Abstract
Background
Sudden cardiac death (SCD) is the leading global cause of mortality, exhibiting increased incidence in diabetics. Ion channel gene perturbations provide a well-established ventricular arrhythmogenic substrate for SCD. However, most arrhythmia susceptibility genes - including the KCNE2 K+ channel β subunit - are expressed in multiple tissues, suggesting potential multiplex SCD substrates.
Methods and Results
Using “whole transcript” transcriptomics, we uncovered cardiac angiotensinogen upregulation and remodeling of cardiac angiotensinogen interaction networks in P21 Kcne2−/− mouse pups, and adrenal remodeling consistent with metabolic syndrome in adult Kcne2−/− mice. This led to the discovery that Kcne2 disruption causes multiple acknowledged SCD substrates of extracardiac origin: diabetes, hypercholesterolemia, hyperkalemia, anemia and elevated angiotensin II. Kcne2 deletion was also prerequisite for aging-dependent QT prolongation, ventricular fibrillation and SCD immediately following transient ischemia, and fasting-dependent hypoglycemia, myocardial ischemia and atrioventricular block.
Conclusions
Disruption of a single, widely expressed arrhythmia susceptibility gene can generate a multisystem syndrome comprising manifold electrical and systemic substrates and triggers of SCD. This paradigm is expected to apply to other arrhythmia susceptibility genes, the majority of which encode ubiquitously expressed ion channel subunits or regulatory proteins.
Keywords: arrhythmia (mechanisms), ion channel, ischemia, potassium, sudden cardiac death, arrhythmia
Introduction
Sudden cardiac death (SCD) is the term given to sudden, unexpected cessation of cardiac function. It is estimated that more than 1 in 1000 people in developed nations succumb to SCD annually, and it is the leading cause of death worldwide1. Distinct from a heart attack, in which the heart continues to beat but blood flow is blocked, in SCD the heart ceases to beat because of catastrophic, sustained ventricular fibrillation (VF). Without defibrillation within minutes, this type of event is fatal; individuals who survive require an implantable cardiac defibrillator to protect against future SCD incidence2. SCD typically occurs without evidence of a myocardial infarction, but is considered to most often require an ischemic substrate or heart failure. Diabetes mellitus is an established risk factor for both myocardial ischemia3 and SCD, the latter even after adjustment for other risk factors that accompany diabetes4. “Dead-in-bed” Syndrome (DIBS), the overnight sudden unexplained death of type I diabetics, may be linked to nocturnal hypoglycemia precipitating lethal ventricular arrhythmias 5; hypoglycemia linked to tight glycemic control is also associated with and predictive of increased mortality in type II diabetics6.
Younger SCD victims may lack detectable ischemic substrates and in these cases in particular, a genetic electrical disorder of cardiac origin is often implicated. More than 25 genes have thus far been identified that are linked or associated with ventricular arrhythmias predisposing to SCD2. These genes all encode cardiac-expressed ion channel subunits or channel interacting proteins; their disruption is considered sufficient to cause monogenic, lethal ventricular arrhythmias by direct perturbation of ion currents orchestrating ventricular myocyte excitability2.
Most arrhythmia susceptibility genes are not, however, expressed exclusively in the heart, suggesting the possibility that monogenic arrhythmia syndromes are complex, multisystem disorders. Here, we report that deletion of the widely expressed KCNE2 K+ channel β subunit gene7, 8 creates a multifactorial substrate for SCD that includes diabetes, dyslipidemia, hyperkalemia and progressive loss of ventricular repolarization reserve. The findings support the idea of multiplex origins even in monogenic ventricular arrhythmias.
Methods
We generated and genotyped the wild-type, heterozygous, Kcne2−/−, Kcne3−/−, and double-knockout Kcne2−/−Kcne3−/− mice as previously described9, 10 and housed and used them according to the US National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal procedures were approved by the Animal Care and Use Committees at Weill Medical College of Cornell University and University of California, Irvine. All adult mice used in this study, aside from breeding males, were female and generated from Kcne2+/− × Kcne2+/− crosses. Except where indicated, two age-groups of adult mice were used, referred to as “4m-old” and “7m-old”. For functional studies, the 4m-old group were of mean age when studied: 4.3 ± 0.1 months, n = 27 (Kcne2+/+) versus 4.2 ± 0.1 months, n = 28 (Kcne2−/−). The 7m-old group were of mean age when studied: 7.4 ± 0.1 months, n = 30 (Kcne2+/+) versus 7.4 ± 0.4 months, n = 42 (Kcne2−/−). The P21 pups studied for the microarray comparison of maternal versus pup genotype effects were all male and were generated from either Kcne2+/− × Kcne2+/− or Kcne2−/− × Kcne2−/− crosses as indicated.
Briefly, statistical analyses were generally conducted as follows: unpaired student’s t-test for comparison of two groups; one-way ANOVA followed by Tukey’s HSD test or Bonferroni test for comparison of multiple groups; repeated measures two-way ANOVA followed by post-hoc Bonferroni correction for ST heights over time. Raw microarray data were analyzed using Partek Express software (Partek, St. Louis, MO). Ingenuity Systems iReport software (Qiagen, Hilden, Germany) was used to independently compare raw microarray data and for pathway and network analysis, using default settings. Unless otherwise described, the Kolmogorov-Smirnov test was used to verify the assumption of normal distribution. Statistical significance was assumed with p < 0.05. Detailed methods and associated references are described in the Online Supplemental Methods section.
Results
Kcne2 deletion causes maternal genotype-dependent and independent cardiac remodeling
Kcne2−/− pups of Kcne2−/− dams exhibit much more substantial cardiac hypertrophy at P21 than do Kcne2−/− pups of Kcne2+/− dams (Figure 1A), primarily arising from complications of maternal hypothyroidism during gestation and lactation11. Specifically, we previously demonstrated that thyroid KCNQ1-KCNE2 potassium channels are required for optimal thyroid hormone biosynthesis; Kcne2 deletion causes hypothyroidism during gestation and lactation in mice but not in non-pregnant/lactating adult mice. The most profound consequences of this are observed pre-weaning in the pups of Kcne2−/− dams, which exhibit alopecia, stunted growth and cardiac hypertrophy. These manifestations are closely associated to maternal hypothyroidism as they can be largely prevented by thyroid hormone treatment of the dam during gestation and lactation, or by surrogacy with wild-type dams at P1. Conversely, these symptoms can be initiated in wild-type pups by surrogacy with Kcne2−/− dams. One of the main mechanistic explanations for these effects is that maternal hypothyroidism impairs milk ejection11.
