Abstract
The daily pattern of blood borne melatonin varies seasonally under the control of a multi-oscillator circadian pacemaker. Here we examine patterns of melatonin secretion and locomotor activity in Siberian and Syrian hamsters entrained to bimodal LDLD8:4:8:4 and LD20:4 lighting schedules that facilitate novel temporal arrangements of component circadian oscillators. Under LDLD, both species robustly bifurcated wheel-running activity in distinct day scotophase (DS) and night scotophase (NS) bouts. Siberian hamsters displayed significant melatonin increases during each scotophase in LDLD, and in the single daily scotophase of LD20:4. The bimodal melatonin secretion pattern persisted in acutely extended 16 h scotophases. Syrian hamsters, in contrast, showed no significant increases in plasma melatonin during either scotophase of LDLD8:4:8:4 or in LD20:4. In this species, detectable levels were observed only when the day scotophase of LDLD was acutely extended to yield 16 h of darkness. Established species differences in the phase lag of nocturnal melatonin secretion relative to activity onset may underlie the above contrast: In non-bifurcated entrainment to 24 h LD cycles, Siberian hamsters show increased melatonin secretion within ~ 2 h after activity onset, whereas in Syrian hamsters, detectable melatonin secretion phase lags activity onset and the L/D transition by at least 4 h. The present results provide new evidence indicating multi-oscillator regulation of the waveform of melatonin secretion, specifically, the circadian control of the onset, offset, and duration of nocturnal secretion.
Keywords: melatonin, Siberian hamster (Phodopus sungorus), Syrian hamster (Mesocricetus auratus), split entrainment, bifurcated circadian rhythms, multiple oscillators
INTRODUCTION
In mammals, an endogenous circadian clock orchestrates 24 h rhythms in behavior, metabolism and the secretion of numerous hormones including pineal melatonin (1, 2). The secretion pattern of melatonin is controlled via a multi-synaptic pathway from the circadian clock in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus to the pineal gland that regulates arylalkylamine N-acetyltransferase (AANAT) (3, 4). Melatonin production and secretion are generally basal during the subjective day and markedly elevated only during subjective night (5–8).
Because of the robust “high-at-night” characteristic of melatonin, this hormone is considered to be a neuroendocrine expression of darkness and a critical modulator of seasonal physiology: Compared to short nights of summer, the SCN entrains a longer bout of melatonin secretion during the long nights of winter that induces a suite of physiological adaptations for winter (e.g., gonadal regression) (8–11). The circadian control and proportional relationship between night length and melatonin secretion duration are highly conserved across species, although precise control and timing of melatonin synthesis nevertheless vary substantially among mammals (11, 12). For example, in the highly photoperiodic Siberian hamster, pineal and plasma melatonin concentrations rise around two hours after dark onset and remain elevated (40–80 pg/mL in plasma) until approximately 30 minutes prior to the entrained dark:light transition, when melatonin concentrations fall to baseline (7, 9, 13). By comparison, in entrainment to typical 24 h laboratory cycles with short or long photophases, Syrian hamsters fail to produce high levels of melatonin until about four hours after activity onset and no sooner than 4–6 h after the light:dark transition (5).
The seasonal variation in melatonin duration has been usefully modeled as an output of a dual-oscillator circadian pacemaker (3, 5, 14–16). The model posits the existence of functionally distinct evening (E) and morning (M) oscillators that underlie circadian regulation of activity duration (α) via their separate entrainment to evening and morning light (16, 17). In addition, these oscillators program the onset (Evening rise) and offset (Morning decline) of melatonin secretion to occur near the beginning and end of the daily scotophase (3, 5). The phase relationship between E & M oscillators thus dictates the duration of subjective night and day, manifest in locomotor activity (α) and melatonin secretion, which are tightly linked (5, 7, 9). Within this framework, two versions are implicit in accounts of the dual oscillator model of melatonin secretion, with each focusing on a different set of empirical observations. In one, here designated the building block model (BB), E and M circadian oscillators may each drive melatonin secretion for a fixed and finite duration (e.g. 5 h). This idea springs from observations of bimodal melatonin profiles in humans and sheep under long nights (15, 18). With sufficiently large E-M phase angles, two relatively shorter (≈ < 5 h) melatonin peaks programmed by E and M do not overlap and would produce a bimodal secretion pattern. Thus, the BB model predicts a minimal duration melatonin peak (e.g., ~ 5 h) when the phase angle between E and M oscillators is very short and progressively longer duration peaks until bimodality emerges. In an alternative version of the dual oscillator model, here termed On/Off (OO), the E oscillator switches melatonin secretion on whilst the M oscillator turns it off, again with the phase angle between E and M determining melatonin peak duration (3). This model is consistent with findings that long photoperiods and advancing light pulses may so severely compress the subjective night that melatonin rhythmicity disappears entirely, even acutely after transfer to constant darkness (3, 5). The BB model fails to account for this observation, whereas the OO model does not easily account for the multiple peaks in 24 hours that have been occasionally reported. Finally, neither of the models disputes the fact that melatonin secretion may also be directly suppressed by light (e.g., masking) through a mechanism that is potentially separable from the entrainment of E and M oscillators (19).
E and M oscillator functions have been provisionally mapped to spatially or temporally segregated cell populations in various species (20–22). In rats housed under 22 h light:dark (LD) cycles, the ventrolateral and dorsomedial SCN become functionally decoupled such that the former remains entrained to the LD cycle whereas the latter free-runs with only relative coordination to the LD cycle (23, 24). Melatonin data collected under these conditions suggest that the ventrolateral SCN mediates the acute light-induced suppression of melatonin, and the dorsomedial SCN mediates circadian entrainment of melatonin.
In an alternative oscillator dissociation paradigm, both Syrian and Siberian hamsters are readily induced to bifurcate locomotor activity into two components that are stably entrained in relation to each of the two daily scotophases of 24 h light:dark:light:dark (LDLD) cycles with permissive dim illumination (< 0.1 lux). This bifurcated entrainment phenomenon, also referred to as LDLD rhythm splitting (25, 26), represents a temporal dissociation of cellular compartments within the SCN that is reliably elicited in nearly all experimental subjects (27–29). In both hamster species, a summer reproductive phenotype is maintained (unpublished observations). We use this LDLD bifurcation protocol to test whether each oscillation is associated with an equal and finite cycle of melatonin expression (the BB model) or whether oscillators separately initiate and terminate melatonin secretion (the OO model). Potential masking effects and interactions between oscillators were further assessed by probing the LDLD patterns of melatonin secretion by acutely extending periods of darkness (from 4 h to 16 h, thereby eliminating one of the two photophases).
