms.
Myelin isolation and sucrose gradient fractionation
Whole spinal cords were harvested from CST WT and KO mice and myelin was isolated according to a modified method of Norton and Poduslo [35] using two rounds of myelin purification by gradient centrifugation was isolated as previously described [36–37]. Purified myelin was layered in a discontinuous sucrose gradient consisting of 0.2M, 1.0M and 2.0M, and centrifuged for 19 hours at 192,000g [44, 54]. Twelve 1mL aliquots were collected per sample and loaded on a dot blot for immunolabeling for neurofascin and flotillin.
Statistical analyses
For the neurofascin cluster quantitation, all counts are reported as mean ± standard deviation and were statistically compared using 2-tailed t-tests; significance was accepted with a p value less than 0.05. For western blot analyses, band densities were standardized against loading controls using NIH Image J [38]. Protein extraction per genotype was calculated as a percent of control (incubated in extraction buffer without detergent). All densities are expressed as mean ± standard deviation and were statistically compared using 2-tailed t-tests; significance was accepted with a p value less than 0.05
RESULTS
Nfasc155 paranodal clusters are significantly more susceptible to detergent extraction in the absence of sulfatide
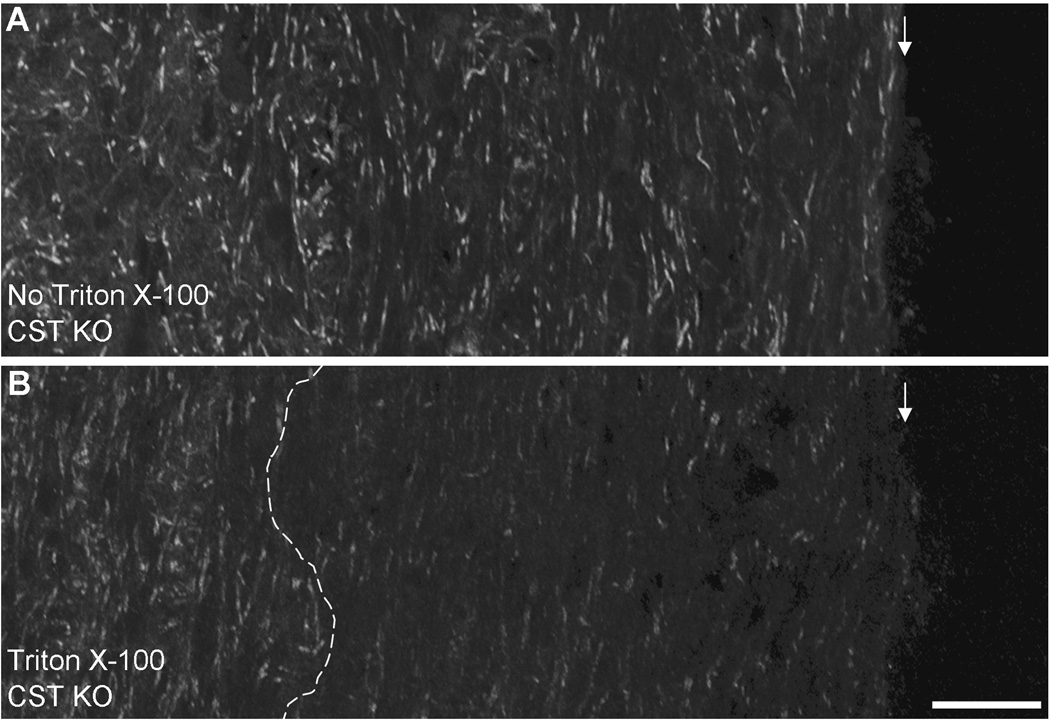
Previous work from our lab demonstrated that CST KO mice contain fewer Nfasc155 paranodal clusters compared to their WT littermates. Here, we further analyzed our data of the CST KO mice and determined that paranodal clusters were also significantly reduced in the CST KO mice between the ages of 15 and 30 days of age (36.8±8.0 clusters per microscopic field at 15 days of age (n=5) compared to 9.5±9.3 clusters per microscopic field at 30 days of age (n=3); p<0.001) without a reduction in protein levels [16]. This indicated that cluster loss was not the consequence of reduced Nfasc155 levels but the result of paranodal domain instability in the absence of sulfatide. To determine a potential mechanism responsible for domain instability, we employed an in situ detergent extraction approached modified from Schafer et al. [23]. As shown in Figure 1B, and consistent with our previous report [24], peripherally positioned Nfasc155 clusters were extracted from CST KO spinal cords following a one-hour incubation in extraction buffer containing detergent. In contrast, more centrally positioned clusters were maintained. As noted by Schafer et al. [23], retention of centrally positioned clusters provides an internal control for the immunocytochemical labeling. Note, when incubated in the extraction buffer without detergent, spinal cords from CST KO mice revealed paranodal labeling in the peripheral and central regions of the tissue (Figure 1A).
Figure 1. Absence of neurofascin labeling indicates depth of detergent penetration.
Following incubation in extraction buffer without detergent (A, no Triton X-100) or with detergent (B, Triton X-100), cervical spinal cords from CST KO mice were immunolabeled using an antibodies directed against the neurofascin proteins. Spinal cords incubated in extraction buffer without detergent revealed abundant labeling of paired clusters consistent with paranodal labeling of Nfasc155. In contrast, spinal cords incubated in extraction buffer with detergent revealed a dramatic reduction of neurofascin labeling in the periphery of the samples. The region lacking neurofascin labeling was never less than 150µm indicating that the detergent penetrated the tissue to at least that depth. Note that central regions of the spinal cord in the Triton X-100 treated sample (B) maintained labeling consistent with samples incubated in the extraction buffer without detergent (A). Magnification bar = 50 µm; white arrows indicate the edge of the tissue; dotted white line in Panel B indicates depth of detergent penetration.