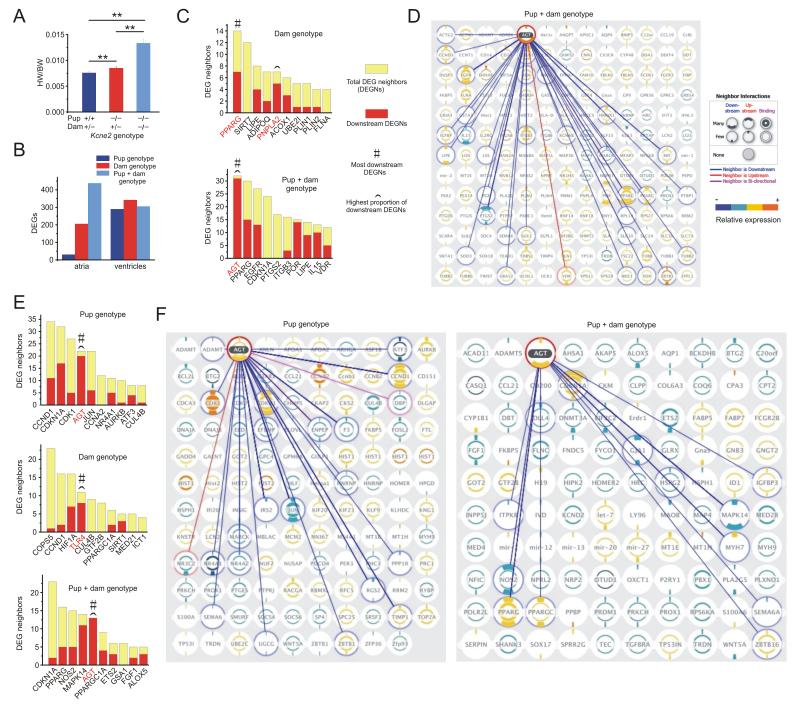
Figure 1.
Kcne2 deletion remodels cardiac angiotensinogen-associated gene interaction networks. A. Effects of pup and maternal Kcne2 genotype on heart weight/body weight of P21 pups (n = 8). **p < 0.01. B. Cardiac DEGs (quantified by microarray analysis, p < 0.05 significance level used for this and all other comparisons, n = 7-8 pups per group) in P21 Kcne2−/− versus Kcne2+/+ pups of either Kcne2+/− or Kcne2−/− dams, grouped as follows: Pup genotype - Kcne2−/− versus Kcne2+/+ pups, all from Kcne2+/− dams; dam genotype - Kcne2+/− versus Kcne2−/− dams, all Kcne2−/− pups; Pup + dam genotype - Kcne2−/− pups from Kcne2−/− dams versus Kcne2+/+ pups from Kcne2+/− dams. C. Top ten ranked (in terms of number of interacting DEGs) atrial DEGs participating in predicted interaction networks with other DEGs, dependent on dam genotype (upper) or pup + dam genotype (lower) (groups as in panel b). Pup genotype alone yielded no significantly altered interaction networks of >2 DEGs. D. Atrial DEG interaction network of angiotensinogen (AGT), the top ranked upstream effector DEG (atria of Kcne2−/− pups from Kcne2−/− dams versus Kcne2+/+ pups from Kcne2+/− dams) showing all its predicted interaction DEGs (connected by lines) and all other DEGs predicted to have one or more interactions with other DEGs (no connecting lines shown). Right, key for interaction hierarchy, number of interacting DEGs, and relative expression. E. Top ten ranked (in terms of number of interacting neighbors) ventricular DEGs participating in predicted interaction networks with other DEGs, dependent on pup genotype (upper), dam genotype (middle) or pup + dam genotype (lower) (groups as in panel B). F. Ventricular DEG interaction network of angiotensinogen (AGT), the top ranked upstream effector DEG (left, pup-genotype dependent; right, pup + dam genotype-dependent) showing all predicted interacting DEGs (connected to AGT by lines) and all other DEGs predicted to have one or more interactions with other DEGs (no connecting lines shown). Key for interaction hierarchy, number of interacting DEGs, and relative expression as shown in panel D.
Here, global whole-transcript microarray analysis revealed differential atrial and ventricular gene network remodeling dependent on pup and/or maternal Kcne2 genotype (Figure 1B); for all differentially expressed genes (DEGs) see Supplementary Spreadsheet 1. Surprisingly, gene interaction hierarchy analysis of predicted DEG interaction networks uncovered AGT, which encodes angiotensinogen, as the most common predicted upstream effector in pup genotype-dependent cardiac remodeling (Figure 1C-F). Dam genotype-dependent, pup genotype-independent predicted pathways were, in contrast, headed by PPARG and PNPLA2 in atria (Figure 1C,D) and TLR4 in the ventricles (Figure 1E,F); the latter finding being consistent with the previously reported role for this gene in ventricular hypertrophy12.
Kcne2 deletion elevates serum angiotensin II and causes adrenal dysfunction and lipid accumulation
Angiotensinogen is cleaved by renin (in response to decreased renal perfusion) to generate angiotensin I, which is converted by ACE to angiotensin II. Cardiac myocyte angiotensinogen expression is increased in an autocrine loop by angiotensin II13 and, accordingly, here we found that serum angiotensin II concentration in Kcne2−/− mice was twice that of Kcne2+/+ littermates (n = 10-14, p = 0.012) (Figure 2A). However, serum aldosterone, which is typically released by the adrenal gland upon angiotensin II stimulation, was unaltered (n = 9, p = 0.95) (Figure 2B). The adrenal unresponsiveness could not be explained by disruption of a direct adrenal role of Kcne2 because it is not expressed in the adrenal glands (Figure 2C). We detected Kcne2 in the lung (Figure 2C), the primary source of angiotensin converting enzyme (ACE), but Kcne2 deletion reduced pulmonary ACE expression (Figure 2D). This is consistent with activation of feedback mechanisms to reduce angiotensin I to II conversion, and suggests against the possibility that pulmonary Kcne2 deficiency increased serum angiotensin II by directly increasing ACE expression in the lung.
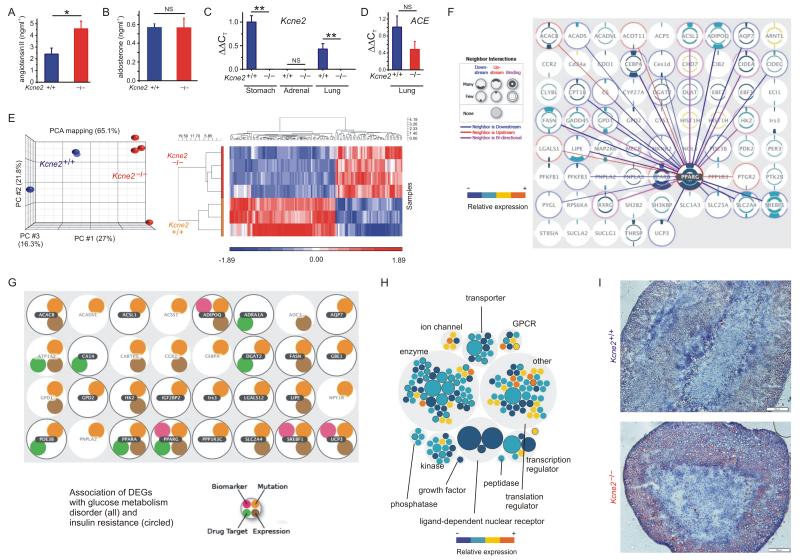
Figure 2.