METHOD
All procedures were approved by UCSD Institutional Animal Care and Use Committee. Male Siberian hamsters (Phodopus sungorus) were selected from a breeding colony established in 1994 and maintained in LD14:10 (lights on 0600 to 2000 PST). For the duration of the study Siberian hamsters were individually housed in polypropylene cages (28 × 19 × 18 cm) equipped with wheels 12 cm in diameter. Male Syrian hamsters (Mesocricetus auratus) were obtained from a breeding colony derived from Harlan stock (Hsd:Han:AURA) and maintained in LD14:10 (lights on 0600 to 2000 PST). For study, Syrian hamsters were individually housed in polypropylene cages (27 × 20 × 15 cm) equipped with 17 cm diameter running wheels. Purina Rodent Chow (#5001 Syrian, #5015 Siberian, St. Louis, MO) and water were available ad libitum. Bedding was Sani-Chip corn cob, and room temperature was maintained at 22 ± 3 °C.
In all experimental conditions, white fluorescent bulbs generated photophase illumination of 100–300 lux at cage lid. Additionally, green light emitting diodes (LEDs) mounted along the perimeter of each photoperiod chamber shelf produced constant and relatively uniform dim scotophase illumination in each wheel cage (< 0.1 lux, peak transmission 560 nm, half bandwidth of 23 nm).
Because standard circadian terminology is specific to traditional LD cycles rather than the LDLD cycles used here, we employ the following definitions: In baseline entrainment to conventional LD14:10 the lights out (L/D) transition is designated Zeitgeber Time 12 (ZT12). Under LDLD, ZT12 is redefined as the beginning of the Night Scotophase (NS), the 4 h dark phase occurring nearest to lights out in the LD14:10 colony. Further, to aide analysis of circadian rhythm bifurcation under LDLD entrainment we distinguish a Night Scotophase (ZT12-16), abbreviated NS, from a Day Scotophase (ZT0-4) labelled DS (Figure 1A and 1C).
Figure 1.
Representative double-plotted wheel-running actograms of male Siberian (A, B) and Syrian (C, D) hamsters with bifurcated activity rhythms in LDLD8:4:8:4 and unbifurcated rhythms in LD20:4. Times of light and dark are represented above actograms with white and black filled rectangles, respectively. Scaling of the ordinate is from 0-177, 0-150, 0-164, and 0-188 counts/min for A-D, respectively. Displayed activity data are shown for 4–5 weeks prior to the first blood sampling.
Experiment #1a: Siberian Hamsters in LDLD8:4:8:4
To induce behavioral bifurcation, male Siberian hamsters (4–6 months old, n = 64) were transferred to individual cages equipped with running wheels as previously described (25). Transfer occurred during the middle of the LD14:10 photophase (approximately 1200 PST), 5 h before the initial 4 h scotophase (NS, 1700-2100 PST) of the new LDLD8:4:8:4 cycle. After 2 weeks on LDLD, blood collection was randomised to occur during one of ten time points (1, 2, 3, 4, or 5 h after the beginning of DS or NS; Figure 2A). Two weeks later, blood was collected a second time at a phase 12 h apart from the first sample.
Figure 2.
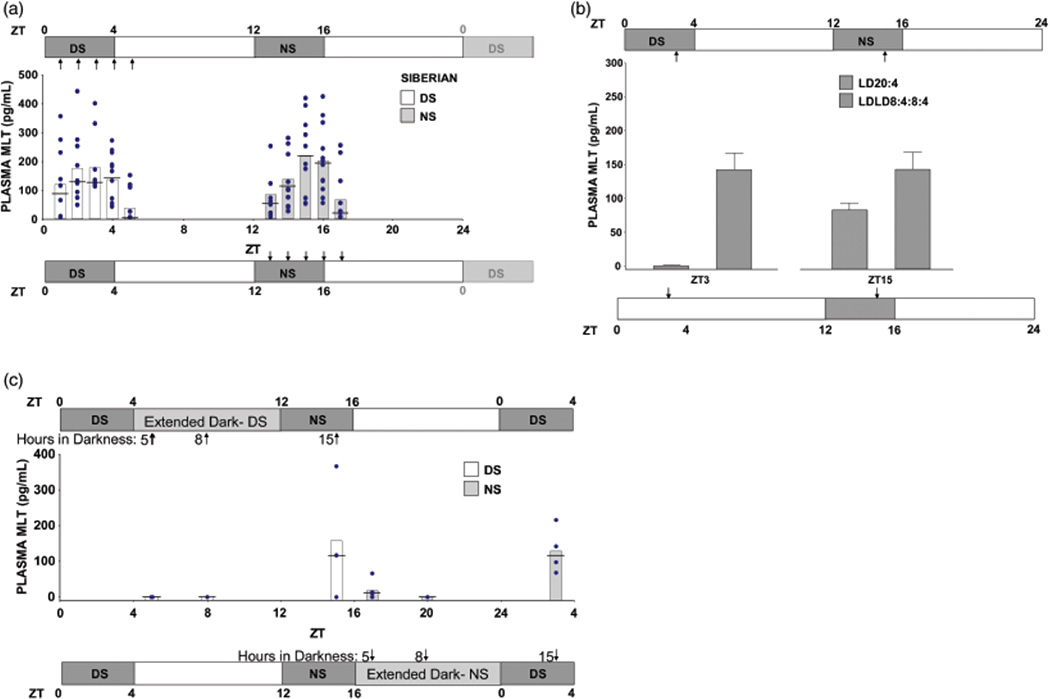
Plasma melatonin concentrations from Siberian hamsters entrained to LDLD8:4:8:4 and LD20:4. Bars display means, horizontal lines denote medians, and circles indicate individual data points. Sampling times (arrows) are illustrated in relation to the light cycle, which is represented with black (scotophases) and white (photophases) boxes with Zeitgeber time (ZT) of light/dark transitions indicated. (A) Melatonin values in LDLD8:4:8:4 (Experiment #1a) in which blood was sampled twice from each bifurcated subject in a counterbalanced design (with the two samples from the same individual always taken at time points 12 h apart). (B) Plasma melatonin concentrations of bifurcated hamsters from Experiment #1b, also in LDLD8:4:8:4, sampled either at ZT15 or ZT3 (n = 10–13) and from unbifurcated hamsters in LD20:4 sampled at both time points in counterbalanced fashion (n =14). Data displayed as mean ± SEM. (C) Plasma melatonin concentrations of bifurcated hamsters in Experiment #1b sampled a second time, exclusively in darkness, at one of three time points (5, 8 or 15 h in darkness) associated with the extension of either the DS (ZT 5, 8, 15) or NS (ZT17, 20, 3) scotophase.
