Abstract
Background
Participation rates in colorectal cancer screening are below recommended European targets.
Aim
To evaluate the effectiveness of an alert in primary care electronic medical records (EMRs) to increase individuals’ participation in an organised, population-based colorectal cancer screening programme when compared with usual care.
Design and setting
Cluster randomised controlled trial in primary care centres of Barcelona, Spain.
Method
Participants were males and females aged 50–69 years, who were invited to the first round of a screening programme based on the faecal immunochemical test (FIT) (n = 41 042), and their primary care professional. The randomisation unit was the physician cluster (n = 130) and patients were blinded to the study group. The control group followed usual care as per the colorectal cancer screening programme. In the intervention group, as well as usual care, an alert to health professionals (cluster level) to promote screening was introduced in the individual’s primary care EMR for 1 year. The main outcome was colorectal cancer screening participation at individual participant level.
Results
In total, 67 physicians and 21 619 patients (intervention group) and 63 physicians and 19 423 patients (control group) were randomised. In the intention-to-treat analysis screening participation was 44.1% and 42.2% respectively (odds ratio 1.08, 95% confidence interval [CI] = 0.97 to 1.20, P = 0.146). However, in the per-protocol analysis screening uptake in the intervention group showed a statistically significant increase, after adjusting for potential confounders (OR, 1.11; 95% CI = 1.02 to 1.22; P = 0.018).
Conclusion
The use of an alert in an individual’s primary care EMR is associated with a statistically significant increased uptake of an organised, FIT-based colorectal cancer screening programme in patients attending primary care centres.
Keywords: colorectal neoplasm, early detection of cancer, electronic medical record, mass screening, primary health care, reminder systems
INTRODUCTION
Colorectal cancer has the highest incident rate of all cancers and is the second leading cause of cancer death in Western countries.1 Evidence from several studies have demonstrated that colorectal cancer screening is effective2–5 and cost effective6 in populations that are at average risk of developing the disease. Searching for occult blood in stools using the guaiac test and, more recently, the faecal immunochemical test (FIT) are predominantly implemented in Europe7 and Australia8, whereas colonoscopy is the dominant screening modality in North America9. Moreover, at population-based level, implementation of organised programmes, in contrast to opportunistic screening, is recommended because they include an administrative structure responsible for service delivery, quality assurance and evaluation.7
The European guidelines for quality assurance in colorectal cancer screening states that the participation rate represents a key quality indicator for ensuring effectiveness and efficiency of population-based screening programmes.7 However, results from current programmes in Spain show that participation is not reaching these recommended figures, with uptake rates varying from 17.2% to 42.3%,10–14 with the exception of the Basque Country (64.3%).14 The uptake rate in the first round for the Colorectal Cancer Screening Program of Barcelona (CCSPB) was 43.6%.15 Differences in programme organisation, sociodemographics, and primary care professional involvement may explain these differences (for example, the involvement of the GP differs depends on certified training, scheduling of colonoscopy, information feedback, or FIT collection in primary care).
The CCSPB is targeted at males and females aged 50–69 years, who are at average risk of developing colorectal cancer; it encompasses a total population of 197 795 individuals.15 Individuals receive a mailed invitation letter, along with an explanatory leaflet about colorectal cancer, screening, and the specific programme. The FIT is delivered and collected at the community pharmacies involved in the programme.
Participants with a positive test (cut-off level of 20 μg of haemoglobin/g of faeces) are invited, by a trained nurse from the screening office, to undergo colonoscopy. Both primary care professionals and pharmacists attended a specific training session at the beginning of each screening round, which explained their role and the functioning of the programme, for example, the standard procedures outlined in the CCSPC.15 Primary care professionals are encouraged to promote colorectal cancer screening and to help the programme refine the target population by identifying individuals with exclusion criteria.
How this fits in
Participation rates in colorectal cancer screening are below recommended targets in many European countries. Incorporating alerts into electronic medical records (EMRs) has proved effective for improving the practice of different preventive care measures, including cancer screening, although limited information has been reported about colorectal cancer, especially in the context of population-based screening programmes. This study presents a clinical trial performed within an organised colorectal cancer screening programme with participation of community pharmacies for the delivering of faecal immunochemical tests. A significant increase in colorectal cancer screening uptake was found among patients attending primary care centres. GPs play a key role in promoting colorectal cancer screening, facilitated by their proximity and relationship with patients, but in busy practices it could be difficult to recommend or order cancer screening. Efforts should continue to develop interventions involving GPs within the population-based programmes.
Direct recommendation by the GP has been identified as one of the strongest predictors for colorectal cancer screening uptake.16 Data obtained in Catalonia indicated that 88.9% of individuals would agree to participate in a colorectal cancer screening programme if their primary care professional suggested it;17 this figure, however, is far from the actual participation rates. In Catalonia, public primary care is organised through primary care centres that each cover a specific population area. Every individual is assigned to a primary care unit, where one physician and one nurse attend to their own patients. Although controversy exists about prompting health professionals to promote colorectal cancer screening,18 the use of new technological strategies may help to increase participation in such programmes.19 In fact, the introduction of specific reminders in electronic medical records (EMRs) has proved effective for improving a range of preventive care measures, including cancer screening.20 However, despite its potential high impact and low cost, electronic reminders remain underutilised in healthcare.