Kcne2 deletion elevates angiotensin II and causes adrenal remodeling and lipid accumulation. A. Mean serum [angiotensin II] of 7m-old Kcne2+/+ and Kcne2−/− mice (n = 10-14). *p = 0.012. B. Mean serum [aldosterone] for 7m-old Kcne2+/+ and Kcne2−/− mice (n = 9). NS, no significant difference between groups. C. Relative expression of Kcne2 transcript in Kcne2+/+ stomach, lung and adrenal tissue (n = 4). **p < 0.01; NS, no significant difference between groups. D. Relative expression of ACE transcript in Kcne2+/+ and Kcne2−/− lung tissue (n = 3). NS, no significant difference between groups. E. Left, principle component analysis (PCA); right, heat map display of global transcript expression differences, after microarray analysis of the adrenal transcriptomes of Kcne2+/+ and Kcne2−/− mice (n = 3-4). Percentage values indicate the percentage of explained variance. F. Adrenal DEG interaction network (DEGs between Kcne2+/+ and Kcne2−/− adrenals) showing all DEGs with one or more predicted DEG interaction neighbors. Predicted interactions are shown between the most common upstream DEG (PPARG) and its neighbors. G. DEGS within the two highest ranking disease processes predicted by analysis of adrenal DEGs in Kcne2−/− versus Kcne2+/+ mice (glucose metabolism disorder, p = 8.5 × 10−10) and insulin resistance, p = 2.1 × 10−10). H. Adrenal DEGs grouped by function, sized by number of interacting neighbors, and colored by relative expression (Kcne2−/− versus Kcne2+/+). DEGs associated with the highest ranking category of disorder based on DEGs (metabolic syndrome) are outlined. I. Representative (n = 4 per genotype) micrographs of Oil Red O stained adrenal gland sections from 6-month-old female Kcne2+/+ and Kcne2−/− mice (scale bars, 20 μm).
Microarray analysis of the adrenal transcriptome (Figure 2E) indicated predominantly downregulation, in adult Kcne2−/− mouse adrenals, of expression of genes and gene networks - with PPARG as the most common upstream element (Figure 2F) - associated with lipid metabolism and uptake (for all differentially expressed transcripts, see Supplementary Spreadsheet 2), glucose metabolism disorders and insulin resistance (Figure 2G) and metabolic syndrome (Figure 2H). The data suggested a compensatory response to hyperstimulation or aberrant adrenal lipid accumulation, a diabetic/hyperlipidemic environment, and/or a defect in steroidogenesis, in Kcne2−/− adrenals.
Adrenal glands of Kcne2−/− mice did not exhibit macroscopic hypertrophy as would be expected in primary hyperaldosteronism, but Kcne2 deletion caused adrenal cortex vacuolation and lipid accumulation consistent with chronic hyperstimulation as occurs with chronic stimulation by angiotensin II (Figure 2I), also consistent with diabetic dyslipidemia14.
Kcne2 deletion causes dyslipidemia, diabetes and anemia
Examining potential causes of the adrenal steatosis, we discovered that Kcne2 deletion causes hypercholesterolemia - raising free LDL cholesterol, but not altering free HDL cholesterol or cholesteryl esters (Figure 3A). Strikingly, Kcne2−/− mice, but not their Kcne2+/+ littermates, also develop diabetes, characterized by fasting hypoglycemia (Figure 3B) and impaired glucose tolerance (Figure 3C,D). We detected Kcne2 expression in mouse pancreas, suggesting Kcne2 deletion may pathologically impair a pancreatic K+ current (Figure 3E).
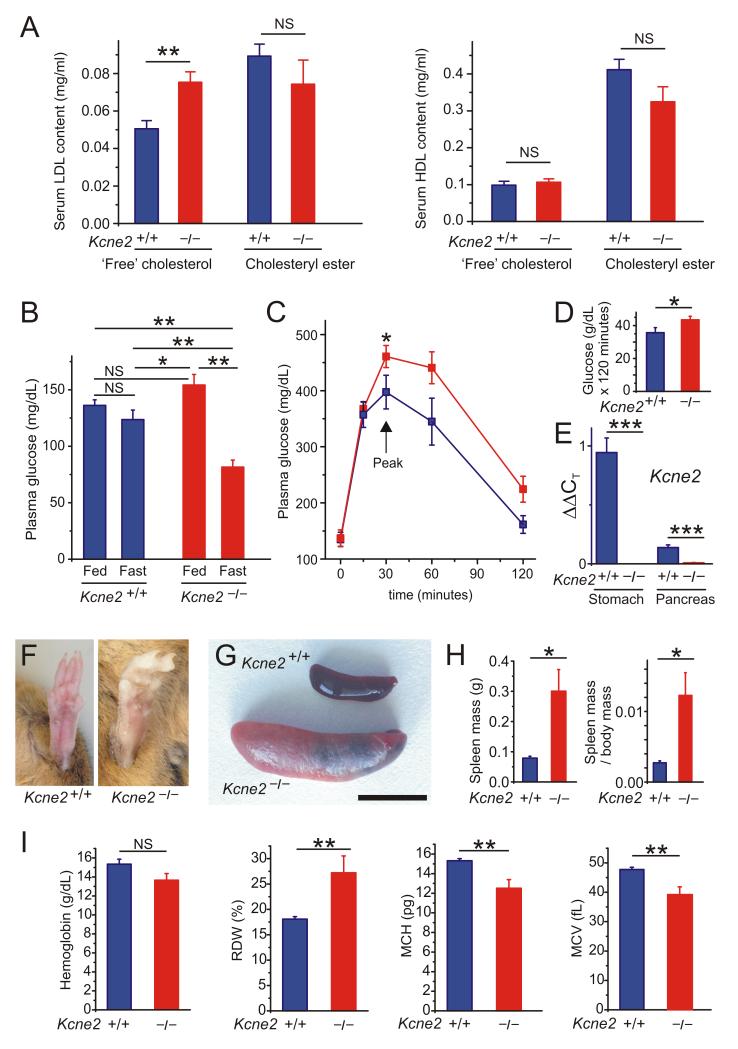
Figure 3.
Kcne2 deletion causes anemia, diabetes and dyslipidemia. A. Mean serum lipid content of 7m-old female Kcne2+/+ and Kcne2−/− mice (n = 11). **p = 0.002; NS, no significant difference between genotypes. B. Mean blood glucose content of 7m-old Kcne2+/+ and Kcne2−/− mice (n = 16-19). *p <0.05; **p < 0.01; NS, no significant difference between groups. C. Glucose tolerance test results: mean blood glucose following glucose injection in 7m-old Kcne2+/+ (n = 11) and Kcne2−/− (n = 16) mice; *p < 0 .05 between genotypes at peak glucose level. D. Integration of area under curve from data in panel C using trapezoidal rule; *p < 0.05. E. Real-time qPCR detection of Kcne2 cDNA in stomach and pancreas of Kcne2+/+ mice with Kcne2−/− mouse tissue as a negative control; ***p<0.001. F. Hind feet of adult Kcne2+/+ and Kcne2−/− mice showing example of pale extremities in the latter. G. Exemplar spleens from adult Kcne2+/+ and Kcne2−/− mice showing splenomegaly in the latter. Scale bar, 1 cm. H. Mean spleen mass and spleen mass/body mass for 7m-old Kcne2+/+ and Kcne2−/− mice (n = 7-9). *p < 0.05. I. Hemoglobin and red blood cell parameters of 4m-old Kcne2+/+ and Kcne2−/− mice (n = 5-7). **p < 0.01. NS, no significant difference between genotypes. RDW, red blood cell distribution width; MCH, mean corpuscular hemoglobin; MVC, mean corpuscular volume.
