Abstract
The KCNE single-span transmembrane subunits are encoded by five-member gene families in the human and mouse genomes. Primarily recognized for co-assembling with and functionally regulating the voltage-gated potassium channels, the broad influence of KCNE subunits in mammalian physiology belies their small size. KCNE2 has been widely studied since we first discovered one of its roles in the heart and its association with inherited and acquired human Long QT syndrome. Since then, physiological analyses together with human and mouse genetics studies have uncovered a startling array of functions for KCNE2, in the heart, stomach, thyroid and choroid plexus. The other side of this coin is the variety of interconnected disease manifestations caused by KCNE2 disruption, involving both excitable cells such as cardiomyocytes, and non-excitable, polarized epithelia. Kcne2 deletion in mice has been particularly instrumental in illustrating the potential ramifications within a monogenic arrhythmia syndrome, with removal of one piece revealing the unexpected complexity of the puzzle. Here, we review current knowledge of the function and pathobiology of KCNE2.
Keywords: cardiac arrhythmia, potassium channel, sudden cardiac death, thyroid, transporter
Introduction
Ion channels are membrane-spanning proteins that facilitate rapid and often selective diffusion of aqueous ions down an electrical gradient and across a hydrophobic barrier. The ability of potassium channels to pass potassium ions while effectively excluding sodium ions, and vice versa for sodium channels, is essential for action potential propagation, and thus cellular excitability. This, in turn, facilitates processes such as neuronal signaling, skeletal muscle contraction and pumping of blood by the heart 1–3. Potassium channels are a particularly diverse group of ion channels, and within these the voltage-gated potassium (Kv) channels themselves boast a forty-strong gene family encoding the various α subunit isoforms 4.
The α, or pore-forming, subunits of Kv channels co-assemble into tetramers that are necessary and sufficient (with important caveats, see below) to generate K+-selective currents in response to membrane depolarization 5. Each α subunit possesses a voltage-sensing domain, a GYG selectivity filter sequence (rarely an alternate sequence is utilized, e.g., GFG for the hERG Kv channel6), and sequence elements that either prohibit co-assembly with α subunits outside of its subfamily, promote co-assembly with same-subfamily subunits, or both. The ability of some Kv α subunits to form heteromeric tetramers with other α subunits within the same subfamily increases the potential diversity of native Kv channels and in some cases appears obligate, e.g., heteromeric KCNQ2-KCNQ3 channels form the muscarinic-regulated M-current in much of the brain7.
As mentioned above, while Kv α subunit tetramers can form functional channels, whether and how often this minimal channel composition occurs in vivo is open to debate. Even when one expresses a single Kv α subunit gene to generate a current for heterologous expression studies, it may require endogenous proteins to function properly, a well-known example being the highly studied KCNQ1 α subunit, which requires calmodulin for correct folding, assembly and function8. More broadly, there are several families of proteins that appear to function primarily as regulatory or ancillary subunits for Kv channels. These can be separated into two types, cytosolic and transmembrane. The first to be discovered were the Kvβ subunits, cytosolic proteins that co-assemble with the cyotosolic domains of the Kv α subunits to modulate their trafficking and functional attributes. This regulation can include addition of a fast-inactivation domain that can plug the Kv channel pore after opening, causing rapid current decay9. Kvβ subunits also function as aldo-keto reductases9, 10, and binding of NADPH stabilizes Kvβ inactivation of Kv1 and Kv4 subfamily channels whereas NAPD+ binding destabilizes inactivation. Other notable cytosolic Kv channel ancillary subunits include the K+ channel interacting proteins (KChIPs) which, e.g., promote surface expression and slow inactivation of Kv4 channels11, and the K+ channel-associated proteins (KChAPs), which promote Kv2, Kv1.3 and Kv4.3 surface expression12.
Transmembrane Kv ancillary subunits include the dipeptidyl aminopeptidase-like (DPPX) subunits, “silent” α subunits, and KCNE subunits, which are the focus of this review. DPPX subunits were discovered to be Kv channel ancillary subunits using an unbiased proteomics approach to find proteins that bind to neuronal Kv4 α subunits to recapitulate the gating properties of native Kv4-generated currents13. They are single-pass transmembrane proteins that regulate Kv4 channel trafficking, membrane targeting and gating kinetics. Some Kv α subunits cannot form functional channels alone but can co-assemble with other Kv α subunits to form functional channels with distinct gating attributes. These silent subunits are typically classified as belonging to subfamilies Kv5, Kv6 and Kv9, are primarily recognized to regulate the widely expressed Kv2.1 α subunit, and may be required for smooth muscle function in the bladder and arteries14, 15.
KCNE2, the focus of this review, is a member of the five-strong human KCNE gene family, members of which each encode a single-pass membrane protein16. KCNE proteins can regulate most if not all known aspects of Kv channel biology, from channel biogenesis itself through to gating, selectivity and conductivity of functional channels at the membrane, and even channel internalization from the membrane17–20. KCNEs form heteromeric complexes with Kv α subunits, with the stoichiometry being 4α:2KCNE subunits21, 22 (quantified for KCNQ1-KCNE1, and still under debate as to whether flexible stoichiometry might occur23) (Figure 1A). While electrophysiological studies in vitro combined with biochemical tools and molecular biological approaches such as site-directed mutagenesis have provided much of what we know about the function of KCNE proteins, much of what we know about their native roles and the pathophysiological outcomes of their disruption has emerged after human and mouse genetics studies.
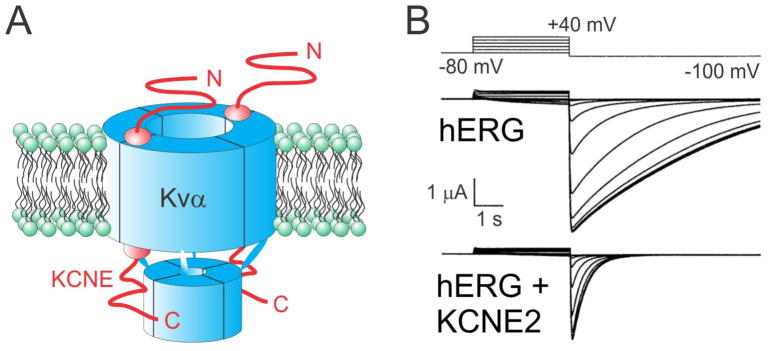
Figure 1. KCNE2 regulates hERG.
A. Cartoon of a Kvα-KCNE subunit complex in the plasma membrane (green) showing a 4:2 subunit stoichiometry.
B. Effect of KCNE2 on hERG in Xenopus oocyte expression studies, measured by two-electrode voltage clamp with 100 mM KCl bath solution. Voltage protocol shown at top. Adapted from 29. KCNE2 reduces hERG unitary and macroscopic conductance and accelerates its deactivation.
KCNE2 in the context of the KCNE gene family
The founding member of the KCNE gene family, KCNE1, was discovered when Takumi and colleagues injected fractionated rat kidney mRNA into Xenopus laevis oocytes. One fraction generated voltage-dependent, K+-selective currents measurable using two-electrode voltage-clamp electrophysiology24. The slow-activating Kv current that was generated by expression of isolated, cloned KCNE1 (originally named MinK or IsK) was recognized to be highly reminiscent of a mammalian cardiac Kv current termed IKs (slow activating K+ current). However, KCNE1 was unlike the other recently cloned Drosophila melanogaster Shaker Kv α subunits and their mammalian orthologs25, 26, and it was unclear how a single transmembrane domain subunit could give rise to K+-selective currents. The mystery was solved when it was discovered that KCNE1 actually activated an endogenous Xenopus oocyte Kv α subunit, KCNQ1 (originally termed KvLQT1) that was also present in human heart. This led to the realization that KCNE1 is actually a β or ancillary subunit for Kv channels27, 28.