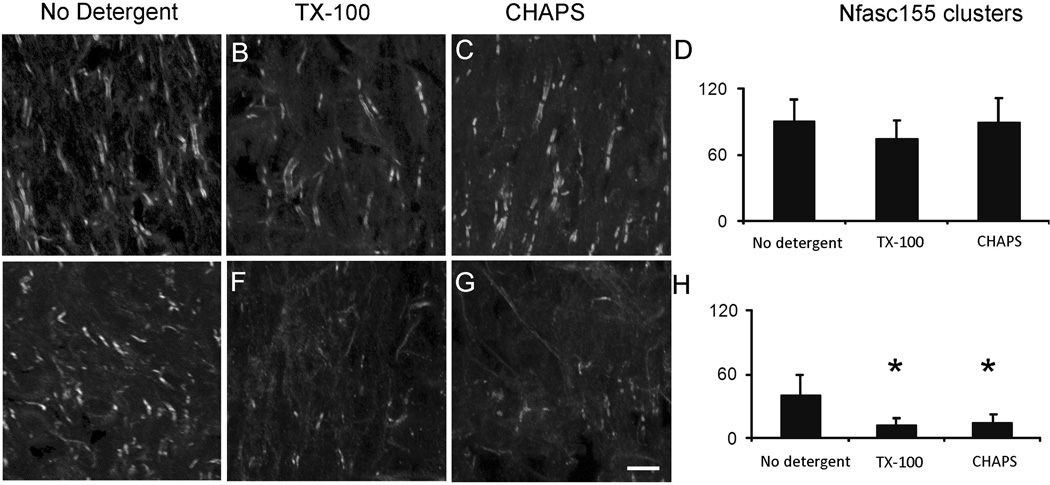
After determining the feasibility of the in situ approach to analyze the stability of protein clusters in the spinal cord (Figure 1), we employed this method to compare the stability of paranodal clusters of Nfasc155 in the presence and absence of sulfatide. As shown in Figure 2A, spinal cords from 15 day old CST WT mice incubated in detergent-free extraction buffer (n=4) revealed 90.0±19.7 paranodal clusters of Nfasc155 per microscopic field. No difference in cluster number was observed in WT spinal cords incubated in extraction buffer containing either the detergent Triton X-100 (74.1±17.5; n=4; p=0.31; Figure 2B&D) or CHAPS (89.3±22.8; n=3; p=0.89; Figure 2C&D). Spinal cords from 15 day CST KO mice (n=4) incubated in buffer without detergent exhibited 39.3±19.9 paranodal clusters per field (Figure 2F&H), which is consistent with the number of clusters observed in spinal cords from 15 day CST KO prior to incubation (36.8±8.0 clusters/field) indicating that incubation in extraction buffer lacking detergent does not result in cluster reduction. Additionally, the number of clusters observed in the spinal cord from 15 day CST KO mice, incubated in buffer without detergent (39.3±19.9 clusters per field), was significantly less than the number of paranodal clusters observed in 15 day old WT spinal cords (90.0±19.7 clusters/filed; p<0.005) incubated in the extraction buffer without detergent. In contrast to the WT spinal cords, incubation of CST KO spinal cords in either Triton X-100 or CHAPS resulted in a significant reduction of Nfasc155 paranodal clusters compared to spinal cords from age matched KO mice incubated in extraction buffer without detergent (39.3±19.9 clusters per field in the absence of detergent versus 11.6±6.8 clusters per field for Triton X-100 incubation; n=5; p<0.001 and 14.1±7.6 clusters per field for CHAPS incubation; n=3; p<0.01; Figure 2 F–H). These findings demonstrate that paranodal cluster extraction is not detergent specific and more importantly, strongly support our hypothesis that paranodal Nfasc155 clusters are significantly less stable in the absence of sulfatide. As no difference was observed between detergents, we limited all subsequent analyses to the detergent Triton X-100.
Figure 2. Neurofascin 155 paranodal clusters are extracted from CST KO but not from CST WT spinal cords.
Spinal cords from WT mice incubated in extraction buffer without detergent (A), with Triton X-100 (B) or with CHAPS (C) revealed no difference in the number of Nfasc155 paranodal clusters (D) indicating that these paranodal clusters are strongly associated in the myelin membrane. In contrast, spinal cords from CST KO mice incubated in extraction buffer containing either Triton X-100 (F) or CHAPS (G) revealed a significant reduction in cluster number (H) compared to spinal cords incubated in extraction buffer lacking detergent (E). These findings indicate that sulfatide is not essential for cluster formation; however, in the absence of sulfatide, Nfasc155 domains are highly vulnerable to degradation. Magnification bar = 15µm
Soluble proteins are extracted from dissected white matter while total protein profiles are not dramatically altered following detergent incubation
Next, we wanted to determine whether the increased vulnerability to detergent extraction was specific to Nfasc155 or if other proteins also displayed an increased susceptibility to extraction in the absence of sulfatide. The importance of this question is emphasized by our work that compromised myelin integrity, evidenced by demyelination, loss of myelin compaction, and the deterioration of glial and neuronal protein domains, is prevalent in sulfatide deficient mice [14–15; 39–41]. Although immunocytochemistry is an effective approach for comparing susceptibility to detergent extraction for proteins that form clusters, it is not suitable for analyzing proteins that do not form distinct aggregates. To circumvent this limitation, we used western blot strategies to analyze levels of both glial and neuronal proteins. In contrast to immunocytochemistry, which reveals the depth of detergent penetration and thus delineates the regions appropriate for quantitation (Figure 1), western blot analysis requires complete penetration of the detergent. Therefore, we modified our methodology in two ways. First, using microdissection, the ventral columns of the cervical spinal cords were harvested and immediately incubated in the extraction buffer, with or without detergent, at 4°C for two hours (see Material and Methods). To determine the effectiveness of detergent penetration, we first compared relative levels of two cytoplasmic proteins, ERK2 and GAPDH, following a two hour incubation of CST WT and KO ventral columns in detergent-free or detergent-containing extraction buffer. ERK2 and GAPDH were chosen since they are universally expressed and, as cytoplasmic proteins, their extraction should provide a less encumbered (i.e. not tethered within the cell) indicator of detergent penetration.