In addition, after observing that Kcne2−/− mice often exhibit paler extremities than their Kcne2+/+ littermates (Figure 3F) we also found that Kcne2−/− mice exhibit splenomegaly (4.2-fold increased mean body mass-corrected spleen mass) (Figure 3G,H). One likely cause for these two observations is anemia. Kcne2 deletion causes achlorhydria because the KCNQ1-KCNE2 K+ channel is expressed on the apical membrane of parietal cells and is required for gastric acid secretion9. One consequence of achlorhydria is anemia because of inadequate iron absorption and vitamin B12 deficiency. Here we quantified blood cell parameters and found that Kcne2−/− mice exhibit anisocytosis and microcytic anemia, specific hallmarks of iron-deficiency anemia; the lowered mean corpuscular hemoglobin was also consistent with this (Figure 3I).
Anemia, hypercholesterolemia and diabetes all diminish myocardial oxygen supply, predispose to chronic myocardial ischemia15, and are each associated with increased risk of human SCD16-18. In addition, fasting-induced hypoglycemia is considered an important risk factor for diabetic SCD in the form of DIBS19, 20.
Kcne2 deletion causes hyperkalemia and QTc prolongation
In normal circumstances, hyperkalemia stimulates the renin-angiotensin-aldosterone system (RAAS), elevating angiotensin II and also aldosterone, increasing sodium reabsorption and potassium excretion by the kidneys, increasing blood pressure. Here, we found that Kcne2−/− mice show significant hyperkalemia (8.2 ± 0.44 mM K+, n = 23) compared to Kcne2+/+ mice (6.4 ± 0.23 mM K+, n = 40; p < 0.01) (Figure 4A). Chronic hyperkalemia would be expected to chronically elevate serum angiotensin II and, in combination with other destructive influences on the adrenal (Figures 2 & 3), lead to impaired aldosterone production (Figure 2B) and inability to restore normokalemia. Interestingly, the high angiotensin II did not elevate blood pressure in Kcne2−/− mice, which was slightly lower than that of age-matched Kcne2+/+ littermates (Supplementary Figure 1), possibly reflecting an inability to excrete sufficient K+.
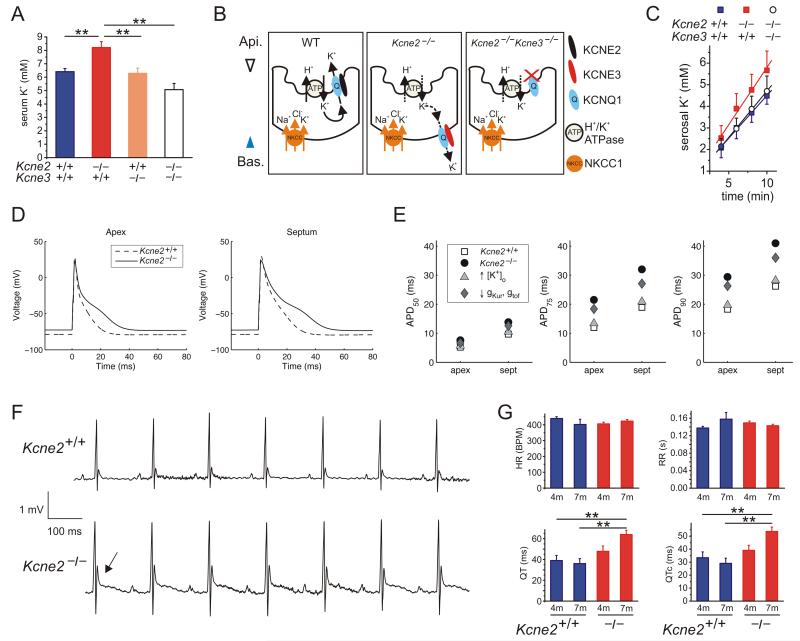
Figure 4.
Kcne2 deletion causes hyperkalemia and age-dependent QTc prolongation. A. Mean serum [K+] for 4m-old Kcne2+/+Kcne3+/+ mice (n = 40) or mice lacking Kcne2 (n = 23), Kcne3 (n = 18), or both Kcne2 and Kcne3 genes (n = 24) as indicated by −/−. **p < 0.01. All other group comparisons showed p > 0.05. B. Schematic (from 10) showing hypothesized short circuit K+ current in the gastric parietal cells of Kcne2−/− mice (middle panel) compared to wild-type mice (left-hand panel), arising from the previously observed switch in KCNQ1 trafficking from apical (wild-type, left-hand panel) to basolateral because of KCNE3 upregulation (middle panel)10. In double-knockout Kcne2−/− Kcne3−/− mice, KCNQ1 is apical but probably nonfunctional because it lacks KCNE2 and KCNE3, which confer constitutive activation (right-hand panel)10. C. Mean K+ flux across whole ex vivo histamine-stimulated stomach mucosae, compared by quantifying the linear phase of serosal K+ accumulation with a constant supply of K+ to the luminal side. Wild-type (Kcne2+/+Kcne3+/+) stomachs (n = 8) were compared to those harvested from Kcne2−/− (n = 9) and Kcne2−/− Kcne3−/− (n = 8) mice. Slopes were not significantly different between genotypes. D. In silico models of Kcne2+/+ and Kcne2−/− mouse ventricle apex and septum myocyte action potentials generated using serum K+ data (panel A) and our previous ventricular myocyte patch clamp recordings23. E. Time taken for 50, 75 and 90% repolarization (APD50, 75 and 90) from models as in panel d, with either Kcne2 deletion-linked myocyte conductance (reduced gKur and gtof), or serum K+ perturbation (increased [K+]o), or both (Kcne2−/−), incorporated and compared to the Kcne2+/+ simulation. F. Exemplar surface electrocardiograms (ECGs) from 7m-old female Kcne2+/+ and Kcne2−/− mice showing abnormal T wave (arrow) in the latter. G. Quantification of parameters from ECGs as in panel F showing age-dependent QT and QTc prolongation in Kcne2−/− mice (n = 8-15). **p < 0.01. All other group comparisons p > 0.05.
KCNQ1-KCNE2 K+ channels generate K+ recycling currents in gastric parietal cells and other epithelia. KCNE2, in parietal cell apical membrane complexes with KCNQ1, is required to return to the stomach lumen K+ ions brought into the cell by the H+/K+ATPase during gastric acid secretion9, 21. We previously found that Kcne2 deletion results in aberrant parietal cell basolateral targeting of KCNQ1 in complexes with pathologically upregulated KCNE310. This could provide a gastric short-circuit current funneling K+ from the stomach through to the blood, robbing the stomach lumen of K+ (consistent with the observed achlorhydria in Kcne2−/− mice) and potentially increasing serum K+ (Figure 4B). To test this hypothesis, we first quantified serum K+ in Kcne3−/− and double-knockout Kcne2−/− Kcne3−/− mice. While Kcne3−/− mice were normokalemic (n = 18), deletion of both Kcne2 and Kcne3 genes caused a statistically non-significant trend toward hypokalemia (serum K+ of 5.1 ± 0.46 mM, n = 24; p > 0.05 vs. wild-type). Consistent with this hypothesis, although not achieving statistical significance when comparing mean slopes between genotypes, using an ex vivo stomach preparation we found that the Kcne2−/− stomach mucosa exhibits a trend toward an increased luminal-to-serosal K+ flux compared to either wild-type or double-knockout Kcne2−/−Kcne3−/− mucosae, as predicted by our model (Figure 4A-C).