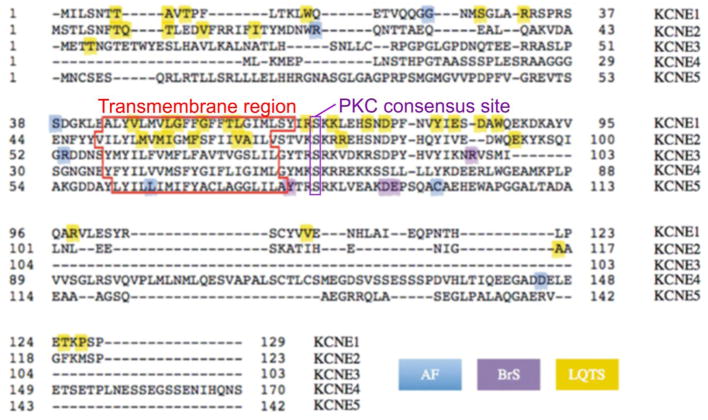
A decade after the discovery of KCNE1, by BLAST searching online databases with stretches of KCNE1 sequence that were known to be functionally important, Steve Goldstein discovered several expressed sequence tags (ESTs) that appeared to encode MinK-related peptides (MiRPs). We cloned three KCNE genes (KCNE2, 3 and 4) and screened for their functional partners using co-expression with an assortment of ion channel α subunits in the Xenopus oocyte expression system29. We found that the KCNE2 gene product, which we originally named MiRP1, alters the functional attributes of co-expressed hERG (KCNH2), a Kv α subunit essential for human ventricular myocyte repolarization29 (Figure 1B). This partnership was not completely unexpected, because KCNE1 had also just been found to regulate hERG, increasing its current density by a still unresolved mechanism30. The Renieri laboratory discovered and cloned an additional member, KCNE1-like or KCNE1-L (now more commonly referred to as KCNE5) 31 around the same time as our initial report on cloning of KCNE2, KCNE3 and KCNE4 genes was published29. The KCNE genes actually share comparatively little homology or identity with one another, although all exhibit a single TM span and a confirmed or putative PKC phosphorylation site in the intracellular, membrane proximal region (Figure 2). KCNE2 appears to have arisen from a gene duplication event, because human KCNE2 is located on chromosome 21q22.1, about 79 kb from KCNE1 and in opposite orientations. The open reading frames of KCNE1 and KCNE2 share 34% identity29.
Figure 2. Sequences and disease associations of the human KCNE family.
Human KCNE protein sequences shown with features highlighted. Disease associations color-coded: AF, atrial fibrillation; BrS, Brugada Syndrome; LQTS, Long QT Syndrome. Adapted from a figure prepared by Dr. Shawn Crump in 89.
Each KCNE isoform is now known to have the capacity to regulate more than one type of Kv α subunit17, and many Kv α subunit isoforms can be regulated by more than one KCNE isoform (perhaps simultaneously). For example, all five known members of the human KCNE family can regulate the KCNQ1 α subunit, with a diverse array of functional outcomes4. It is postulated that all five types of bipartite KCNQ1-KCNE complexes may occur in human heart, contributing to current diversity and regulation, but that has not been confirmed4, 32. In addition, it has been suggested that tripartite, KCNQ1-KCNE1-KCNE2 complexes contribute to native cardiac IKs33.
KCNE2 in the stomach
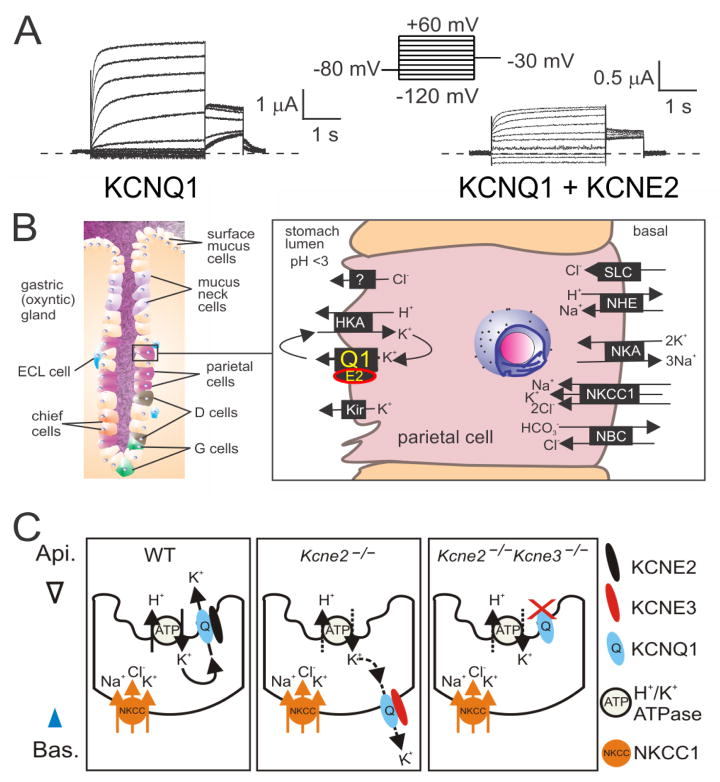
Discovery of the gastric role of the KCNQ1 α subunit was a surprise made possible by mouse genetics. Lee and colleagues found that Kcnq1−/− mice exhibited gastric hyperplasia, and that this stemmed from an inability to secrete gastric acid34. One of the aspects that made this so surprising was that KCNQ1 forms voltage-gated channels how could it function efficiently in a non-excitable cell such as the parietal cell, which secretes gastric acid through its apically located H+/K+-ATPase? Several subsequent studies implicated regulation of KCNQ1 by KCNE2, which had recently been found to impart striking changes in gating attributes upon KCNQ135–39. KCNQ1-KCNE2 channels form constitutively active channels, probably by virtue of left-shifted voltage dependence of activation and greatly slowed deactivation (Figure 3A). Although peak macroscopic currents through KCNQ1-KCNE2 are much smaller than for KCNQ1 channels lacking co-assembled KCNE subunits, the mixed complexes remain open at hyperpolarized voltages and do not significantly inactivate, so can easily operate at the mildly negative membrane potentials visited by parietal cells (around −40 to −20 mV).
Figure 3. KCNQ1 and KCNE2 form constitutively active gastric K+ channels.
A. Effects of KCNE2 on KCNQ1 in Xenopus oocyte expression studies measured by two-electrode voltage clamp with 4 mM KCl bath solution. Voltage protocol shown at top. KCNE2 greatly slows KCNQ1 deactivation and/or left-shifts its voltage dependence of activation, and decreases current density.
B. The role of KCNQ1-KCNE2 in parietal cells. Adapted from 4. D cell, somatostatin-producing cell; E2, KCNE2; ECL, enterochromaffin-like; G cell, Guard cell; HKA, H+/K+-ATPase; Kir, inward rectifier K+ channels; NBC, Na+/HCO3− co-transporter; NHE, sodium/hydrogen exchanger; NKA, Na+/K+-ATPase; NKCC1, Na+/K+/2Cl− co-transporter 1; Q1, KCNQ1; SLC, solute carrier transporter.
C. KCNE3 upregulation caused by Kcne2 gene deletion rewires parietal cells. In the absence of KCNE2, KCNE3 traffics KCNQ1 to the basolateral membrane, where it cannot perform its normal function of allowing K+ back into the stomach lumen. When both Kcne2 and KCne3 are germline-deleted, KCNQ1 defaults to the apical membrane but does not function properly without KCNE2. From 4.
Crucially, KCNE2 also imparts upon KCNQ1 the property of being activated by low extracellular pH, whereas KCNQ1 without any KCNEs is inhibited by external protons40. This enables KCNQ1-KCNE2 to function as an apical K+ recycling conduit in parietal cells, returning to the stomach lumen K+ ions that were swapped for protons by the gastric H+/K+-ATPase to acidify the stomach (Figure 3B).
When we generated the Kcne2−/− mouse line, the most obvious pathology was that the stomachs of the mice were massively hyperplastic41. By one year of age, Kcne2 deletion causes an 8-fold increase in stomach mass. The stomach wall thickens several fold and develops cysts, in a condition termed gastritis cystica profunda42. This condition is sometimes observed in people, most commonly following gastric surgery, and it predisposes to gastric cancer. We also found that KCNE2 expression is disrupted and reduced in human gastric carcinoma and adenocarcinoma42. After we had discovered this condition in Kcne2−/− mice, a group in Japan found that in areas of cysts in this condition in a human patient with gastric adenocarcinoma, KCNE2 expression is reduced43. Impaired gastric acid secretion leads to bacterial overgrowth and inflammation, which can develop into metaplasia and gastric cancer, and we observed neoplasia in Kcne2−/− mouse gastric tissue42. However, loss of gastric acid may not be the whole story, because it was previously shown that KCNE2 downregulation can increase human gastric cancer cell proliferation in vitro, in the absence of effects on stomach pH. In that study and in our in vivo study of Kcne2 deletion, increased nuclear cyclin D1 localization was observed42, 44. Together these data suggest KCNE2 can directly influence the cell cycle; whether this involves regulation of a channel, and which one, are still unknown.
We were astonished at how severely Kcne2 deletion affected gastric acid secretion. Kcne2−/− mice are achlorhydric, with a resting gastric pH of 6.5 and no noticeable lowering of stomach pH when stimulated with histamine. Using pH-sensitive dyes to dynamically monitor pH in individual parietal cells ex vivo, we found that proton-loaded parietal cells from Kcne2−/− mice were unable to restore their pH which remained essentially constant upon histamine or carbachol stimulation, after cessation of proton loading in contrast, wild-type parietal cells restored their pH at a rate of almost 0.1 pH/minute41.
Our electron microscopy studies revealed that parietal cell morphology, and in particular the secretory canaliculus, was severely disrupted by Kcne2 deletion. However, in Kcne2+/− mice, parietal cell morphology appeared normal and yet there was still a ~50% disruption of gastric acid secretion. This suggested that the mechanism for loss of gastric acid secretion in Kcne2−/− mice was not secondary to disrupted cellular morphology, but vice versa41.