The aim of this study was to evaluate the effectiveness of an electronic alert in individuals’ primary care EMR to increase their participation in an organised, FIT-based colorectal cancer screening programme.
METHOD
The Colo-Alert study was a single-blinded, cluster randomised controlled trial comparing an electronic alert to primary care professionals in individual’s EMR to increase uptake in an organised, FIT-based colorectal cancer screening programme to usual care of a primary care professional. It was performed within the population-based CCSPB.
This project was registered at ClinicalTrials.gov (NCT01877018), and the study protocol published elsewhere.21
Sample and recruitment
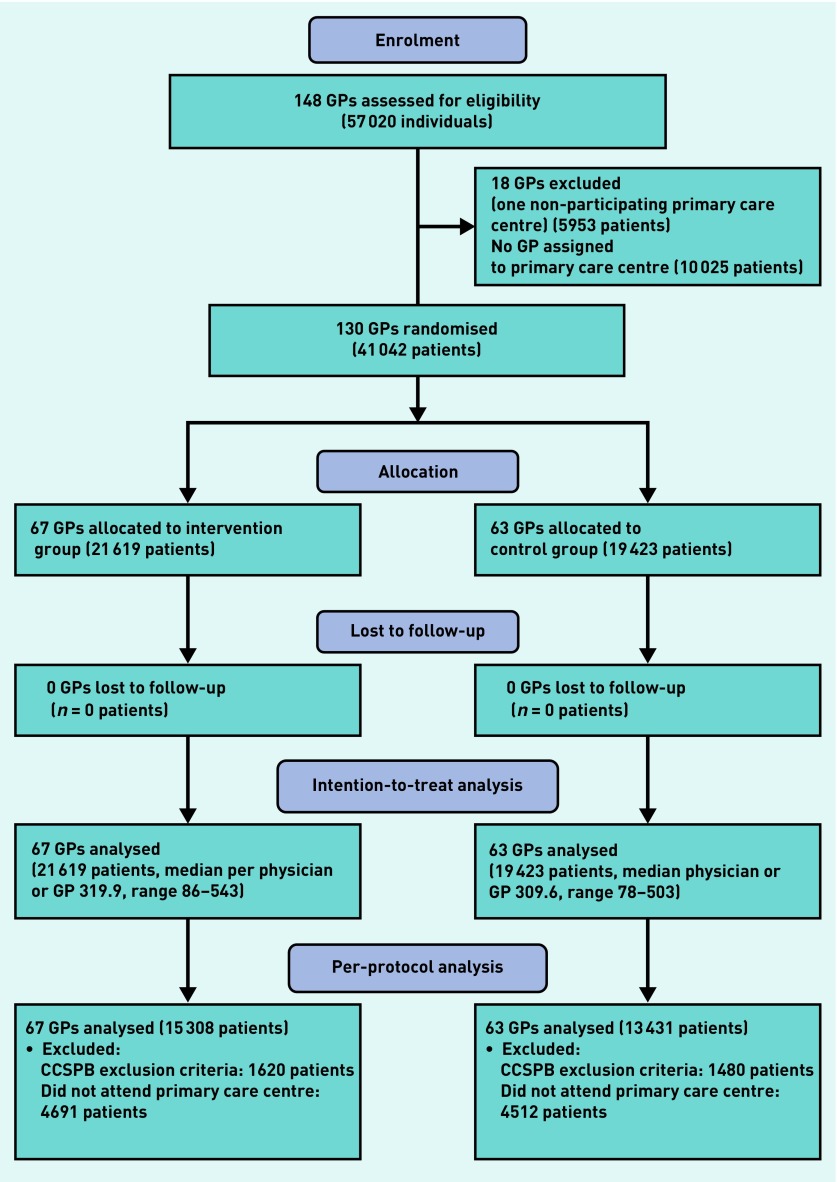
The study was performed in 10 primary care centres. Participants were males and females aged 50–69 years, who were invited to the first round of the CCSPB and had a GP assigned to their participating centre, together with their respective primary care professional. Primary care centres were successively involved in the CCSPB; the recruitment period ran from July 2011 until May 2012. Follow-up lasted for 1 year after invitation for each centre. Figure 1 outlines the study process.
Figure 1.
CONSORT flowchart of the study process. CCSPB = Colorectal Cancer Screening Program of Barcelona
Patient characteristics were obtained from the EMR and provided by the Primary Care Services Information System at the beginning of the study. Patients’ baseline characteristics are summarised in Table 1. Anonymity of data was guaranteed throughout the whole study process.
Table 1.