Experiment #1b: Siberian Hamsters in Extended Darkness
A separate population of male Siberian hamsters (n = 32) was transferred at 4–6 months old from LD14:10 to LDLD8:4:8:4 repeating the protocol established in Experiment #1a. Blood collection commenced six weeks later with samples taken either at ZT3 or ZT15 (1 h before lights on), in partial replication of Experiment #1a, to verify that melatonin was again elevated in the DS and NS. Five weeks later, DS and NS were acutely and separately extended by eliminating the subsequent photophase, and blood was again collected spanning the "extended darkness" (ZT5, ZT8, and ZT15 for extended DS; ZT17, ZT20, and ZT3 for extended NS; Figure 2B). Hamsters first sampled during the DS (ZT3) were subject to blood collection in extended NS, and animals first sampled during the NS (ZT15) were exposed to extended DS during the second blood collection.
LD20:4 controls (n = 16) were maintained for six weeks prior to a first blood collection at either ZT15 (in dark) or ZT3 (in light), when melatonin is expected to be high and low, respectively. Three weeks later, the bleed times were reversed. Order of collection time was randomised, and results were combined. The longer spacing between blood collections in Experiment #1b versus #1a reflected practical considerations rather than differing research objectives.
To provide benchmarks for experimental melatonin titres obtained in LDLD8:4:8:4 and LD20:4, blood collections in the daytime (ZT6; n = 6) and nighttime (ZT15; n = 6) were carried out in a separate set of Siberian hamsters (5–7 months old) maintained in LD14:10.
Experiment #2a: Syrian Hamsters in LDLD8:4:8:4
Three month old Syrian hamsters (n = 40) were moved from LD14:10 to individual running wheel cages at the beginning of the DS (1000-1400 PST) of the new LDLD8:4:8:4 schedule (NS, 2200-0200 PST; Figure 1C). During week 8, blood was collected at one of six time points (3, 4, or 5 h after lights off for each scotophase; Figure 3A). Two weeks later, blood was collected a second time at a phase 12 h apart from the first sampling time. Bleed order (DS or NS) was randomised and data were combined according to designated ZT. Individuals from a control group housed in LD20:4 (n = 12) were sampled at ZT15, ZT16, or ZT17 to compare with data from the twice daily scotophases of LDLD.
Figure 3.
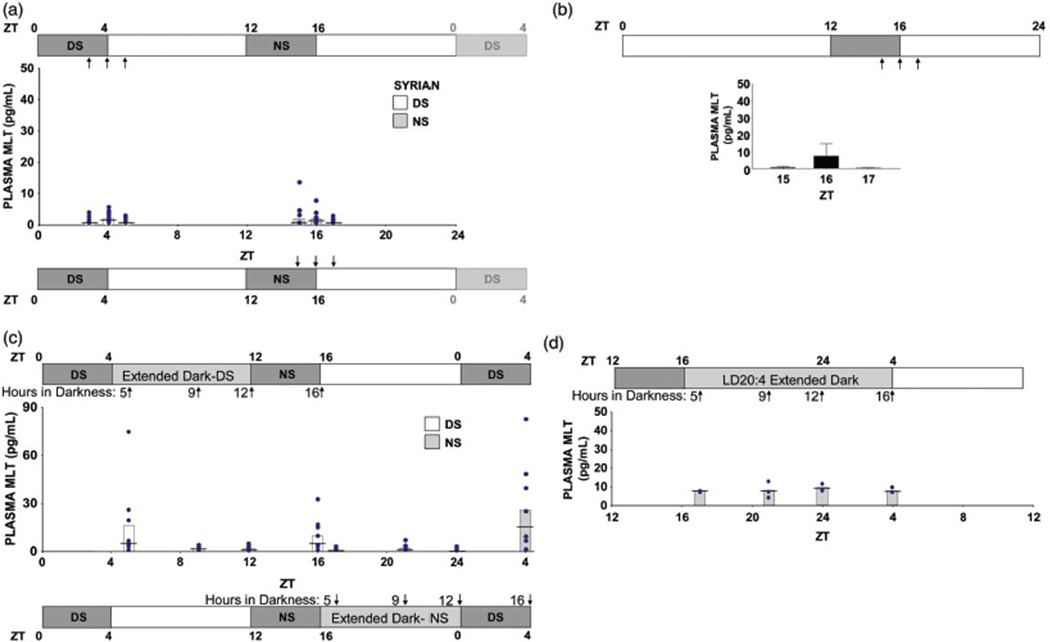
Means, medians, and individual data points of plasma melatonin concentrations from Syrian hamsters during or after LDLD8:4:8:4 and LD20:4. Conventions as in Figure 2. (A) Melatonin values from Experiment #2a in which blood was sampled twice from each bifurcated subject in LDLD8:4:8:4 in a counterbalanced design. (B) Mean ± SEM of plasma melatonin concentrations from unbifurcated hamsters in LD20:4 sampled once at one of three time points (n = 4). (C) Plasma melatonin concentrations of bifurcated hamsters from Experiment #2b in LDLD8:4:8:4 sampled after extension of each scotophase. The two samples from individual hamsters were collected at times 12 h apart in a counter-balanced design. (D) Melatonin concentrations of unbifurcated hamsters entrained to LD20:4 and then sampled 5, 9, 12 and 16 h into an acute extension of the usual 4 h scotophase.
Experiment #2b: Syrian Hamsters in Extended Darkness
Hamsters from Experiment #2a were retained in LDLD, and blood was collected a third and fourth time during weeks 12 and 14, respectively (once after acutely extending the DS by replacing the entire photophase between DS and NS with darkness, and again, two weeks later after the same manipulation in the opposite half of the LDLD cycle). Blood was collected 5, 9, 12, and 16 h into each extended dark phase (ZTs 17, 21, 0 and 4 for the extended NS, and ZTs 5, 9, 12, and 16 for the extended DS; Figure 3B). In order to include a within-subjects analysis, blood collection during hour 5 of extended darkness was done with hamsters previously bled at ZT5 and ZT17 of Experiment #2a. Similarly, blood collection during hour 16 of extended darkness was taken from hamsters previously sampled at ZT4 and ZT16 in Experiment #2a. The remaining hamsters were randomly assigned to have blood collected after 9 or 12 h of extended darkness. Additionally, a second blood sample from LD20:4 controls (n = 12) of Experiment #2a was taken after 5, 9, 12, or 16 h of acutely extended darkness.