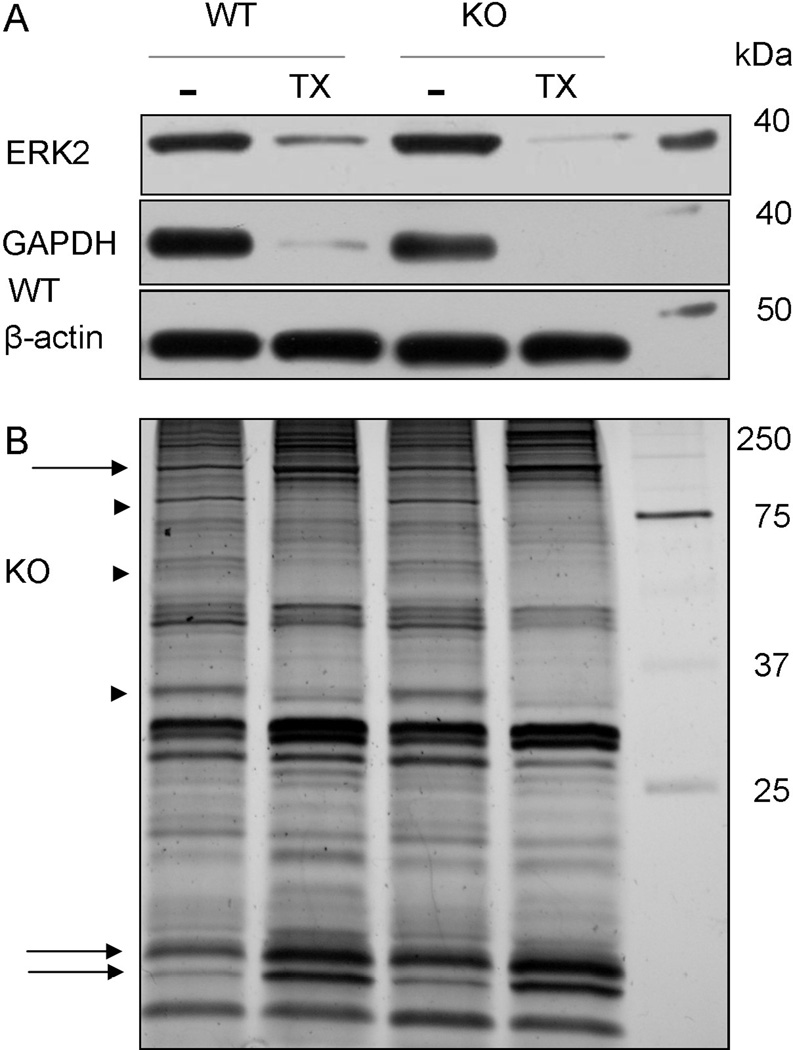
As shown in Figure 3A, western blot analyses indicated that ERK2 and GAPDH were nearly completely extracted by incubation in extraction buffer containing detergent from both WT and KO spinal cord ventral columns. Although these cytoplasmic proteins were readily depleted, we also wanted to determine whether the two hour extraction protocol resulted in global protein degradation or extraction, which would implicate a non-specific protein disruption. To test this possibility, we used silver stain to compare total protein banding patterns between genotypes and between treatments (i.e. with and without detergent). Protein assays revealed an approximate 40% reduction of total protein from both the WT and CST KO ventral columns (data not shown); however, all samples presented clearly defined bands without smears indicating the absence of protein degradation (Figure 3B). In addition, the protein banding patterns were similar among the samples; however, in lanes containing WT and KO detergent-treated samples, several bands were absent or less intense (arrowheads; Figure 3B) while other bands appeared more intense (arrows; Figure 3B) as compared to their genotype-matched samples incubated without detergent. We interpret that a reduction in band intensity indicates that some proteins were extracted to a greater extent than proteins as a whole while the apparent increased intensity indicates that proteins were less susceptible to detergent extraction compared to all proteins. Taken together, our ERK2 and GAPDH extraction findings combined with the silver stained protein banding patterns indicate that the two hour extraction of isolated cervical ventral columns is a suitable approach to analyze in situ protein solubilities without inducing protein degradation.
Figure 3. Extended incubation in extraction buffer with detergent extracts cytoplasmic proteins without protein degradation.
Dissected ventral columns from WT and CST KO mice were incubated in extraction buffer (4°C) for two hours. Western blot analyses indicate that the detergent completely penetrated the tissue as the cytoplasmic proteins ERK2 and GAPDH were efficiently extracted (A). Silver staining revealed clearly defined protein bands for all samples indicating that protein degradation was not prevalent. Additionally, the protein profiles between genotypes were similar; however, several differences were observed between detergent treated and untreated samples including bands that were lost/reduced with detergent treatment (arrow heads) and bands that revealed an apparent increase in intensity following detergent incubation (arrows).
Neurofascin proteins are susceptible to detergent extraction
Since our immunocytochemical studies were limited to Nfasc155 (Figure 2), we initially employed our western blot approach to quantify levels of this protein following extraction of harvested ventral columns. Our laboratory has previously reported that Nfasc155 is not a single protein but exists in two forms, which we have named Nfasc155 high (H) and Nfasc155 low (L) based on their apparent electrophoretic molecular masses [16]. Presently, Nfasc155H and Nfasc155L cannot be distinguished by in vivo immunocytochemical labeling; thus, findings from the immunocytochemical analyses refer to total Nfasc155. In contrast, western blot analysis provided the additional advantage of comparing extraction susceptibilities of the two forms of Nfasc155 as well as the extraction susceptibility of the neuronal form of the neurofascin family of proteins known as Nfasc186.
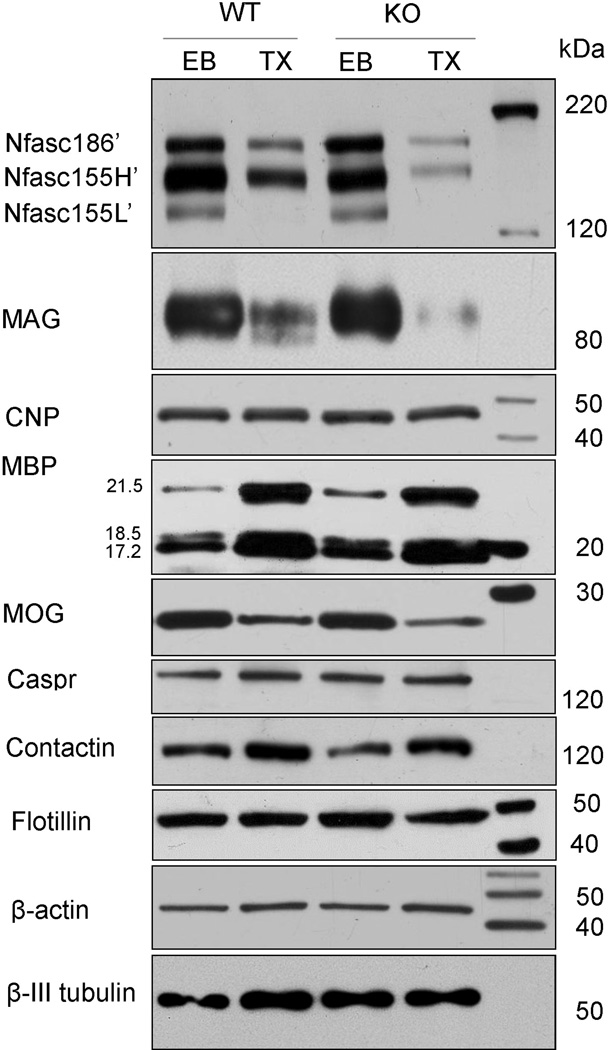
In Figure 4, the three neurofascin proteins are referred to as Nfasc186’, Nfac155H’ and Nfasc155L’ indicating the deglycosylated (PNGase F treated) form of each protein. Nfasc186’ and Nfasc155H’ were partially extracted from the WT ventral columns following detergent incubation (33.5±3.0% and 22.2±10.0%, respectively; n=3; Table I) while Nfasc155L’ was nearly completely extracted (95.2±8.4%; Table I) compared to WT columns incubated in extraction buffer without detergent (n=3). Nfasc186’ and Nfasc155H’ were significantly more susceptible to detergent extraction in the absence of sulfatide with both proteins revealing a reduction of approximately 60% (62.5±6.1% and 60.5±6.7%, respectively; n=3; p=0.005 and 0.008; Table I). Similar to the WT samples, incubation in the extraction buffer with detergent completely extracted Nfasc155L’ from KO tissues (99.0±1.7%; Table I). Together, these findings confirm our immunocytochemical data by demonstrating that in the absence of sulfatide Nfasc155 is highly susceptible to detergent extraction. Moreover, our western blot data also reveal a distinct difference in extraction susceptibility between the two Nfasc155 proteins as Nfasc155H is significantly more susceptible to detergent extraction in the absence of sulfatide while Nfasc155L is highly soluble and its solubility is sulfatide independent. Finally, yet somewhat surprising, the absence of the myelin lipid sulfatide also resulted in the significant increase in the extraction of Nfasc186, the neuronal isoform of neurofascin.
