Abstract
Diabetic kidney disease represents a considerable burden; around one-third of patients with type 2 diabetes develop chronic kidney disease. In health, the kidneys play an important role in the regulation of glucose homeostasis via glucose utilization, gluconeogenesis and glucose reabsorption. In patients with diabetes, renal glucose homeostasis is significantly altered with an increase in both gluconeogenesis and renal tubular reabsorption of glucose. Environmental factors, both metabolic (hyperglycaemia, obesity and dyslipidaemia) and haemodynamic, together with a genetic susceptibility, lead to the activation of pro-oxidative, pro-inflammatory and pro-fibrotic pathways resulting in kidney damage. Hyperfiltration and its haemodynamic-driven insult to the kidney glomeruli is an important player in proteinuria and progression of kidney disease towards end-stage renal failure. Control of glycaemia and blood pressure are the mainstays to prevent kidney damage and slow its progression. There is emerging evidence that some hypoglycaemic agents may have renoprotective effects which are independent of their glucose-lowering effects. Sodium-glucose co-transporter-2 (SGLT-2) inhibitors may exert a renoprotective effect by a number of mechanisms including restoring the tubuloglomerular feedback mechanism and lowering glomerular hyperfiltration, reducing inflammatory and fibrotic markers induced by hyperglycaemia thus limiting renal damage. Simultaneous use of an SGLT-2 inhibitor and blockade of the renin-angiotensin-aldosterone system may be a strategy to slow progression of diabetic nephropathy more than either drug alone. The use of dipeptidyl peptidase-4 inhibitors and glucagon-like peptide 1 receptor agonists may exert a renoprotective effect by reducing inflammation, fibrosis and blood pressure. Given the burden of diabetic kidney disease, any additional renoprotective benefit with hypoglycaemic therapy is to be welcomed. Large randomized controlled trials are currently underway investigating if these new anti-diabetic agents can provide renoprotection in diabetes.
Keywords: diabetic kidney disease, DPP-4 inhibitor, GLP-1 receptor agonists, renoprotection, SGLT-2 inhibitor
INTRODUCTION
Type 2 diabetes is a progressive disease. Typically the development of insulin resistance precedes hyperglycaemia and a diagnosis of diabetes by around a decade. Progressive worsening of hyperglycaemia from mild dysregulation of glucose metabolism (impaired fasting glycaemia and impaired glucose tolerance) to frank hyperglycaemia is paralleled by the development of micro- and macrovascular complications over time [1].
The chronic vascular complications of diabetes result in considerable morbidity and mortality. Patients with diabetes are two- to four-times more likely to develop cardiovascular disease (CVD) than those without diabetes, indeed CVD is the leading cause of premature death and disability in patients with diabetes [2]. Microvascular complications result in a considerable burden too, in almost all high-income countries, diabetes is a leading cause of blindness and renal failure [2].
The aim of treatment is to prevent and/or delay the progression of complications; it has been demonstrated that tight glycaemic control reduces macrovascular and microvascular complications [3–5].
It is becoming increasingly clear that lifetime exposure to glycaemia is important in the development of complications [6]. It appears that long-term exposure to glycaemia (also known as glycaemic legacy or metabolic memory) translates into poor future outcomes. Large-scale studies of tight glycaemic control in patients with established diabetes (8–11.5 years) with sub-optimal glycaemic control (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation, Action to Control Cardiovascular Risk in Diabetes and Veterans Affairs Diabetes Trial-VADT trials), failed to demonstrate the same level of benefit as the UK Prospective Diabetes Study (UKPDS) [3] which enrolled patients with newly diagnosed diabetes [7–9].
The mechanisms of target organ damage resulting from hyperglycaemia are similar in patients with type 1 and 2 diabetes, however, in patients with type 2 diabetes, diagnosis often occurs relatively late in the course of the disease and around one-half of patients present with microvascular complications. Data from UKPDS reveal that 21% of patients presented with retinopathy, 20% with erectile dysfunction and 12% with neuropathy on study enrolment [10]. Around one in five patients (18%) present with nephropathy at diagnosis [11].
It is clear that early diagnosis and appropriate treatment of glycaemia to achieve tight glycaemic control once the diagnosis is made will reduce glycaemic legacy, and the development of complications and their devastating impact on morbidity and mortality. This paper focuses on diabetic nephropathy (DN) and discusses the role of the kidney on glucose metabolism in patients with diabetes, the impact of diabetes on chronic kidney disease (CKD) and the putative additional renoprotective benefit of hypoglycaemic therapy in the prevention of CKD.
THE ROLE OF THE KIDNEY IN DIABETES
Renal glucose homeostasis in normal physiology
In normal physiology, the kidneys play an important role in the regulation of glucose homeostasis via glucose utilization, gluconeogenesis and glucose reabsorption [12].
The kidney utilizes glucose as substrate; ∼5–10% of the glucose released into the circulation after an overnight fast and ∼10–15% in the post-prandial state is used by the kidney.
Renal gluconeogenesis is responsible for ∼50% of glucose released into the circulation after an overnight fast. In the post-prandial state, overall endogenous gluconeogenesis is reduced by ∼60% [12]. In trying to dissect the role of the liver and kidney in the post-prandial state, studies have shown a marked suppression (80%) of hepatic glucose production, while in contrast renal gluconeogenesis increases by 2-fold; it has been postulated that an increase in post-prandial renal gluconeogenesis may drive liver glycogen repletion in parallel to a substantial suppression of hepatic glucose production [13].