The findings (Figures 1-4) present a picture of a single gene deletion causing interactive disruption of multiple epithelia, and consequent cardiac gene network remodeling, creating a multifactorial systemic substrate comprising a panoply of known risk factors for SCD. We next examined potential electrical substrates. Hyperkalemia depolarizes cardiac myocyte membranes, counteracting the effects of repolarizing K+ currents, and is in itself proarrhythmic 22. In the mouse ventricular myocardium, Kcne2 regulates Ito and IK,slow by forming complexes with, and augmenting currents through, the Kv4.2 and Kv1.5 Kv α subunits, respectively23. We previously demonstrated that Kcne2 deletion reduces ventricular myocyte Ito and IK,slow density in young adult (3-4-month old) mice, and although it does not alter baseline QT or QTc interval, it compromises ventricular myocyte repolarization sufficiently to predispose mice to drug-induced QTc prolongation23.
Here, modeling in silico the effects on ventricular action potentials of the hyperkalemia in Kcne2−/− mice, the hyperkalemia was found to be a significant component in prolonging action potential duration and was predicted to depolarize the resting membrane potential of myocytes in both the ventricular apex and septum by 6.1 mV (from −78.7 to −72.6 mV; Figure 4D,E). Next, we recapitulated the previously observed lack of effects on baseline ventricular repolarization on 4m-old mice23, but examination of older mice (7m-old) revealed an abnormally high-amplitude T wave and a striking delay in returning of the T wave to baseline (Figure 4F) quantified as an 85% increase in heart rate-corrected QT interval (QTc) in Kcne2−/− mice compared to age-matched wild-type littermates (n = 8-15) (Figure 4G). The age-dependent QTc lengthening cannot readily be explained by a “pure” electrical defect caused solely by loss of KCNE2 from ventricular ion channels, and instead is consistent with a more complex etiology involving chronic pathologic changes such as are observed in the cardiac remodeling associated with diabetes and hypercholesterolemia in human subjects and in mouse models24, 25, the molecular underpinnings of which can be detected as early as P21 in Kcne2−/− mice (Figure 1).
Kcne2 deletion generates an electrical substrate for post-ischemic sudden cardiac death
The systemic defects reported above would be predicted to generate myocardial ischemia in aging mice or humans, and in combination with the profound QTc prolongation (Figure 4G) would be expected to predispose to dangerous ventricular tachycardias. Kcne2−/− mice from heterozygous crosses exhibit hypothyroidism and cardiac hypertrophy at 12-15 months11, complicating attempts to correlate specific systemic abnormalities with cardiac events at these advanced ages. Therefore, here we instead standardized ischemia using surgery in 7m-old female mice from Kcne2+/− crosses, that are euthyroid11 and do not yet exhibit cardiac hypertrophy (Supplementary Figure 2), to directly compare the role of electrical and other perturbations caused by Kcne2 deletion in an ischemic context, but without the pathologic structural remodeling that occurs in these mice after 12 months because of hypothyroidism11.
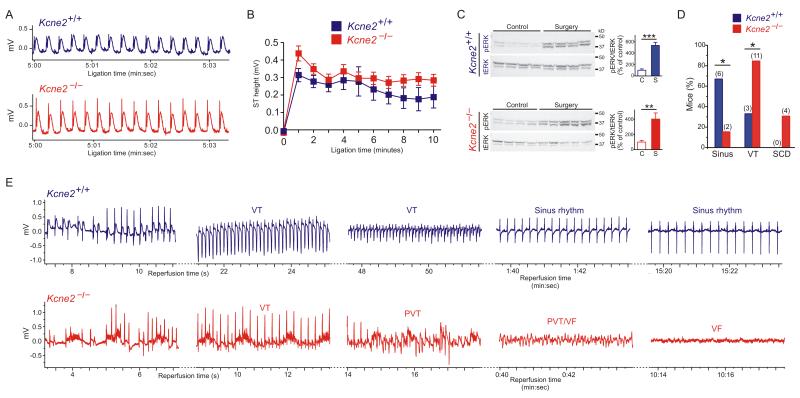
Electrocardiographic analysis during a 10-minute left anterior descending coronary artery ligation revealed similar ST elevation 7m-old Kcne2−/− mice compared to age-matched Kcne2+/+ littermates (Figure 5A,B); myocardial ERK activation measured in hearts isolated after 20 minutes of reperfusion suggested similar ischemic insult in both genotypes (Figure 5C). While two-thirds of Kcne2+/+ mice remained in sinus rhythm throughout reperfusion and the remainder exhibited monomorphic ventricular tachycardia (VT) returning to sinus rhythm within 20 minutes, only 2/13 Kcne2−/− mice remained in sinus rhythm, the rest exhibiting VT. Most strikingly, in 4/13 Kcne2−/− mice monomorphic VT degenerated into polymorphic VT, VF and SCD; this was not observed in Kcne2+/+ mice (Figure 5D,E). Additionally, two Kcne2−/− mice died during the ligation period, with ECGs consistent with acute heart failure (no fibrillation); these are not included in the analysis in Figure 5 because they did not reach the reperfusion stage.
Figure 5.
Kcne2 deletion predisposes to SCD early in reperfusion. A. Representative ECGs recorded during coronary artery ligation of 7m-old female Kcne2+/+ and Kcne2−/− mice (n = 8-12). B. Mean ST heights from traces as in panel A, p > 0.05 between genotypes at all time points (n = 8-12). Groups were not significantly different (by repeated measures ANOVA). C. Left, western blots of ventricular phosphorylated ERK (pERK) and total ERK (tERK) from mice as in panel B, isolated from control (non-operated) mice or after ischemia/reperfusion (surgery); right, pERK/tERK protein band density measured from blots on left (n = 4). **p < 0.01, ***p < 0.001 for control (C) vs. surgery (S). D. Cardiac arrhythmia incidence and mortality during post-ischemia reperfusion in 7m-old female Kcne2+/+ (n = 9) and Kcne2−/− (n = 13) mice. Sinus, remained in sinus rhythm; VT, ventricular tachycardia; SCD, sudden cardiac death arising from VT/VF without recovery. Numbers of animals per category are indicated in parentheses. *p < 0.05 between genotypes. E. Exemplar ECGs of the most severe arrhythmias by genotype, recorded during post-coronary artery ligation reperfusion of 7m-old female Kcne2+/+ and Kcne2−/− mice (n = 9-13). Kcne2+/+ mice always returned to sinus rhythm, unlike Kcne2−/− mice. VT, ventricular tachycardia; PVT, polymorphic ventricular tachycardia; VF, ventricular fibrillation.