Figure 4. Nfasc155H, Nfasc186 and MAG but not CNP, MBP, or MOG exhibit an increased susceptibility to detergent extraction in the absence of sulfatide.
Dissected ventral columns from WT and CST KO mice, incubated in extraction buffer without detergent (EB), exhibited the expected profile of the neurofascin family of proteins. Following incubation in buffer containing the detergent Triton X-100 (TX), Nfasc155L was completely extracted independent of sulfatide. In contrast, Nfasc186 and Nfasc155H were extracted from WT samples but the extent of extraction was significantly greater in the absence of sulfatide. These findings indicate that Nfasc155H and Nfasc186 exist in detergent soluble and insoluble populations under WT conditions and that the population that is normally detergent insoluble is dependent on sulfatide. Similar to Nfasc155H, MAG was also significantly more readily extracted in the absence of sulfatide. In contrast, levels of CNP were not reduced following detergent extraction from either the WT or CST KO samples while levels of MBP appeared increased (see Discussion). MOG levels were reduced in both WT and KO samples following detergent extraction but the extent of reduction was not different between genotypes. These findings suggest that sulfatide is required for maintaining stable associations between MAG and the myelin membrane while membrane associations of the myelin proteins CNP, MBP and MOG are not dependent on this lipid. Finally, levels of the neuronal paranodal proteins Caspr and contactin were not readily extracted by detergent incubation and no difference was observed between genotypes. β-actin and β-III tubulin were used as loading controls.
Table I.
Percent protein reduction following detergent extraction
| WT | KO | |
|---|---|---|
| Nfasc186 | 33.5± 3.0 | 62.5± 6.1* |
| Nfasc155H | 22.2±10.0 | 60.5± 6.7* |
| Nfasc155L | 95.2± 8.4 | 99.0± 1.7 |
| MAG | 39.0± 15.1 | 77.7± 15.6* |
| MOG | 58.1±15.7 | 54.4± 26.1 |
| CNP | 9.9± 6.4 | 6.0± 4.0 |
Values (mean ± s.d.) indicate percent of protein reduction as compared to spinal cords incubate without detergent;
indicates p<0.05
MAG, but not CNP, MBP or MOG, is significantly more susceptible to detergent extraction in the absence of sulfatide
In addition to the neurofascin proteins, we also analyzed the vulnerability to detergent extraction of the myelin proteins myelin-associated glycoprotein (MAG), cyclic nucleotide phosphodiesterase (CNP), myelin basic proteins (MBP) and myelin/oligodendrocyte glycoprotein (MOG). Following incubation without detergent, levels of MAG were similar between the CST KO mice and their WT littermates. Following incubation with detergent, MAG was partially extracted from both WT and KO samples; however, the extent of extraction was significantly greater from the mutant tissue (39.0±15.1% extracted from WT (n=3) compared to 77.7±15.6% extracted from KO (n=3; p=0.037)) (Figure 5; Table I). Together, our findings demonstrate that the absence of sulfatide increases detergent solubility of MAG. In contrast to Nfasc186, Nfasc155H and MAG, CNP exhibited minimal susceptibility to detergent extraction for either WT or KO spinal cord columns (9.9±6.4%, n=3 and 6.0±4.0%, n=3; respectively; p=0.47; Figure 4). Similarly, MBP was not extracted as levels of the MBP isoforms were not reduced in either the WT or KO samples following detergent incubation. Not only were the MBP isoforms not reduced following extraction but band densities were increased for both genotypes following the detergent incubation (75.9±38.6% and 86.5±57.6% increase for all isoforms for WT and KO samples); however, these apparent increases were not statistically different between genotypes (p=0.39). In contrast to CNP and MBP, MOG was partially extracted by detergent incubation; however, the extent of extraction did not differ between genotypes (58.1±15.7% and 54.4±26.1% for WT (n=3) and KO (n=3) samples respectively; p=0.94; Figure 4; Table I) indicating that MOG is susceptible to detergent extraction but extraction is independent of sulfatide. Together, our data indicate that sulfatide plays a role in maintaining membrane associations of Nfasc155H and MAG, but is not involved in maintaining membrane associations of Nfasc155L, MBP, CNP and MOG indicating that both sulfatide-dependent and independent mechanisms regulate the maintenance of myelin integrity.
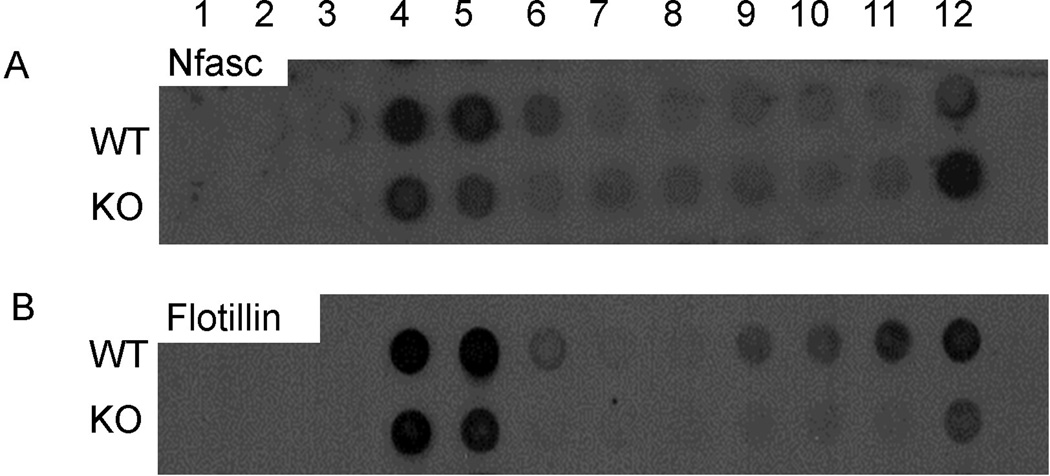
Figure 5. Myelin neurofascin from CST KO spinal cords is isolated from the subfraction with the highest sucrose concentration.
Purified myelin was isolated from spinal cords of CST WT and KO mice and subfractionated by a sucrose gradient. Subfractions were probed with antibodies against in neurofascin (A) and flotillin (B), a putative indicator of membrane raft fractions. Consistent with previous reports, neurofascin from the WT accumulated in the higher fractions (lower sucrose concentrations) of the gradient. In contrast, a dramatic shift was observed with regard to the distribution of neurofascin from the CST KO sample as neurofascin accumulated in the lowest fraction (fraction 12). (B) In the CST WT samples, flotillin was observed in both the higher fractions (fractions 4 and 5) and the lower fractions (fractions 9–12); however, in the CST KO samples, flotillin was primarily observed in fractions 4 and 5 with a small portion of the protein accumulating in fraction 12.