To provide long-day benchmark values of melatonin for comparison with LDLD8:4:8:4 and LD20:4, middle of the night (ZT17) plasma melatonin levels were obtained separately from Syrian hamsters (n = 6) housed under LD14:10.
ANALYSES
Wheel Running Activity
Wheel running activity was recorded by Dataquest III and VitalView software, compiled into 6-min bins. Data were analyzed using Clocklab (Actimetrics, Evanston IL, USA), SPSS (LEAD technologies Inc. Chicago IL, USA), and Microsoft Excel (Microsoft, Belleview, WA, USA). As in past studies, the entrainment to LDLD was unambiguously bifurcated (i.e., animals express two daily bouts of activity approximately 12 h apart, one associated with each scotophase) or unbifurcated (i.e., activity is concentrated in one scotophase). Activity onset and offset were determined by eye fit of wheel running activity from the 10 days prior to the first blood collection. Activity duration (α) was calculated as the difference in time between activity onset and activity offset associated with each scotophase.
Plasma Melatonin Collection
Samples were collected relative to clock hour (± 10 min), except that collections for the 4th hour of darkness (ZT4, ZT16) all occurred in the 15 min prior to lights on. Hamsters were lightly anaesthetised (Siberian hamsters with isoflurane; Syrian hamsters with ~60 mg/kg sodium pentobarbital) and bled with the aid of a dim red flashlight (<1 lux). Blood (~0.3 ml) was removed retro-orbitally (preferably from the right eye) using heparinised capillary tubes, then transferred to polypropylene tubes containing 40 µl heparin (1000 units/ml). Blood tubes were placed on ice, centrifuged and ~ 125–200 µl of plasma was stored at −80 °C for later assay.
Plasma Melatonin Assay
Plasma melatonin (pg/ml) concentrations were measured using Bühlmann Melatonin Direct Immunoassy assay kits (000-RK-MDI, ALPCO, ltd., Windham, NH) with the Kennaway G280 anti-melatonin antibody as previously described (30). Reduced sample volumes raised the functional least detectable value to approximately 10.4 pg/ml for Siberian and 2.6 pg/ml for Syrian plasma (for 20 vs. 80 μl). Kit controls gave intra- and inter-assay CVs of approximately 6 and 11%. Comparing low and high Siberian plasma controls by linear regression (n = 11 pairs detectable by both PT and column extraction methods) yielded slope = 1.07, R2 = 0.91, p < 0.0001, providing further validation of the method.
Data Analysis
A mixed-model 2-way ANOVA was carried out for each experiment to determine main and interaction effects of scotophase (DS and NS) as well as sampling time on observed melatonin concentrations. Non-parametric Kruskal-Wallis tests were also used to compare main effects of sampling time on melatonin concentration when data were not normally distributed because of a high frequency of non-detectable levels. A 2-independent samples t-test was used in Experiment #1b to compare melatonin concentrations between NS and DS in hamsters entrained to LDLD8:4:8:4. 2-tailed Wilcoxon's matched-pairs signed-ranks tests were run in Experiment #2a-b to detect within-subject differences in melatonin concentrations in Syrian hamsters sampled at the same ZT between Experiment #2a and #2b. Effects were considered statistically significant at p < 0.05. Plasma melatonin values were excluded from analysis in rare instances where the coefficient of variation of duplicate determinations exceeded 20% or when an individual’s bifurcation status was not maintained throughout both blood collections. Finally, a cohort of Siberian hamsters maintained in LD20:4 exhibited melatonin concentrations with an elevated baseline (exceeding 50 pg/mL during the light phase and peaking at 190 mg/mL at night), suggesting an error in sample collection or preparation, and insufficient sample was available for re-assay. Instead, an independent group of LD20:4 hamsters was included in Experiment #1b, which produced expected nighttime and daytime melatonin values (see below). Data from the initial cohort were considered aberrant for unknown reasons and thus are not further reported.
RESULTS
Experiment #1a: Siberian Hamsters in LDLD8:4:8:4
LDLD8:4:8:4 running wheel entrainment
Of 64 subjects, 54 initially adopted stable bifurcated activity rhythms in LDLD8:4:8:4, confirmed visually by observing two daily bouts of wheel running activity that temporally coincided with the DS and NS (Figure 1A-B). As in previous reports, both components were characterised by activity onsets that followed lights off and neither phase angle of entrainment nor activity duration differed between the two scotophases (Table 1). The blood collection protocol did not disrupt the bifurcated pattern of activity, which resumed within one cycle of this procedure.
Table 1.
Wheel-running parameters (mean ± SEM) of entrained Hamsters under light-dark:light-dark cycles and LD 20:4 controls
| Phase Angle of Activity | Night/Day | Night/Day | ||||
|---|---|---|---|---|---|---|
| Onset (h before lights off) |
Difference |
Activity Duration (h) |
Difference |
|||
| Night | Day | P | Night | Day | P | |
| Siberian Hamsters | ||||||
| LDLD 8:4:8:4 (n = 54) | −0.67 ± 0.06 | −0.56 ± 0.04 | 3.07 ± 0.07 | 3.14 ± 0.06 | ||
| LD 20:4 controls (n = 15) | 0.74 ± 0.32 | - | n/a | 4.09 ± 0.43 | - | n/a |
| Syrian Hamsters | ||||||
| LDLD 8:4:8:4 (n = 29) | −1.38 ± 0.03 | −0.93 ± 0.03 | < 0.05 | 2.88 ± 0.10 | 3.37 ± 0.07 | < 0.05 |
| LD 20:4 controls (n = 12) | 1.45 ± 0.11 | - | n/a | 6.07 ± 0.25 | - | n/a |
Plasma melatonin
Benchmark nighttime high (ZT15) and daytime low (ZT6) melatonin concentrations in LD14:10 were 208.1 ± 64.8 pg/mL, (mean ± SEM, n = 6) and 12.8 ± 5.2 pg/mL (n = 6), respectively. In behaviorally bifurcated animals in LDLD8:4:8:4, mean melatonin concentration peaked at 3 h (>150 pg/mL) into both scotophases and were lowest 1 h into each photophase (Figure 2A), yielding a statistically significant effect of sampling time (F(4,35) = 11.4, p < 0.001) with no main effect of scotophase (F(1,35) = 0.6, ns) and no scotophase×sampling time interaction (F(4,35) = 1.8, ns, Figure 2A).