Conducting further studies on the mechanisms of KCNQ1 regulation by KCNE2 in parietal cells, we discovered that when KCNE2 is absent, KCNQ1 aberrantly traffics to the basolateral side of the cell, where it cannot replenish stomach luminal K+. After finding that parietal cell KCNE3 expression, which is typically very low, was upregulated by Kcne2 deletion, we constructed double-knockout Kcne2−/−Kcne3−/− mice and examined KCNQ1 localization. In double knockouts, KCNQ1 is apically expressed in parietal cells, as in wild-type45. Thus, KCNE2 is not normally required for apical KCNQ1 targeting, but in the absence of KCNE2, upregulated KCNE3 misdirects KCNQ1 basolaterally (Figure 3C). This reflects the situation further down the digestive tract; in the normal intestine, basolateral KCNQ1-KCNE3 channels regulate cAMP-stimulated chloride secretion46. Is this remodeling part of, or at least related to, other forms of gastric metaplasia also characterized by adoption of intestinal characteristics?
Interestingly, the gastric phenotype of Kcne2−/−Kcne3−/− mice was worse than that of single-knockout Kcne2−/− mice. Thus, even when KCNQ1 is apically targeted, it cannot perform its role in supporting the apical proton pump without co-assembled KCNE245. This probably reflects both the loss in constitutive activation and the protection from inhibition by extracellular low pH that KCNE2 provides. While there may be other, non pH-related mechanisms for the more severe gastric hyperplasia in Kcne2−/−Kcne3−/− mice compared to Kcne2−/− mice, we suggest that basolateral KCNQ1-KCNE3 channels in Kcne2−/− mice, while not able to return K+ directly to the stomach, can facilitate K+ exit from parietal cells into the bloodstream, partly mitigating the loss of downhill K+ gradient from stomach lumen to parietal cell47. This may explain the slightly less severe loss of gastric acidification in Kcne2+/−Kcne3−/− mice compared to Kcne2+/−Kcne3+/+ mice.
Although loss of KCNE2 expression has been observed in human gastric cancers and around gastric cysts, association between human hypochlorhydria and KCNE2 polymorphisms have not yet been reported. However, human KCNQ1 mutations have been associated with impaired gastric acid secretion, iron deficiency anemia stemming from hypochlorhydria and gastric cancer. In one family, longer QT intervals correlated with higher gastrin levels, an indicator of poorer gastric acidification48–50. It is likely, as with Long QT syndrome, that given enough time and patients, KCNE2 polymorphisms associated with at least subclinical hypochlorhydria will emerge, and possibly rarely associate with familial gastric cancer particularly if homozygous recessive cases of KCNE2 loss-of-function mutations occur (as is observed for hearing loss in LQTS patients with KCNE1 mutations and Jervell and Lange-Nielsen syndrome). We also discovered, unsurprisingly because low gastric pH is required for efficient iron absorption, that Kcne2−/− mice exhibit iron-deficiency anemia47, a finding later confirmed by others51. Again, it will be of interest to see whether human KCNE2 polymorphisms, as with KCNQ1, correlate with anemia.
KCNE2 in the thyroid
While breeding the Kcne2−/− mouse line, we noticed that deviations from the typical Kcne2+/− x Kcne2+/− crosses had effects not entirely explained by pup genotype. Thus, pups from Kcne2−/− dams, regardless of the genotype of the sire or even the pups themselves, exhibited slow development, alopecia, cardiomegaly and reduced cardiac ejection fraction (a measure of pumping ability). Litters from Kcne2−/− dams were also on average half the size of those from heterozygous or wild-type dams. This effect was explained when we discovered that Kcne2 deletion caused hypothyroidism, especially in gestating or lactating dams52. Accordingly, surrogacy of Kcne2−/− pups with wild-type dams starting at P1, or thyroid hormone supplementation of dams during gestation and pups or dams during lactation, prevented the alopecia and dwarfism and partially alleviated the cardiac hypertrophy. Conversely, surrogacy of Kcne2+/+ pups by Kcne2−/− dams caused these phenotypes. The dams’ genotype was so important because the hypothyroidism impaired milk ejection, and because the little milk that was delivered by Kcne2−/− dams was deficient in thyroxine (T4). The milk ejection defect was acutely ameliorated by oxytocin injection52.
We found that KCNQ1-KCNE2 channels are expressed on the basolateral side of thyroid epithelial cells, and that thyroid-stimulating hormone (TSH) stimulates protein expression of KCNQ1 and KCNE2, and increases in the FRTL5 thyroid epithelial cell line a K+ current with the electrophysiological and pharmacological characteristics of KCNQ1-KCNE2. Using positron emission tomography to directly visualize 124I movement in lactating dams and the pups that fed from them, we found that Kcne2 deletion caused a defect in thyroid iodide accumulation in dams and pups, and in wild-type pups surrogated by Kcne2−/− dams52.
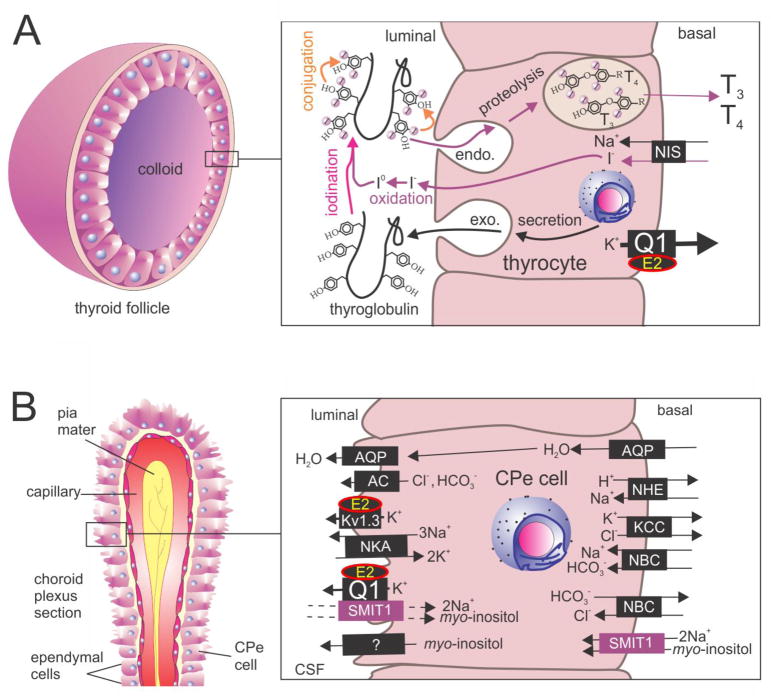
We subsequently found that KCNQ1-KCNE2 channels are specifically required for thyroid iodide uptake by the sodium/iodide symporter (NIS) as opposed to organification of iodide once it has been taken up (Figure 4A) 53. The next challenge will be to elucidate the mechanism by which KCNQ1-KCNE2 assists NIS in iodide uptake; in contrast to the gastric H+/K+-ATPase, NIS does not utilize K+ in its transport cycle, instead it utilizes the downhill Na+ gradient to electrogenically bring in one iodide ion for every two sodium ions54.
Figure 4. KCNQ1 and KCNE2 form constitutively active thyroid and choroid plexus K+ channels.
A. KCNQ1-KCNE2 in thyroid epithelial cells. Adapted from 86. E2, KCNE2; Q1, KCNQ1; NIS, sodium/iodide symporter; T3, triiodothyronine; T4, thyroxine.
B. Model of the role of KCNQ1-KCNE2 in choroid plexus epithelial cells. Updated from 86. AC, anion channel; AQP, aquaporin; CPe, choroid plexus epithelium; E2, KCC, K+Cl− co-transporter; KCNE2; Kir, inward rectifier K+ channels; NBC, Na+/HCO3− co-transporter; NHE, sodium/hydrogen exchanger; NKA, Na+/K+-ATPase; Q1, KCNQ1; SMIT1, Na+-dependent myo-inositol transporter.
KCNE2 and the discovery of K+ channel-solute transporter complexes
We noticed that Kcne2−/− mice are somewhat prone to handling-induced seizures, and found that they have an increased susceptibility to pentylenetetrazole-induced seizures, showing shorter latency to first seizure, increased seizure severity and increased mortality compared to their wild-type littermates55. KCNE2 is reportedly expressed in neuronal populations, as judged by in situ hybridization56, but we could not detect KCNE2 protein in mouse neurons by immunohistochemistry57. Although this may be an antibody sensitivity issue, and further studies on the potential neuronal roles of KCNE2 are worth consideration, we did find one area of the brain in which KCNE2 is highly enriched: the choroid plexus epithelium. This epithelium lines the ventricles of the brain, constitutes the blood:CSF barrier, and, indeed, is the primary site of cerebrospinal fluid (CSF) production and secretion. We found that KCNE2 is highly expressed at the apical side of the choroid plexus epithelium, facing the CSF, and it is readily detected there with rabbit polyclonal antibodies, with Kcne2 knockout tissue providing a clear negative control57.