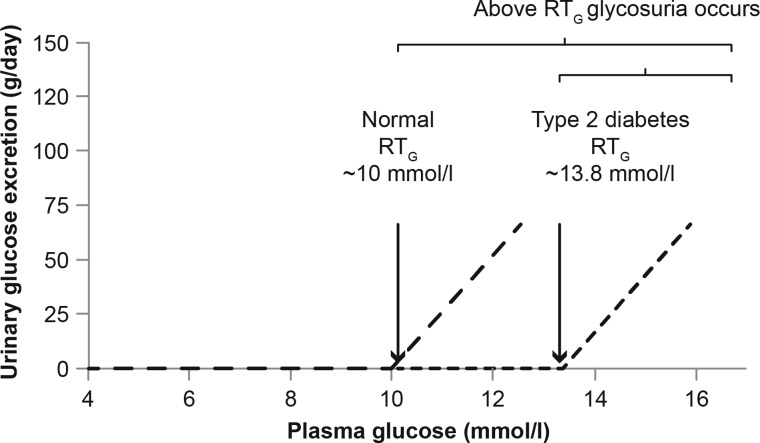
The kidney returns glucose filtered in the pre-urine to the circulation via its re-absorption at the level of the proximal convoluted tubule. Renal glucose reabsorption is the main contributor to glucose homeostasis in the kidney; in normal physiology, around 180 g of glucose is filtered and reabsorbed by the kidneys each day which is considerably more than the 15–55 g produced via gluconeogenesis and the 25–35 g metabolized by the kidney [12]. Reabsorption of glucose occurs in the proximal convoluted tubule and is mediated by two sodium-glucose co-transporters (SGLTs), specifically SGLT-2 (which contributes to 90% of all glucose reabsorbed) and SGLT-1 (10% contribution) [14]. SGLT-mediated glucose transport is an active process [15]. In patients without diabetes, if plasma glucose concentration rises over ∼10 mmol/L the capacity of the SGLT-1 and SGLT-2 transporters is exceeded, the transporters are unable to reabsorb the excess filtered glucose and glycosuria occurs [12].
Renal glucose homeostasis in diabetes
In patients with diabetes, renal glucose homeostasis is significantly altered. There is an increase in gluconeogenesis, in both the fasting and post-prandial state, due in part to insulin resistance and reduced post-prandial release of insulin [16]. In the post prandial state, renal gluconeogenesis accounts for most of the endogenous glucose production [12].
In patients with type 2 diabetes renal tubular reabsorption of glucose is increased [17]. The observed increase in renal tubular glucose reabsorption in patients with diabetes is secondary to an upregulation of the expression of SGLT-2 transporters. Hyperglycaemia and angiotensin II have all been reported to upregulate the expression of SGLT-2 transporters [18, 19]. The increased number of SGLT-2 transporters results in increased glucose reabsorption and the renal threshold (RTG) for glycosuria shifts to the right (Figure 1). However, despite the increased capacity for glucose reabsorption, untreated hyperglycaemia results in plasma glucose which is often above the renal threshold leading to glycosuria.
FIGURE 1:
The renal threshold (RTG) in patients with and without type 2 diabetes.
The kidney also plays an important role in the metabolism of insulin [20]. In health, around 25% of insulin is metabolized by the kidney each day. In patients with diabetes and kidney damage, the half-life of circulating insulin is increased secondary to impairment of the renal clearance of insulin; this is particularly important in patients with co-existing diabetes and CKD receiving exogenous insulin, who have a significantly reduced requirement for insulin and an elevated risk of hypoglycaemia.
THE IMPACT OF DIABETES ON CKD
DN is characterized by persistent albuminuria, declining glomerular filtration rate (GFR), increasing blood pressure and an elevated risk of CVD. DN is associated with increased CV morbidity and mortality and is the main cause of renal failure [21, 22].
Data from UKPDS reveals the natural history of kidney damage from diagnosis of type 2 diabetes [23]. At diagnosis, 92.7% of patients had no evidence of nephropathy, 6.5% had microalbuminuria and 0.7% had macroalbuminuria. The rate of deterioration was ∼2–3% each year, and by 10 years post-diagnosis 24.9% of patients had microalbuminuria. Modelling revealed that 38.3% would have persistent microalbuminura or worse 25 years post-diagnosis.
Diabetic CKD is relatively common in type 2 diabetes, data from a UK-based Primary Care database study revealed that around one-third of patients with type 2 diabetes had impaired kidney function (CKD stage: 3–5 or GFR <60 mL/min/1.732), with most patients (24.8%) having grade 3 CKD (GFR: 30–59 90 mL/min/1.732) [24]. More recent data from the US used the National Health and Nutrition Examination Survey (NHANES) to estimate the prevalence of CKD defined by Kidney Disease Improving Global Outcomes (KDIGO) as eGFR <60 mL/min/1.732 or urinary albumin excretion (UAE) >30 mg/g in patients with type 2 diabetes. Overall, 43.5% of patients had CKD based on eGFR or UAE, rising to 61% in those aged 65 years and above. The prevalence of CKD by eGFR alone (<60 mL/min/1.732) was 22% overall and 43.1% in those aged 65 years and above [25]. Clearly, the increased prevalence in older patients reflects their glycaemic legacy.
Given the anticipated increase in prevalence of diabetes from 382 million world-wide in 2013 to 592 million by 2035, an increase of 55% over 20 years [2], the numbers of patients with CKD are set to increase considerably.
Like all diabetic complications, CKD has a significant impact on all-cause mortality and morbidity. Mortality rates 10 years post-onset of nephropathy in UKPDS 64 were 29.2% in those with microalbuminuria, 34.9% in those with macroalbuminuria and 91.5% in those with elevated plasma creatinine or renal replacement therapy compared with 12.9% in patients with no evidence of nephropathy [23].