Experiment #1b: Siberian Hamsters in Extended Darkness
Wheel running
Of 32 hamsters exposed to LDLD8:4:8:4, 23 adopted bifurcated activity rhythms with activity patterns comparable to those reported in Table 1 (data not shown). Of 16 controls, 15 entrained with a stable phase angle to LD20:4 (one did not adopt stable wheel running entrainment relative to lights off and was therefore excluded).
Plasma melatonin in extended dark
In the first blood collection designed to replicate Experiment #1a, melatonin concentrations obtained from bifurcated hamsters under LDLD exceeded mean values of 135 pg/mL in both scotophases (ZT15 and ZT3), yielding no significant difference (t(21) = 0.029, ns; Figure 2B) between DS and NS. In LD20:4 controls, there was a statistically significant effect of sampling time as melatonin was elevated at night (>80 pg/mL) and near or below detectable levels during the day (t(14) = 4.54, p < 0.001, Figure 2B). A post-hoc analysis revealed that a related but different pattern was evident in the small population of unbifurcated hamsters sampled in LDLD8:4:8:4: Those sampled 3 h into the scotophase that contained locomotor activity had modest but clearly elevated melatonin concentrations (60.0 ± 17.7 pg/mL, n = 6), whereas those sampled 3 h into the scotophase lacking any locomotor activity had undetectable values (n = 2; data not shown).
Following extension of darkness after DS or NS, melatonin concentrations were relatively low at phases that were previously illuminated (ZT5, ZT8, ZT17, ZT20) and elevated at times that were previously dark (ZT3, ZT15), yielding a significant effect of sampling time (F(2,17) = 9.51, p < 0.001) but no main effect of scotophase (DS vs. NS) (F(1,17) = 0.02, ns), and no scotophase x sampling time interaction (F(2,17) = 0.21, ns; Figure 2C).
Experiment #2a: Syrian Hamsters in LDLD8:4:8:4 and LD20:4
LDLD8:4:8:4 and LD20:4 running wheel entrainment
Bifurcated activity rhythms were adopted by 39 of 40 Syrian hamsters in LDLD8:4:8:4 (Fig. 1C). Of these 39 hamsters, 10 did not maintain bifurcated activity rhythms throughout both sampling times of Experiment #2a (not shown) and were not bled if not bifurcated. All 12 Syrian controls housed in LD20:4 entrained with a stable unimodal activity rhythm (Fig. 1D). In bifurcated hamsters, activity began after each lights off, occurring later in NS than in DS and with shorter duration. In LD20:4 controls, activity onset anticipated lights off by more than 1 h (Table 1). Again, blood collection did not disrupt the bifurcated entrainment state.
Plasma melatonin concentration
As a benchmark, nocturnal melatonin elevations averaged 47 ± 10 (SEM) pg/ml (n = 6) in plasma collected from Syrian hamsters at ZT18-20 under LD14:10. In Syrian hamsters with bifurcated entrainment in LDLD8:4:8:4, melatonin was undetectable in the majority of samples (43 of 56). There was also no influence of either sampling time or scotophase on observed melatonin levels (F(2,25) = 1.429, ns; F(1,25) = 0.198, ns, respectively, Figure 3A) and no interaction between sampling time and scotophase (F(2,25)= 1.067, ns). Because of the frequency of non-detectable melatonin concentrations, ANOVAs were supplemented with non-parametric analyses revealing a significant rise in plasma melatonin in bifurcated hamsters during the final minutes of the DS (Kruskal-Wallis, H(2) = 8.83, p < 0.025), but not during the NS (H(2) = 3.38, ns). In hamsters entrained to LD20:4, there was no elevation in plasma melatonin associated with the single 4 h scotophase (H(2) = 0.47, ns; Figure 3B).
Experiment #2b: Syrian Hamsters in Extended Darkness
LDLD8:4:8:4 and LD20:4 running wheel entrainment
Prior to blood sampling in extended dark, 32 hamsters were stably bifurcated in LDLD8:4:8:4 throughout the final phase of the experiment and all 12 LD20:4 controls remained stably entrained. The replacement of a single photophase with darkness did not disrupt bifurcated entrainment. Blood collection was typically followed by a transient reduction or absence of wheel running for 24–48 h, but the return to normal activity levels gave no indication of phase shifting or destabilization of the prior entrainment state.
Plasma melatonin concentration under LDLD8:4:8:4 extended dark
There was no main effect of scotophase (DS vs. NS) on melatonin concentration in extended dark (F(1,25) = 0.004, ns), but there was a main effect of sampling time as well as a significant interaction between these factors F(3,25) = 4.696, p < 0.010; F(3,25)= 3.053, p < 0.047, respectively, Figure 3B). Following dark extension of the NS, melatonin concentrations were undetectable at times corresponding to the former photophase but were elevated at ZT4 (4th h of prior DS). Following dark extension of the DS, melatonin was detectable at ZT5, representing the beginning of the former photophase. Subsequently, there was a decline at ZT9 and ZT12, before rising modestly again at ZT16 (corresponding to last hour of prior NS).
Planned comparisons
Due to the high frequency of non-detectable melatonin values throughout Experiment 2, Wilcoxon's matched-pairs signed-ranks analyses were used in Experiment #2a-b to detect differences in melatonin concentrations of Syrian hamsters in the entrained LDLD state compared to the same ZT in extended darkness. There were significant (within-subject) increases in plasma melatonin concentration in hamsters bled in extended dark when compared to prior melatonin concentrations obtained during equivalent sampling times in LDLD8:4:8:4 at ZT4, ZT5, and ZT16, but not at ZT17 (T = 3, p < 0.039; T = 1, p < 0.008; T = 2, p < 0.023; T = 15.5, ns, respectively; n = 8 in all groups).