Kcne2 deletion causes fasting-induced ischemia, AV block and SCD
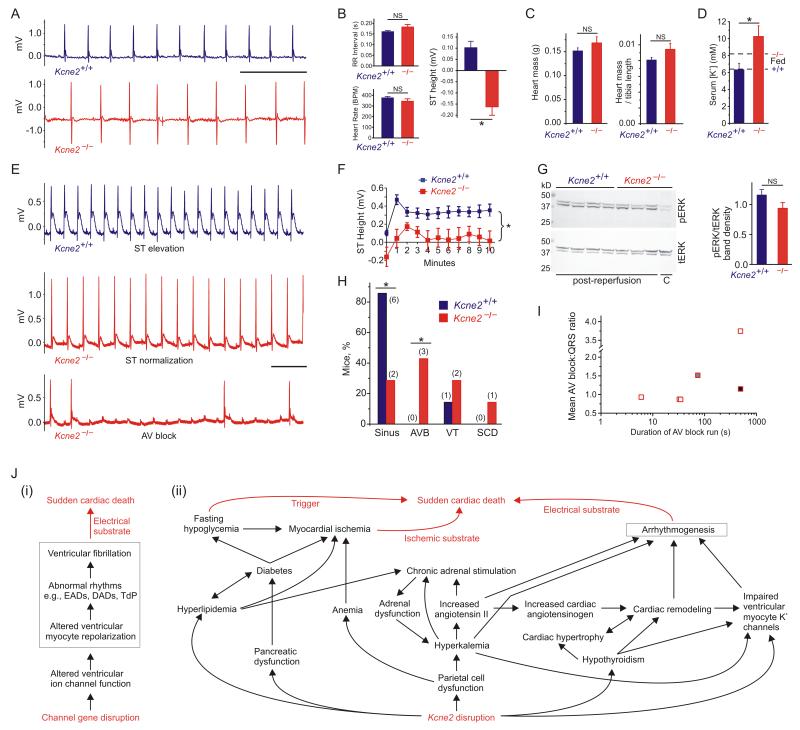
Because of the heightened risk of SCD in hypoglycemic diabetics, we repeated the electrocardiographic studies in a further cohort of 7m-old female mice that had been fasted overnight. Strikingly, fasted Kcne2−/− mice, but not Kcne2+/+ mice, showed marked ST segment depression (Figure 6A,B), a hallmark of myocardial ischemia26 (an additional Kcne2−/− mouse, but no Kcne2+/+ mice, died suddenly during transfer from its cage before an ECG could be recorded). The acute ischemia was caused by overnight fasting, as ST depression was not observed in fed Kcne2−/− mice (Figure 5A,B). Suggesting against a structural defect, cardiac hypertrophy was not detectable (Figure 6C), contrary to what is observed in Kcne2−/− mice at 12-15 months11. Overnight fasting further increased serum [K+] in Kcne2−/− but not Kcne2+/+ mice (Figure 6D); this would be predicted to further prolong the QTc but accurate QTc quantification was not possible because of the ST depression.
Figure 6.
Kcne2 deletion causes fasting-dependent ischemia and AV block during ischemia. A. Representative ECGs recorded from overnight-fasted 7m-old female Kcne2+/+ and Kcne2−/− mice (n = 7). Scale bar, 500 ms. B. Mean electrocardiographic parameters measured from traces recorded from overnight-fasted mice as in panel A (n = 7). *p < 0.05; NS, no significant difference. C. Mean heart mass (absolute and normalized to tibia length) for mice as in panel B. NS, no significant difference. D. Mean post-reperfusion serum [K+] for fasted 7m-old female Kcne2+/+ and Kcne2−/− mice (n = 7). *p < 0.05. Values for fed mice (dashed lines) from Figure 4 A are shown for comparison (n = 23-40). E. Representative ECGs recorded during coronary artery ligation of overnight fasted 7m-old female Kcne2+/+ and Kcne2−/− mice (n = 7). AV block, atrioventricular block. Scale bar, 500 ms. F. Mean ST heights from traces as in panel e, *p < 0.05 between genotypes by repeated measures ANOVA (n = 7). G. Left, western blots of ventricular pERK and tERK from mice as in panel B, isolated from control (C, non-operated) mice or after ischemia/reperfusion (post-reperfusion); right, pERK/tERK protein band density in post-reperfusion mouse ventricles measured from blots on left (n = 3-4). NS, no significant difference. H. Cardiac arrhythmia incidence and mortality during ischemia and post-ischemia reperfusion in 7m-old female Kcne2+/+ and Kcne2−/− mice as in panel f (n = 7). Sinus, remained in sinus rhythm; AVB, atrioventricular block; VT, ventricular tachycardia then recovery to sinus; SCD, sudden cardiac death after AVB. Numbers of animals per category are indicated in parentheses. *p < 0.05. I. Mean AV block:QRS ratio plotted versus duration of runs of AV block for the three Kcne2−/− mice summarized in panel H that exhibited AV block. Each shade indicates a different mouse; one mouse (open squares) exhibited 4 distinct runs of AV block. J. (i) Existing model for the mechanism of monogenic predisposition to SCD. (ii) Multifactorial etiology of Kcne2-associated arrhythmogenesis and SCD supported by the findings herein. EADs, early after-depolarizations; DADs, delayed after-depolarizations; TdP, torsades de pointe.
While overnight fasting did not alter the outcome of coronary artery ligation in Kcne2+/+ mice (Figure 6E,F), overnight-fasted Kcne2−/− mice showed significantly depressed ST height compared to that of Kcne2+/+ mice throughout ligation (Figure 6F) despite exhibiting equivalent cardiac ERK activation (Figure 6G). Kcne2−/− mice also developed severe atrioventricular (AV) block, which was not observed in the absence of fasting (Figure 6E,H,I). This severity of AV block would strongly predispose to syncope and SCD in a human subject27. Notably, hyperkalemia, which was markedly exacerbated here in Kcne2−/− mice by overnight fasting, is associated with potentially lethal AV block, particularly in human subjects with existing factors causing myocardial ischemia28. Interestingly, hypoglycemia more typically results in hypokalemia, itself arrhythmogenic29, highlighting the degree of dysregulation occurring in multiple homeostatic systems because of Kcne2 deletion. Indeed, Kcne2 deletion appears to disrupt RAAS to the extent that normal homeostatic mechanisms are lost, probably because of chronic hyperkalemia in combination with diabetic dyslipidemia.
Summing all observed mortality in 7m-old mice immediately before or during ischemia/reperfusion experiments (30 minute duration) in this study, we observed a significant predisposition to sudden death in Kcne2−/− mice (8/23 Kcne2−/− mice versus 0/16 Kcne2+/+ mice; p = 0.008 by one-tailed Fisher’s exact test).
Discussion
The current model for monogenic arrhythmogenesis (Figure 6J (i)) relies heavily upon a “myocentric” mechanism, but given the new findings for KCNE2 this may be too simplistic a model, which ignores the extracardiac effects of disrupting arrhythmia susceptibility genes, the large majority of which are also expressed outside the heart. While extrapolation of mouse models to human disease must be conducted with extreme caution, 35% of Kcne2−/− mice in the present study succumbed to sudden death immediately before, during or immediately after surgery to induce transient ischemia – supporting the hypothesis that more subtle, single-allele effects of human KCNE2 mutations could be significant when considering large populations. We suggest a new model for KCNE2-linked arrhythmogenesis in mice, elements of which may be applicable to some human arrhythmias (Figure 6J (ii)), and aspects of which are likely to apply to other broadly expressed arrhythmia susceptibility genes – especially KCNQ1, which partners KCNE1, 2 and/or 3 in a variety of epithelial cell types7.