Participants’ baseline characteristics
| Characteristics | Total n = 41 042 | Intervention n = 21 619 | Control n = 19 423 |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 19 039 (46.4) | 9971 (46.1) | 9068 (46.7) |
| Female | 22 003 (53.6) | 11 648 (53.9) | 10 355 (53.3) |
|
| |||
| Mean age, years (SD) | 58.7 (5.6) | 58.8 (5.6) | 58.7 (5.6) |
|
| |||
| Age groups, n (%) | |||
| 50–54 | 11 991 (29.2) | 6195 (28.7) | 5796 (29.8) |
| 55–69 | 10 765 (26.2) | 5590 (25.9) | 5175 (26.6) |
| 60–64 | 9910 (24.1) | 5411 (25.0) | 4499 (23.2) |
| 65–69 | 8376 (20.4) | 4423 (20.5) | 3953 (20.4) |
|
| |||
| Smoking, n (%) | |||
| Never smokers | 14 028 (34.2) | 7429 (34.4) | 6599 (34.0) |
| Smokers | 6908 (16.8) | 3703 (17.1) | 3205 (16.5) |
| Ever smokers | 8105 (19.7) | 4507 (20.8) | 3598 (18.5) |
| Unknown | 12 001 (29.2) | 5980 (27.7) | 6021 (31.0) |
|
| |||
| Alcohol consumption, n (%) | |||
| Non drinkers | 16 352 (39.8) | 8792 (40.7) | 7560 (38.9) |
| Low-risk drinkers | 9349 (22.8) | 5008 (23.2) | 4341 (22.3) |
| High-risk drinkers | 760 (1.9) | 413 (1.9) | 347 (1.8) |
| Unknown | 14 581 (35.5) | 7406 (34.3) | 7175 (36.9) |
|
| |||
| Body mass index, n (%) | |||
| Low weight | 539 (1.3) | 284 (1.3) | 255 (1.3) |
| Normal weight | 5351 (13.0) | 2847 (13.2) | 2504 (12.9) |
| Overweight | 9193 (22.4) | 4898 (22.7) | 4295 (22.1) |
| Obesity | 6474 (15.8) | 3450 (16.0) | 3024 (15.6) |
| Morbid obesity | 583 (1.4) | 291 (1.3) | 292 (1.5) |
| Unknown | 18 902 (46.1) | 9849 (45.6) | 9053 (46.6) |
|
| |||
| Socioeconomic deprivation index quintiles, n (%) | |||
| Q1 | 7648 (18.6) | 4188 (19.4) | 3460 (17.8) |
| Q2 | 7724 (18.8) | 3893 (18.0) | 3831 (19.7) |
| Q3 | 7511 (18.3) | 4128 (19.1) | 3383 (17.4) |
| Q4 | 7706 (18.8) | 3942 (18.2) | 3764 (19.4) |
| Q5 | 7547 (18.4) | 3832 (17.7) | 3715 (19.1) |
| Unknown | 2906 (7.1) | 1636 (7.6) | 1270 (6.5) |
|
| |||
| Visits to PCC during the study period, n (%) | |||
| 0 | 10 163 (24.8) | 5183 (24.0) | 4980 (25.6) |
| 1 | 3295 (8.0) | 1731 (8.0) | 1564 (8.0) |
| 2 | 3246 (7.9) | 1748 (8.1) | 1498 (7.7) |
| 3 | 3081 (7.5) | 1629 (7.5) | 1452 (7.5) |
| 4 | 2841 (6.9) | 1509 (7.0) | 1332 (6.9) |
| ≥ 5 | 18 416 (44.9) | 9819 (45.4) | 8597 (44.3) |
|
| |||
| Clinical risk group, n (%) | |||
| Without dominant chronic illness | 15 827 (38.6) | 8459 (39.1) | 7368 (37.9) |
| With dominant chronic illness | 23 132 (56.4) | 12 136 (56.1) | 10 996 (56.6) |
| Unknown | 2083 (5.1) | 1024 (4.7) | 1059 (5.5) |
PCC = primary care centre. SD = standard deviation.
The control group comprised all study participants assigned to primary care professionals who did not receive any electronic reminder to increase participation; they, therefore, followed the standard CCSPB protocol.15
Interventions and procedures
All study participants assigned to health professionals who received an alert to increase participation constituted the intervention group. In addition to the standard CCSPB procedures, an electronic reminder was included in individuals’ primary care EMR for 1 year, identifying those subjects who had been invited to the CCSPB (intervention targeted at cluster level).
When a non-participant of the CCSPB was identified in any medical appointment, both GPs and nurses were asked to encourage participation by means of brief, structured counselling.
The socioeconomic deprivation index was expressed as quintiles: the first quintile (Q1) represents the least deprived while the fifth quintile (Q5) represents the most deprived.22 The clinical risk group, defined in nine initial categories, was collapsed into two categories:
without dominant chronic illness (healthy, acute illness, minor chronic illness, or multiple minor chronic illness); and
with dominant chronic illness (one dominant chronic illness, two chronic illnesses, three chronic illnesses, neoplasm, or catastrophic illness).23
Outcomes
The outcome of the study was participation in the CCSPB, which was defined as individuals returning the FIT during the follow-up period. At the beginning of the study, the CCSPB technical office provided a list of individuals invited to participate in the programme and their corresponding primary care centre; at the end of study, it provided information regarding participation.
Sample size
The appropriateness of including the entire population (130 GPs with 41 042 patients, belonging to primary care centres whose patients were invited to participate in the CCSPB from July 2011 until May 2012) was considered. This sample size allowed the smallest increase in participation — 3.7 percentage points between the two groups — to be detected, taking into account the clusters and assuming a low colorectal cancer screening uptake rate of 30% in the control group.