Neuronal paranodal proteins are not more vulnerable to detergent extraction in the absence of sulfatide
Since our findings from both the immunocytochemical and western blot analyses indicated compromised paranodal domains in the absence of sulfatide, we also investigated the effect that the lack of sulfatide has on the susceptibility to detergent extraction of neuronal paranodal proteins. No difference in levels of Caspr was observed between WT (n=3) and KO (n=3) samples (Figure 4) indicating that the susceptibility of Caspr to detergent extraction is not dependent on sulfatide. Moreover, the maintenance of the paranodal protein Caspr suggests that the extraction of Nfasc155H is not a non-specific, secondary consequence of paranode disruption. In further confirmation that the paranode is not indiscriminately susceptible to detergent extraction in the absence of sulfatide, western blot analysis also revealed no reduction in the levels of contactin (Figure 4), the other member of the neuronal heterocomplex that mediates interaction with Nfasc155 [4; 26], following detergent extraction.
Neurofascin is observed in detergent insoluble glycoprotein complexes but sucrose subfractionation reveals differential distribution in the WT versus KO spinal cords
One explanation of the differential vulnerability of Nfasc155 to detergent extraction is that Nfasc155 is recruited into microdomains known as membrane rafts [23]. Although the analysis of rafts is technically challenging, one method routinely used to study these microdomains is the isolation of detergent insoluble glycoprotein complexes (DIGs) by sucrose gradient fractionation [24]. Therefore, we used this approach to determine whether myelin neurofascin would be present in putative raft fractions from WT mice and whether the distribution of myelin neurofascin would be altered in the CST KO mice. As shown in Figure 5, myelin neurofascin was observed in subfractions 4–6, which is consistent with previous reports [23]. Interestingly, myelin neurofascin isolated from the CST KO spinal cord was also observed in fractions 4 and 5. In contrast to the WT sample, the subfraction of the KO sample that revealed the greatest level of protein was subfraction 12 indicating a dramatic shift in the distribution of myelin neurofascin in the absence of sulfatide. Interestingly, flotillin, a putative marker of DIGs [24], also revealed an altered distribution; however the distribution of flotillin differed from the distribution of neurofascin. While the absence of sulfatide decreased the level of Nfasc in fractions 4–6, it increased the proportion of flotillin in these fractions. These findings suggest that sulfatide plays a role in recruitment of Nfasc into rafts while recruitment of flotillin into rafts dos not require this lipid. Finally, we used our in situ extraction approach to analyze the effect of detergent extraction on flotillin in the intact spinal cord. As shown in Figure 4, extraction of flotillin was not significantly different between genotypes (p=0.169) and thus, consistent with our findings with the sucrose fractionation approach, stability of membrane association of flotillin is independent of sulfatide.
DISCUSSION
We have developed an in situ detergent extraction approach to compare the stability of Nfasc155 paranodal clusters in the spinal cords of CST WT and KO mice. As Nfasc155 forms paranodal clusters, we used immunocytochemistry to evaluate cluster stability. Our findings demonstrate that Nfasc155 clusters are significantly more susceptible to detergent extraction in the absence of sulfatide. Additionally, our laboratory has recently demonstrated that Nfasc155 is not a single protein but exists in two forms that we have identified as Nfasc155H and Nfasc155L [16]. As these forms cannot be distinguished by immunocytochemistry, we used western blot strategies to compare extraction vulnerability of these distinct forms in the WT and KO mice. Nfasc155L was highly soluble independent of sulfatide. Nfasc155H was partially extracted in the presence of sulfatide; however, in the absence of sulfatide, Nfasc155H was significantly more susceptible to extraction. In addition to Nfasc155H, we also determined that MAG and MOG are susceptible to detergent extraction; however, whereas extraction of MAG is facilitated by the absence of sulfatide, the extraction of MOG is sulfatide independent. Interestingly, MBP and CNP are not readily susceptible to extraction either in the presence or absence of sulfatide. Together, these findings demonstrate that myelin proteins employ distinct mechanisms to maintain their membrane association and that these distinct mechanisms are both sulfatide dependent and independent
Stability of Nfasc155 domains is sulfatide dependent
We have previously reported that paranodal clusters of Nfasc155 are lost with age in the CST KO mice [15–16]. We have also reported that the myelin paranode structurally deteriorates with age in these animals [15]; however, it remains to be determined how sulfatide is responsible for maintaining both structural and molecular organization of the myelin paranode. Previous work by Schafer et al. [23] demonstrated that mice that lack both galactocerebroside and sulfatide form unstable Nfasc155 membrane domains. Here, we demonstrate that the stability of myelin Nfasc155 domains is sulfatide dependent and that galactocerebroside [13] is not sufficient to maintain these domains. Interestingly, changes in sulfatide composition have been reported in MS independent of demyelination [29]. Moreover, paranodal ultrastructure is compromised in MS and the altered structure observed in MS (everted paranodal loops [30]) strongly resembles the paranodal abnormalities that develop in the sulfatide KO mice [15].
Plausible hypotheses as to how sulfatide regulates paranode stability include altered expression and/or trafficking of protein(s) that modulate membrane associations of paranodal neurofascin, and compromised interactions with membrane stabilizing molecules and/or components of the cytoskeleton [42–45]. Another intriguing possibility focuses on the concept of membrane rafts, small membrane domains involved in a variety of cellular functions including adhesion and protein distribution. The raft possibility is particularly intriguing based on the sucrose gradient subfractionation results (Figure 5) and differential detergent solubility observed with the in situ extraction as triton solubilization is a well-documented approach for raft analysis [reviewed in 24].
Although the mechanisms that regulate raft formation remain to be fully elucidated, a feature commonly associated with the recruitment of proteins into membrane rafts is the addition of long, saturated fatty acid chains [46–49]. Interestingly, the neurofascin proteins, which have been implicated as components of membrane rafts [23], have the potential for such a modification with the addition of palmitate residues [47–48]. The sites of palmitoylation of the neurofascin proteins are highly conserved cysteine residues located within the transmembrane domain. Mutation of these cysteine residues inhibits palmitoylation and membrane domain incorporation [50] strongly suggesting that neurofascin palmitoylation facilitates recruitment into membrane rafts, bolsters membrane associations and stabilizes protein domains. Moreover, we have recently observed that treatment with hydroxyl amine, a chemical known to cleave the thioester bonds that link palmitate groups to proteins [52], facilitates extraction of Nfasc155 but has no effect on the solubility of Caspr [24] consistent with the requirement of the palmitate group to anchor Nfasc155 in the myelin sheath. In contrast, the solubility of Caspr, a protein that has not been demonstrated to be palmitoylated, was unaltered by the hydroxyl amine treatment suggesting that stability of Caspr membrane domains is independent of such post translational modifications.
Although the solubility of Nfasc155H was significantly increased by the absence of sulfatide, Nfasc155L was completely extracted independent of sulfatide. It remains to be determined why these two forms exhibit differential solubilities; however, differential palmitoylation is a possibility. Interestingly, MAG, whose solubility is also sulfatide dependent, is palmitoylated [53] and a putative membrane raft protein [44; 54]. Taken together, since both Nfasc155 and MAG have been localized to the CNS paranode [4; 26; 28; 55–56] and mediate axon-glial binding [4; 26; 57–58], we propose that paranode deterioration in the CST KO mice results from compromised stability of Nfasc155H and MAG domains.