KCNQ1 gene variants are strongly linked to type II diabetes30, yet the mechanism has not been delineated, nor has a dissection of the potential arrhythmogenic contribution of KCNQ1-linked diabetes in humans, or systemic effects of Kcnq1 deletion in mice, been reported, to our knowledge (although Kcne1 disruption has been shown to affect K+ homeostasis in mice, with the suggestion this could contribute to arrhythmogenesis31). However, a recent study showed that hypergastrinemia, a marker of parietal cell dysfunction, correlated well with QTc prolongation in KCNQ1-linked Jervell and Lange-Nielsen Syndrome 32. Interestingly, we previously found that even single-allele disruption of Kcne2 causes hypochlorhydria in mice, intermediate between wild-type mice and the achlorhydria we found in Kcne2−/− mice9. Furthermore, we previously found that the gastric effects of Kcne2 deletion worsened with age10, providing indirect support for the hypothesis that extracardiac effects of Kcne2 disruption could contribute to age-dependent worsening of the cardiac phenotype. KCNQ1 and KCNE1 are also reportedly expressed in neuronal networks and the brainstem, and KCNQ1-linked seizures suggested as being arrhythmogenic, potentially explaining some cases of sudden unexplained death in epilepsy33; an analogous situation was recently reported for Rett syndrome34.
Diabetes, hypercholesterolemia and anemia all ultimately reduce myocardial oxygen supply, leading to myocardial ischemia, and are recognized risk factors in SCD, especially in combination. In addition, nocturnal hypoglycemia in diabetics predisposes to SCD; cardiac events in overnight-fasted Kcne2−/− mice reported here provide insights into the type of catastrophic cardiac events that might lead to the DIBS form of SCD in a hypoglycemic diabetic. Particularly striking is that a single gene disruption provides the systemic substrate (diabetes, hypercholesterolemia, elevated angiotensin II), the electrical substrate (impaired ventricular repolarization caused by direct ventricular channel subunit loss, hyperkalemia, and probably remodeling in response to the elements of the ischemic substrate) and also a trigger (hypoglycemia, causing in this case acute ischemia, acute exacerbation of hyperkalemia, and probably AV block).
While results obtained from knockout mice cannot be directly extrapolated to explain human disease states, the AV block observed in fasted Kcne2−/− mice suggests parallels to arrhythmogenesis mechanisms linked to increased mortality in large-scale studies of diabetes35 and thought to underlie nocturnal SCD in non-diabetics36. ST depression (as we observed in fasted Kcne2−/− mice) was also observed in type 2 diabetics in whom hypoglycemia was induced by insulin infusion37. The general consensus is that there must be an underlying genetic component involving a ventricular ion channel in DIBS, to provide an electrical substrate in addition to systemic substrates and the trigger of, e.g., hypoglycemia20. We now show that a single gene disruption can underlie all of these factors, at least in a mouse model.
One of the mysteries surrounding monogenic arrhythmia syndromes has been why some individuals die young from SCD whereas others live without incident into advanced age. Our findings suggest that lifestyle can play a more important role than could be rationalized solely by considering the myocentric arrhythmogenesis mechanism. Individuals that maintain healthy diet and exercise regimes, for example, would be expected to delay the systemic effects of a pathologic KCNE2 gene variant and thus minimize the ischemic substrate while also diminishing aspects of the electrical substrate and potentially eliminating the hypoglycemic trigger. It would require long-term study of a large cohort of individuals to determine if any aspects of the KCNE2-linked multisystem arrhythmogenesis model apply in human arrhythmias. Rare KCNE2 mutations were very recently reported in two independent post-mortem studies of human sudden unexplained death but underlying mechanisms were not elucidate; indeed, rigorous investigation of the mechanisms involved in such human sudden unexplained death cases would be incredibly challenging. In these studies, four mutations were found; in three cases the sentinel event was sudden unexplained nocturnal death, in the other the sentinel event was syncope during exertion38, 39. Interestingly, the predicted KCNE2 protein mutation, T10M, of this latter case was similar to that in a patient we previously reported in whom the sentinel event was also syncope and who subsequently experienced ventricular fibrillation following exertion40. Unfortunately, many studies of this nature preclude the very patients that could be affected by a multisystem syndrome such as we demonstrate in Kcne2−/− mice. This is because individuals exhibiting structural heart disease, diabetes and hyperlipidemia are often excluded from arrhythmia genetic association studies as these conditions are, understandably, considered as confounding variables, rather than potential manifestations of the channel gene sequence variant.
In summary, new findings from the Kcne2−/− mouse suggest that a more holistic view of the etiology of monogenic cardiac arrhythmias could provide a clearer picture of the mechanisms underlying SCD, if results from this mouse model are found to recapitulate aspects of human cardiac arrhythmogenesis. This could include enhancing the ability to recognize individuals with an inherited predisposition to SCD, particularly in the context of diabetes, and to design more effective avoidance, management, and therapy strategies that lessen the overall SCD substrate and reduce the probability of a triggering event. Strategies might involve routine genetic screening of diabetics for pathologic SNPs in known monogenic arrhythmia genes (particularly KCNQ1 and KCNE2), especially before embarking upon aggressive glycemic control regimens.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the NIH: HL079275 (G.W.A./D.J.C), HL101190 (D.J.C./G.W.A).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Fishman GI, Chugh SS, Dimarco JP, Albert CM, Anderson ME, Bonow RO, et al. Sudden cardiac death prediction and prevention: Report from a national heart, lung, and blood institute and heart rhythm society workshop. Circulation. 2010;122:2335–2348. doi: 10.1161/CIRCULATIONAHA.110.976092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George AL., Jr Molecular and genetic basis of sudden cardiac death. J Clin Invest. 2013;123:75–83. doi: 10.1172/JCI62928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein FH. Hyperglycemia. A risk factor in coronary heart disease. Circulation. 1967;36:609–619. doi: 10.1161/01.cir.36.4.609. [DOI] [PubMed] [Google Scholar]
- 4.Balkau B, Jouven X, Ducimetiere P, Eschwege E. Diabetes as a risk factor for sudden death. Lancet. 1999;354:1968–1969. doi: 10.1016/S0140-6736(99)04383-4. [DOI] [PubMed] [Google Scholar]