CVD is the leading cause of death in patients with diabetes, however, in patients with co-existing diabetes and CKD, CVD and mortality rates are significantly higher than in patients with diabetes alone [26]. Data from a large (>1 million) sample of the US Medicare population aged 65 years or older revealed that rates of congestive heart failure were 18.5 per 100 patient years in patients with diabetes alone versus 52.3 per 100 patient years in patients with diabetes and CKD and rates for atherosclerotic vascular disease overall (first occurrence of acute myocardial infarction, peripheral vascular disease and stroke/transient ischaemic attack) were 25.3 versus 49.1. Overall mortality rates were more than doubled in patients with co-existing diabetes and CKD compared with diabetes alone (8.1 per 100 patient years versus 19.9 per 100 patient years) [26].
Recently the Bergamo Nephrologic Diabetes Complication Trial (BENEDICT) follow-up study has shown that even in what would be termed normoalbuminuric range by current cut-off values, any degree of measurable albuminuria associates continuously with significant CV risk that is lost with early ACE inhibitor therapy in type 2 diabetes [27].
Mortality rates are also significantly higher in patients with diabetes and co-existing hypertension and proteinuria. In one study, standardized mortality ratios were 11-times higher in men and 18-times higher in women with type 1 diabetes and co-existing hypertension and proteinuria compared with the general population. Corresponding figures in type 2 diabetes were five times higher in men and eight times higher in women. Diabetic patients with proteinuria alone had higher standardized mortality ratios than those with hypertension alone [28].
PATHOPHYSIOLOGY OF DIABETIC KIDNEY DISEASE
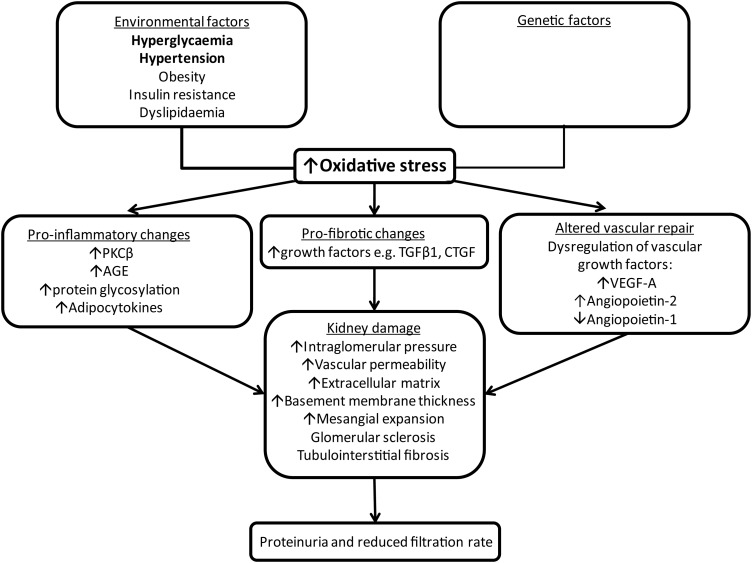
The pathophysiology of diabetic kidney disease is complex and multi-factorial. Environmental factors such as metabolic and haemodynamic perturbations and a genetic susceptibility lead to the activation of pro-oxidative, pro-inflammatory and pro-fibrotic pathways (Figure 2); these lead to increased levels of reactive oxygen species, activation of protein kinase C β, up-regulation of transforming growth factor (TGF)-β1, dysregulation of vascular growth factors (e.g. VEGF-A, angiopoietins), formation of advanced glycation end products (AGE) and adipocytokines [29]. These changes result in deposition of extracellular matrix, thickening of the glomerular basement membrane and mesangial expansion and eventually glomerular sclerosis and tubulointerstitial fibrosis [30, 31].
FIGURE 2:
Pathophysiological mechanisms of kidney damage in diabetes.
Haemodynamic changes lead to hyperfiltration, an early event in the course of type 2 diabetes, probably prior to diagnosis in many cases [32, 33], which is followed by a gradual and progressive decline in GFR.
There is conflicting evidence around the prognostic value of hyperfiltration [34], however, a recent study of 600 patients with type 2 diabetes with normo- and microalbuminura followed up for a median of 4 years revealed that long-term decline in GFR and progression to micro- and macroalbuminuria was more rapid in patients with persistent hyperfiltration at study entry compared with those with normal filtration or hyperfiltration ameliorated by blood pressure and metabolic control [35]. Overall, 90 subjects (15%) had hyperfiltration at baseline, a significant proportion of these individuals (23.4%) progressed to micro- or macroalbuminuria versus only 10.6% of the 502 with normal filtration or ameliorated hyperfiltration.
It is believed that hyperfiltration results in glomerular death which leads to higher filtration rates and eventual loss of remaining glomeruli resulting in a decline in GFR and ultimately end stage renal disease [36].