Stability of Nfasc186 domains is also sulfatide dependent
Schafer et al. [23] previously reported that Nfasc186 is, at least partially, detergent soluble in WT rodent optic nerve. Therefore, partial solubility of Nfasc186 from WT spinal cords is consistent with previous findings; however, the significant increase in the solubility of neuronal neurofascin in the absence of sulfatide was not expected. Presently, it remains unknown how sulfatide regulates the stability of Nfasc186 domains. One possibility is that since sulfatide is synthesized in neurons [59–61], the absence of neuronal sulfatide results in compromised neuronal raft stability. A second possibility is that the formation and stability of Nfasc186-containing membrane domains is dependent on ligand-receptor interactions between the glial and neuronal membranes, which is consistent with membrane raft formation in several systems including oligodendrocytes [23–24; 43; 62]. Interestingly, we previously reported that Nfasc186 is frequently not detected in MS samples but is routinely detected in non-MS tissues [16]. The explanation for this remains to be determined but unstable membrane domains is consistent with altered fatty acid composition of sulfatide [29].
Myelin proteins maintain membrane associations by sulfatide dependent and independent mechanisms
While sulfatide is required for maintaining paranode integrity by regulating protein domain stability, our findings also demonstrate that the membrane association of other myelin proteins is independent of this lipid. As shown in Figure 4, CNP, MBP and MOG reveal no difference in relative levels between genotypes following detergent extraction. The insolubility of CNP is consistent with the previous findings of Pfeiffer and colleagues [44; 63], who postulated that CNP is associated with DIGs. Incorporation into these membrane domains is consistent with our current findings as CNP is not detergent-extracted from the WT spinal cords (Figure 4). However, recruitment into membrane rafts is largely driven by the addition of long, saturated carbon chains that facilitate membrane associations [64]. CNP establishes and maintains its association with the myelin membrane through the addition of a lipid residue, known as a prenyl group, to its carboxy terminus [65]. Prenyl groups, however, do not display the typical features of the lipid residues associated with raft recruitment as prenyl groups are short, highly branched and bulky [66]. Moreover, the absence of sulfatide, a sphingolipid and a known component of at least some populations of myelin membrane rafts [54], does not increase CNP’s susceptibility to extraction (Figure 4). Therefore, the insoluble nature of CNP may be independent of raft incorporation, or at least independent of sulfatide-dependent rafts, but may result from membrane linkage via a carboxyl terminal prenyl group [65] that does not require sulfatide.
In contrast to CNP, some controversy exists with regard to MBP’s association with raft domains [44; 54; 67–68]. Here, we report that MBP is not extracted from either WT or KO tissues. As sulfatide is concentrated in the outer leaflet of the membrane bilayer [17–18] and MBP is a cytoplasmic protein, it is not surprising that the mechanism that regulates the association between MBP and the myelin membrane is independent of sulfatide. Interestingly, the three isoforms observed in this study (21.5, 18.5 and 17.2 kD isoforms) appear to increase following detergent extraction indicating that the MBP isoforms resist extraction to a greater degree than proteins in general. Thus, the MBP isoforms maintain a strong association with the membrane and this association is independent of sulfatide. In contrast to CNP, which revealed no difference in band intensities, and MBP, which revealed an increase in intensities, MOG revealed a decrease in intensities following extraction but this decrease was independent of sulfatide since both genotypes presented a reduction of ~50%.
In summary, we have developed an in situ extraction method that allows for the analysis of the relative strength of membrane associations of both clustered and non-clustered proteins. Furthermore, we have used this method to study the consequence of sulfatide depletion on membrane associations of prominent myelin proteins. Our findings indicate that myelin proteins maintain membrane associations both by sulfatide dependent and independent mechanisms. Based on our findings we propose that sulfatide regulates paranode stability by maintaining the membrane associations of Nfasc155H and MAG. These findings provide a plausible explanation as to why CST KO mice are incapable of maintaining paranodal domains throughout life. Importantly, since fatty acid composition of sulfatide is altered in MS [29], our findings provide a possible explanation for the paranodal degeneration [30] and CNS dysfunction that is prevalent in this disease.
ACKNOWLEDGEMENTS
The authors wish to thank Drs. Manzoor Bhat, Peter Brophy, and Matt Rasband for their generous gifts of antibodies. This work was funded by a grant from the National Institute of Health-National Institute of Neurologic Disease and Stroke (R03 NS066186 (JLD)). All microscopy was performed at the VCU Department of Anatomy and Neurobiology Microscopy Facility, supported, in part, with funding from an National Institute of Health Center core grant (5P30NS047463).
Footnotes
The authors report no conflict of interests.
LITERATURE CITED
- 1.Hirano A, Dembitzer HM. The transverse bands as a means of access to the periaxonal space of the central myelinated nerve fiber. J Ultrastruct Res. 1969;28(1):141–149. doi: 10.1016/s0022-5320(69)90012-4. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbluth J. Abnormal axoglial junctions in the myelin-deficient rat mutant. J Neurocytol. 1987;16(4):497–509. doi: 10.1007/BF01668504. [DOI] [PubMed] [Google Scholar]
- 3.Dupree JL, Coetzee T, Blight A, Suzuki K, Popko B. Myelin galactolipids are essential for proper node of Ranvier formation in the CNS. J Neurosci. 1998;18(5):1642–1649. doi: 10.1523/JNEUROSCI.18-05-01642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc AM, Girault JA, Brophy PJ, Lubetzki C. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol. 2002;12(3):217–220. doi: 10.1016/s0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- 5.Gollan L, Salomon D, Salzer JL, Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. J Cell Biol. 2003;163(6):1213–1218. doi: 10.1083/jcb.200309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnon C, Bel C, Goutebroze L, Maigret B, Girault JA, Faivre-Sarrailh C. PGY repeats and N-glycans govern the trafficking of paranodin and its selective association with contactin and neurofascin-155. Mol Biol Cell. 2007;18(1):229–241. doi: 10.1091/mbc.E06-06-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaxton C, Pillai AM, Pribisko AL, Labasque M, Dupree JL, Faivre-Sarrailh C, Bhat MA. In vivo deletion of immunoglobulin domains 5 and 6 in neurofascin (Nfasc) reveals domain-specific requirements in myelinated axons. J Neurosci. 2010;30(14):4868–4876. doi: 10.1523/JNEUROSCI.5951-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30(2):369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- 9.Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30(2):385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 10.Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, Macklin WB, Meek S, Smith AJ, Cottrell DF, Brophy PJ. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48(5):737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Pillai AM, Thaxton C, Pribisko AL, Cheng JG, Dupree JL, Bhat MA. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J Neurosci Res. 2009;87(8):1773–1793. doi: 10.1002/jnr.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamins JA, Hadden T, Skoff RP. Cerebroside sulfotransferase in Golgi-enriched fractions from rat brain. J Neurochem. 1982;38(1):233–241. doi: 10.1111/j.1471-4159.1982.tb10875.x. [DOI] [PubMed] [Google Scholar]