Electrophysiological analyses comparing the effects of Kcne2 deletion on choroid plexus epithelial cells revealed alteration of currents sensitive to XE991 and to margatoxin, but not dendrotoxin, suggestive of regulation of a KCNQ isoform and of Kv1.3, but not Kv1.1, by KCNE2 in the choroid plexus57. This was confirmed by immunofluorescence (identifying the KCNQ isoform as KCNQ1) and also by subsequent co-immunoprecipitation studies55. K+ channels on the apical side of the choroid plexus epithelium have been implicated in controlling CSF K+ and Cl− and we indeed found that Kcne2 deletion hyperpolarized the choroid epithelial cells by 7 mV, and also that CSF chloride levels were marginally increased by Kcne2 deletion. This did not seem sufficient to generate increased seizure predisposition, however, and CSF potassium concentration and pH, two criteria that can dictate seizure susceptibility, were not altered in Kcne2−/− mice57.
We therefore took an unbiased approach to investigate the possible complexes of Kcne2 deletion on CSF composition. Using mass spectrometry based metabolomics, we looked for possible changes in CSF metabolites. Incredibly, this analysis revealed only one component that was both unambiguously assignable to a known metabolite and differentially expressed between genotype: myo-inositol55. The cyclic polyol myo-inositol is of immense biological importance, as one of the principle osmolytes in the body, and also as a precursor for signaling molecules including phosphatidylinositol phosphates. These include PIP2, which happens to regulate the function of a great many membrane proteins, notably ion channels and markedly, KCNQ139.
CSF myo-inositol levels are highly regulated, and myo-inositol is actively concentrated in the CSF from the blood, probably largely across the choroid plexus epithelium. It was previously suggested that this occurs via basolateral uptake from the blood through sodium-dependent myo-inositol transporters, probably SMIT1, encoded by SLC5A3, although visualization of SMIT1 protein in the choroid plexus had not been achieved58, 59. The mechanism by which myo-inositol was then transported across the apical side of the choroid plexus epithelium and into the CSF itself was not determined. We found that SMIT1 is, indeed, expressed at the basolateral side, but unexpectedly that it is also expressed at the apical side. This result was supported by use of Slc5a3−/− mouse choroid plexus epithelium and kidney tissue as a negative control, and wild-type kidney as a positive control. Expression of SMIT1 at the apical side tallied with our previous findings for KCNE2, and we discovered that SMIT1, KCNE2 and KCNQ1 form complexes together, the first report in the literature of a K+ channel-solute transporter complex (Figure 4B) 55.
Reconstituting these complexes in Xenopus oocytes, we found that KCNQ1 and SMIT1 augment one another activity, doubling both K+ current and myo-inositol uptake. However, when KCNE2 was included in the complex, the channel function was double compared to KCNQ1-KCNE2 alone, but the myo-inositol transport activity was strongly inhibited. Transporter function was also robustly impaired by co-expression of a constitutively open KCNQ1 variant bearing an R231A voltage sensor charge neutralization that we previously found to hold open the pore, probably by locking the S4 helix in the activated state regardless of membrane potential55. This suggested that it was the constitutively active nature of KCNQ1-KCNE2 channels, and not their low conductance compared to homomeric KCNQ1, that inhibited SMIT1 activity. This is counterintuitive because SMIT1 operates by using the downhill sodium gradient to bring in myo-inositol along with sodium, an electrogenic transport that would be expected to be more efficient if there were a nearby channel permitting egress of cations, even if they were potassium ions instead of sodium. We conclude from this, together with biochemical evidence of physical complex formation, that SMIT1 is sensitive to conformation of KCNQ1-KCNE2, and something about the conformation adopted by KCNQ1 either with the R231A mutant or with co-assembled KCNE2, inhibits SMIT1 myo-inositol efflux activity. KCNQ1-specific inhibitors also inhibit co-assembled SMIT1 activity; this suggests that as would be expected, K+ efflux through KCNQ1 does help SMIT1 bring in more substrate, but only when the KCNQ1 conformation is amenable. We also found that KCNQ4 does not co-assemble with SMIT1, at least in Xenopus oocytes, and that KCNQ4 does not alter SMIT1 activity (or vice versa). This suggests that merely having any Kv channel in the cell is not sufficient to augment SMIT1 activity, and that the channel-transporter crosstalk requires at least the proximity afforded by physical interaction.
Coming back to the seizures in Kcne2−/− mice, the dual basolateral and apical localization of choroid plexus SMIT1, the apical location of SMIT1-KCNQ1-KCNE2 complexes, and the finding that KCNE2 inhibits transport activity in these complexes, suggests a model in which these apical channel transporter complexes may be responsible for regulating CSF myo-inositol to ensure it does not get too high, or at times when variations in composition might be needed for neuroprotection or another reason. Removal of KCNE2 from these complexes might result in overactivity, and thus too much myo-inositol being removed from the CSF, hence the reduction in CSF myo-inositol in the Kcne2−/− mice. Another possible model to consider is that these complexes are actually responsible for myo-inositol efflux from the choroid plexus epithelium, and that Kcne2 deletion impairs the ability of these complexes to facilitate myo-inositol efflux from the cell into the CSF; this would require a radical functional versatility of SMIT1 to be endowed by channel co-assembly, and although attractive is not currently specifically supported by any experimental data. KCNQ1 also forms complexes with SMIT2, resulting in KCNQ1 inhibition and no discernible change in SMIT2 transport activity, although, again, R231A-KCNQ1 inhibits SMIT2 transport activity55. We do not yet know the physiological significance, if any, of SMIT2-KCNQ1 complexes, an area of ongoing research in the author’s lab.
KCNE2 in the heart
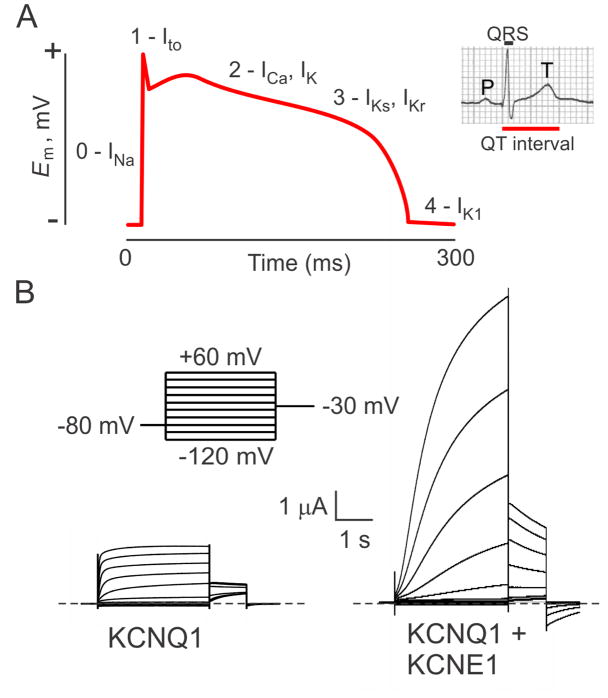
The prior sections describe the importance of KCNE2 in various epithelia, but when we originally cloned it we were focused on its potential roles in the heart29. Rhythmic cardiac contraction relies upon exquisite timing and tight regulation of the duration and morphology of cardiac myocyte action potentials, requiring the concerted efforts of several different classes of potassium channel. In human ventricular myocytes, the delayed rectifier currents IKr (generated by hERG) and IKs (generated by KCNQ1) are the main repolarizing currents. These unusual Kv currents repolarize cardiomyocytes after a plateau phase that lasts several hundred milliseconds (Figure 5A) 28, 60, 61.
Figure 5. KCNQ1 and the human ventricular myocyte action potential.
A. Idealized ventricular action potential showing when specific ionic currents are influential. Inset, the QT interval on a human body surface electrocardiogram. Adapted from 89.
B. Effect of KCNE1 on KCNQ1 in Xenopus oocyte expression studies measured by two-electrode voltage clamp with 4 mM KCl bath solution. Voltage protocol shown upper left. KCNE1 slows KCNQ1 activation, right-shifts the voltage dependence of activation, and increases unitary conductance.
To achieve the slow activation required for the lengthy cardiac action potentials of human ventricular myocytes, KCNQ1 forms complexes with at least one type of KCNE subunit, KCNE1 (Figure 5B) 28. KCNQ1 may also be regulated by KCNE2 in the heart, although detection of the “background” current that KCNQ1-KCNE2 complexes generate has yet to be definitively reported in cardiomyocytes. This lack of detection could very well result from the relatively small conductance exhibited by KCNQ1-KCNE2, and the fact that it would likely coexist with other KCNQ1-containing complexes with similar (although not identical) pharmacologic characteristics, making molecular identification challenging. In addition, mixed KCNQ1-KCNE1-KCNE2 or other combinations may occur, further complicating the issue33.