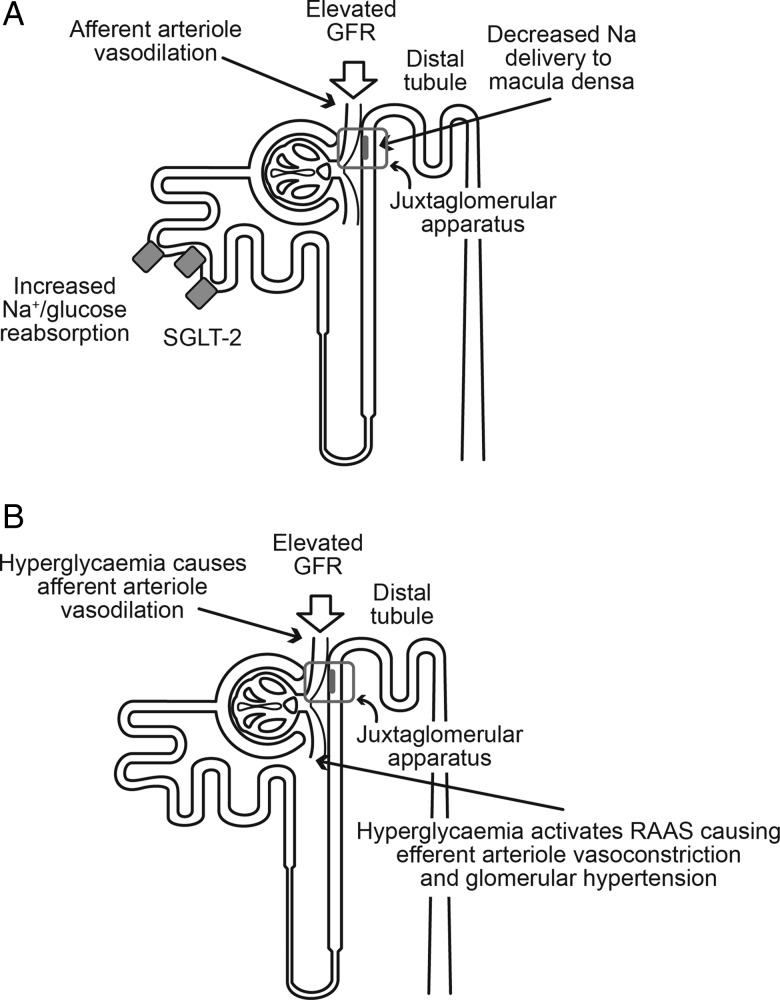
In diabetes, the mechanisms underlying glomerular hyperfiltration encompass glomerular haemodynamic and tubular hypotheses (Figure 3).
FIGURE 3:
Glomerular hyperfiltration of early diabetic renal disease: (A) tubuloglomerular feedback and (B) glomerular haemodynamic hypothesis.
The glomerular haemodynamic hypothesis is believed to occur via hyperglycaemia-mediated dysregulation of the glomerular afferent and efferent arteriolae tone, with a higher reduction in afferent arteriolar tone compared with efferent arteriolar tone mainly driven by a local (glomerular) upregulation of angiotensin II. This results in a steady transmission of systemic pressure to the glomerular capillary leading to severe structural glomerular damage. The mechanisms behind this dysregulation are complex but it is worth remembering that the efferent glomerular arteriole is 10–100 times more sensitive to the vasoconstrictive action of angiotensin II than the afferent one and this may contribute to the imbalance in arteriolar tone and the secondary higher intraglomerular capillary pressure observed in diabetes [37].
The tubular hypothesis proposes that glomerular hyperfiltration is controlled via activation of the tubular reabsorption of sodium and chloride via the tubuloglomerular feedback mechanism. In tubuloglomerular feedback, the macula densa, specialized cells in the distal convoluted tubule next to the glomerulus and the juxtaglomerular cells, sense sodium. In normal physiology, the tubuloglomerular feedback maintains stable glomerular filtration by modulation of the tone of the afferent glomerular arteriole. When the macula densa senses an increase in distal tubular sodium delivery promotes appropriate vasoconstriction of the afferent glomerular arteriolae. On the contrary, a reduction in the amount of sodium in the distal tubuli will be sensed by the macula densa and autoregulatory vasodilation of the afferent glomerular arteriolae will occur.
In diabetes, upregulation of glucose and sodium reabsorption by SGLT-2 in the S1 and S2 segments of the proximal tubule leads to a reduction in sodium levels sensed by the macula densa and consequent vasodilation of the afferent glomerular arteriolae with hyperfiltration [36]. Animal work suggests that the cytokine TGF-β1 may regulate SGLT-2 expression, rather than elevated glucose levels themselves [38].
PREVENTION OF CKD
Given the considerable burden of DN, in terms of morbidity (CVD and renal failure) and mortality, early intervention to slow the development of DN is good clinical practice.
Control of glycaemia and blood pressure
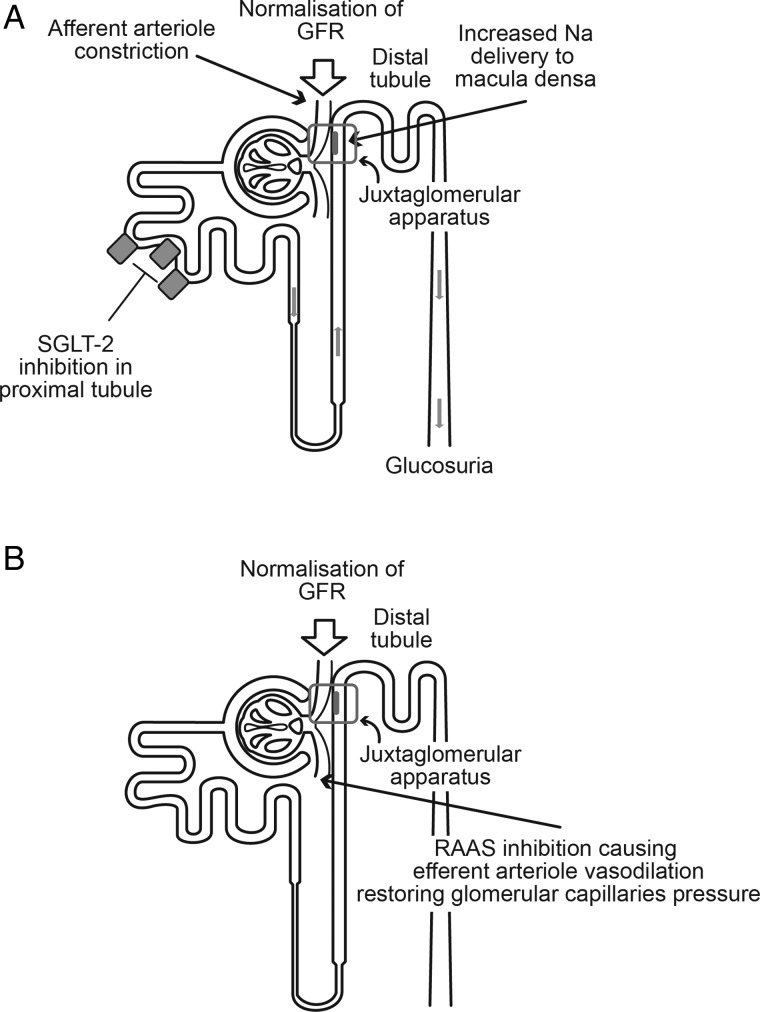
Control of glycaemia and blood pressure are the mainstays to prevent kidney damage and slow its progression [39]. It has been definitively demonstrated that tight control of glycaemia significantly reduces the risk of microalbuminuria and kidney damage in patients with diabetes [3, 4]. In terms of blood pressure control, the use of ACE inhibitors or angiotensin II receptor blockers is recommended [39], due to their effect on the renin-angiotensin-aldosterone system (RAAS) (Figure 4B). Large clinical studies, such as Microvascular Heart Outcomes Prevention Evaluation (Micro-HOPE), Reduction in ENdpoints with the Angiotensin Antagonist Losartan, Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria, Irbesartan Diabetic Nephropathy Trial and BENEDICT have demonstrated that RAAS blockade is renoprotective [40–44].