- 5.Weston PJ, Gill GV. Dead-in-bed syndrome in diabetes mellitus. Lancet. 1997;350:1032–1033. doi: 10.1016/s0140-6736(05)64083-4. [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr., Bigger JT, Buse JB, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott GW. Kcne2 and the k (+) channel: The tail wagging the dog. Channels (Austin) 2012;6:1–10. doi: 10.4161/chan.19126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, et al. Mirp1 forms ikr potassium channels with herg and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 9.Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, et al. The kcne2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem. 2006;281:23740–23747. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 10.Roepke TK, King EC, Purtell K, Kanda VA, Lerner DJ, Abbott GW. Genetic dissection reveals unexpected influence of beta subunits on kcnq1 k+ channel polarized trafficking in vivo. FASEB J. 2011;25:727–736. doi: 10.1096/fj.10-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roepke TK, King EC, Reyna-Neyra A, Paroder M, Purtell K, Koba W, et al. Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nat Med. 2009;15:1186–1194. doi: 10.1038/nm.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrentraut H, Weber C, Ehrentraut S, Schwederski M, Boehm O, Knuefermann P, et al. The toll-like receptor 4-antagonist eritoran reduces murine cardiac hypertrophy. Eur J Heart Fail. 2011;13:602–610. doi: 10.1093/eurjhf/hfr035. [DOI] [PubMed] [Google Scholar]
- 13.Mascareno E, Dhar M, Siddiqui MA. Signal transduction and activator of transcription (stat) protein-dependent activation of angiotensinogen promoter: A cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci U S A. 1998;95:5590–5594. doi: 10.1073/pnas.95.10.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wexler BC, Lutmer RF. Adrenal glandular lipids and circulating corticosterone in severely diabetic rats. Br J Exp Pathol. 1975;56:299–306. [PMC free article] [PubMed] [Google Scholar]
- 15.Pepine CJ, Nichols WW. The pathophysiology of chronic ischemic heart disease. Clin Cardiol. 2007;30:I4–9. doi: 10.1002/clc.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 17.Tada T, Shiba N, Watanabe J, Matsuki M, Kagaya Y, Shinozaki T, et al. Prognostic value of anemia in predicting sudden death of patients with diastolic heart failure. Int J Cardiol. 2008;128:419–421. doi: 10.1016/j.ijcard.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: The prospective randomized amlodipine survival evaluation (praise) J Am Coll Cardiol. 2003;41:1933–1939. doi: 10.1016/s0735-1097(03)00425-x. [DOI] [PubMed] [Google Scholar]
- 19.Nordin C. The case for hypoglycaemia as a proarrhythmic event: Basic and clinical evidence. Diabetologia. 2010;53:1552–1561. doi: 10.1007/s00125-010-1752-6. [DOI] [PubMed] [Google Scholar]
- 20.Tu E, Twigg SM, Semsarian C. Sudden death in type 1 diabetes: The mystery of the ‘dead in bed’ syndrome. Int J Cardiol. 2010;138:91–93. doi: 10.1016/j.ijcard.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Lee MP, Ravenel JD, Hu RJ, Lustig LR, Tomaselli G, Berger RD, et al. Targeted disruption of the kvlqt1 gene causes deafness and gastric hyperplasia in mice. J Clin Invest. 2000;106:1447–1455. doi: 10.1172/JCI10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J. 2011;18:233–245. [PubMed] [Google Scholar]
- 23.Roepke TK, Kontogeorgis A, Ovanez C, Xu X, Young JB, Purtell K, et al. Targeted deletion of kcne2 impairs ventricular repolarization via disruption of i(k,slow1) and i(to,f) FASEB J. 2008;22:3648–3660. doi: 10.1096/fj.08-110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thandavarayan RA, Watanabe K, Ma M, Gurusamy N, Veeraveedu PT, Konishi T, et al. Dominant-negative p38alpha mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am J Physiol Heart Circ Physiol. 2009;297:H911–919. doi: 10.1152/ajpheart.00124.2009. [DOI] [PubMed] [Google Scholar]
- 25.Bugger H, Chen D, Riehle C, Soto J, Theobald HA, Hu XX, et al. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice. Diabetes. 2009;58:1986–1997. doi: 10.2337/db09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deedwania PC, Carbajal EV. Role of myocardial oxygen demand in the pathogenesis of silent ischemia during daily life. Am J Cardiol. 1992;70:19F–24F. doi: 10.1016/0002-9149(92)90185-2. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Wellens HJ, Josephson ME. Paroxysmal atrioventricular block. Heart Rhythm. 2009;6:1229–1234. doi: 10.1016/j.hrthm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Michaeli J, Bassan MM, Brezis M. Second degree type ii and complete atrioventricular block due to hyperkalemia. J Electrocardiol. 1986;19:393–396. doi: 10.1016/s0022-0736(86)81068-8. [DOI] [PubMed] [Google Scholar]
- 29.Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes. 2003;52:1469–1474. doi: 10.2337/diabetes.52.6.1469. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda K, Miyake K, Horikawa Y, Hara K, Osawa H, Furuta H, et al. Variants in kcnq1 are associated with susceptibility to type 2 diabetes mellitus. Nat Genet. 2008;40:1092–1097. doi: 10.1038/ng.207. [DOI] [PubMed] [Google Scholar]
- 31.Arrighi I, Bloch-Faure M, Grahammer F, Bleich M, Warth R, Mengual R, et al. Altered potassium balance and aldosterone secretion in a mouse model of human congenital long qt syndrome. Proc Natl Acad Sci U S A. 2001;98:8792–8797. doi: 10.1073/pnas.141233398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice KS, Dickson G, Lane M, Crawford J, Chung SK, Rees MI, et al. Elevated serum gastrin levels in jervell and lange-nielsen syndrome: A marker of severe kcnq1 dysfunction? Heart Rhythm. 2011;8:551–554. doi: 10.1016/j.hrthm.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 33.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: Kcnq1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 2009;1:2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCauley MD, Wang T, Mike E, Herrera J, Beavers DL, Huang TW, et al. Pathogenesis of lethal cardiac arrhythmias in mecp2 mutant mice: Implication for therapy in rett syndrome. Sci Transl Med. 2011;3:113ra125. doi: 10.1126/scitranslmed.3002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Movahed MR, Hashemzadeh M, Jamal MM. Increased prevalence of third-degree atrioventricular block in patients with type ii diabetes mellitus. Chest. 2005;128:2611–2614. doi: 10.1378/chest.128.4.2611. [DOI] [PubMed] [Google Scholar]
- 36.Serafini A, Dolso P, Gigli GL, Fratticci L, Cancelli I, Facchin D, et al. Rem sleep brady-arrhythmias: An indication to pacemaker implantation? Sleep Med. 2012;13:759–762. doi: 10.1016/j.sleep.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Lindstrom T, Jorfeldt L, Tegler L, Arnqvist HJ. Hypoglycaemia and cardiac arrhythmias in patients with type 2 diabetes mellitus. Diabet Med. 1992;9:536–541. doi: 10.1111/j.1464-5491.1992.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Zhao Q, Su T, Tang S, Lv G, Liu H, et al. Postmortem molecular analysis of kcnq1, kcnh2, kcne1 and kcne2 genes in sudden unexplained nocturnal death syndrome in the chinese han population. Forensic Sci Int. 2013;231:82–87. doi: 10.1016/j.forsciint.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 39.Tester DJ, Medeiros-Domingo A, Will ML, Haglund CM, Ackerman MJ. Cardiac channel molecular autopsy: Insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc. 2012;87:524–539. doi: 10.1016/j.mayocp.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon E, Panaghie G, Deng L, Bee KJ, Roepke TK, Krogh-Madsen T, et al. A kcne2 mutation in a patient with cardiac arrhythmia induced by auditory stimuli and serum electrolyte imbalance. Cardiovasc Res. 2008;77:98–106. doi: 10.1093/cvr/cvm030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.