Our initial screens of KCNE2 activity revealed that it modulates hERG currents when co-expressed either in Xenopus oocytes or in CHO cells. hERG-KCNE2 currents were, macroscopically, 40% smaller than those of homomeric hERG, and this was recapitulated at the single channel level. KCNE2 also accelerated hERG deactivation (Figure 1B) 29. Sequencing of the DNA from white blood cells isolated from individuals with cardiac arrhythmias revealed several KCNE2 mutations in people with inherited Long QT syndrome, which manifests as prolongation of the electrocardiogram QT interval (Figure 5A) and is an indicator of impaired ventricular repolarization. These KCNE2 gene variants were not found in the asymptomatic population and caused loss of function of heterologously expressed hERG-KCNE2 channels29.
One of the defining features of hERG is that it is especially sensitive to drug block, predisposing to drug-induced arrhythmia61. hERG channels are inhibited by a large range of structurally diverse compounds because of unique features of hERG gating and also hydrophobic residues lining the pore that do not appear in other Kv channels62, 63. We found that some KCNE2 polymorphisms increase sensitivity of hERG-KCNE2 channels to inhibition by the pharmacological agents that precipitated the arrhythmias in the individuals harboring the polymorphism, a crucial piece of evidence that hERG-KCNE2 channels exist in human ventricles29. Another piece of evidence for KCNE2 modulation of hERG in human ventricles emerged when one of our geneticist colleagues found a T10M KCNE2 mutation in a young woman who had ventricular fibrillation possibly related to electrolyte imbalance after running the New York marathon. This individual had historic episodes of auditory-induced syncope, an event almost exclusively linked to hERG mutations in other populations, but both her copies of hERG were normal64. In addition, another group was able to transfer increased sensitivity to IKr block by macrolide antibiotics to the atria of pigs by atrial-specific adenovirus-mediated expression of Q9E-KCNE265. This polymorphism, found in 3% of African-Americans, was detected in a patient with clarithromycin-induced Long QT syndrome, and we found that it increases sensitivity of hERG-KCNE2 channels to block by clarithromycin29. Despite all this evidence and other independent reports66–69, there is still some resistance in the field to acceptance of hERG modulation in the heart by KCNE2.
We investigated the cardiac roles of KCNE2 in the mouse by generating Kcne2 null mice and performing an array of functional and biochemical analyses70. In adult mouse ventricles, ventricular myocyte action potentials are dramatically shorter than those of human heart, reflecting a 5–10-fold higher heart rate in the mouse compared to man. IKr is absent, while IKs may be weakly expressed and is difficult to detect and is not a major repolarizing current in mouse ventricles. Therefore, study of the mouse heart does not help us understand the importance of hERG-KCNE2 or KCNQ1-KCNE2 complexes in the heart. Instead, adult mouse ventricular repolarization is primarily orchestrated by the rapidly activating and inactivating Ito, which is generated by Kv4 α subunits, and the rapidly activating, slowly inactivating IKslow, which is generated by Kv1.5 and Kv2.171.
Although mouse and human hearts are fundamentally different with respect to the K+ currents that dictate their action potential duration and morphology, several studies have demonstrated that the same KCNE isoforms modulate myocardial repolarization of either species, because each KCNE isoform can associate with and functionally regulate multiple types of Kv channel70, 72. Thus, just as KCNE2 gene variants associate with human Long QT syndrome (LQTS), Kcne2 deletion in mice also delays ventricular repolarization70. In adult mouse ventricles, KCNE2 co-assembles with both Kv4.2 and Kv1.5, augmenting their currents. Kv4.2 was previously discovered as a partner for KCNE2 in vitro, and it was found that KCNE2 slows its inactivation and increases peak currents73. KCNE2 is required for efficient trafficking and/or retention of Kv1.5 at the intercalated discs of mouse ventricular myocytes, and given that KCNE2 does not appear to change its gating properties, loss of this trafficking effect may be the sole mechanism underlying the reduced Kv1.5 current in Kcne2−/− mouse ventricles. While 4-month-old Kcne2−/− mice did not exhibit baseline prolongation of QTc (QT interval corrected for heart rate), when provoked by sevoflurane anesthesia, Kcne2 deletion did impair ventricular repolarization in mice of this age, at a dose that did not affect Kcne2+/+ mouse QTc70. Interestingly, cardiac KCNE2 is highly regulated by direct genomic action of estrogen, with KCNE2 transcript expression being increased ten-fold at the end of gestation in mice. This is suggested to augment Ito and therefore help compensate for other, QT-prolonging effects of estrogen at the culmination of pregnancy74.
Extracardiac consequences of Kcne2 deletion and their effects on the heart
In a more recent study, we recapitulated the lack of effect of Kcne2 deletion on baseline QTc at 4 months, but found that in older mice (7 months) Kcne2 deletion did cause QTc prolongation47. Monogenic cardiac arrhythmia syndromes are typically considered solely from the perspective of direct disruption of cardiac myocyte ion currents generated by channel complexes containing the mutated gene. However, this is a narrow view considering that most of the 25 genes associated with ventricular arrhythmia syndromes that predispose to sudden cardiac death75 encode cardiac ion channel subunits or proteins that regulate them, but which are also expressed in other tissues in addition to the heart. This suggests the possibility of a complexity to arrhythmia syndromes that cannot be fully appreciated by focusing just on cardiac myocytes, and it is also a very difficult hypothesis to test in human populations because of the inherent genetic diversity within these populations, and also variations in environment, lifestyle and other risk factors. In fact, these variations are often eliminated by excluding individuals from arrhythmia cohorts if they exhibit, for example, structural heart disease, diabetes, or other chronic extracardiac conditions. But what if the same gene variant causes or predisposes to multiple pathologies in different tissues?
In a single colony of C57BL/6 laboratory mice, which is genetically more homogeneous than a typical human population, and in which environment and nutrition can be controlled, and age- and sex-matched littermates directly compared, Kcne2 deletion causes a battery of extracardiac consequences that can impact cardiac structure and function47. First, as described above, Kcne2 deletion causes hypothyroidism most prominently in pups from Kcne2−/− dams and this alters cardiac structure and impairs contractility52. Second, also described above, Kcne2 deletion increases seizure susceptibility55. In other mouse models, and in human cases of Sudden Death in Epilepsy (SUDEP), it is suggested that seizures can trigger fatal arrhythmias by disrupting neural control of heart rhythm76.
Also in Kcne2−/− mice, parietal cell KCNQ1 mis-trafficking diverts K+ into the blood, causing arrhythmogenic hyperkalemia47. The achlorhydria caused by loss of KCNE2 from KCNQ1-KCNE2 complexes causes iron-deficiency anemia, which in human populations can contribute to myocardial ischemia. Kcne2−/− mice also exhibit chronic stimulation and steatosis of the adrenal glands, and elevated Angiotensin II, which appears to contribute to cardiac gene remodeling from an early age in Kcne2−/− pups. Kcne2 deletion also causes dyslipidemia, glucose intolerance and fasting hypoglycemia47. While not all the underlying mechanisms for these conditions are known, some or all of them may contribute to increased likelihood of cardiac ischemia in aging mice, and potentially also in people with KCNE2 gene variants, although this would be expected to be much less severe unless accompanied by other risk factors that acted synergistically. In mice, Kcne2 deletion increases vulnerability to ventricular fibrillation occurring during experimentally imposed post-ischemic reperfusion, and predisposes to sudden cardiac death under these conditions. Finally, fasting of Kcne2−/− mice sends their blood sugar down and their blood K+ even higher than at baseline, and predisposes to atrioventricular block after experimentally imposed post-ischemia reperfusion47. Loss of KCNE2 from cardiomyocyte channel complexes is almost certainly a major factor in these arrhythmias, but so, also, other factors appear to play a role - at least in the mouse. Interestingly, recent population genetics studies have indicated that KCNE2 polymorphisms are associated with lung dysfunction, early-onset myocardial infarction, and atherosclerosis77–79 could some of these relate to the pathologies we observe in Kcne2−/− mice?
Conclusions and other roles for KCNE2
In addition to the many native functions described above for KCNE2, most of which appear to involve regulation of KCNQ1, KCNE2 has some more exotic capabilities. Not limited to Kv channels, KCNE2 can regulate the hyperpolarization-activated, cyclic nucleotide-gated (HCN) pacemaker channels, which are essential for setting cardiac rhythm and are enriched in the Purkinje fibers and sinoatrial myocytes. Indeed, some pathogenic KCNE2 mutations may act by disrupting function of KCNE2-HCN complexes80–82. Furthermore, despite KCNE2 expression in the brain being difficult to detect by immunohistochemistry, we found that Kcne2 deletion also impairs HCN channel function in cortical-thalamic relay neurons, suggesting the possibility of HCN-KCNE2 complexes in mammalian brain83. KCNE2 can also reportedly regulate the cardiac voltage-gated calcium channel, Cav1.2, forming complexes with reduced activity compared to channels lacking KCNE2, possibly mediated through the Cav1.2 N-terminal inhibitory module. The inhibitory effect was increased by the atrial fibrillation-associated KCNE2 mutation, R27C84.