FIGURE 4:
Glomerular hyperfiltration of early diabetic renal disease: effects of (A) SGLT-2 inhibition and (B) RAAS inhibition.
Potential renoprotective properties of hypoglycaemic agents; an ongoing question
There is emerging evidence and discussion around the potential for some hypoglycaemic agents to have renoprotective effects, independent of their glucose lowering effects (Figure 4A).
SGLT-2 inhibitors
SGLT-2 inhibitors are novel hypoglycaemic agents, which reduce glycaemia using an insulin-independent mechanism. In vitro work in human proximal tubular cells suggests that the SGLT-2 inhibitor, empagliflozin, reduces inflammatory and fibrotic markers induced by hyperglycaemia and may limit glucose-induced damage of the proximal tubule. The specific mode of action is thought to be blockage of glucose entry into the cell [38]. SGLT-2 inhibitors block the reabsorption of glucose and sodium via the SLGT-2 transporter in the proximal tubule, which decreases the capacity for renal glucose reabsorption and reduces the renal threshold at which glucose is excreted [45, 46] resulting in net loss of excess glucose in the urine [47]. In addition to their action in reducing glycaemia, the SGLT-2 inhibitors also result in a modest reduction in blood pressure and body weight. Given their mode of action, the SGLT-2 inhibitors are only effective in patients with effective renal function.
Animal models of diabetes have demonstrated that SGLT-2 inhibition restores tubuloglomerular feedback. A study using dapagliflozin resulted in reduced reabsorbtion of glucose in the proximal tubule leading to an increase in distal delivery of glucose and sodium and a decrease in GFR of ∼15–20% [48]. The impact of SGLT-2 blockade on GFR has also been demonstrated in a study in SGLT-2 knockout mice with streptozotocin-induced diabetes, which revealed a reduction in glycaemia and prevention of glomerular hyperfiltration [49]. A further study by the same group assessed the effect of the SLGT-2 inhibitor empaglifozin in type 1 diabetic Akita mice characterized by an upregulation of kidney SGLT-2. This study revealed an improvement in glycaemia paralleled by a reduction of diabetes-mediated hyperfiltration, together with a reduction in albuminuria, kidney weight and markers of inflammation [50].
A study in 40 patients with type 1 diabetes, 27 with hyperfiltration (GFR ≥135 mL/min/1.73 m2) and 13 without hyperfiltration, revealed that treatment with empagliflozin for 8 weeks reduced eGFR in patients with hyperfiltration by 19% (baseline: 172 ± 23, 8 weeks: 139 ± 25 mL/min/1.73 m2) under euglycaemic clamp conditions and 24% (baseline: 186 ± 33; 8 weeks: 142 ± 29 mL/min/1.73 m2) in hyperglycaemic clamp conditions, P < 0.01 for both [51]. Patients with type 1 diabetes without hyperfiltration were unaffected. Patients in the hyperfiltation group experienced a modest but significant reduction in systolic blood pressure (baseline: 111 ± 10 mmHg; 8 weeks: 108 ± 9 mmHg, P < 0.05). This was accompanied by a significant increase in circulating mediators of the RAAS.
Indeed, blockade of SGLT-2 would favour increased sodium levels presented to the macula densa and secondary autoregulatory vasoconstriction of afferent glomerular arteriolae to counteract the vascular imbalance driven by local angiotensin II characterized by glomerular hypertension as seen in diabetes [36]. It is worth noting that natriuresis paralleled by volume depletion will activate the systemic RAAS; indeed, aldosterone levels are increased in patients with type 1 diabetes following treatment with SGLT-2 inhibitor [51] and net sodium urine excretion is not changed.