DISCUSSION
The present study documents in Siberian hamsters a novel melatonin secretion pattern characterised by two discrete and equally robust bouts of melatonin secretion entrained by light and separated by approximately 12 h. The two melatonin peaks during LDLD8:4:8:4 were statistically indistinguishable from values obtained in the single dark period of LD20:4. A degree of bimodality in melatonin rhythm profiles has been previously demonstrated in various species, including sheep, humans, mice and hamsters under various conditions (15, 18, 31, 32). However, in all these cases, the multiple peaks were either temporally overlapping and/or asymmetric in magnitude. In contrast to Siberian hamsters, Syrian hamsters entrained to the same conditions produced melatonin concentrations that were uniformly low or non-detectable. Additionally, following LDLD8:4:8:4 or LD20:4 entrainment, acutely extending the 4 h scotophases to 16 h revealed only modestly elevated melatonin levels at select times. As these elevations were not equivalent to peak (mid-scotophase) levels from Syrian hamsters entrained to LD14:10, neither LDLD8:4:8:4 or LD20:4 are permissive for robust melatonin production in this species.
To date, entrainment bifurcation in rodents has been characterised most extensively in terms of locomotor activity and probed with respect to central clock organization (25, 33). Those studies have determined that the bifurcated state reflects a temporal dissociation of functionally defined oscillators that interact strongly under constant conditions to favor a non-bifurcated state (25, 33). The bifurcated activity pattern is maintained indefinitely, however, under LDLD conditions that prevent the oscillators from re-joining. Like other circadian pacemaker dissociation paradigms (splitting under constant light, forced desynchrony in 22 h LD cycles), this paradigm likely reflects a functional reorganization of the cellular network in the SCN (24, 28, 34, 35). At least in Siberian hamsters, the present study extends the characterization of the bifurcated state to include a strongly circadian neuroendocrine output, i.e., melatonin synthesis. Further, as a gold-standard marker of subjective night, the melatonin secretion profile corroborates a key inference regarding the bifurcated circadian pacemaker: namely, that the bifurcated state includes two short and separate subjective nights (periods of high melatonin, high sensitivity to resetting, high locomotor activity) that are separated by two short and separate subjective days (periods of low melatonin, resetting insensitivity, locomotor inactivity). This bifurcated entrainment pattern does not represent negative masking by light of one long subjective 16 h night spanning both activity bouts. The latter possibility is excluded by the decline in melatonin secretion following each short scotophase, when a complete absence of melatonin between activity bouts was observed: This pattern persisted even when each photophase is replaced by darkness. In addition, melatonin expression resumed at the expected time (e.g., the last 4 h) during extension of darkness.
Pittendrigh and Daan's (17) model of a complex circadian pacemaker, derived from component evening and morning oscillators ((23, 36, 37), has been invoked to explain a number of key observations including seasonal variations in melatonin signal duration and dissociable phase-resetting effects of light pulses on melatonin onset versus offset (3, 5). In one version of the model, two essentially equivalent circadian oscillators (traditionally designated E and M) individually produce a finite duration of nocturnal melatonin synthesis (and other manifestations of subjective night). Thus, seasonal variation of elevated melatonin duration results from the flexible phase angle and degree of temporal overlap among the “building blocks” orchestrated through their differential entrainment. The expression of essentially equivalent melatonin production in each of the two daily scotophases of LDLD as observed here (Figure 2) is thus readily accommodated in this model by the complete temporal separation of two building blocks, formerly associated with E and M, into separate DS and NS components. The 2 oscillator building block model can also account qualitatively for the Siberian melatonin response to LD20:4 with or without acutely extended dark: In LD20:4 the two blocks may overlap completely, producing a single short duration of melatonin production, consistent with our observation of elevated melatonin at ZT15 but not at ZT3 (Figure 2B). The building block model is likewise consistent with the absence of robust melatonin production at ZTs 17, 20, 5, and 8 of extended dark, time points coincident with light (8 h L) prior to acute extension of the DS or NS.
The data from Syrian hamsters, in contrast, fail to support the above model, although the long lag between activity onset and the rise in melatonin secretion characteristic of this species complicates evaluation under entrained LDLD8:4:8:4 and LD20:4 conditions. If melatonin secretion begins 4 or more h after activity onset, its rise near the end of the 4 h scotophase may be non-detectable either because of acute suppression by, or entrainment effects of, the following photophase. However, with extended darkness we found only modest elevations at ZT5 and ZT16 (5th and 16th h of extended DS) with titres well below nighttime values from Syrian hamsters entrained to LD14:10. Additionally, the observation of no significant melatonin elevation either in LD20:4 or in extended darkness after LD20:4 must reflect unspecified consequences of circadian entrainment to this ultra-long photoperiod. That is, acute masking by light does not explain the absence of melatonin in LD20:4. We note that our finding of no melatonin elevation in LD20:4 replicates a previous study (38).
In an alternative complex pacemaker formulation, two distinct oscillators initiate and terminate melatonin secretion, respectively, with their phase angle dictating the overall waveform of the signal. If these hypothetical oscillators were to correspond to the two activity bouts of the bifurcated pacemaker, the model would predict a melatonin rise at the beginning of one activity bout and melatonin offset coinciding with the other activity bout. Disregarding the issue of whether it is the DS or NS component which corresponds to the ON or the OFF oscillator, this model predicts that dark extension of either the NS or DS would produce an immediate appearance of a single, longer melatonin peak. As this pattern was not observed, this formulation of the two-oscillator model must be rejected. Instead, our results from Siberian hamsters suggest that each activity bout of the bifurcated activity rhythm may itself reflect a complex oscillator capable of discretely programming both an onset and an offset of melatonin secretion.
Syrian hamsters displayed non-symmetric response to extended darkness, where melatonin secretion was marginally elevated by one additional hour of darkness at ZT5 (extending DS) but not at ZT17 (extending NS) and was again marginally elevated only at the end (at 16 h) of DS- and not NS-extension. This non-equivalence between the bifurcated oscillators is evident also in non-endocrine measures (Table 1) that replicate previous findings of differing phase angles of entrainment and activity durations comparing the two bifurcated bouts (33). Non-equivalence of DS and NS oscillators has been previously demonstrated in other behavioral and SCN measures (29, 33) (28, 34). Nightly melatonin secretion is initiated by noradrenergic stimulation from sympathetic afferents to the pineal gland, which increases activity of AANAT and HIOMT (acetylserotonin O-methyltransferase), two rate-limiting enzymes in melatonin biosynthesis. Rodent species differ in the details of these adrenergic mechanisms including the necessity of de novo transcription and the temporal gating of adrenergic stimulation of melatonin synthesis by, as yet, not fully specified transcriptional, translational, and post-translational mechanisms (39–41). Conversely, the morning termination of melatonin secretion is understood to reflect not just the withdrawal of adrenergic stimulation but also the actions of inhibitory transcription factors (e.g., inducible cAMP-early repressor, ICER, DREAM) (42)). It remains to be determined more exactly how these ON and OFF molecular factors relate to the present findings and more precisely, how they interact with or are driven by circadian neural signals to yield exquisite seasonally appropriate regulation of melatonin phase and duration (43). In rats, sensitivity of ICER protein levels to the duration of adrenergic stimulation on the night prior has been proposed as one mechanism by which the duration of melatonin secretion is regulated (44).