KCNE2 can also regulate Kv channels that are expressed in the brain, but we do not yet know whether these complexes occur in vivo, either. We discovered that KCNE2 has striking effects on N-type-inactivating Kv channels, which form rapidly decaying currents because of open pore block by an N-terminal ball domain physically occluding the pore after activation. We found that KCNE2 co-assembles with Kv1.4 and Kv3.4, both of which are N-type α subunits, and retains them intracellularly, preventing their surface activity. These retained complexes could be rescued by same-subfamily delayed rectifier (slow-inactivating) α subunits, and the resulting tripartite complexes traveled to the surface to pass currents. Thus, Kv3.1 or Kv3.2 rescued Kv3.4-KCNE2 complexes, whereas Kv1.1 rescued Kv1.4-KCNE2 complexes18, 19, 85. What could be the reason for this type of complexity? N-type inactivation represents a powerful regulating force to determine action potential duration and frequency. Therefore, it is important to control this process. Channels formed by homomeric assemblies of e.g., Kv3.4 are especially rapidly inactivating because they possess four inactivating domains (1 for each α subunit in the tetramer), only one of which is required to block the pore. Channels formed by, e.g., two Kv3.4 and two Kv3.1 α subunits, dictated by KCNE2 retaining Kv3.4 until Kv3.1 came along to rescue surface expression, would exhibit roughly half the inactivation rate of homomeric Kv3.4, because with only two inactivation domains they have half the likelihood of an inactivation domain binding the pore at a given moment. KCNE2 (and KCNE1) can therefore dictate α subunit composition of Kv channels - a nice example of the tail wagging the dog86 - while KCNE1 (and possibly KCNE2, although this has not to our knowledge been reported) also brokers clathrin-mediated, dynamin-dependent endocytosis of KCNQ1, a process used to regulate IKs density and enhanced by PKC phosphorylation of KCNE120, 87. The established and postulated roles for KCNE2 in channel trafficking are summarized in Figure 6.
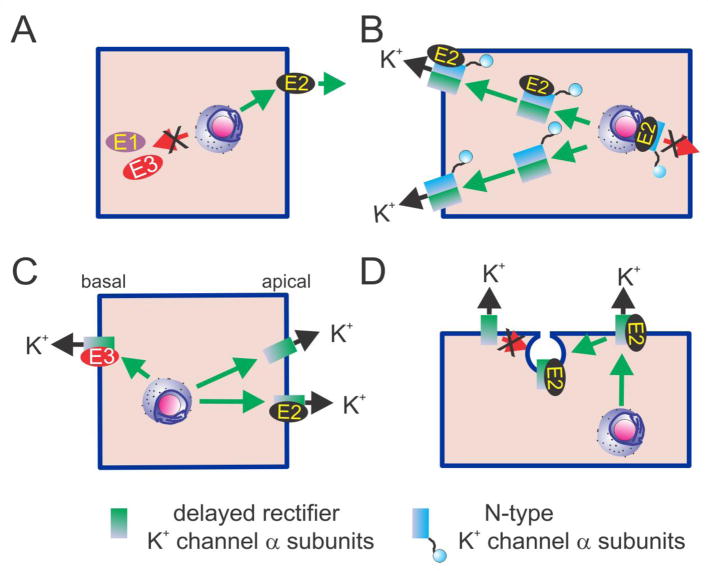
Figure 6. Role of KCNE2 in determining Kv α subunit trafficking and subunit composition.
A. KCNE2 (E2) can reach the plasma membrane without the assistance of α subunit partners, whereas KCNE1 (E1) and KCNE3 (E3) require them17.
B. KCNE2 (E2) can retain N-type inactivating α subunits (Kv1.4, Kv3.3 and Kv3.4) in the Golgi and/or endoplasmic reticulum, preventing surface expression of homomeric N-type channels. Same-subfamily delayed-rectifier α subunits can rescue the N-type α subunits, ensuring mixed-α complexes reach the cell surface. It is not clear whether KCNE2 travels to the surface within these mixed complexes (upper left)18,19.
C. In some polarized cell types, KCNEs can dictate α subunit localization. In parietal cells, KCNQ1 traffics to the apical surface alone or with KCNE2 (E2), but if KCNE3 (E3) is expressed in the absence of KCNE2, the resultant KCNQ1-KCNE3 channels travel to the basolateral membrane45.
D. KCNE2 (E2) and KCNE1 (not shown) can mediate forms of α subunit turnover not necessarily observed for the α subunit alone. KCNE2 accelerates hERG protein degradation68, possibly involving increased hERG internalization from the membrane.
KCNE2 carries out essential functions in a diverse set of tissues - despite being a small, single-transmembrane protein it exerts wide influence because of its ubiquitous expression and partnering promiscuity. Proteins such as KCNE2 and the other KCNE subunits are tempting therapeutic targets because it would seem increasing their function could impart multiple therapeutic effects in various disorders. However, because their primary role is to regulate larger channel proteins, and they have differential effects depending on the channel involved, they are extremely challenging as drug targets. More encouragingly, KCNE proteins, including KCNE2, alter the pharmacological profile of some Kv α subunits23, 88, and could perhaps provide heightened specificity if we can understand exactly which KCNE-α complexes occur and where, what are their precise functions, and which pathologic effects can be linked to which complexes in which tissues or cell types. This is an extraordinarily challenging task for all the KCNE subunits, which will be facilitated by tools including tissue-specific and inducible knockout mouse models, improved KCNE detection methods, further human genetics studies in cohorts with diseases other than or in addition to cardiac arrhythmias, and multiscale computational approaches sufficiently sophisticated to model the effects of a germline KCNE gene variant in multiple tissues and the ensuing multisystem sequelae.
Highlights.
KCNE2 modulates Kv channel gating, trafficking, composition and pharmacology
KCNE2 is promiscuous and widely expressed in excitable and epithelial tissues
KCNE2-KCNQ1 complexes regulate secretory processes in multiple epithelia
Kcne2−/− mice exhibit cardiac arrhythmia, achlorhydria, and hypothyroidism
Human KCNE2 sequence variants associate with cardiac arrhythmias and atherosclerosis
Acknowledgments
This review and the corresponding Gene Wiki article are written as part of the Cardiac Gene Wiki Review series--a series resulting from a collaboration between the journal GENE, the Gene Wiki Initiative, and the BD2K initiative. The Cardiac Gene Wiki Initiative is supported by National Institutes of Health (GM089820 and GM114833). Additional support for Gene Wiki Reviews is provided by Elsevier, the publisher of GENE. The author is grateful for financial support from the National Institutes of Health (HL079275, DK41544 and GM115189). The corresponding Gene Wiki entry for this review can be found here: https://en.wikipedia.org/wiki/KCNE2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Catterall WA. The molecular basis of neuronal excitability. Science. 1984;223(4637):653–61. doi: 10.1126/science.6320365. [DOI] [PubMed] [Google Scholar]
- 2.Hille B, Armstrong CM, MacKinnon R. Ion channels: from idea to reality. Nature medicine. 1999;5(10):1105–9. doi: 10.1038/13415. [DOI] [PubMed] [Google Scholar]
- 3.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. The Journal of physiology. 1952;117(4):500–44. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott GW. Biology of the KCNQ1 potassium channel. New Journal of Science. 2014 [Google Scholar]
- 5.MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350(6315):232–5. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- 6.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396(6712):687–90. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh S, Nunziato DA, Pitt GS. KCNQ1 assembly and function is blocked by long-QT syndrome mutations that disrupt interaction with calmodulin. Circulation research. 2006;98(8):1048–54. doi: 10.1161/01.RES.0000218863.44140.f2. [DOI] [PubMed] [Google Scholar]
- 9.Bahring R, Milligan CJ, Vardanyan V, Engeland B, Young BA, Dannenberg J, et al. Coupling of voltage-dependent potassium channel inactivation and oxidoreductase active site of Kvbeta subunits. The Journal of biological chemistry. 2001;276(25):22923–9. doi: 10.1074/jbc.M100483200. [DOI] [PubMed] [Google Scholar]
- 10.Weng J, Cao Y, Moss N, Zhou M. Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase. The Journal of biological chemistry. 2006;281(22):15194–200. doi: 10.1074/jbc.M513809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403(6769):553–6. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 12.Wible BA, Yang Q, Kuryshev YA, Accili EA, Brown AM. Cloning and expression of a novel K+ channel regulatory protein, KChAP. The Journal of biological chemistry. 1998;273(19):11745–51. doi: 10.1074/jbc.273.19.11745. [DOI] [PubMed] [Google Scholar]
- 13.Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37(3):449–61. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- 14.Stocker M, Hellwig M, Kerschensteiner D. Subunit assembly and domain analysis of electrically silent K+ channel alpha-subunits of the rat Kv9 subfamily. Journal of neurochemistry. 1999;72(4):1725–34. doi: 10.1046/j.1471-4159.1999.721725.x. [DOI] [PubMed] [Google Scholar]
- 15.Bocksteins E, Labro AJ, Mayeur E, Bruyns T, Timmermans JP, Adriaensen D, et al. Conserved negative charges in the N-terminal tetramerization domain mediate efficient assembly of Kv2.1 and Kv2.1/Kv6.4 channels. The Journal of biological chemistry. 2009;284(46):31625–34. doi: 10.1074/jbc.M109.039479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbott GW, Goldstein SA. A superfamily of small potassium channel subunits: form and function of the MinK-related peptides (MiRPs) Quarterly reviews of biophysics. 1998;31(4):357–98. doi: 10.1017/s0033583599003467. [DOI] [PubMed] [Google Scholar]