- 13.Honke K, Hirahara Y, Dupree J, Suzuki K, Popko B, Fukushima K, Fukushima J, Nagasawa T, Yoshida N, Wada Y, Taniguchi N. Paranodal junction formation and spermatogenesis require sulfoglycolipids. Proc Natl Acad Sci U S A. 2002;99(7):4227–4232. doi: 10.1073/pnas.032068299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishibashi T, Dupree JL, Ikenaka K, Hirahara Y, Honke K, Peles E, Popko B, Suzuki K, Nishino H, Baba H. A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation. J Neurosci. 2002;22(15):6507–6514. doi: 10.1523/JNEUROSCI.22-15-06507.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53(4):372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- 16.Pomicter AD, Shroff SM, Fuss B, Sato-Bigbee C, Brophy PJ, Rasband MN, Bhat MA, Dupree JL. Novel forms of neurofascin 155 in the central nervous system: alterations in paranodal disruption models and multiple sclerosis. Brain. 2010;133(Pt 2):389–405. doi: 10.1093/brain/awp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cestaro B, Marchesini S, Cervato G, Viani P, Vesely S. Bilayer-micelle transition in phosphatidylcholine-sulfatide mixtures. Ital J Biochem. 1984;33(6):381–391. [PubMed] [Google Scholar]
- 18.Taylor CM, Marta CB, Bansal R, Pfeiffer S. The transport, assembly, and function of myelin lipids. In: Lazzarini R, editor. Myelin biology and disorders. New York: Elsevier Academic Press; 2003. pp. 57–88. [Google Scholar]
- 19.Oliveira RG, Maggio B. Compositional domain immiscibility in whole myelin monolayers at the air-water interface and Langmuir-Blodgett films. Biochim Biophys Acta. 2002;1561(2):238–250. doi: 10.1016/s0005-2736(02)00350-4. [DOI] [PubMed] [Google Scholar]
- 20.Maggio B, Borioli GA, Del Boca M, De Tullio L, Fanani ML, Oliveira RG, Rosetti CM, Wilke N. Composition-driven surface domain structuring mediated by sphingolipids and membrane-active proteins: above the nano- but under the micro-scale: Mesoscopic biochemical/structural cross-talk in biomembranes. Cell Biochem Biophys. 2007;50(2):79–109. doi: 10.1007/s12013-007-9004-1. [DOI] [PubMed] [Google Scholar]
- 21.Rosetti CM, Maggio B. Protein-induced surface structuring in myelin membrane monolayers. Biophys J. 2007;93(12):4254–4267. doi: 10.1529/biophysj.107.112441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosetti CM, Maggio B, Oliveira RG. The self-organization of lipids and proteins of myelin at the membrane interface. Molecular factors underlying the microheterogeneity of domain segregation. Biochim Biophys Acta. 2008;1778(7–8):1665–1675. doi: 10.1016/j.bbamem.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Schafer DP, Bansal R, Hedstrom KL, Pfeiffer SE, Rasband MN. Does paranode formation and maintenance require partitioning of neurofascin 155 into lipid rafts? J Neurosci. 2004;24(13):3176–3185. doi: 10.1523/JNEUROSCI.5427-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupree JL, Pomicter AD. Myelin, DIGs, and membrane rafts in the central nervous system. Prostaglandins Other Lipid Mediat. 2010;91(3–4):118–129. doi: 10.1016/j.prostaglandins.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Schnaar RL, Collins BE, Wright LP, Kiso M, Tropak MB, Roder JC, Crocker PR. Myelin-associated glycoprotein binding to gangliosides. Structural specificity and functional implications. Ann N Y Acad Sci. 1998;845:92–105. doi: 10.1111/j.1749-6632.1998.tb09664.x. [DOI] [PubMed] [Google Scholar]
- 26.Tait S, Gunn-Moore F, Collinson JM, Huang J, Lubetzki C, Pedraza L, Sherman DL, Colman DR, Brophy PJ. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. J Cell Biol. 2000;150(3):657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trapp BD, Quarles RH. Presence of the myelin-associated glycoprotein correlates with alterations in the periodicity of peripheral myelin. J Cell Biol. 1982;92(3):877–882. doi: 10.1083/jcb.92.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartsch U, Kirchhoff F, Schachner M. Immunohistological localization of the adhesion molecules L1, N-CAM, and MAG in the developing and adult optic nerve of mice. J Comp Neurol. 1989;284:451–462. doi: 10.1002/cne.902840310. [DOI] [PubMed] [Google Scholar]
- 29.Marbois BN, Faull KF, Fluharty AL, Raval-Fernandes S, Rome LH. Analysis of sulfatide from rat cerebellum and multiple sclerosis white matter by negative ion electrospray mass spectrometry. Biochim Biophys Acta. 2000;1484(1):59–70. doi: 10.1016/s1388-1981(99)00201-2. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki K, Andrews JM, Waltz JM, Terry RD. Ultrastructural studies of multiple sclerosis. Lab Invest. 1969;20(5):444–454. [PubMed] [Google Scholar]
- 31.Shroff SM, Pomicter AD, Chow WN, Fox MA, Colello RJ, Henderson SC, Dupree JL. Adult CST-null mice maintain an increased number of oligodendrocytes. J Neurosci Res. 2009;87(15):3403–3414. doi: 10.1002/jnr.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogawa Y, Rasband MN. Proteomic analysis of optic nerve lipid rafts reveals new paranodal proteins. J Neurosci Res. 2009;87(15):3502–3510. doi: 10.1002/jnr.21984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heffer-Lauc M, Viljetić B, Vajn K, Schnaar RL, Lauc G. Effects of detergents on the redistribution of gangliosides and GPI-anchored proteins in brain tissue sections. J Histochem Cytochem. 2007;55(8):805–812. doi: 10.1369/jhc.7A7195.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepherd MN, Pomicter AD, Velazco CS, Henderson SC, Dupree JL. Paranodal reorganization results in the depletion of transverse bands in the aged central nervous system. Neurobiol Aging. 2012;33(1):203, e13–e24. doi: 10.1016/j.neurobiolaging.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norton WT, Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21(4):749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- 36.Sato C, Larocca JN, Bálsamo N, Pasquini JM, Soto EF. Neonatal malnutrition in the rat affects the delivery of sulfatides from microsomes and their entry into myelin. Neurochem Res. 1985;10(2):179–189. doi: 10.1007/BF00964566. [DOI] [PubMed] [Google Scholar]