With regard to the present results, these mechanistic models imply, at a minimum, that the SCN is capable of triggering noradrenergic signals at least twice daily in Siberian hamsters entrained to LDLD and that ICER tone is permissive for melatonin secretion at least twice per cycle. The lack of bimodal melatonin rhythms in Syrian hamsters in LDLD, however, need not imply the absence of a bimodal adrenergic signal as this species is already known to show daytime refractoriness of melatonin biosynthesis to α- and β-adrenergic stimulation (41). Finally, it remains to be determined whether the bimodal melatonin secretion pattern in Siberian hamsters under LDLD occurs in individual pinealocytes or whether separate cell populations in the pineal secrete melatonin unimodally but at alternate circadian phases.
In conclusion, this study extends the characterization of a non-invasive environmental manipulation of circadian waveform and establishes that, in at least one mammalian species, the melatonin rhythm may be entrained to express a stable bimodal pattern. To the extent that suppression of melatonin by light may contribute to adverse health outcomes in shift workers and others with chronobiological disorders (45), a deeper mechanistic understanding of the flexible regulation of melatonin secretion patterns may spur development of more effective strategies for dealing with human shiftwork and jetlag.
Acknowledgments
We would like to thank the McGill Hall veterinary technicians, Robert Sundberg, Antonio Mora, and Melissa Scholl for excellent animal care. Funded by NIH grant NICHD 36460 and NSF grant IBN-0346391.
REFERENCES
- 1.Maywood ES, O'Neill JS, Chesham JE, Hastings MH. Minireview: The circadian clockwork of the suprachiasmatic nuclei--analysis of a cellular oscillator that drives endocrine rhythms. Endocrinol. 2007;148:5624–5634. doi: 10.1210/en.2007-0660. [DOI] [PubMed] [Google Scholar]
- 2.Liu AC, Lewis WG, Kay SA. Mammalian circadian signaling networks and therapeutic targets. Nat Chem Biol. 2007;3:630–639. doi: 10.1038/nchembio.2007.37. [DOI] [PubMed] [Google Scholar]
- 3.Illnerova H. The suprachiasmatic nucleus and rhythmic pineal melatonin production. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: the Mind's Clock. New York: Oxford University Press; 1991. pp. 197–216. [Google Scholar]
- 4.Moore RY, Klein DC. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 1974;71:17–33. doi: 10.1016/0006-8993(74)90188-7. [DOI] [PubMed] [Google Scholar]
- 5.Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol A. 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- 6.Borjigin J, Samantha Zhang L, Calinescu AA. Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol. doi: 10.1016/j.mce.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yellon SM, Tamarkin L, Pratt BL, Goldman BD. Pineal melatonin in the Djungarian hamster: photoperiodic regulation of a circadian rhythm. Endocrinol. 1982;111:488–492. doi: 10.1210/endo-111-2-488. [DOI] [PubMed] [Google Scholar]
- 8.Bartness TJ, Goldman BD. Mammalian pineal melatonin: a clock for all seasons. Experientia. 1989;45:939–945. doi: 10.1007/BF01953051. [DOI] [PubMed] [Google Scholar]
- 9.Darrow JM, Goldman BD. Circadian regulation of pineal melatonin and reproduction in the Djungarian hamster. J Biol Rhythms. 1985;1:39–54. doi: 10.1177/074873048600100106. [DOI] [PubMed] [Google Scholar]
- 10.Elliott JA. Circadian rhythms and photoperiodic time measurement in mammals. Fed Proc. 1976;35:2339–2346. [PubMed] [Google Scholar]
- 11.Tamarkin L, Baird CJ, Almeida OFX. Melatonin: a coordinating signal for mammalian reproduction? Science. 1985;227:714–720. doi: 10.1126/science.3881822. [DOI] [PubMed] [Google Scholar]
- 12.Stehle JH, von Gall C, Schomerus C, Korf HW. Of rodents and ungulates and melatonin: creating a uniform code for darkness by different signaling mechanisms. J Biol Rhythms. 2001;16:312–325. doi: 10.1177/074873001129002033. [DOI] [PubMed] [Google Scholar]
- 13.Lerchl A, Schlatt S. Serotonin content and melatonin production in the pineal gland of the male Djungarian hamster (Phodopus sungorus) J Pineal Res. 1992;12:128–134. doi: 10.1111/j.1600-079x.1992.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 14.Illnerova H, Vanecek J. Extension of the rat pineal N-acetyltransferase rhythm in continuous darkness and on short photoperiod. Brain Res. 1983;261:176–179. doi: 10.1016/0006-8993(83)91301-x. [DOI] [PubMed] [Google Scholar]
- 15.Wehr TA, Schwartz PJ, Turner EH, Feldman-Naim S, Drake CL, Rosenthal NE. Bimodal patterns of human melatonin secretion consistent with a two-oscillator model of regulation. Neurosci Lett. 1995;194:105–108. doi: 10.1016/0304-3940(95)11740-n. [DOI] [PubMed] [Google Scholar]
- 16.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J Comp Physiol A. 1976;106:333–355. [Google Scholar]
- 17.Daan S, Berde C. Two coupled oscillators: simulations of the circadian pacemaker in mammalian activity rhythms. J Theor Biol. 1978;70:297–313. doi: 10.1016/0022-5193(78)90378-8. [DOI] [PubMed] [Google Scholar]
- 18.Arendt J, Symons AM, Laud C. Pineal function in the sheep: evidence for a possible mechanism mediating seasonal reproductive activity. Experientia. 1981;37:584–586. doi: 10.1007/BF01990063. [DOI] [PubMed] [Google Scholar]
- 19.Brainard GC, Richardson BA, Hurlbut EC, Steinlechner S, Matthews SA, Reiter RJ. The influence of various irradiances of artificial light, twilight, and moonlight on the suppression of pineal melatonin content in the Syrian hamster. J Pineal Res. 1984;1:105–119. doi: 10.1111/j.1600-079x.1984.tb00202.x. [DOI] [PubMed] [Google Scholar]