An intracluster correlation coefficient (ICC) of ρ = 0.022 was assumed with an average cluster size of 318.2. This resulted in a design effect of eight; dividing the intended sample size (41 042 patients) by the design effect resulted in an effective sample size of 2565 patients in each group. An α risk of 0.05 and β risk of 0.20 was assumed.
Randomisation
The GP (cluster) was the unit of randomisation. Allocation of GP to the intervention or control group was carried out through a random allocation (1:1 ratio). Nurses were allocated to the intervention and control group according to the allocation of the corresponding GP with whom they shared a patient’s care. If a nurse shared a patient’s care with a GP allocated to a different study group, the nurse was allocated to the group of the GP with which they shared most patients. Patients were allocated to the intervention or control group according to the allocation of their corresponding GP.
Given the nature of the intervention, it was not possible to carry out blinding of the primary care professionals but patients did not receive any information regarding the development of the study (that is, no informed consent was requested). The CCSPB’s technical office was blinded to the study group in which individuals were allocated.
Statistical analysis
A descriptive analysis was carried out between groups with respect to each baseline variable. The categorical variables were expressed as absolute number and percentage, and quantitative variables were shown as mean and standard deviation if normally distributed. The relationship between participation in the CCSPB and individuals’ baseline characteristics, adjusted by study group, was assessed using a clustered standard errors method in a logistic regression model (one model for each characteristic).
The intention-to-treat analysis included all individuals randomised to each group, whereas the per-protocol analysis was limited to those attended by their GP or nurse within the follow-up period and without exclusion criteria for participating in the CCSPB. The study includes patient participants who were eligible for the population-based screening programme.
Nevertheless, some participants were excluded by the programme afterwards (Figure 1), since any of the exclusion criteria of the programme, unidentified before sending the invitation letter, was notified to the programme during the screening process (for example, normal colonoscopy performed in last 5 years, colorectal cancer high risk by family history, presence of red flag symptoms).
In this study all initial patients included in the study in the intention-to-treat analysis were analysed; and excluded were those patients with any exclusion criteria identified by the programme during the screening process and also those patients who did not visit primary care during the followup period, in the per-protocol analysis (Figure 1).
Differences in participation between the intervention and control groups were established by a clustered standard errors method in a multiple logistic regression analysis: both unadjusted and adjusted by age, sex, smoking, alcohol consumption, body mass index, socioeconomic deprivation index, number of visits to primary care centre, and clinical risk group. Results of the analysis were reported as odds ratios (ORs) with 95% confidence intervals (95% CIs). The adjusted analyses were performed including only those patients for whom all baseline variables were available (complete cases) and including all cases after missing data had been imputed according to the Imputation by the chained equation function of Stata.24 All results in Tables 2 and 3 have been analysed taking into account the correlation between outcomes of patients who belong to the same GP, using clustered robust standard errors method in a logistic regression analysis. More specifically, the Huber-White (also called Sandwich) standard errors method has been used. All analyses were performed using Stata (version 13.1).
Table 2.
Screening programme uptake, by patient characteristica
| Characteristics | Total n = 41 042 | Non-participation n = 23 307 | Participation n = 17 735 | OR (95% CI)b | P-valueb | |
|---|---|---|---|---|---|---|
| Study group, n (%) | ||||||
| Control | 19 423 (47.3) | 11 227 (57.8) | 8196 (42.2) | 1c | ||