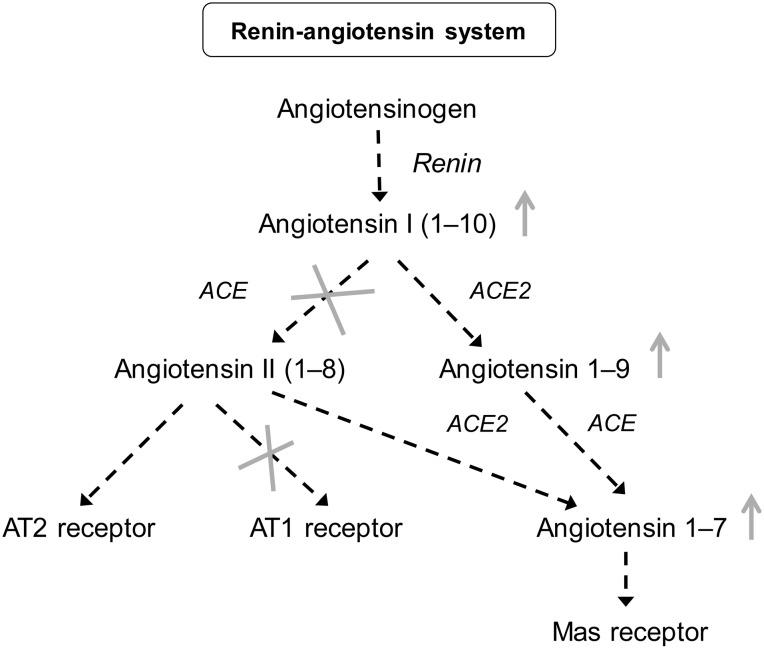
The use of SGLT-2 inhibitors in patients with diabetes normally treated with RAAS inhibitors may confer some therapeutic advantages in the treatment of diabetic kidney disease, potentially by activating the non-classical RAAS cascade [52] (Figure 5).
FIGURE 5:
Rationale for potential benefit of SGLT-2 inhibition on top of treatment with RAAS inhibitors. The use of SGLT-2 inhibitors in patients with diabetes treated with RAAS inhibitors may favour an increase in angiotensin 1-7/1-9. ACE, angiotensin converting enzyme [52].
The RAAS cascade can be separated into two major types of action: the classical and non-classical cascades. The classical cascade results in the production of angiotensin II which binds to the AT1 receptor, leading to vasoconstriction, cell proliferation, inflammation, increased oxidative stress and cell apoptosis. The non-classical pathway, results in the production of angiotensin (1–7) which has a vasodilatory, anti-proliferative, anti-inflammatory and anti-oxidative stress effect [52].
Evidence from an animal study suggests that the use of SGLT-2 inhibitor plus an ACE-inhibitor slows the progression of DN to a greater extent than the use of each agent alone [53], an event likely to be linked to up-regulation of the angiotensin (1–7) pathway.
Dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists
There is also some emerging data for a renoprotective effect with the dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists. The DPP-4 enzyme cleaves polypeptides with a proline/alanine in the penultimate position at the aminoterminal position. DPP-4 inhibitors exert their hypoglycaemic effects by preventing the breakdown of short-lived endogenous incretins, such as GLP-1 and gastric inhibitory polypeptide. The resultant increased incretin levels lead to an increase in insulin and inhibition of glucagon release. Recent studies have proposed a potential renoprotective property of DPP-4 inhibitors in humans and future studies are ongoing for further validation [54]. A study in an experimental animal model of DN revealed that after 4-weeks treatment with linagliptin, diabetic kidney fibrosis was ameliorated [55]. The study used an insulin-deficient model of diabetes and demonstrated an effect on fibrosis with linagliptin which was independent of glycaemic control. Work with cultured endothelial cells revealed that that linagliptin inhibited TGF-β2-induced endothelial-to-mesenchymal transition mediated via microRNA (miRNA) 29 induction. miRNAs are a class of single-stranded endogenous RNAs that regulate gene expression at the post-transcriptional level, miRNA 29s is involved in the regulation of fibrosis.
In an animal model of type 1 diabetes, a GLP-1 receptor agonist was reported to ameliorate renal inflammation, albuminuria and glomerular structural changes, an effect which was reported to be independent of changes in blood pressure, blood glucose and weight loss [56].
GLP-1 receptor agonists suppress the potent inflammatory mediator nuclear factor kappa B and reduce monocyte chemoattractant protein-1 (MCP-1) and cell adhesion molecules such vascular cell adhesion molecule-1, intracellular cell adhesion molecule-1 which have been associated with abnormalities in vascular function as well as progression of diabetic renal disease [57, 58].
In addition, sustained elevated GLP-1 levels as seen with DPP-4 inhibitors or GLP-1 receptor agonists, or post-prandially with SGLT-2 inhibition [59] have been proposed to promote atrial natriuretic peptide (ANP) secretion from atrial cardiomyocytes. ANP induces cyclic guanosine monophosphate-mediated smooth muscle relaxation and natriuresis, leading to a reduction in blood pressure [60, 61].
In animal studies GLP-1 receptor agonists improve cardiac and vascular function through both glucose-dependent and -independent pathways [62, 63].
GLP-1 receptor agonists have also been shown to markedly reduce proximal tubule sodium reabsorption and reduce angiotensin II levels which may represent other potential renoprotective mechanisms [64, 65]. The GLP-1-mediated increase in ANP has not been observed in humans, and more studies will have to be carried out to establish these proposed experimental mechanisms [66].
CONCLUSION
Diabetes and kidney disease commonly co-exist and patients with diabetes and kidney disease have poorer outcomes in terms of mortality and morbidity than those with diabetes alone. It is well documented that tight control of blood pressure and of glycaemia can slow the progression of nephropathy.
Some of the newer oral hypoglycaemic agents appear to have potential renoprotective effects. SGLT-2 inhibitors may exert a renoprotective effect by a number of mechanisms: by lowering glomerular hyperfiltration, limiting hyperglycaemia-induced damage to the proximal tubule, reducing blood pressure and causing weight loss. DPP-4 inhibitors and GLP-1 receptor agonists may exert a renoprotective effect by reducing inflammation, fibrosis and blood pressure and improving cardiac and vascular function.