- 37.Sato C, Yu RK. Myelin galactolipid synthesis in different strains of mice. J Neurochem. 1987;49(4):1069–1074. doi: 10.1111/j.1471-4159.1987.tb09995.x. [DOI] [PubMed] [Google Scholar]
- 38.Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophot Internat. 2004;11(7):36–42. [Google Scholar]
- 39.Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86(2):209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 40.Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147(6):1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dupree JL, Mason JL, Marcus JR, Stull M, Levinson R, Matsushima GK, Popko B. Oligodendrocytes assist in the maintenance of sodium channel clusters independent of the myelin sheath. Neuron Glia Biol. 2004;(3):179–192. doi: 10.1017/S1740925X04000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marta CB, Taylor CM, Coetzee T, Kim T, Winkler S, Bansal R, Pfeiffer SE. Antibody cross-linking of myelin oligodendrocyte glycoprotein leads to its rapid repartitioning into detergent-insoluble fractions, and altered protein phosphorylation and cell morphology. J Neurosci. 2003;23(13):5461–5471. doi: 10.1523/JNEUROSCI.23-13-05461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor CM, Coetzee T, Pfeiffer SE. Detergent-insoluble glycosphingolipid/cholesterol microdomains of the myelin membrane. J Neurochem. 2002;81(5):993–1004. doi: 10.1046/j.1471-4159.2002.00884.x. [DOI] [PubMed] [Google Scholar]
- 45.Yap CC, Vakulenko M, Kruczek K, Motamedi B, Digilio L, Liu JS, Winckler B. Doublecortin (DCX) mediates endocytosis of neurofascin independently of microtubule binding. J Neurosci. 2012;32(22):7439–7453. doi: 10.1523/JNEUROSCI.5318-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson TE, Tillack TW. Organization of glycosphingolipids in bilayers and plasma membranes of mammalian cells. Annu Rev Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- 47.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochem. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 48.Babiychuk EB, Draeger A. Biochemical characterization of detergent-resistant membranes: a systematic approach. Biochem J. 2006;397(3):407–416. doi: 10.1042/BJ20060056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He M, Jenkins P, Bennett V. Cysteine 70 of ankyrin-g is s-palmitoylated and is required for function of ankyrin-g in membrane domain assembly. J Biol Chem. 2012;287(52):43995–44005. doi: 10.1074/jbc.M112.417501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren Q, Bennett V. Palmitoylation of neurofascin at a site in the membrane-spanning domain highly conserved among the L1 family of cell adhesion molecules. J Neurochem. 1998;70(5):1839–1849. doi: 10.1046/j.1471-4159.1998.70051839.x. [DOI] [PubMed] [Google Scholar]
- 51.Levental I, Lingwood D, Grzybek M, Coskun U, Simons K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc Natl Acad Sci U S A. 2010b;107(51):22050–22054. doi: 10.1073/pnas.1016184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drisdel RC, Green WN. Labeling and quantifying sites of protein palmitoylation. Biotechniques. 2004;36(2):276–285. doi: 10.2144/04362RR02. [DOI] [PubMed] [Google Scholar]
- 53.Pedraza L, Owens GC, Green LA, Salzer JL. The myelin-associated glycoproteins: membrane disposition, evidence of a novel disulfide linkage between immunoglobulin-like domains, and posttranslational palmitylation. J Cell Biol. 1990;111:2651–2661. doi: 10.1083/jcb.111.6.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arvanitis DN, Min W, Gong Y, Heng YM, Boggs JM. Two types of detergent-insoluble, glycosphingolipid/cholesterol-rich membrane domains from isolated myelin. J Neurochem. 2005;94(6):1696–1710. doi: 10.1111/j.1471-4159.2005.03331.x. [DOI] [PubMed] [Google Scholar]
- 55.Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Erb M, Flueck B, Kern F, Erne B, Steck AJ, Schaeren-Wiemers N. Unraveling the differential expression of the two isoforms of myelin-associated glycoprotein in a mouse expressing GFP-tagged S-MAG specifically regulated and targeted into the different myelin compartments. Mol Cell Neurosci. 2006;31:613–627. doi: 10.1016/j.mcn.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Vyas AA, Schnaar RL. Brain gangliosides: functional ligands for myelin stability and the control of nerve regeneration. Biochim. 2001;83(7):677–682. doi: 10.1016/s0300-9084(01)01308-6. [DOI] [PubMed] [Google Scholar]
- 58.Vyas AA, Patel HV, Fromholt SE, Heffer-Lauc M, Vyas KA, Dang J, Schachner M, Schnaar RL. Gangliosides are functional nerve cell ligands for myelin-associated glycoprotein (MAG), an inhibitor of nerve regeneration. Proc Natl Acad Sci USA. 2002;99(12):8412–8417. doi: 10.1073/pnas.072211699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohlschutter A, Herschkowitz NN. Sulfatide synthesis in neurons: a defect in mice with a hereditary myelination disorder. Brain Res. 1973;50:379–385. doi: 10.1016/0006-8993(73)90739-7. [DOI] [PubMed] [Google Scholar]
- 60.DeVries GH, Zmachinski CJ. The lipid composition of rat CNS axolemma-enriched fractions. J Neurochem. 1980;34:424–430. doi: 10.1111/j.1471-4159.1980.tb06613.x. [DOI] [PubMed] [Google Scholar]
- 61.Pernber Z, Molander-Melin M, Berthold CH, Hansson E, Fredman P. Expression of the myelin and oligodendrocyte progenitor marker sulfatide in neurons and astrocytes of adult rat brain. J Neurosci Res. 2002;69:86–93. doi: 10.1002/jnr.10264. [DOI] [PubMed] [Google Scholar]
- 62.Krämer EM, Klein C, Koch T, Boytinck M, Trotter J. Compartmentation of fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J Biol Chem. 1999;274(41):29042–29049. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- 63.Kim T, Pfeiffer SE. Myelin glycosphingolipid/cholesterolenriched microdomains selectively sequester the non-compact myelin proteins CNP and MOG. J Neurocytol. 1999;28:281–293. doi: 10.1023/a:1007001427597. [DOI] [PubMed] [Google Scholar]
- 64.Levental I, Grzybek M, Simons K. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochem. 2010;49(30):6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- 65.Esposito C, Scrima M, Carotenuto A, Tedeschi A, Rovero P, D'Errico G, Malfitano AM, Bifulco M, D'Ursi AM. Structures and micelle locations of the nonlipidated and lipidated C-terminal membrane anchor of 2',3'-cyclic nucleotide-3'-phosphodiesterase. Biochemistry. 2008;47(1):308–319. doi: 10.1021/bi701474t. [DOI] [PubMed] [Google Scholar]
- 66.Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem. 1999;274(6):3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 67.Krämer EM, Koch T, Niehaus A, Trotter J. Oligodendrocytes direct glycosyl phosphatidylinositol-anchored proteins to the myelin sheath in glycosphingolipid-rich complexes. J Biol Chem. 1997;272(14):8937–8945. doi: 10.1074/jbc.272.14.8937. [DOI] [PubMed] [Google Scholar]
- 68.Arvanitis DN, Yang W, Boggs JM. Myelin proteolipid protein, basic protein, the small isoform of myelin-associated glycoprotein, and p42MAPK are associated in the Triton X-100 extract of central nervous system myelin. J Neurosci Res. 2002;70(1):8–23. doi: 10.1002/jnr.10383. [DOI] [PubMed] [Google Scholar]