| Intervention | 21 619 (52.7) | 12 080 (55.9) | 9539 (44.1) | 1.08 (0.97 to 1.20) | 0.146 | |
|
| ||||||
| Sex, n (%) | ||||||
| Male | 19 039 (46.4) | 11 287 (59.3) | 7752 (40.7) | 1c | ||
| Female | 22 003 (53.6) | 12 020 (54.6) | 9983 (45.4) | 1.21 (1.16 to 1.26) | <0.001 | |
|
| ||||||
| Age groups, years, n (%) | ||||||
| 50–54 | 11 991 (29.2) | 7231 (60.3) | 4760 (39.7) | 1c | ||
| 55–59 | 10 765 (26.2) | 6155 (57.2) | 4610 (428) | 1.13 (1.07 to 1.20) | <0.001 | |
| 60–64 | 9910 (24.2) | 5278 (53.3) | 4632 (46.7) | 1.32 (1.23 to 1.41) | <0.001 | |
| 65–69 | 8376 (20.4) | 4643 (55.4) | 3733 (44.6) | 1.21 (1.12 to 1.30) | <0.001 | |
|
| ||||||
| Smoking, n (%) | ||||||
| Never smokers | 14 028 (48.3) | 7184 (51.2) | 6844 (48.8) | 1c | ||
| Smokers | 6908 (23.8) | 4268 (61.8) | 2640 (38.2) | 0.65 (0.61 to 0.70) | <0.001 | |
| Ever smokers | 8105 (27.9) | 3946 (48.7) | 4159 (51.3) | 1.10 (1.01 to 1.20) | 0.020 | |
|
| ||||||
| Alcohol consumption, n (%) | ||||||
| Non drinkers | 16 352 (61.8) | 8675 (53.1) | 7677 (46.9) | 1c | ||
| Low-risk drinkers | 9349 (35.3) | 4723 (505) | 4626 (49.5) | 1.10 (1.04 to 1.17) | 0.001 | |
| High-risk drinkers | 760 (2.9) | 463 (60.9) | 297 (39.1) | 0.73 (0.62 to 0.86) | <0.001 | |
|
| ||||||
| Body mass index, n (%) | ||||||
| Low weight | 539 (2.4) | 335 (62.1) | 204 (37.9) | 1c | ||
| Normal weight | 5351 (24.2) | 2784 (52.0) | 2567 (48.0) | 1.51 (1.24 to 1.84) | <0.001 | |
| Overweight | 9193 (41.5) | 4641 (50.5) | 4552 (49.5) | 1.61 (1.34 to 1.94) | <0.001 | |
| Obesity | 6474 (29.2) | 3382 (50.2) | 3092 (47.8) | 1.50 (1.24 to 1.82) | <0.001 | |
| Morbid obesity | 583 (2.6) | 346 (59.3) | 237 (40.7) | 1.12 (0.88 to 1.43) | 0.356 | |
|
| ||||||
| Socioeconomic deprivation index quintiles, n (%) | ||||||
| Q1 | 7648 (20.1) | 4659 (60.9) | 2989 (39.1) | 1c | ||
| Q2 | 7724 (20.2) | 4272 (55.3) | 3452 (44.7) | 1.27 (1.16 to 1.39) | <0.001 | |
| Q3 | 7511 (19.7) | 3786 (50.4) | 3725 (49.6) | 1.53 (1.41 to 1.68) | <0.001 | |
| Q4 | 7706 (20.2) | 4169 (54.1) | 3537 (45.9) | 1.33 (1.21 to 1.46) | <0.001 | |
| Q5 | 7547 (19.8) | 4761 (63.1) | 2786 (36.9) | 0.92 (0.82 to 1.03) | 0.168 | |
|
| ||||||
| Visits to PCC during the study period, n (%) | ||||||
| 0 | 10 163 (24.8) | 7222 (71.1) | 2941 (28.9) | 1c | ||
| 1 | 3295 (8.0) | 2023 (61.4) | 1272 (38.6) | 1.53 (1.39 to 1.69) | <0.001 | |
| 2 | 3246 (7.9) | 1881 (57.9) | 1365 (42.1) | 1.75 (1.59 to 1.92) | <0.001 | |
| 3 | 3081 (7.5) | 1647 (53.5) | 1434 (46.5) | 2.10 (1.92 to 2.30) | <0.001 | |
| 4 | 2841 (6.9) | 1462 (51.5) | 1379 (48.5) | 2.28 (2.04 to 2.54) | <0.001 | |
| ≥ 5 | 18 416 (44.9) | 9072 (49.3) | 9344 (50.7) | 2.48 (2.31 to 2.66) | <0.001 | |
|
| ||||||
| Clinical risk group, n (%) | ||||||
| Without dominant chronic illness | 15 827 (40.6) | 9090 (57.4) | 6737 (42.6) | 1c | ||
| With dominant chronic illness | 23 132 (59.4) | 12 465 (53.9) | 10 667 (46.1) | 1.15 (1.10 to 1.21) | <0.001 | |
PCC = primary care centre.
Based on intention-to-treat analysis.
Each row is a logistic regression model adjusted by study group with clustered robust standard error method.
Reference group.
Table 3.
Screening programme uptake, by analysis type
| n | OR | 95% CI | P-value | |
|---|---|---|---|---|
| Intention-to-treat analysis | ||||
| Unadjusted | ||||
| Control group | 19 423 | 1a | ||
| Intervention group | 21 619 | 1.08 | 0.97 to 1.20 | 0.146 |
| Adjustedb (only complete cases) | ||||
| Control group | 8185 | 1a | ||
| Intervention group | 9394 | 1.09 | 0.99 to 1.19 | 0.064 |
| Adjustedb (missing-data imputationc) | ||||
| Control group | 19 423 | 1a | ||
| Intervention group | 21 619 | 1.06 | 0.98 to 1.14 | 0.129 |
|
| ||||
| Per-protocol analysisd | ||||
| Unadjusted | ||||
| Control group | 13 431 | 1a | ||
| Intervention group | 15 308 | 1.09 | 0.99 to 1.19 | 0.065 |
| Adjustedb (only complete cases) | ||||
| Control group | 7100 | 1a | ||
| Intervention group | 8181 | 1.11 | 1.02 to 1.22 | 0.018 |
| Adjustedb (missing-data imputationc) | ||||
| Control group | 13 431 | 1a | ||
| Intervention group | 15 308 | 1.08 | 1.01 to 1.15 | 0.033 |
OR = odds ratio. Control group is the reference category.
Reference category.
Adjusted by sex, age, smoking, alcohol consumption, body mass index, socioeconomic deprivation index, number of visits to primary care centre, and clinical risk group.