Haemodynamic changes in the diabetic kidney lead to hyperfiltration which eventually results in kidney damage [36]. It has been shown that patients with type 2 diabetes and hyperfiltration have more rapid progression of kidney disease than those without [35]. Agents which have a positive haemodynamic effect and reduce hyperfiltration may allow us to combat DN earlier in the natural history of the disease.
Studies are underway to investigate the potential cardiovascular or cardio-renal longer-term effects in patients with type-2 diabetes include for SGLT2 agonists CREDENCE (ClinicalTrials.gov Identifier-CTI: NCT02065791), CANVAS-R (CTI: NCT01989754], DECLARE (CTI:NCT01730534), EMPA-REG (CTI: NCT01131676), for DPP-IV agonists CAROLINA (CTI: NCT01243424), TECOS (CTI: NCT00790205), and for GLP-1 receptor agonists ELIXA (CTI: NCT01147250), LEADER (CTI: NCT01179048), EXSCEL (CTI: NCT01144338).
Given the burden of diabetic kidney disease, any additional cardio-renal protective benefits with hypoglycaemic therapy is to be welcomed.
CONFLICT OF INTEREST STATEMENT
Janssen provided an educational grant to King's College London to support the writing of this paper. Janssen had no editorial control over the content. Tricia Dixon of JB Medical Ltd provided medical writing support. This manuscript has not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 2004; 88: 787–835, ix [DOI] [PubMed] [Google Scholar]
- 2.IDF Diabetes Atlas 6E. International Diabetes Foundation. 2013
- 3.Stratton IM, Adler AI, Neil HA et al. . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J 2000; 321: 405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329: 977–986 [DOI] [PubMed] [Google Scholar]
- 5.Gaede P, Vedel P, Larsen N et al. . Multifactorial intervention and in patients with type 2 diabetes. N Engl J Med 2003; 348: 383–393 [DOI] [PubMed] [Google Scholar]
- 6.Bianchi C, Del PS. Metabolic memory and individual treatment aims in type 2 diabetes—outcome-lessons learned from large clinical trials. Rev Diabet Stud 2011; 8: 432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duckworth W, Abraira C, Moritz T et al. . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139 [DOI] [PubMed] [Google Scholar]
- 8.Patel A, MacMahon S, Chalmers J et al. . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572 [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Miller ME, Byington RP et al. . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UKPDS. UK Prospective Diabetes Study 6. Complications in newly diagnosed type 2 diabetic patients and their association with different clinical and biochemical risk factors. Diabetes Res 1990; 13: 1–11 [PubMed] [Google Scholar]
- 11.The Hypertension in Diabetes study group. Hypertension in Diabetes Study (HDS): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens 1993; 11: 309–317 [DOI] [PubMed] [Google Scholar]
- 12.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 2010; 27: 136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer C, Dostou JM, Welle SL et al. . Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab 2002; 282: E419–E427 [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Davidson JA, Del PS. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab 2012; 14: 5–14 [DOI] [PubMed] [Google Scholar]
- 15.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med 2007; 261: 32–43 [DOI] [PubMed] [Google Scholar]
- 16.Meyer C, Woerle HJ, Dostou JM et al. . Abnormal renal, hepatic, and muscle glucose metabolism following glucose ingestion in type 2 diabetes. Am J Physiol Endocrinol Metab 2004; 287: E1049–E1056 [DOI] [PubMed] [Google Scholar]
- 17.Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest 1971; 28: 101–109 [DOI] [PubMed] [Google Scholar]
- 18.Rahmoune H, Thompson PW, Ward JM et al. . Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005; 54: 3427–3434 [DOI] [PubMed] [Google Scholar]
- 19.Bautista R, Manning R, Martinez F et al. . Angiotensin II-dependent increased expression of Na+-glucose cotransporter in hypertension. Am J Physiol Renal Physiol 2004; 286: F127–F133 [DOI] [PubMed] [Google Scholar]
- 20.Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia 1984; 27: 351–357 [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Chertow GM, Fan D et al. . Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 22.Collins AJ, Foley RN, Chavers B et al. . United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012; 59: A7., e1-A7,420 [DOI] [PubMed] [Google Scholar]
- 23.Adler AI, Stevens RJ, Manley SE et al. . Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003; 63: 225–232 [DOI] [PubMed] [Google Scholar]
- 24.Middleton RJ, Foley RN, Hegarty J et al. . The unrecognized prevalence of chronic kidney disease in diabetes. Nephrol Dial Transplant 2006; 21: 88–92 [DOI] [PubMed] [Google Scholar]
- 25.Bailey RA, Wang Y, Zhu V et al. . Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on Kidney Disease: Improving Global Outcomes (KDIGO) staging. BMC Res Notes 2014; 7: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley RN, Murray AM, Li S et al. . Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 2005; 16: 489–495 [DOI] [PubMed] [Google Scholar]
- 27.Ruggenenti P, Porrini E, Motterlini N et al. . Measurable urinary albumin predicts cardiovascular risk among normoalbuminuric patients with type 2 diabetes. J Am Soc Nephrol 2012; 23: 1717–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SL, Head J, Stevens L et al. . Excess mortality and its relation to hypertension and proteinuria in diabetic patients. The World Health Organization multinational study of vascular disease in diabetes. Diabetes Care 1996; 19: 305–312 [DOI] [PubMed] [Google Scholar]
- 29.Gnudi L. Cellular and molecular mechanisms of diabetic glomerulopathy. Nephrol Dial Transplant 2012; 27: 2642–2649 [DOI] [PubMed] [Google Scholar]
- 30.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013; 93: 137–188 [DOI] [PubMed] [Google Scholar]