- 20.Rohling J, Wolters L, Meijer JH. Simulation of day-length encoding in the SCN: from single-cell to tissue-level organization. J Biol Rhythms. 2006;21:301–313. doi: 10.1177/0748730406290317. [DOI] [PubMed] [Google Scholar]
- 21.Jagota A, de la Iglesia HO, Schwartz WJ. Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nat Neurosci. 2000;3:372–376. doi: 10.1038/73943. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki N, Honma S, Ono D, Tanahashi Y, Honma K. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc Natl Acad Sci U S A. 2007;104:7664–7669. doi: 10.1073/pnas.0607713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz MD, Wotus C, Liu T, Friesen WO, Borjigin J, Oda GA, de la Iglesia HO. Dissociation of circadian and light inhibition of melatonin release through forced desynchronization in the rat. Proc Natl Acad Sci U S A. 2009;106:17540–17545. doi: 10.1073/pnas.0906382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Iglesia HO, Cambras T, Schwartz WJ, Diez-Noguera A. Forced desynchronization of dual circadian oscillators within the rat suprachiasmatic nucleus. Curr Biol. 2004;14:796–800. doi: 10.1016/j.cub.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 25.Gorman MR, Elliott JA. Entrainment of 2 subjective nights by daily light:dark:light:dark cycles in 3 rodent species. J Biol Rhythms. 2003;18:502–512. doi: 10.1177/0748730403260219. [DOI] [PubMed] [Google Scholar]
- 26.Gorman MR. Exotic photoperiods induce and entrain split circadian activity rhythms in hamsters. J Comp Physiol A. 2001;187:793–800. doi: 10.1007/s00359-001-0250-1. [DOI] [PubMed] [Google Scholar]
- 27.Gorman MR, Elliott JA, Evans JA. Plasticity of hamster circadian entrainment patterns depends on light intensity. Chronobiol Int. 2003;20:233–248. doi: 10.1081/cbi-120018576. [DOI] [PubMed] [Google Scholar]
- 28.Yan L, Silver R, Gorman M. Reorganization of suprachiasmatic nucleus networks under 24-h LDLD conditions. J Biol Rhythms. 2010;25:19–27. doi: 10.1177/0748730409352054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorman MR, Steele NA. Phase angle difference alters coupling relations of functionally distinct circadian oscillators revealed by rhythm splitting. J Biol Rhythms. 2006;21:195–205. doi: 10.1177/0748730406287665. [DOI] [PubMed] [Google Scholar]
- 30.Evans JA, Elliott JA, Gorman MR. Circadian effects of light no brighter than moonlight. J Biol Rhythms. 2007;22:356–367. doi: 10.1177/0748730407301988. [DOI] [PubMed] [Google Scholar]
- 31.Nakahara D, Nakamura M, Iigo M, Okamura H. Bimodal circadian secretion of melatonin from the pineal gland in a living CBA mouse. Proc Natl Acad Sci U S A. 2003;100:9584–9589. doi: 10.1073/pnas.1631069100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerchl A, Nieschlag E. Interruption of nocturnal pineal melatonin synthesis in spontaneous recrudescent Djungarian hamsters (Phodopus sungorus) J Pineal Res. 1992;13:36–41. doi: 10.1111/j.1600-079x.1992.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 33.Evans JA, Elliott JA, Gorman MR. Dynamic interactions between coupled oscillators within the hamster circadian pacemaker. Behav Neurosci. 2010;124:87–96. doi: 10.1037/a0018088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe T, Naito E, Nakao N, Tei H, Yoshimura T, Ebihara S. Bimodal clock gene expression in mouse suprachiasmatic nucleus and peripheral tissues under a 7-hour light and 5-hour dark schedule. J Biol Rhythms. 2007;22:58–68. doi: 10.1177/0748730406295435. [DOI] [PubMed] [Google Scholar]
- 35.de la Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290:799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- 35.Daan S, Albrecht U, van der Horst GT, Illnerova H, Roenneberg T, Wehr TA, Schwartz WJ. Assembling a clock for all seasons: are there M and E oscillators in the genes? J Biol Rhythms. 2001;16:105–116. doi: 10.1177/074873001129001809. [DOI] [PubMed] [Google Scholar]
- 37.Gorman MR, Yellon SM, Lee TM. Temporal reorganization of the suprachiasmatic nuclei in hamsters with split circadian rhythms. J Biol Rhythms. 2001;16:552–563. doi: 10.1177/074873001129002240. [DOI] [PubMed] [Google Scholar]
- 38.Tamarkin L, Reppert SM, Klein DC, Pratt B, Goldman BD. Studies on the daily pattern of pineal melatonin in the Syrian hamster. Endocrinol. 1980;107:1525–1529. doi: 10.1210/endo-107-5-1525. [DOI] [PubMed] [Google Scholar]
- 39.Karolczak M, Korf HW, Stehle JH. The rhythm and blues of gene expression in the rodent pineal gland. Endocrine. 2005;27:89–100. doi: 10.1385/ENDO:27:2:089. [DOI] [PubMed] [Google Scholar]
- 40.Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep. 2009;61:383–410. doi: 10.1016/s1734-1140(09)70081-7. [DOI] [PubMed] [Google Scholar]
- 41.Simonneaux V, Sinitskaya N, Salingre A, Garidou ML, Pevet P. Rat and Syrian hamster: two models for the regulation of AANAT gene expression. Chronobiol Int. 2006;23:351–359. doi: 10.1080/07420520500521962. [DOI] [PubMed] [Google Scholar]
- 42.Maronde E, Pfeffer M, Glass Y, Stehle JH. Transcription factor dynamics in pineal gland and liver of the Syrian hamster (Mesocricetus auratus) adapts to prevailing photoperiod. J Pineal Res. 2007;43:16–24. doi: 10.1111/j.1600-079X.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- 43.Mason R. The effects of continuous light exposure on Syrian hamster suprachiasmatic (SCN) neuronal discharge activity in vitro. Neurosci Lett. 1991;123:160–163. doi: 10.1016/0304-3940(91)90920-o. [DOI] [PubMed] [Google Scholar]
- 44.Foulkes NS, Duval G, Sassone-Corsi P. Adaptive inducibility of CREM as transcriptional memory of circadian rhythms. Nature. 1996;381:83–85. doi: 10.1038/381083a0. [DOI] [PubMed] [Google Scholar]
- 45.Schernhammer E, Schulmeister K. Light at night and cancer risk. Photochem Photobiol. 2004;79:316–318. doi: 10.1562/sa-03-28.1. [DOI] [PubMed] [Google Scholar]