Missing-data imputation for smoking, alcohol consumption, body mass index, socioeconomic deprivation index, and clinical risk group.
Performed in patients who attended a primary care centre at least once during the study period and without CCSPB exclusion criteria identified during the screening process.
RESULTS
After randomisation, 67 GPs and their respective 21 619 patients were allocated to the intervention group, while 63 GPs and their 19 423 patients were allocated as controls. As shown in Table 1, both groups were similar with respect to baseline characteristics. Overall, 20.9% of activated alerts were responded by primary care professionals. Adjusting for primary care frequentation (that is excluding the individuals that did not visit their primary care centre during the follow-up period) this figure rises to 27.5%.
Intention-to-treat analysis
Participation in the CCSPB of individuals allocated to the intervention group was not significantly higher than that of those allocated to the control group (44.1% versus 42.2% respectively) (OR 1.08, 95% CI = 0.97 to 1.20, P = 0.146) (Table 2). No statistically significant improvements in colorectal cancer screening participation were found when clustering and other confounding variables were accounted for in the analysis (Tables 2 and 3).
Per-protocol analysis
When the analysis was restricted to those individuals who could benefit from the electronic reminder, the participation rate was higher, but not statistically significant, in the intervention group (OR 1.09, 95% CI = 0.99 to 1.19; P = 0.065) (Table 3). After adjusting for potential confounders, either in complete cases (OR 1.11, 95% CI = 1.02 to 1.22, P = 0.018) or on missing-data imputation (OR 1.08, 95% CI = 1.01 to 1.15; P = 0.033) (Table 3), colorectal cancer screening uptake showed a statistically significant increase in the intervention group.
DISCUSSION
Summary
These results show that an electronic alert in patients’ primary care EMRs was not associated with a statistically significant increased participation in the first round of an organised, FIT-based colorectal cancer screening programme. Nevertheless, when the analysis was restricted to those individuals attending their primary care professional during the follow-up period and fulfilling the screening programme entry criteria, a beneficial effect (up to 11% increase in participation) was observed.
Strengths and limitations
To the authors’ knowledge, this is the first study evaluating the impact of an electronic reminder in primary care to increase uptake in an organised, population-based colorectal cancer screening programme. Strengths of this study include a large sample size, its randomised design, and an accurate interpretation of results based on both intention-to-treat and per-protocol analyses by adjusting for cluster effect and potential confounding factors.
In addition, this trial included a broadly representative sample of the population at average risk of developing colorectal cancer, in terms of age, sex, socioeconomic status, and comorbidity, as well as an evaluation of several colorectal cancer risk factors (that is, smoking, alcohol, and obesity), which allowed for the impact of the alert to be assessed in each subset of individuals. The study was also conducted under conditions of routine clinical practice and, therefore, its results may be applicable to countries with a public health system in which population-based colorectal cancer screening programmes involve primary care professionals.
However, the authors are aware of some limitations of the study. There were larger-than-expected pre-randomisation exclusions. Nevertheless, as most of them were due to administrative issues (patients with a primary care professional not assigned to a participating centre) or the primary care professional’s reluctance to participate in the study, it is unlikely that they may represent any selection bias.
In addition, colorectal cancer screening participation differs, depending on the approach and test offered;25 results of this study are limited to organised, FIT-based programmes; these are the most widely implemented in Europe,26 but including only one test or approach could, nonetheless, be considered a limitation. Finally, because of socioeconomic differences identified between the participating centres (a recognised determinant of screening participation) the GP was the unit of randomisation; as a consequence, a negligible interprofessional contamination cannot definitively be ruled out as intervention and control professionals coexisted in the same centres.
Comparison with existing literature
There is growing desire in Europe to improve participation in organised colorectal cancer screening programmes specifically, as most countries do not reach recommended European guidelines figures.27,28 To achieve this goal, several strategies and interventions have been proposed (for example, postal or telephone reminders, including the GP’s signature in the invitation letter, or mailing FIT samples), but they are influenced by the characteristics of such programmes.29 Interventions based on invitation letter or GP involvement, among others, were consistently effective in screening participation when organised programmes were compared with opportunistic approaches.30
Prompting health professionals during a medical encounter aims to reduce missed opportunities to recommend, order, or deliver cancer screening services.31,32 A systematic review, including 61 clinical trials in which various alerts were evaluated, found an average increase of 12–14% in delivering preventive care measures.20 However, results of a recent Cochrane review specifically assessing the usefulness of synchronous electronic reminders suggest that their impact is small or modest (median absolute improvement of 4.2%), and probably less than would have been expected in the context of an increasingly wider use of EMRs.33 In eight comparisons, individuals allocated to the intervention group experienced a median absolute improvement of 2.5%, in comparison with the 1.9% found in the current study. However, this review included only one study in the context of colorectal cancer screening.34
Another systematic review on the effectiveness of provider reminders to increase breast, cervical, and colorectal cancer screening performance concluded that they have a beneficial effect with respect to the use of mammography, cytology, flexible sigmoidoscopy, and faecal occult blood testing.31 In the last scenario, six studies were evaluated, with an average increase of 10.5% on screening.31 Finally, a recent meta-analysis, including five clinical trials assessing the role of physician reminders to increase participation in colorectal cancer screening using faecal occult blood testing, found a non-statistically significant higher participation in the intervention group.18 In one of the evaluated studies, an electronic reminder was specifically assessed, although this was done in a non-population-based scenario in which both faecal occult blood testing and endoscopic tests were offered.35 These authors found that screening among individuals whose physicians received an electronic alert did not significantly increase compared with those whose doctors did not receive the reminder (41.9% versus 40.2% respectively).35 These results were very similar to those found in this current study, despite the fact that the GPs’ response rate to the alert (20.9%) was lower than in others.36
Implications for research and practice
The use of an electronic alert in individuals’ primary care EMRs was not associated with a statistically significant increased uptake of an organised, FIT-based colorectal cancer screening programme that had a moderate baseline participation rate. Nevertheless, when the analysis was restricted to those individuals who could benefit from the electronic reminder, colorectal cancer screening uptake showed a statistically significant increase in the intervention group, after adjusting for potential confounders. Strategies to improve primary care professionals’ responses to the alert could improve the potential benefits of electronic reminders (for example, by involving primary care professionals in the alert design and working, limit alerts to a shorter period of time, arranging specific primary care visits addressing colorectal cancer screening, thus avoiding competition with other medical consultations, or improve communication and information feedback between primary care and population-based screening programmes).