- 17.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47 (6):787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Kanda VA, Lewis A, Xu X, Abbott GW. KCNE1 and KCNE2 inhibit forward trafficking of homomeric N-type voltage-gated potassium channels. Biophysical journal. 2011;101(6):1354–63. doi: 10.1016/j.bpj.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanda VA, Lewis A, Xu X, Abbott GW. KCNE1 and KCNE2 provide a checkpoint governing voltage-gated potassium channel alpha-subunit composition. Biophysical journal. 2011;101(6):1364–75. doi: 10.1016/j.bpj.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda VA, Purtell K, Abbott GW. Protein kinase C downregulates I(Ks) by stimulating KCNQ1-KCNE1 potassium channel endocytosis. Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8(10):1641–7. doi: 10.1016/j.hrthm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Kim LA, Rajan S, Xu S, Goldstein SA. Charybdotoxin binding in the I(Ks) pore demonstrates two MinK subunits in each channel complex. Neuron. 2003;40(1):15–23. doi: 10.1016/s0896-6273(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 22.Plant LD, Xiong D, Dai H, Goldstein SA. Individual IKs channels at the surface of mammalian cells contain two KCNE1 accessory subunits. Proceedings of the National Academy of Sciences of the United States of America; 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu H, Lin Z, Mattmann ME, Zou B, Terrenoire C, Zhang H, et al. Dynamic subunit stoichiometry confers a progressive continuum of pharmacological sensitivity by KCNQ potassium channels. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(21):8732–7. doi: 10.1073/pnas.1300684110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242(4881):1042–5. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 25.Papazian DM, Schwarz TL, Tempel BL, Jan YN, Jan LY. Cloning of genomic and complementary DNA from Shaker, a putative potassium channel gene from Drosophila. Science. 1987;237(4816):749–53. doi: 10.1126/science.2441470. [DOI] [PubMed] [Google Scholar]
- 26.Tempel BL, Jan YN, Jan LY. Cloning of a probable potassium channel gene from mouse brain. Nature. 1988;332(6167):837–9. doi: 10.1038/332837a0. [DOI] [PubMed] [Google Scholar]
- 27.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384(6604):78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 28.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384(6604):80–3. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 29.Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97(2):175–87. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 30.McDonald TV, Yu Z, Ming Z, Palma E, Meyers MB, Wang KW, et al. A minK-HERG complex regulates the cardiac potassium current I(Kr) Nature. 1997;388(6639):289–92. doi: 10.1038/40882. [DOI] [PubMed] [Google Scholar]
- 31.Piccini M, Vitelli F, Seri M, Galietta LJ, Moran O, Bulfone A, et al. KCNE1-like gene is deleted in AMME contiguous gene syndrome: identification and characterization of the human and mouse homologs. Genomics. 1999;60(3):251–7. doi: 10.1006/geno.1999.5904. [DOI] [PubMed] [Google Scholar]
- 32.Bendahhou S, Marionneau C, Haurogne K, Larroque MM, Derand R, Szuts V, et al. In vitro molecular interactions and distribution of KCNE family with KCNQ1 in the human heart. Cardiovascular research. 2005;67(3):529–38. doi: 10.1016/j.cardiores.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Jiang M, Xu X, Wang Y, Toyoda F, Liu XS, Zhang M, et al. Dynamic partnership between KCNQ1 and KCNE1 and influence on cardiac IKs current amplitude by KCNE2. The Journal of biological chemistry. 2009;284(24):16452–62. doi: 10.1074/jbc.M808262200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, et al. Targeted disruption of the Kvlqt1 gene causes deafness and gastric hyperplasia in mice. The Journal of clinical investigation. 2000;106(12):1447–55. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tinel N, Diochot S, Borsotto M, Lazdunski M, Barhanin J. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. The EMBO journal. 2000;19 (23):6326–30. doi: 10.1093/emboj/19.23.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dedek K, Waldegger S. Colocalization of KCNQ1/KCNE channel subunits in the mouse gastrointestinal tract. Pflugers Archiv : European journal of physiology. 2001;442(6):896–902. doi: 10.1007/s004240100609. [DOI] [PubMed] [Google Scholar]
- 37.Grahammer F, Herling AW, Lang HJ, Schmitt-Graff A, Wittekindt OH, Nitschke R, et al. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology. 2001;120(6):1363–71. doi: 10.1053/gast.2001.24053. [DOI] [PubMed] [Google Scholar]
- 38.Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y. Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. American journal of physiology Gastrointestinal and liver physiology. 2002;282(2):G277–87. doi: 10.1152/ajpgi.00200.2001. [DOI] [PubMed] [Google Scholar]
- 39.Heitzmann D, Grahammer F, von Hahn T, Schmitt-Graff A, Romeo E, Nitschke R, et al. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. The Journal of physiology. 2004;561(Pt 2):547–57. doi: 10.1113/jphysiol.2004.075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heitzmann D, Koren V, Wagner M, Sterner C, Reichold M, Tegtmeier I, et al. KCNE beta subunits determine pH sensitivity of KCNQ1 potassium channels. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2007;19(1–4):21–32. doi: 10.1159/000099189. [DOI] [PubMed] [Google Scholar]
- 41.Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, et al. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. The Journal of biological chemistry. 2006;281(33):23740–7. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 42.Roepke TK, Purtell K, King EC, La Perle KM, Lerner DJ, Abbott GW. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PloS one. 2010;5(7):e11451. doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuwahara N, Kitazawa R, Fujiishi K, Nagai Y, Haraguchi R, Kitazawa S. Gastric adenocarcinoma arising in gastritis cystica profunda presenting with selective loss of KCNE2 expression. World journal of gastroenterology : WJG. 2013;19(8):1314–7. doi: 10.3748/wjg.v19.i8.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanglin P, Lina Z, Zhiguo L, Na L, Haifeng J, Guoyun Z, et al. KCNE2, a down-regulated gene identified by in silico analysis, suppressed proliferation of gastric cancer cells. Cancer letters. 2007;246(1–2):129–38. doi: 10.1016/j.canlet.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Roepke TK, King EC, Purtell K, Kanda VA, Lerner DJ, Abbott GW. Genetic dissection reveals unexpected influence of beta subunits on KCNQ1 K+ channel polarized trafficking in vivo. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(2):727–36. doi: 10.1096/fj.10-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, et al. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403(6766):196–9. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 47.Hu Z, Kant R, Anand M, King EC, Krogh-Madsen T, Christini DJ, et al. Kcne2 Deletion Creates a Multisystem Syndrome Predisposing to Sudden Cardiac Death. Circulation Cardiovascular genetics. 2014 doi: 10.1161/CIRCGENETICS.113.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tranebjaerg L, Samson RA, Green GE. Jervell and Lange-Nielsen Syndrome. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, et al., editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- 49.Rice KS, Dickson G, Lane M, Crawford J, Chung SK, Rees MI, et al. Elevated serum gastrin levels in Jervell and Lange-Nielsen syndrome: a marker of severe KCNQ1 dysfunction? Heart rhythm : the official journal of the Heart Rhythm Society. 2011;8(4):551–4. doi: 10.1016/j.hrthm.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 50.Winbo A, Sandstrom O, Palmqvist R, Rydberg A. Iron-deficiency anaemia, gastric hyperplasia, and elevated gastrin levels due to potassium channel dysfunction in the Jervell and Lange-Nielsen Syndrome. Cardiology in the young. 2013;23(3):325–34. doi: 10.1017/S1047951112001060. [DOI] [PubMed] [Google Scholar]