- 31.De CS, Menzaghi C, Prudente S et al. . Role of insulin resistance in kidney dysfunction: insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant 2013; 28: 29–36 [DOI] [PubMed] [Google Scholar]
- 32.Okada R, Yasuda Y, Tsushita K et al. . Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant 2012; 27: 1821–1825 [DOI] [PubMed] [Google Scholar]
- 33.Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant 2012; 27: 1708–1714 [DOI] [PubMed] [Google Scholar]
- 34.Jerums G, Premaratne E, Panagiotopoulos S et al. . The clinical significance of hyperfiltration in diabetes. Diabetologia 2010; 53: 2093–2104 [DOI] [PubMed] [Google Scholar]
- 35.Ruggenenti P, Porrini EL, Gaspari F et al. . Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care 2012; 35: 2061–2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanton RC. Sodium glucose transport 2 (SGLT2) inhibition decreases glomerular hyperfiltration: is there a role for SGLT2 inhibitors in diabetic kidney disease? Circulation 2014; 129: 542–544 [DOI] [PubMed] [Google Scholar]
- 37.Gnudi L, Thomas SM, Viberti G. Mechanical forces in diabetic kidney disease: a trigger for impaired glucose metabolism. J Am Soc Nephrol 2007; 18: 2226–2232 [DOI] [PubMed] [Google Scholar]
- 38.Panchapakesan U, Pegg K, Gross S et al. . Effects of SGLT2 inhibition in human kidney proximal tubular cells—renoprotection in diabetic nephropathy? PLoS One 2013; 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molitch ME, DeFronzo RA, Franz MJ et al. . Nephropathy in diabetes. Diabetes Care 2004; 27 (Suppl 1): S79–S83 [DOI] [PubMed] [Google Scholar]
- 40. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in peoplewith diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000; 355: 253–259 [PubMed] [Google Scholar]
- 41.Brenner BM, Cooper ME, de Zeeuw D et al. . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869 [DOI] [PubMed] [Google Scholar]
- 42.Parving HH, Lehnert H, Brochner-Mortensen J et al. . The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878 [DOI] [PubMed] [Google Scholar]
- 43.Lewis EJ, Hunsicker LG, Clarke WR et al. . Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860 [DOI] [PubMed] [Google Scholar]
- 44.Remuzzi G, Macia M, Ruggenenti P. Prevention and treatment of diabetic renal disease in type 2 diabetes: the BENEDICT study. J Am Soc Nephrol 2006; 17: S90–S97 [DOI] [PubMed] [Google Scholar]
- 45.DeFronzo RA, Hompesch M, Kasichayanula S et al. . Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 2013; 36: 3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sha S, Devineni D, Ghosh A et al. . Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab 2011; 13: 669–672 [DOI] [PubMed] [Google Scholar]
- 47.Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab 2010; 95: 34–42 [DOI] [PubMed] [Google Scholar]
- 48.Thomson SC, Rieg T, Miracle C et al. . Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 2012; 302: R75–R83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vallon V, Rose M, Gerasimova M et al. . Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol 2013; 304: F156–F167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallon V, Gerasimova M, Rose MA et al. . SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol 2014; 306: F194–F204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cherney DZ, Perkins BA, Soleymanlou N et al. . Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–597 [DOI] [PubMed] [Google Scholar]
- 52.Burrell LM, Johnston CI, Tikellis C et al. . ACE2, a new regulator of the renin-angiotensin system. Trends Endocrinol Metab 2004; 15: 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kojima N, Williams JM, Takahashi T et al. . Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 2013; 345: 464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Groop PH, Cooper ME, Perkovic V et al. . Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 2013; 36: 3460–3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanasaki K, Shi S, Kanasaki M et al. . Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes 2014; 63: 2120–2131 [DOI] [PubMed] [Google Scholar]
- 56.Kodera R, Shikata K, Kataoka HU et al. . Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 2011; 54: 965–978 [DOI] [PubMed] [Google Scholar]
- 57.Chaudhuri A, Ghanim H, Vora M et al. . Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab 2012; 97: 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab 2009; 94: 3171–3182 [DOI] [PubMed] [Google Scholar]
- 59.Polidori D, Sha S, Mudaliar S et al. . Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care 2013; 36: 2154–2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim M, Platt MJ, Shibasaki T et al. . GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 2013; 19: 567–575 [DOI] [PubMed] [Google Scholar]
- 61.Wang B, Zhong J, Lin H et al. . Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab 2013; 15: 737–749 [DOI] [PubMed] [Google Scholar]
- 62.Ban K, Hui S, Drucker DJ et al. . Cardiovascular consequences of drugs used for the treatment of diabetes: potential promise of incretin-based therapies. J Am Soc Hypertens 2009; 3: 245–259 [DOI] [PubMed] [Google Scholar]
- 63.Gallwitz B. Preclinical and clinical data on extraglycemic effects of GLP-1 receptor agonists. Rev Diabet Stud 2009; 6: 247–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skov J. Effects of GLP-1 in the kidney. Rev Endocr Metab Disord 2014; 15: 197–207 [DOI] [PubMed] [Google Scholar]
- 65.Skov J, Dejgaard A, Frokiaer J et al. . Glucagon-like peptide-1 (GLP-1): effect on kidney hemodynamics and renin-angiotensin-aldosterone system in healthy men. J Clin Endocrinol Metab 2013; 98: E664–E671 [DOI] [PubMed] [Google Scholar]
- 66.Lovshin JA, Barnie A, DeAlmeida A et al. . Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic Peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 2015; 38: 132–139 [DOI] [PubMed] [Google Scholar]