Given that low-intensity interventions targeting a large number of individuals may have a relevant impact on health promotion according to the five-dimension RE-AIM framework (reach, efficacy/effectiveness, adoption, implementation, and maintenance),37 further research on this topic should still be considered.
Acknowledgments
The authors thank all members of Barcelona Colorectal Cancer Screening Programme’s executive committee for the design and implementation of the study, the Catalan Health Institute’s Primary Care Services Information System for its participation in the design and management of the electronic medical record alert, and all health professionals and patients in the participating centres. A list of the members of the PROCOLON group are available from the authors.
Funding
This project was funded by a government grant from Carlos III Health Institute; with record PI10/01994 code, co-financed by the European Union through the European Regional Development Fund (ERDF).
Ethical approval
This project obtained ethical approval from the Jordi Gol Primary Care Research Institute’s Ethics and Clinical Research Committee (P10/31).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v10, Cancer Incidence and Mortality Worldwide. Lyon, France: International Agency for Research on Cancer; IARC CancerBase No. 11. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx [accessed 25 May 2016]. [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343(22):1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 4.Hewitson P, Glasziou P, Watson E, et al. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103(6):1541–1549. doi: 10.1111/j.1572-0241.2008.01875.x. [DOI] [PubMed] [Google Scholar]
- 5.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 6.Heitman SJ, Hilsden RJ, Au F, et al. Colorectal cancer screening for average-risk North Americans: an economic evaluation. PLoS Med. 2010;7(11):e1000370. doi: 10.1371/journal.pmed.1000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segnan N, Patrick J, von Karsa L, editors. European guidelines for quality assurance in colorectal cancer screening and diagnosis. 1st edn. Luxembourg: Publications Office of the European Communities; 2010. [Google Scholar]
- 8.Australian Institute of Health and Welfare and the Australian Government Department of Health Analysis of colorectal cancer outcomes for the Australian National Bowel Cancer Screening Program. Asia Pac J Clin Oncol. 2016;12:22–32. doi: 10.1111/ajco.12452. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society Colorectal Cancer Facts & Figures 2014–2016. 2014. http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.pdf (accessed on 23 May 2016).
- 10.Peris M, Espinàs JA, Muñoz L, et al. Lessons learnt from a population-based pilot programmeme for colorectal cancer screening in Catalonia (Spain) J Med Screen. 2007;14(2):81–86. doi: 10.1258/096914107781261936. [DOI] [PubMed] [Google Scholar]
- 11.Pérez Riquelme F, Navarro Sánchez C, Chirlaque López MD, et al. [Report on the prevention of colon and rectal cancerin the Murcia region] In: Pérez Riquelme F, Cruzado Quevedo J, Gutiérrez García JJ, editors. [The prevention of colon and rectal cancer in the Murcia region] Murcia: Consejería de Sanidad de la Región de Murcia; 2008. [In Spanish] Serie informes No: 50. [Google Scholar]
- 12.Málaga López A, Salas Trejo D, Sala Felis T, et al. [Programme of screening for colorectal cancer in the Valencia community, Spain. results of the first round (2005–2008)]. [In Spanish] Rev Esp Salud Pública. 2010;84(6):731–743. [PubMed] [Google Scholar]
- 13.Brugos-Llamazares V, González de Aledo Linos A, Vada-Sánchez J, et al. [Results of the screening program for colorectal cancer screening in Cantabria, Spain, during the period November 2008–March 2010]. [In Spanish] Rev Esp Salud Pública. 2010;84(6):757–770. doi: 10.1590/s1135-57272010000600007. [DOI] [PubMed] [Google Scholar]
- 14.Portillo I, Idígoras I, Ojembarrena E, et al. [Main results of the colorectal cancer screening program in the Basque Country (Spain)]. [In Spanish] Gac Sanit. 2013;27(4):358–361. doi: 10.1016/j.gaceta.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Burón A, Grau J, Andreu M, et al. [Colorectal Cancer Early Screening Program of Barcelona, Spain: indicators of the first round of a program with participation of community pharmacies]. [In Spanish] Med Clin (Barc) 2015;145(4):141–146. doi: 10.1016/j.medcli.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Cardarelli R, Thomas JE. Having a personal health care provider and receipt of colorectal cancer testing. Ann Fam Med. 2009;7(1):5–10. doi: 10.1370/afm.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos M, Taltavull M, Piñeiro P, et al. [Attitudes of primary health care users to a colorectal cancer screening program]. [In Spanish] Gac Sanit. 2013;27(6):516–520. doi: 10.1016/j.gaceta.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui MRS, Sajid MS, Khatri K, et al. The role of physician reminders in faecal occult blood testing for colorectal cancer screening. Eur J Gen Pract. 2011;17(4):221–228. doi: 10.3109/13814788.2011.601412. [DOI] [PubMed] [Google Scholar]