- 51.Salsbury G, Cambridge EL, McIntyre Z, Arends MJ, Karp NA, Isherwood C, et al. Disruption of the potassium channel regulatory subunit KCNE2 causes iron-deficient anemia. Experimental hematology. 2014;42(12):1053–8. e1. doi: 10.1016/j.exphem.2014.07.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roepke TK, King EC, Reyna-Neyra A, Paroder M, Purtell K, Koba W, et al. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nature medicine. 2009;15 (10):1186–94. doi: 10.1038/nm.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purtell K, Paroder-Belenitsky M, Reyna-Neyra A, Nicola JP, Koba W, Fine E, et al. The KCNQ1-KCNE2 K+ channel is required for adequate thyroid I- uptake. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012 doi: 10.1096/fj.12-206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I- symporter. Mechanism, stoichiometry, and specificity. The Journal of biological chemistry. 1997;272(43):27230–8. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- 55.Abbott GW, Tai KK, Neverisky DL, Hansler A, Hu Z, Roepke TK, et al. KCNQ1, KCNE2, and Na+-Coupled Solute Transporters Form Reciprocally Regulating Complexes That Affect Neuronal Excitability. Science signaling. 2014;7(315):ra22. doi: 10.1126/scisignal.2005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tinel N, Diochot S, Lauritzen I, Barhanin J, Lazdunski M, Borsotto M. M-type KCNQ2-KCNQ3 potassium channels are modulated by the KCNE2 subunit. FEBS letters. 2000;480(2–3):137–41. doi: 10.1016/s0014-5793(00)01918-9. [DOI] [PubMed] [Google Scholar]
- 57.Roepke TK, Kanda VA, Purtell K, King EC, Lerner DJ, Abbott GW. KCNE2 forms potassium channels with KCNA3 and KCNQ1 in the choroid plexus epithelium. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(12):4264–73. doi: 10.1096/fj.11-187609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo W, Shimada S, Tajiri H, Yamauchi A, Yamashita T, Okada S, et al. Developmental regulation of Na+ / myo-inositol cotransporter gene expression. Brain research Molecular brain research. 1997;51(1–2):91–6. doi: 10.1016/s0169-328x(97)00220-9. [DOI] [PubMed] [Google Scholar]
- 59.Spector R, Lorenzo AV. Myo-inositol transport in the central nervous system. The American journal of physiology. 1975;228(5):1510–18. doi: 10.1152/ajplegacy.1975.228.5.1510. [DOI] [PubMed] [Google Scholar]
- 60.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. The Journal of general physiology. 1990;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 62.Sanguinetti MC, Chen J, Fernandez D, Kamiya K, Mitcheson J, Sanchez-Chapula JA. Physicochemical basis for binding and voltage-dependent block of hERG channels by structurally diverse drugs. Novartis Foundation symposium. 2005;266:159–66. discussion 66–70. [PubMed] [Google Scholar]
- 63.Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440(7083):463–9. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- 64.Gordon E, Panaghie G, Deng L, Bee KJ, Roepke TK, Krogh-Madsen T, et al. A KCNE2 mutation in a patient with cardiac arrhythmia induced by auditory stimuli and serum electrolyte imbalance. Cardiovascular research. 2008;77(1):98–106. doi: 10.1093/cvr/cvm030. [DOI] [PubMed] [Google Scholar]
- 65.Perlstein I, Burton DY, Ryan K, Defelice S, Simmers E, Campbell B, et al. Posttranslational control of a cardiac ion channel transgene in vivo: clarithromycin-hMiRP1-Q9E interactions. Human gene therapy. 2005;16(7):906–10. doi: 10.1089/hum.2005.16.906. [DOI] [PubMed] [Google Scholar]
- 66.Ackerman MJ, Tester DJ, Jones GS, Will ML, Burrow CR, Curran ME. Ethnic differences in cardiac potassium channel variants: implications for genetic susceptibility to sudden cardiac death and genetic testing for congenital long QT syndrome. Mayo Clinic proceedings Mayo Clinic. 2003;78(12):1479–87. doi: 10.4065/78.12.1479. [DOI] [PubMed] [Google Scholar]
- 67.Isbrandt D, Friederich P, Solth A, Haverkamp W, Ebneth A, Borggrefe M, et al. Identification and functional characterization of a novel KCNE2 (MiRP1) mutation that alters HERG channel kinetics. J Mol Med (Berl) 2002;80(8):524–32. doi: 10.1007/s00109-002-0364-0. [DOI] [PubMed] [Google Scholar]
- 68.Zhang M, Wang Y, Jiang M, Zankov DP, Chowdhury S, Kasirajan V, et al. KCNE2 protein is more abundant in ventricles than in atria and can accelerate hERG protein degradation in a phosphorylation-dependent manner. Am J Physiolo Heart Circ Physiol. 2012;302(4):H910–22. doi: 10.1152/ajpheart.00691.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Um SY, McDonald TV. Differential association between HERG and KCNE1 or KCNE2. PloS one. 2007;2(9):e933. doi: 10.1371/journal.pone.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roepke TK, Kontogeorgis A, Ovanez C, Xu X, Young JB, Purtell K, et al. Targeted deletion of kcne2 impairs ventricular repolarization via disruption of I(K,slow1) and I(to,f) The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22(10):3648–60. doi: 10.1096/fj.08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nerbonne JM, Nichols CG, Schwarz TL, Escande D. Genetic manipulation of cardiac K(+) channel function in mice: what have we learned, and where do we go from here? Circulation research. 2001;89(11):944–56. doi: 10.1161/hh2301.100349. [DOI] [PubMed] [Google Scholar]
- 72.Temple J, Frias P, Rottman J, Yang T, Wu Y, Verheijck EE, et al. Atrial fibrillation in KCNE1-null mice. Circulation research. 2005;97(1):62–9. doi: 10.1161/01.RES.0000173047.42236.88. [DOI] [PubMed] [Google Scholar]
- 73.Zhang M, Jiang M, Tseng GN. minK-related peptide 1 associates with Kv4. 2 and modulates its gating function: potential role as beta subunit of cardiac transient outward channel? Circulation research. 2001;88(10):1012–9. doi: 10.1161/hh1001.090839. [DOI] [PubMed] [Google Scholar]
- 74.Kundu P, Ciobotaru A, Foroughi S, Toro L, Stefani E, Eghbali M. Hormonal regulation of cardiac KCNE2 gene expression. Molecular and cellular endocrinology. 2008;292(1–2):50–62. doi: 10.1016/j.mce.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.George AL., Jr Molecular and genetic basis of sudden cardiac death. The Journal of clinical investigation. 2013;123(1):75–83. doi: 10.1172/JCI62928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Science translational medicine. 2009;1(2):2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kathiresan S, Voight BF, Purcell S, Musunuru K, Ardissino D, Mannucci PM, et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nature genetics. 2009;41(3):334–41. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sabater-Lleal M, Malarstig A, Folkersen L, Soler Artigas M, Baldassarre D, Kavousi M, et al. Common genetic determinants of lung function, subclinical atherosclerosis and risk of coronary artery disease. PloS one. 2014;9(8):e104082. doi: 10.1371/journal.pone.0104082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soler Artigas M, Loth DW, Wain LV, Gharib SA, Obeidat M, Tang W, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nature genetics. 2011;43(11):1082–90. doi: 10.1038/ng.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nawathe PA, Kryukova Y, Oren RV, Milanesi R, Clancy CE, Lu JT, et al. An LQTS6 MiRP1 mutation suppresses pacemaker current and is associated with sinus bradycardia. Journal of cardiovascular electrophysiology. 2013;24(9):1021–7. doi: 10.1111/jce.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qu J, Kryukova Y, Potapova IA, Doronin SV, Larsen M, Krishnamurthy G, et al. MiRP1 modulates HCN2 channel expression and gating in cardiac myocytes. The Journal of biological chemistry. 2004;279(42):43497–502. doi: 10.1074/jbc.M405018200. [DOI] [PubMed] [Google Scholar]
- 82.Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, et al. MinK-related peptide 1: A beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circulation research. 2001;88(12):E84–7. doi: 10.1161/hh1201.093511. [DOI] [PubMed] [Google Scholar]
- 83.Ying SW, Kanda VA, Hu Z, Purtell K, King EC, Abbott GW, et al. Targeted Deletion of Kcne2 Impairs HCN Channel Function in Mouse Thalamocortical Circuits. PloS one. 2012;7(8):e42756. doi: 10.1371/journal.pone.0042756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu W, Deng J, Wang G, Zhang C, Luo X, Yan D, et al. KCNE2 modulates cardiac L-type Ca channel. Journal of molecular and cellular cardiology. 2014 doi: 10.1016/j.yjmcc.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 85.Kanda VA, Abbott GW. KCNE Regulation of K(+) Channel Trafficking - a Sisyphean Task? Frontiers in physiology. 2012;3:231. doi: 10.3389/fphys.2012.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Abbott GW. KCNE2 and the K (+) channel: The tail wagging the dog. Channels (Austin) 2012;6(1) doi: 10.4161/chan.19126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu X, Kanda VA, Choi E, Panaghie G, Roepke TK, Gaeta SA, et al. MinK-dependent internalization of the IKs potassium channel. Cardiovascular research. 2009;82(3):430–8. doi: 10.1093/cvr/cvp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panaghie G, Abbott GW. The impact of ancillary subunits on small-molecule interactions with voltage-gated potassium channels. Current pharmaceutical design. 2006;12(18):2285–302. doi: 10.2174/138161206777585175. [DOI] [PubMed] [Google Scholar]
- 89.Crump SM, Abbott GW. Arrhythmogenic KCNE gene variants: current knowledge and future challenges. Frontiers in genetics. 2014;5:3. doi: 10.3389/fgene.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]