- 19.Pignone MP, Lewis CL. Using quality improvement techniques to increase colon cancer screening. Am J Med. 2009;122(5):419–420. doi: 10.1016/j.amjmed.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dexheimer JW, Talbot TR, Sanders DL, et al. Prompting clinicians about preventive care measures: a systematic review of randomized controlled trials. J Am Med Inform Assoc. 2008;15(3):311–320. doi: 10.1197/jamia.M2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guiriguet-Capdevila C, Muñoz-Ortiz L, Rivero-Franco I, et al. Can an alert in primary care electronic medical records increase participation in a population-based screening programme for colorectal cancer? COLO-ALERT, a randomised clinical trial. BMC Cancer. 2014;14:232. doi: 10.1186/1471-2407-14-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Domínguez-Berjón F, Borrell C, Cano-Serral G, et al. [Constructing a deprivation index based on census data in large Spanish cities (the MEDEA Project)]. [In Spanish] Gac Sanit. 2008;22(3):179–187. doi: 10.1157/13123961. [DOI] [PubMed] [Google Scholar]
- 23.Averill RF, Goldfield NI, Eisenhandler J, et al. Development and evaluation of clinical risk groups Final Report to the National Institutes of Standards and Technology, US Department of Commerce. http://solutions.3m.com/3MContentRetrievalAPI/BlobServlet?lmd=1225920653000&assetId=%201180606514454&assetType=MMM_Image&blobAttribute=ImageFile (accessed 23 May 2016).
- 24.Royston P. Multiple imputation of missing values. Stata J. 2004;4(3):227–241. [Google Scholar]
- 25.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366(8):697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 26.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64(10):1637–1649. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 27.Senore C, Inadomi J, Segnan N, et al. Optimising colorectal cancer screening acceptance: a review. Gut. 2015;64(7):1158–1177. doi: 10.1136/gutjnl-2014-308081. [DOI] [PubMed] [Google Scholar]
- 28.Von Karsa L, Patnick J, Segnan N. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First edition — executive summary. Endoscopy. 2012;44(Suppl 3):SE1–8. doi: 10.1055/s-0032-1309822. [DOI] [PubMed] [Google Scholar]
- 29.Camilloni L, Ferroni E, Cendales BJ, et al. Methods to increase participation in organised screening programs: a systematic review. BMC Public Health. 2013;13:464. doi: 10.1186/1471-2458-13-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferroni E, Camilloni L, Jimenez B, et al. How to increase uptake in oncologic screening: a systematic review of studies comparing population-based screening programmes and spontaneous access. Prev Med. 2012;55(6):587–596. doi: 10.1016/j.ypmed.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Baron RC, Melillo S, Rimer BK, et al. Intervention to increase recommendation and delivery of screening for breast, cervical, and colorectal cancers by healthcare providers: a systematic review of provider reminders. Am J Prev Med. 2010;38(1):110–117. doi: 10.1016/j.amepre.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 32.Sabatino SA, Lawrence B, Elder R, et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: nine updated systematic reviews for the guide to community preventive services. Am J Prev Med. 2012;43(1):97–118. doi: 10.1016/j.amepre.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Shojania KG, Jennings A, Mayhew A, et al. The effects of on-screen, point of care computer reminders on processes and outcomes of care. Cochrane Database Syst Rev. 2009;8(3):CD001096. doi: 10.1002/14651858.CD001096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tape TG, Campbell JR. Computerized medical records and preventive health care: success depends on many factors. Am J Med. 1993;94(6):619–625. doi: 10.1016/0002-9343(93)90214-a. [DOI] [PubMed] [Google Scholar]
- 35.Sequist TD, Zaslavsky AM, Marshall R, et al. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169(4):364–371. doi: 10.1001/archinternmed.2008.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nease DE, Jr, Ruffin MT, 4th, Klinkman MS, et al. Impact of a generalizable reminder system on colorectal cancer screening in diverse primary care practices: a report from the prompting and reminding at encounters for prevention project. Med Care. 2008;46(9 Suppl 1):S68–73. doi: 10.1097/MLR.0b013e31817c60d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–1327. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]